Abstract
Vanadium pentoxide (V2O5) and multiwalled carbon nanotubes added V2O5 composite (MWCNT/V2O5) films were prepared by hydrothermal technique. The influence of various levels of MWCNT on the electrochromic properties of MWCNT/V2O5 films was investigated. X-ray diffraction analysis revealed that the films are polycrystalline in nature and belong to orthorhombic crystal system of V2O5. Raman and FTIR spectral analyses confirmed the formation of V2O5 phase and the influence of MWCNT on the vibrational frequencies of V2O5 films. V2O5 film added with 1 wt.% MWCNT showed enhanced transmittance and the bandgap of V2O5 increases with further increase in the addition of MWCNT in V2O5 films. The electrochromic studies revealed electrochromic reversibility of 82% (1 wt.% MWCNT added film) and 82.5% (5 wt.% MWCNT added film) whereas after 100 cycles the films added with 1 wt.% and 5 wt.% of MWCNT acquired excellent electrochromic reversibility of 92% and 88%, respectively. Further the switching kinetics of the film added with 1 wt.% MWCNT is 1.8 s for coloration and 2.4 s for bleaching processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Electrochromic materials find wide range of applications in the large area display, energy efficient glazing and smart windows [1]. Transition metal oxides exhibit color changes and high coloration efficiency because of their electrochromic characteristics that arise from the reversible redox reaction of the transition metal ions. This reaction process involves electron–ion intercalation/deintercalation due to the applied potential [2]. Among the transition metal oxides such as tungsten oxide (WO3), titanium dioxide (TiO2), vanadium pentoxide (V2O5), nickel oxide (NiO) and iridium oxide (IrO2), vanadium pentoxide has attracted great attention in the area of electrochromic applications because of its multiple oxidation states (V2+ to V5+), multi electrochromic behavior and high reversibility [3]. Various deposition techniques such as spray pyrolysis [4], spin coating [5], hydrothermal [6], sputtering [7] and doctor blade [8] methods were employed to deposit V2O5 films. However, V2O5 has its limitation to use it in electrochromic devices and lithium ion (Li+) batteries due to its low electronic conductivity, slow ion diffusion rate and poor cycling stability [9, 10].
The literature survey reveals that various researchers have proposed different approaches for enhancing the electrical conductivity, structural stability, Li+ ions intercalation and deintercalation properties and the electrochemical properties of V2O5. The effect of adding various transition metal ions and carbonaceous materials such as single walled carbon nanotubes (SWCNT), multi walled carbon nanotubes (MWCNT) and graphene possessing unique structure, superior electronic, optical and mechanical properties to modify the properties of V2O5 was investigated [11, 12]. Metallic dopants introduced in V2O5 matrix replace the vanadium atoms and enhance the electrical properties. In general, the metal dopant atom forming an octahedral chain makes the material structure more stable during electrochemical cycling and improves the system’s reversibility [13]. Wei et al. [14] reported that Ti doping with the V2O5 film makes the films amorphous and gives more space for Li+ ions to intercalate and deintercalate which increases the transmittance and improves cyclic switching stability. Panagopoulou et al. [13] reported that 15 at.% Mg doped V2O5 films improved coloration efficiency to 44.5 cm2 C−1 at 750 nm and to 71.3 cm2 C−1 at 520 nm. Li et al. [15] reported that 3D porous structured V2O5 and Fe0.1V2O5.15 thin films deposited by electrostatic spray deposition technique improved the stability and cycling performance of V2O5 due to incorporation of Fe3+ into V2O5 matrix. Zhang et al. [16] reported that V2O5/graphene nanocomposite films prepared by dip-coating technique show multi color electrochromism and improved cycling stability and response time with enhanced optical modulatory range (from 20.6 to 30.8%). Zhi et al. [17] reported that V2O5/reduced graphene oxide (V2O5/rGO) nanocomposite films deposited by a combined process of sol–gel and spin–coating method exhibit better cycling stability, faster switching speed, improved optical modulatory range and high coloration efficiency. Generally, CNTs show unique one-dimensional tubular structure, large surface area, high electrical conductivity and electrochemical stability which make them suitable candidates for energy storage and energy conversion applications [18]. Further, the combination of CNTs and V2O5 shows enhanced electrochemical performances with high discharge rates [19]. Thus the nanocomposites provide favorable diffusion pathways for both the electrons and lithium ions, which are essential in high rate rechargeable lithium-ion batteries and electrochromic devices [19].
