Abstract
The European Union’s as well as India’s reduction of hazardous materials (RoHS) directives state that producers of certain categories of electrical and electronic equipments will not be able to offer for sale any product that contains any of hazardous substances: Cd, Pb, Hg, Cr6+, polybrominated biphenyls (PBB), polybrominated diphenyl ethers (PBDE), bis(2-ethylhexyl) phthalate (DEHP), butyl benzyl phthalate (BBP), dibutyl phthalate (DBP) and diisobutyl phthalate (DIBP) beyond the specified limits. Allowable concentration levels in any homogeneous material contained within a product are extremely low: 0.01% for Cd and 0.1% for other substances by weight. These substances when present in quantities in excess of the permissible limits are considered hazardous and damaging to the environment and human health. With the introduction of the RoHS Directive many manufacturing companies in the world are pursuing chemical testing as a means to identify and quantify these hazardous substances. This article presents various testing methods that are currently available to the manufacturing firms who need to generate data to prove that their products are compliant to the RoHS directive. The utility of portable X-ray fluorescence spectrometer (XRF) and also the potential of laser-induced breakdown spectrometer (LIBS) for rapid screening applications is described. For quantitative determination of Pb, Cd, Hg and Cr, the role of instrumental analytical techniques such as atomic absorption spectrometry, XRF, instrumental neutron activation analysis, inductively coupled plasma optical emission spectrometry, the inductively coupled plasma mass spectrometry (ICP-MS), LIBS and the potential of the new analytical technique, microwave plasma atomic emission spectrometry is discussed. Applicability of hyphenated techniques such as HPLC-ICP-MS for Cr6+, GC–MS for the determination of PBB, PBDE and phthalates, and the importance of certified reference materials, challenges and future trends are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the increasing use of electronic devices in today’s world, electronic waste (e-waste) containing huge amounts of hazardous substances is getting accumulated quickly in very vast amounts and polluting landfills and ground water which is an emerging threat to human health and environment. In an effort to protect the mankind and the environment, European Union has brought a new directive into place with effective from 1st July, 2006. This directive requires reduction of hazardous materials (RoHS) to restrict the use of Pb, Cd, Hg, Cr6+ and two halide-containing flame retardants, polybrominated biphenyls (PBB), polybrominated diphenyl ethers (PBDE), and four phthalates, bis(2-ethylhexyl) phthalate (DEHP), butyl benzyl phthalate (BBP), dibutyl phthalate (DBP) and diisobutyl phthalate (DIBP), in electronic and electrical equipments as these substances are dangerous in terms of occupational exposure during manufacturing processes and disposal (Table 1). In 2011 EU has brought out RoHS2, with the intention to phase in various types of electronics under the regulation over the course of several years to come [1]. Both RoHS directives apply to all electrical household equipments, consumer electronics, information technology (IT) and telecommunication equipments, lamps and lights, electronic tools, toys, electronic medical equipments, monitoring and control instruments as well as slot- or vending machines (Table 2). The four phthalates have been added from February, 2015 and they will be restricted from 22 July 2019 for all electrical and electronic equipments (Table 2) apart from Category 8 (medical devices) and Category 9 (monitoring and control equipment) that will have an additional 2 years to comply by 22 July 2021 [2]. Engineers involved in design and manufacturing of these equipments are obliged to implement only RoHS compatible components in assembly process, and producers of these categories of electrical or electronic equipments will not be able to sell any product that contains any of the banned hazardous substances beyond permissible limits in the 25 European Union member nations. The RoHS directive was also enforced and became law in other countries such as US, China and India. India enacted ‘E-waste Rules 2011’ since 1st May, 2012, and further amended 'E-waste Management Rule 2016' since 1st October 2016 to restrict the hazardous substance in the electronics and electrical products. The clause V of the Rule includes RoHS directive. The Rule was equivalent of the EU’s RoHS directive and restricts the same ten substances at the same maximum concentration levels as in the EU. Batteries and radioactive waste are not dealt with under the Rules 2011, as they are already regulated by existing Indian laws [3, 4]. In other words, the RoHS Directive is forcing the world industry to create unique solutions and find out better alternatives to these substances in their products. The regulation also puts forward specific testing, documentation, and verification obligations on both the manufacturers of these goods and on importers and distributors as well. As with the EU RoHS directive, there are over 80 exemptions. These include products for military use or designed to be sent into space, Cd in solar panels, and implantable medical devices, and some of them will automatically expire after 5 or 7 years unless renewed [5]. There are some minor variances between Indian RoHS and the EU RoHS although the list of the hazardous materials and limits for Indian RoHS are the same as EU RoHS. A well compiled comparison of EU and India RoHS positions is presented [6]. To comply with the RoHS requirements, companies must attest that their products do not contain any of the ten identified substances at concentrations greater than that allowed by the directive. If the company chooses to pursue testing to make this identification, the testing must be conducted at the homogeneous material level. RoHS directive can’t be enforced without standard testing methods. The International Electrotechnical Commission (IEC) which is a worldwide organization for standardization (http://www.iec.ch/) has prepared ‘IEC 62321 document’ for this purpose. However, in that document some of the recent developments in the analytical technology are not included. The purpose of this document is to describe various testing methods along with recent advancements that are currently available to the electronic and electrical equipment manufacturing firms to determine the levels of regulated substances, namely, Pb, Hg, Cd, Cr6+, PBB, PBDE and the four phthalates in their products to prove that their products are compliant to the RoHS directive. Figure 1 presents the flow-chart of the analytical techniques that can be used to enforce RoHS regulations. Unfortunately, no single analytical technique exists that can test for the complete range of RoHS substances. Testing for RoHS substances can be divided into destructive and nondestructive methods. There are definite advantages and disadvantages of both types of evaluation methods. Here an attempt is made to present a comprehensive overview on these aspects which includes the latest developments in the analytical instrumentation, advantages and disadvantages of different analytical techniques for both types of evaluations. International traceability and the challenges involved in the implementation of the RoHS regulations are also illustrated and explained with some practical examples.
2 Rapid Screening by Portable Analytical Instruments
At every stage, starting from the raw materials to production of the finished products, it is necessary to carry out the compliance tests. Speed and accuracy are vital for any test utilized for the screening process. Screening tests should be easy to set up, perform and provide rapid results, ideally within a few minutes, to minimize cost and loss of productivity. Portable analytical instruments like XRF, Raman, LIBS provide required speed and precision, which are vital for any test utilized for the screening process. In recent times it has been proved that these portable techniques like XRF, Raman, LIBS are capable of providing highly accurate data very rapidly. For example, portable XRF provided extremely precise and accurate data of several trace elements in different materials when comparisons were made with those of the data of CRMs and data obtained by other established techniques such as ICP-AES and ICP-MS [7,8,9].
2.1 Rapid Screening for Pb, Hg, Cd and Cr
XRF analysis has been a well-established non-destructive analytical technique for elemental analysis for over 60 years and is commonly cited as the best means to quickly screen components. Use of table-top or handheld XRFs can be useful in detecting gross amounts of RoHS substances in different electronic components, such as the presence of bromine in plastics and high Cd or Pb (as high as 30,000 µg/g) levels in plastics. Especially, handheld ED-XRF is used for analysis of Pb, Cd, Hg and Br, routinely for checking various locations on a component or piece of equipment to determine whether that item is in compliance to RoHS regulations or not. This offers the best combination of low detection limits (2–20 µg/g for these four elements) speed, reliability, and versatility compared to any other RoHS screening technique(s). In general, portable instruments are becoming more and more popular because they are available with smart features such as Touch-screen, Wi-Fi and Bluetooth connectivity for fast data downloads, easy to use, light weight, short analysis time, require little or no sample preparation and have sufficient sensitivity and specificity suitable for screening the contaminant of interest at the expected concentration levels. Rapid screening can also support rapid response—once a suite of portable instruments is in place. Laser-induced breakdown spectrometer (LIBS) is also being utilized in place of portable XRF for detecting inorganic contaminants by some laboratories since portable LIBS requires no sample preparation and offers excellent detection limits (Pb, 2 µg/g; Cd, 1 µg/g; Cr, 0.1 µg/g and Hg, 10 µg/g) (Table 3). However, portable XRF is currently more popular for RoHS applications as a rapid screening technique because it’s very old technique and LIBS is only getting popularized now. Since XRF can only be used to measure total Cr and Br content, rather than Cr6+ and Br in certain compounds, those materials which were tested positive for these two elements have to be analyzed further by other appropriate techniques. Materials failing the rapid screening should be sent to laboratory for more precise analysis by techniques such as flame atomic absorption spectrometry (F-AAS), inductively coupled plasma optical emission spectrometry (ICP-OES) and inductively coupled plasma mass spectrometry (ICP-MS). Figure 1 presents a flow-chart of the analytical techniques that are normally used to enforce RoHS regulations.