The advantage of indulging hydrothermal technique for depositing inorganic material films involves the crystal growth process under the supercritical state of water and also the periodic change in physical and chemical properties of these materials [20]. Thus by altering the growth parameters of hydrothermal technique one can achieve the formation of single phase materials and control effectively their surface morphology and crystalline nature to tune the properties for required applications.
Comprehensive approaches towards modifying the V2O5 morphology by the addition of MWCNT employing hydrothermal technique are proved to be effective in making V2O5 for electrochemical applications. This composite structure of V2O5 can improve the electronic conductivity, the lithium-ion diffusion and cyclic stability. Furthermore, the resulting microstructured V2O5 morphology containing nanorods can be expected to increase the active areas for Li+ diffusion in the composites; thus leading to fast response time for bleaching and coloring, better structural stability and cyclic stability. In the present study, we report a simple and unique approach to grow V2O5 and MWCNT/V2O5 composites via the hydrothermal technique for different concentrations of MWCNT (0, 1, 3 and 5 wt%). Further, we demonstrate that these MWCNT/V2O5 composites have the ability to overcome the limitations of V2O5 and achieving improved electrochemical performance as cathode materials for electrochromic device applications.
2 Experimental section
2.1 Preparation of V2O5 and V2O5/MWCNT composite films by hydrothermal method
The V2O5 and V2O5/MWCNT composite films were prepared by hydrothermal method onto the fluorine doped tin oxide (FTO) glass substrates. V2O5 powder (0.2 g) was taken in a beaker containing 50 ml of double distilled water and stirred well. The process was performed in an oil bath at 80 °C. Then 1.97 g of oxalic acid was added to the above solution and stirred for 1 h to obtain VOC2O4 solution [21]. This solution was added with 500 ml double distilled water taken in a beaker. Then NH4OH was added slowly to this VOC2O4 solution to adjust the solution pH ~ 2. This forms the aqua precursor solution. Precursor solution of 40 ml was utilized from 550 ml stock solution to deposit V2O5 films. To coat the V2O5/MWCNT composite films, MWCNT of 1, 3 and 5 wt.% was added in separate experiments with 40 ml of the aqua precursor stock solution and was ultra-sonicated for 30 min. The FTO glass substrate of 1 cm × 1 cm size was placed into the solution taken in a Teflon lined autoclave. The substrate was supported with a glass microslide such that the conducing side faces upwards and inclined at about 45° angle to the wall of Teflon. The hydrothermal temperature was fixed at ~ 230 °C and the duration of reaction was 4 h. Then the autoclave was naturally cooled to room temperature. The films obtained were greenish in color which indicates the formation of VO2 phase [21]. Further these films were air annealed at 500 °C for 1 h to obtain the V2O5 films. The V2O5/MWCNT films deposited from 0 wt.%, 1 wt.%, 3 wt.% and 5 wt.% MWCNT added precursor solutions are respectively named as films VC0, VC1, VC3 and VC5.
2.2 Characterization studies
An X-ray diffractometer (X’pert PRO Panalytical powder X-ray diffraction system) was used to scan the samples with CuKα radiation (λ = 1.5405 Å) in Bragg–Brentano geometry (θ/2θ coupled) in the 2θ range (20°–90°) to study the structural properties. Field emission scanning electron microscopy (FEI Quanta FEG200) was used to study the surface morphology. UV–Vis–NIR spectrophotometer (Specord-200) was employed to measure the transmittance of the films in the 300–1100 nm wavelength range. The room temperature electrical studies were carried out employing Ecopia HMS-3000 with a magnetic field of 0.57 T (van der Pauw configuration). The electrochromic cell consists of a conventional three electrodes, in which the V2O5/MWCNT composite films deposited onto FTO glass substrates, platinum and Ag/AgCl respectively served as working electrode, counter electrode and the reference electrode. Lithium perchlorate (LiClO4) (1 M) in propylene carbonate (100 ml) was employed as the electrolyte solution. The cyclic voltammetry (CV), chronoamperometry (CA), chronocoulometry (CC) and impedance measurements were carried out using CHI604E electrochemical workstation.
3 Results and discussion
3.1 X-ray diffraction analysis
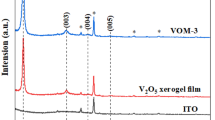
The X-ray diffraction (XRD) pattern of V2O5 film (VC0) and V2O5/MWCNT composites (VC1–VC5) deposited onto FTO glass substrates by hydrothermal technique along with that of the bare FTO glass substrate are presented in Fig. 1a. XRD pattern of polycrystalline V2O5 film and V2O5/MWCNT composite films exhibit orthorhombic crystal system of V2O5. All the films show XRD peaks at 2θ ~ 15.3°, 17.6°, 20.2° and 21.5° due to respectively (200), (002), (001) and (101) planes that match well with the standard data (JCPDS card no. 41-1426). The XRD pattern of MWCNT powder, presented in Fig. 1b, match well with the reported XRD pattern [22]. Figure 1a shows that the peak intensity of (001) plane is reduced by adding 1, 3 and 5 wt.% of MWCNT. This is due to the influence of addition of MWCNT on the crystallinity of the deposited films. With the addition of 3 wt.% of MWCNT the growth of the film along (001) is reduced and favored the growth along (002) plane. Addition of 5 wt.% of MWCNT reveals the saturated chemical interaction between the MWCNT and V2O5. This results in the growth of film along (001) and the peak intensity is relatively increased when compared to 1 and 3 wt.% MWCNT/V2O5 composite films which show similar behavior as that of V2O5 film. The crystallite size (D) was estimated using Scherrer equation [23],
where λ (1.5406 Å) is the X-ray wavelength; β is the full width at half maximum (FWHM) and θ is the Bragg diffraction angle, K = 0.9 is the so-called Scherrer constant. K depends on the crystallite shape and the size distribution indices the diffraction line [24]. The crystallite size (D) of the films VC0, VC1, VC3 and VC5 obtained from (002) plane are 24.0 nm, 21.0 nm, 14.0 nm and 21.0 nm, respectively. The crystallinity decreases with the addition of MWCNT in the matrix of V2O5.
a XRD pattern of the films VC0, VC1, VC3 and VC5 and FTO glass substrate; b XRD pattern of MWCNT powder; c Raman spectra of the films VC0, VC1, VC3 and VC5; d Zoomed peak at ~ 143 cm−1 shows Raman shift; e D and G Raman band peaks of MWCNT observed for the film VC1; and f FTIR spectra of the films VC0, VC1, VC3 and VC5 and the inset represents the shift in the peak at 1000 cm−1 (Color figure online)
Raman spectra of the films VC0, VC1, VC3 and VC5 are shown in Fig. 1c. The peaks observed at 143, 195, 285, 302, 406, 483, 526, 700 and 995 cm−1 confirm the formation of V2O5 [25, 26]. The Raman frequencies of the present work are compared with the reported values in Table 1. The peak at 995 cm−1 corresponds to V5+=O. The peak positioned at 700 cm−1 is due to stretching mode of v(V–O–V)s (doubly coordinated oxygen) which results from corner-shared oxygen common to two pyramids [27]. The peak at 526 cm−1 is due to the stretching mode of v(V–O)s (triply coordinated oxygen) which results from the edge shared oxygen atoms that is common to three pyramids. Peak at 483 cm−1 is attributed to the bending vibration of the doubly coordinated oxygen bridging (V–O–V) and the peak at 298 cm−1 corresponds to triply coordinated oxygen (V–O) bond. The peaks at 406 and 285 cm−1 are representing the bending vibration of the V=O bonds. The prominent peak at 143 cm−1 and a weak peak at 195 cm−1 are attributed to the lattice vibrations of V2O5 [25, 26].
Raman frequencies obtained for the films VC0, VC1, VC3 and VC5 match well with the earlier reports of V2O5 [25, 26]. The influence of addition of MWCNT in V2O5 matrix is seen in the peak shifts and decrement in the Raman peak intensities as shown in Fig. 1c. The peak shift observed at 143 cm−1 (Fig. 1d) represents the disturbance in the layered structures of V2O5 due to the addition of MWCNT. The Raman peak of film VC5 shows shift towards lower values when compared to film VC1 and VC3 which indicates the saturated chemical interaction between V2O5 and MWCNT. The Raman frequencies given in Table 1 show that the incorporation of MWCNT (1, 3 and 5 wt.%) in V2O5 does not effectively influence the magnitude of vibrational frequencies of V2O5. The V2O5/MWCNT composite film (film VC1) shows two characteristics peaks of carbon at 1333 and 1585 cm−1 [28] (also marked in Fig. 1e) as D and G bands for sp3 carbons (defect regions) and sp2 carbons (graphitic regions) respectively [28]. The intensity of the peak at 995 cm−1 of the films varies and no appreciable shift in the frequency is observed (Fig. 1c).