2.2 Rapid Screening for PBDEs, PBBs and Phthalates
PBDE and PBB are two groups of substances that are used as flame retardants in plastics and textiles. In relation to the PBDE and PBB substance groups, both of which contain bromine, the XRF screening can be used to measure the presence of bromine, but this method cannot be used to determine the type of brominated compound. But a positive XRF screening should be supplemented with a laboratory test such as by using GC–MS to ascertain that bromine is present in the form of PBDE or PBB. Thin-layer chromatography screening method has been very useful for PBDE and PBB [10]. On the other hand, portable FTIR can be used for a very rapid screening of plastic products and components to determining the presence and the levels of phthalates as each phthalate has a distinct FTIR spectrum. Ting et al. [11] have described a GC–MS screening method for phthalates in children’s toys. This method is simple, fast, and effective, with ample sensitivity to quantify the 4 restricted phthalates in children’s toys at 100 µg/g (limit of quantitation = 100 µg/g) which is 10 times lower than the legal allowable level of 1000 µg/g.
3 Analytical Methods for Inorganic Analysis (Cd, Pb, Hg, Cr)
Currently an array of state-of-the-art instrumental analytical techniques are available for the implementation of RoHS directive. Some of them require sample preparation methods such as simple digestion using hot plate or microwave assisted acid digestion, or extraction by organic solvent via Soxhlet apparatus and hot plate-assisted water or alkaline solution sample preparation step. Later on, a measurement technique such as ultraviolet/visible (UV/VIS) spectrometry, F-AAS, graphite furnace AAS (GF-AAS), XRF, ICP-OES, ICP-MS, instrumental neutron activation analysis (INAA), LIBS and microwave plasma atomic emission spectrometry (MP-AES). Table 3 presents comparative detection limits for all the RoHS substances by different analytical techniques considered in this review. Each of these techniques and their suitability for the analysis of heavy metals regulated by RoHS directives will be considered in detail.
3.1 UV–VIS Spectrophotometry
UV/Visible spectrophotometry has become popular because it is relatively cheap and simple. Spectrophotometry uses the intensity of color as a measure of the amount of a material in solution. Spectrophotometry uses light in the visible, ultraviolet, and near infrared ranges and can be used to determine the concentration of several metals although some desired constituents are self-colored, and sometimes it involves the use of ligands which selectively bind to metals to produce colored complexes with a higher molar absorptivity to enable sensitive determination of the metals of interest [12]. Spectrophotometry is equally popular for the determination of Cr6+ in the implementation of RoHS directives [13]. Though spectrophotometry is an established technique for RoHS applications, essentially, it’s a destructive technique and some procedures are lengthy and take longer times [14].
3.2 Atomic Absorption Spectrometry (F-AAS and GF-AAS)
AAS is based on the principle that when a beam of electromagnetic radiation is passed through a substance in a flame, free atoms will absorb light at frequencies or wavelengths characteristic of the element of interest. The amount of absorbance is the measure of concentration of a particular analyte. Graphite furnace-AAS uses pyrolytic graphite coated tube (PGT) in a furnace to vaporize the sample instead of flame. Both flame and graphite furnace atomic absorption spectrometric methods are extremely popular for the determination of Pb, Cd, Hg and Cr in electrical and electronic equipments. F-AAS is a widely accepted analytical technique for the determination of metals at μg/ml level and below in different types of materials [15,16,17]. GF-AAS is a technique which offers better sensitivity and involves injection of a small amount of solution to be analyzed into a small graphite tube [18] and thus is suitable for the analysis of metals at ultra-trace levels (ng/ml) particularly useful when only a very small amount of sample is available for testing. Direct solid sampling method was proposed for GF-AAS with calibration against aqueous standards. The limits of detection of 0.1 µg/g for Cd and 0.6 µg/g for Pb are more than adequate for the purpose [19]. Recently the most advanced high-resolution continuum source GF-AAS was used for the determination of Cr and Sb in polymers from electrical and electronic equipment [20]. Cold vapor-AAS is especially valuable for measuring low amounts of mercury by absorption at 254 nm with a detection limit of 9 ng/ml [21] and this method was successfully used for the precise determination of mercury in plastic certified reference materials (CRMs) [22].