The Fourier transform infrared (FTIR) spectra recorded in the range 500–3800 cm−1 for the V2O5 film and V2O5/MWCNT composite films are displayed in Fig. 1f. Vibrational mode of vanadyl group observed in the FTIR spectra of the films, given in the inset of Fig. 1f, show a slight variation in the values. The bands observed at 1013, 810 and 590 cm−1 are assigned to the V=O stretching, V–O–V bending and V–O stretching modes, respectively [29]. The band at 1414 cm−1 belongs to bending mode of C–H [30] and the bands appearing at 1570 cm−1 and 1654 cm−1 correspond to OH vibrations [29, 31]. The bands observed at 2888 and 2993 cm−1 are attributed to symmetry and asymmetry stretching modes of CH2 respectively which may be originated from the residual organics in the products or from MWCNTs [26]. The peak at 3666 cm−1 belongs to the stretching mode of OH [31]. The interaction between the V2O5 and MWCNTs increases the peak intensity for addition of 1 wt.% MWCNT whereas the peak intensity decreases with further increase in the MWCNT concentration. However 5 wt.% of MWCNT in V2O5 (VC5) shows the peak intensity is nearly equal to that of VC0 film, thus indicating the saturated chemical interactions between V2O5 and MWCNTs [32]. Thus, Raman and FTIR spectral studies coupled with XRD results confirm strong influence of 1 wt.% MWCNT on V2O5 films. Whereas 5 wt.% MWCNT added film (VC5) shows saturated chemical interactions and hence exhibits similar properties as that of film VC0.
3.2 Surface morphology
The SEM images showing the surface morphology of the films VC0, VC1, VC3 and VC5 are given in Fig. 2. The magnified SEM images of the selected part of film VC0, VC1, VC3 and VC5 are shown in Fig. 2a2–d2—(1 μm × 1 μm) respectively. The surface of the V2O5 film (film VC0) reveals the formation of hollow microspheres structures containing nanorods (Fig. 2a1, a2). The surface morphology of the film VC1 reveals that 1 wt.% MWCNT in V2O5 yields relatively less number of microspheres formed by nanorods on the surface. This may be due to the influence of MWCNTs on the V2O5 nucleations (Fig. 2b1, b2). When the concentration of MWCNT is increased to 3 wt.% (Fig. 2c1, c2), the surface morphology of the film VC3 shows formation of intense nanorods and less number of microspheres containing nanorods. The MWCNT (5 wt.%) in the film VC5 modified the shape of morphology structures into microplates (Fig. 2d1, d2) as nearly those shapes formed on the film VC0. The SEM images of the film VC1 (Fig. 2e) and film VC3 (Fig. 2f) (200 nm magnification) evidently exhibit the formation of nanorods, with varying size and density, below the hollow microspheres owing to the addition of MWCNT. Thus the size, shape and morphology of the nanorods and the microspheres of V2O5 formed by nanorods on the films are effectively modified by the various levels of MWCNT concentration added in the precursor solution.
Surface morphology of the films VC0, VC1, VC3 and VC5 with 10 μm × 10 μm magnification are presented in (a1), (b1), (c1) and (d1) respectively. Whereas the magnified surface morphology of the selected part of the film VC0, VC1, VC3 and VC5 are shown in (a2), (b2), (c2) and (d2) with 1 μm × 1 μm magnification respectively. In this figure (e) and (f) represents the SEM images of the film VC1 and film VC3 respectively with 200 nm × 200 nm magnification (Color figure online)
3.3 Optical analysis
The optical transmittance spectra of V2O5 films and V2O5/MWCNT composite films recorded in the 350–1100 nm wavelength range are displayed in Fig. 3a. Table 2 presents the average visible transmittance (AVT) value obtained in the region (500–800 nm) and the wavelength at which maximum transmittance (Tmax.%) occurred for the films VC0, VC1, VC3 and VC5. The AVT and Tmax.% acquired by the film VC1 is relatively high when compared to that of the other films due to formation of the hollow microspheres structures consisting nanorods (Fig. 2e). The shift in the absorption edge may be due to surface modification with varying MWCNT content in the films. The direct bandgap (Eg) of the coated films VC0, VC1, VC3 and VC5 was evaluated using Tauc plot relation [33]. The Eg values vary between 2.34 and 2.49 eV (Fig. 3b). The widening of Eg in films VC1, VC3 and VC5 is due to Burstein–Moss effect. The film VC1 shows increase in the carrier concentration (Table 2) when compare with that of the other films which in turn increases the Eg [34, 35]. Thus the film VC1 exhibits relatively higher Tmax.% due to formation of hollow microspheres containing nanorod structures with respect to other films.
3.4 Electrical transport properties
The Hall measurement setup was employed to study the electrical transport properties of the films. Carrier concentration, mobility and resistivity of the coated films are presented in Table 2. The film VC1 (addition of 1 wt.% MWCNTs in the precursor solution) shows increment in the carrier concentration. The slight increment in the mobility and conductivity observed in the films VC1, VC3 and VC5 when compare with that of film VC0 is due to influence of MWCNT in V2O5. The addition of 1 wt.% of MWCNT in V2O5 (film VC1) shows effective variation in the electrical transport properties. This may be due to the formation of hollow microspheres by the nanorods structures which allow the electrons to flow freely.
3.5 Electrochemical analysis
3.5.1 Cyclic voltammetry
Cyclic voltammetry (CV) measurements were performed to investigate the anodic and cathodic behavior of electrochromic property of V2O5 and V2O5/MWCNT composite films. Figure 4 shows the CVs of films CV0, CV1, CV3 and CV5 measured at 5 mV s−1 sweep rate in a potential range from − 0.5 to 0.95 V (vs Ag/AgCl). When the potential is swept towards − 0.55 V, the intercalation of Li+ and electrons into V2O5 matrix forms lithium vanadate bronze (LixV2O5) and shows color changes from yellow to green to blue. Further when the potential is reversed Li+ and electrons deintercalate at which LixV2O5 is oxidized to original state of V2O5 and changes the color from blue to green to yellow. The Li+ intercalation and deintercalation and the redox reaction between V4+ and V5+ is given by the equation V2O5 + xLi+ + xe− ↔ LixV2O5 [36]. Figure 4 reveals that the anodic and cathodic peak potential is shifted towards more negative and more positive values respectively in the films VC1 and VC3. The film VC5 shows increase in anodic peak potential and decrease in the cathodic peak potential due to saturated chemical interactions between MWCNTs and V2O5.
The diffusion coefficient of Li+ was obtained using well known Randles–Servick’s equation [37]
where ip is the peak current density, n is the number of electrons involved in the redox process, A is the area of the working electrode, Co is the concentration of Li+ and υ is the scan rate. The calculated values of diffusion coefficient for Li+ in V2O5 and V2O5/MWCNT composite films are listed in Table 3.
The electrochemical stability of the films determined by cyclic voltammetry measurements with potential varying between − 0.2 and 0.75 V (for films VC0, VC1 and VC3) and between − 0.5 and 1 V (for film VC5) at a scan rate of 10 mV s−1 from 1 to 100 cycles is shown in Fig. 5. The current density increases from 1st cycle to 100th cycle for the films VC0 and VC5. The films VC1 and VC3 show slight increase in current density without significant changes in the shape of the CVs. The film VC5 shows less stability as has been observed in the film VC0; this may be due to the formation of microplates like structure which hinders the Li+ ions intercalation/deintercalation owing to the capture of Li+ ions inside the compact microplate structures as compared to hollow microsphere structures formed in the films VC1 and VC3.
3.5.2 Chronoamperometry
The switching kinetics of the films VC0, VC1, VC2 and VC3 are important especially for the practical application. Figure 6a shows the switching time for the films VC0, VC1, VC3 and VC5 and the values are compared with the literature values in Table 4. Figure 6b displays the response time observed in the films VC0, VC1 and VC3 corresponding to the change from bleached state to colored states whereas Fig. 6c reveals the response time for the films corresponding to change from colored state to bleaching state. The response time for the film VC5 is shown in Fig. 6d. The film VC1 (1 wt% of MWCNT) exhibits fastest switching time of 1.8 s for the change from colored to bleached state and 2.4 s switching time for the change from bleached state to colored state. Switching time observed for the film VC1 is relatively faster when compared with the switching times of other films in the present work (VC0, VC3 and VC5) and that of the films reported in the literature [13, 17, 38,39,40,41,42,43]. These values are compared in Table 4. The switching times calculated after 100 cycles in the CVs measurements for all the films are presented in Fig. 7a–d and the details are given in Table 4. After 100 cycles of CVs, the coloration and bleaching times for the film VC1 show fastest response when compared to that of the other films. Because of the higher trap of Li+ in the V2O5 matrix, the reaction time for all deposited films after 100 cycles is elevated.