3.3 X-ray Fluorescence Spectrometry (Both WD-XRF and ED-XRF)
XRF is an analytical technique that uses the interaction of X-rays with a material to determine its elemental composition. XRF is suitable for solids, liquids and powders, and in most circumstances, is non-destructive. A sample specimen is excited by a primary X-ray source, electrons from the inner electron shells to get knocked-off and electrons from outer electron shells drop-into fill the resultant voids emitting a fluorescence radiation characteristic in its energy distribution for a particular element and this fluorescence radiation is counted/measured by the detector. In recent times, XRF analysis has become increasingly attractive and popular when compared to other techniques, especially due to the ease of sample preparation and on several occasions, it doesn’t need any sample preparation. It can be classified into wavelength-dispersive X-ray fluorescence (WD-XRF) and energy-dispersive X-ray fluorescence (ED-XRF) according to the methods of excitation, dispersion, and detection [23, 24]. Both forms, namely, WD-XRF and ED-XRF techniques have been successfully applied for the implementation of RoHS regulations. Since it is a non-contact analysis, problems such as memory effects and contamination errors commonly experienced in solution analysis, are not encountered. As it is a non-destructive technique, it is possible to reuse the sample after measurements. Though XRF is an elemental analysis tool and as such it is possible to obtain values for the heavy metals (Pb, Cd, Hg, and Cr), but not specifically for Cr6+ as XRF cannot differentiate between oxidation states of a particular metal. It can’t also detect the restricted PBB, PBDE and phthalates and can only provide a total value for bromine in brominated flame retardants. The usefulness of ED-XRF is depicted in Table 4 where the results for RoHS substances in individual parts of a commercial mobile phone are presented. All components of the particular commercial mobile phone tested failed to comply the RoHS limits except back cover. This shows the gravity of the situation in the country.
3.4 Instrumental Neutron Activation Analysis (INAA)
INAA is a method for the determination of many elements at low levels in a wide variety of materials [25]. A small sample of 5–100 mg is subjected to a neutron flux in a nuclear reactor. The stable nuclei absorb neutrons and become unstable radioactive nuclides and the resultant radioactive nuclides decay with emission of particles or, more importantly gamma rays, which are characteristic of the elements from which they were emitted. The energy of the emitted gamma rays is used to identify the nuclide and the intensity of the radiation can be used to determine its abundance levels. Semiconductor radiation detectors are normally used for quantitative measurement. During the last 60 years, this analytical technique has been extensively utilized for the determination of trace and minor elements in many types of geological and environmental materials [26, 27]. The advantages include, (1) the method is non-destructive, hence the same sample can be used for other measurements (However, once irradiated the sample comes under radioactive sample category and hence not possible often!); this feature is extremely important as some of the RoHS regulated samples such as plastics can yield hazardous chemicals up on decomposition, (2) sample size can be very small, often as little as a milligram; (3) detection limits for many elements are in the ng/g range; (4) no sample preparation is required; and (5) over 40 elements including Pb, Cd, Cr, Hg and Br can be measured simultaneously. INAA was utilized for measuring Cd, Cr and Br in polypropylene samples for the development of polypropylene certified reference materials (CRMs) of these metals in polypropylene [28, 29]. Despite the above advantages, INAA is certainly not a popular analytical technique as it is time-consuming, not independent, requires a reactor nearby and involves longer cooling times for certain elements. Hence, the application of INAA for RoHS purpose is limited, particularly in a country like India where there is only one nuclear reactor for such purpose.