a After 100 cycles of CVs, switching time calculated for the films VC0, VC1, VC3 and VC5, b after 100 cycles of CVs, response time calculated for the films VC0, VC1, VC3 and VC5 to get in colored state, c after 100 cycles of CVs, response time for the films VC0, VC1, VC3 and VC5 to get in bleached state, and d after 100 cycles of CVs, response time for the films VC5 (Color figure online)
3.5.3 Chronocoulometry
Chronocoulometry measurements of the films VC0, VC1, VC3 and VC5 were carried out before and after 100 cycles of CV at the voltage swept from − 0.5 to 1 V with steps of 30 s to calculate the intercalation and deintercalation of Li+. The results are presented in Fig. 8a, b. In the forward condition, the films are colored because of the intercalation of charges due to diffusion process and the reduction of V5+ to V4+. Whereas in the reverse condition the films are bleached because of deintercalating the Li+ due to applying positive potential which changes the oxidation states of vanadium from V4+ to V5+. The electrochromic reversibility of films can be calculated using the ratio of the deintercalated charge (Qdi) to the intercalated charge (Qi) (for coloration/bleaching after 30 s) using the following relation [44]
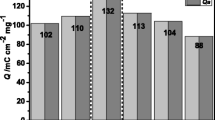
The calculated percentage of electrochromic reversibility for the coated films before and after 100 cycles is listed in Table 5. The electrochromic reversibility obtained before performing 100 cycles for the films VC0 and VC5 shows excellent reversibility of about 95.8% and 97.2% respectively due to good crystallinity as observed from XRD results (Fig. 1a) and dense microspheres morphology of the films. The films VC1 and VC3 respectively show about 82.1% and 82.5% electrochromic reversibility due to relatively less crystalline nature and less number of active microspheres. After 100 cycles of CVs, the estimated electrochromic reversibility decreases from 95.8 to 91% for the film VC0 whereas for film VC5 it decreases from 97.2 to 86% which indicates that some of the Li+ are trapped into microspheres during the diffusion process. Further the films VC1 and VC3 show relatively enhanced electrochromic reversibility from 82.1 to 92% and from 82.5 to 88% respectively due to interaction of MWCNT with V2O5.
3.5.4 Impedance measurements
The charge transfer process in the deposited films is investigated by the electrochemical impedance spectroscopy (Fig. 9). Nyquist plot of the films shows a semicircle and an inclined line respectively in the high frequency region and in low frequency region. The semicircle represents the migration of Li+ between the electrode and electrolyte interface whereas straight line indicates the Li+ diffusion into the bulk V2O5 material [45]. The free diffusion of the Li+ from an electrolyte as well as the electrons from the working electrode into the electrochromic layers results in the linear impedance spectrum that indicates the process of diffusion of Li+ and electrons into and out of the working electrode, i.e. V2O5 films [46]. The steeper slopes observed from low frequency tail for VC0, VC1, and VC3 films represent higher Li+ conductivity in the electrode materials i.e., low value of charge transfer resistance indicates the presence of highly conductive ion migration during the intercalation/deintercalation process when compared to that of the film VC5. The films VC0, VC1 and VC3 exhibit relatively faster switching time when compared to that of film VC5.
4 Conclusions
The V2O5/MWCNT composites films were successfully deposited by the hydrothermal technique on the FTO glass substrates. The peaks shift observed in Raman and FTIR analyses is due to the effect of MWCNT in V2O5/MWCNT composites films. The numbers of microspheres formed on the surface of the film V2O5/MWCNT (1 wt% MWCNT) is reduced and modified due to addition of 1 wt.% and 3 wt.% of MWCNT in the synthesis process. Enhanced transmission is observed for V2O5/MWCNT (1 wt.%) composite film (VC1) due to formation of hollow microspheres structures consisting nanorods and a slight increase in the bandgap value when compare with that of VC0. The switching kinetics of the film VC1 shows fastest coloration and bleaching time of 1.8 s and 2.8 s respectively due to increase in the conductivity and mobility of the film. The films VC1 and VC3 show excellent electrochromic reversibility of 92% and 88% respectively after 100 cycles in CV measurements; thus the improved stability of the films VC1 and VC3 is due to formation of hollow microsphere containing nanorods. The fastest switching kinetics and high reversibility (after 100 cycles) observed in the film VC1 represent its suitability for electrochromic device application.
Data availability
The raw data are available at the corresponding author and can be presented for reasonable requests.
References
G. Cai, J. Wang, P. See Lee, Acc. Chem. Res. 49, 1469 (2016)
P. Jittiarporn, S. Badilescu, M.N. Al Sawafta, L. Sikong, T. Vo-Van, J. Sci. Adv. Mater. Devices 2, 286 (2017)
A. Jin, W. Chen, Q. Zhu, Z. Jian, Electrochim. Acta. 55, 6408 (2010)
H. Khmissi, S.A. Mahmoud, A.A. Akl, Optik 227, 165979 (2021)
V. Balasubramani, J. Chandrasekaran, V. Manikandan, R. Marnadu, P. Vivek, P. Balrajuc, Inorg. Chem. Commun. 119, 108072 (2020)
C. Xiong, A.E. Aliev, B. Gnade, K.J. Balkus Jr., ACS Nano 2, 293 (2008)
M. Panagopoulou, D. Vernardou, E. Koudoumas, D. Tsoukalas, Y.S. Raptis, Electrochim. Acta 321, 134743 (2019)
I. Mjejri, L.M. Manceriu, M. Gaudon, A. Rougier, F. Sediri, Solid State Ion. 292, 8 (2016)
X. Ren, Y. Zhai, L. Zhu, Y. He, A. Li, C. Guo, L. Xu, ACS Appl. Mater. Interfaces 8, 17205 (2016)
L. Chen, X. Gu, X. Jiang, N. Wang, J. Yue, H. Xu, J. Yang, Y. Qian, J. Power Sources 272, 991 (2014)
J.H. Yao, Z.L. Yin, Z.G. Zou, Y.W. Li, RSC Adv. 7, 32327 (2017)
G.P. Pandey, T. Liu, J. Emery Brown, Y. Yang, Y. Li, X. Susan Sun, Y. Fang, J. Li, ACS Appl. Mater. Interfaces 8, 9200 (2016)
M. Panagopoulou, D. Vernardou, E. Koudoumas, N. Katsarakis, D. Tsoukalas, Y.S. Raptis, J. Phys. Chem. C 121, 70 (2017)
Y. Wei, J. Zhou, J. Zheng, C. Xu, Electrochim. Acta 166, 277 (2015)
S.R. Li, S.Y. Ge, Y. Qiao, Y.M. Chen, X.Y. Feng, J.F. Zhu, C.H. Chen, Electrochim. Acta 64, 81 (2012)
X. Zhang, H. Sun, Z. Li, J. Xu, S. Jiang, Q. Zhu, A. Jin, G.S. Zakharova, J. Electrochem. Soc. 160, H587 (2013)
M. Zhi, W. Huang, Q. Shi, B. Peng, K. Ran, J. Electrochem. Soc. 163, H891 (2016)
X.M. Liu, Z.D. Huang, S. Oh, P.C. Ma, P.C.H. Chan, G.K. Vedam, K. Kang, J.K. Kim, J. Power Sources 195, 4290 (2010)
M. Qin, J. Liu, S. Liang, Q. Zhang, X. Li, Y. Liu, M. Lin, J. Solid State Electrochem. 18, 2841 (2014)
T. Adschiri, Y. Hakuta, K. Arai, Ind. Eng. Chem. Res. 39, 4901 (2000)
L. Hongjiang, G. Yanfeng, Z. Jiadong, L. Xinling, C. Zhang, C. Chuanxiang, L. Hongjie, K. Minoru, J. Solid State Chem. 214, 79 (2014)
A. Fallah-Shojaei, K. Tabatabaeian, F. Shirinia, S. Zoha Hejazia, RSC Adv. 4, 9509 (2014)
P. Scherrer, Nachr. Ges. Wiss. Göttingen 26, 98 (1918)
J.I. Langford, A.J.C. Wilson, J. Appl. Cryst. 11, 102 (1978)
W.C. Fang, J. Phys. Chem. C 112, 11552 (2008)
T. Kim, H. Kim, T.S. You, J. Kim, J. Alloys Compd. 727, 522 (2017)
A. Bilal, G.R. Khan, K. Asokan, RSC Adv. 5, 52602 (2015)
H. Zhao, L. Pan, S. Xing, J. Luo, J. Xu, J. Power Sources 222, 21 (2013)
J. Shin, H. Jung, Y. Kim, J. Kim, J. Alloys Compd. 589, 322 (2014)
C. Xiong, E.A. Ali, G. Bruce, J.B. Kenneth, ACS Nano 2, 293 (2008)
W. Chen, L. Qiang Mai, J. Feng Peng, Q. Xu, Q. Yao Zhu, J. Mater. Sci. 39, 2625 (2004)
Q. He, X. Xiangdong, W. Meng, S. Minghui, J. Yadong, Y. Jie, A. Tianhong, Phys. Chem. Chem. Phys. 18, 1422 (2016)
J.I. Pankove, Optical Processes in Semiconductors (Prentice-Hall Inc., Englewood Cliffs, 1971), p.36
E. Burstein, Phys. Rev. 93, 632 (1954)
T.S. Moss, Proc. Phys. Soc. Sect. B 67, 775 (1954)
B. Klaus, T. Gambke, G. Sparschuh, in: R.E. Hummel, K.H. Guenther (Eds.), Optically Active Thin Film Coatings, Handbook of Optical Properties: Thin Films for Optical Coatings, (CRC Press, Boca Raton, 1995), p. 123
C.G. Granqvist, Handbook of Inorganic Electrochromic Materials (Elsevier, Amsterdam, 1995), pp.1–3
E. Eren, G. Yurdabak Karaca, C. Alver, A. Uygun Oksuz, Eur. Polym. J. 84, 345 (2016)
Z. Tong, J. Hao, K. Zhang, J. Zhao, B.L. Suc, Y. Li, J. Mater. Chem. C 2, 3651 (2014)
U. Tritschler, F. Beck, H. Schlaad, H. Cölfen, J. Mater. Chem. C 3, 950 (2015)
K. Che Cheng, F.R. Chen, J. Jung Kai, Sol. Energy Mater. Sol. Cells 90, 1156 (2006)
Z. Tong, H. Yang, L. Na, H. Qu, X. Zhang, J. Zhao, Y. Li, J. Mater. Chem. C 3, 3159 (2015)
W. He, Y. Liu, Z. Wana, C. Jia, RSC Adv. 6, 68997 (2016)
S. Poongodi, P. Suresh Kumar, Y. Masuda, D. Mangalaraj, N. Ponpandian, C. Viswanathana, S. Ramakrishna, RSC Adv. 5, 96416 (2015)
W. Man, H. Lu, L. Ju, F. Zheng, M. Zhang, M. Guo, RSC Adv. 5, 106182 (2015)
K.S. Usha, R. Sivakumar, C. Sanjeeviraja, S. Vasant, V. Ganesan, T.Y. Wange, RSC Adv. 6, 79668 (2016)
Acknowledgements
One of the authors (M.M.M) thanks SRM Institute of Science and Technology, Kattankulathur, for the award of SRM University Fellowship to carry out the research work. The authors thank Prof. D. John Thiruvadigal, Dean of Sciences and Dr. A. Karthigeyan, Head, Department of Physics and Nanotechnology, SRM Institute of Science and Technology for extending the facilities available in the department. The authors also thank DST – SERB – (YSS/2015/000651) for extending the facility of electrochemical workstation. The authors also thank CeNSE, IISc under INUP at IISc which have been sponsored by DeitY, MCIT, Government of India to carry out Raman studies and SEM analysis. The authors thank Dr. B. Neppolian, Professor and Dean Research, Research Institute, SRM Institute of Science and Technology, Kattankulathur for extending UV–Vis–IR Spectrophotometer facility.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
MMM: Conceptualization, Methodology, Formal analysis, Writing- Original draft preparation SM: Conceptualization, formal analysis, Investigation, Writing—Review & Editing KR: Validation, Formal analysis, Writing—Review & Editing, VG: Resources, Writing—Review & Editing, RR: Resources, Writing—Review & Editing KS: Resources, Writing—Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Margoni, M.M., Mathuri, S., Ramamurthi, K. et al. Electrochromic properties of hydrothermally grown microstructured V2O5 and MWCNT/V2O5 composite films. J Mater Sci: Mater Electron 33, 24819–24833 (2022). https://doi.org/10.1007/s10854-022-09193-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-09193-7