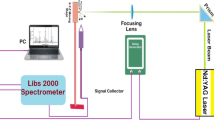
3.5 Laser Induced Breakdown Spectroscopy (LIBS)
LIBS technique is another important development in this direction which gives improved detection limits for light elements such as Mg, Al and Si compared to XRF and it is a well-suited elemental technique for RoHS compliance testing. LIBS is a type of atomic emission spectroscopy which uses a highly energetic laser pulse as the excitation source. LIBS can analyze any matter regardless of its physical state, be it solid, liquid or gas. Because all elements emit light of characteristic frequencies when excited to sufficiently high temperatures. The intensity of the emitted light at a particular wavelength is the measure of concentration of a particular element and this technique can (in principle) detect all elements. LIBS technique was used for the analysis of Pb in lead-frames and the data obtained correlated very well with data obtained from ICP-OES [30]. Handheld LIBS analyzers are also available making this technique more attractive particularly to RoHS applications as measurements on materials can be made wherever needed, without the need to take samples to a lab for lengthy preparation and analysis. In fact, all RoHS substances—Pb, Hg, Cd and Cr can be determined with LIBS. Since limits of these elements normally encountered in different materials are very high, compared to the detection limits of the instrument, LIBS technique is a perfect means for electrical and electronics manufacturers to monitor and demonstrate their product’s compliance [31].
3.6 Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
The development of ICP-OES [32, 33] has provided sensitive multi-element analytical technique to detect several elements including those related to RoHS directive. A liquid sample is converted to an aerosol and transported to the high temperature ICP. In the plasma the sample undergoes desolvation, vaporization, atomization and ionization. The atoms and ions then absorb energy from the plasma which causes electrons within them to move from one energy level to another. When the electrons fall back to ground state, light of wavelengths specific to each element are emitted. The measured emission intensities are then compared to the intensities of standard samples of known concentrations to obtain the respective elemental concentrations in an unknown sample. The technique can simultaneously measure up to 60 elements with high sensitivity and wide linear dynamic range [34]. ICP-OES technique is well suited for quantitative determination of Co, Cr and Pb in polymers using a high pressure asher digestion with detection limits in the range of 0.3–3.5 ng/g for all these elements [35]. Axial mode ICP-OES was used for the determination of full suite of RoHS elements including mercury in raw plastic matrices. Table 5 presents ICP-OES results obtained on the concentrations (µg/g) of different hazardous substances obtained in different parts of compact fluorescent tube lights (CFL) of various commercial brands available in the Indian market currently. Pass/Fail is indicated in the last column which indicates that only about 28% of the tested CFLs that are complaint to RoHS directive. These results suggest that vast majority of the CFLs sold in the Indian market do not comply to the RoHS regulation.
3.7 Inductively Coupled Plasma Mass Spectrometry (ICP-MS, LA-ICP-MS, HR-ICP-MS and HPLC-ICP-MS)
Since the first linking of an ICP ion source with a quadrupole mass spectrometer in 1980, development of commercial instruments in 1983, further advances such as the development of high resolution-ICP-MS (HR-ICP-MS) and ICP time of flight mass spectrometry (ICP-TOF–MS), during the last three decades have brought this technique to a point where it can deliver detection limits of one part in 1015 for a majority of elements in the periodic table [36, 37]. The sample is ionized in a high temperature ICP, the positively charged ions are then separated using a mass spectrometer (quadrupole, time of flight or a magnetic sector) and quantified by an electron multiplier detector [38]. HR-ICP-MS technique can resolve several interferences very easily for an accurate quantitative determination of elements such as Cr and several other elements without the need of any collision or reaction cell mechanisms [39]. Currently, ICP-MS is probably the second most popular technique after XRF, for RoHS testing. ICP-MS with collision–reaction interface technology has been successfully used to determine Cd, Cr, Hg and Pb in plastics from waste electrical and electronic equipments [40]. Some workers [41] determined the ultra-trace amounts (ng/g) of mercury in steel by ICP-MS after ion-exchange chromatographic separation.
Laser Ablation-ICP-MS (LA-ICP-MS) is yet another potential analytical tool for the analysis of materials for hazardous substances (Pb, Cd, Cr and Hg). Sample is ablated by a series of pulsed lasers and the material is transported to ICP for subsequent analysis. The laser beam diameter can be accurately set by the instrument software to produce variable “spot” sizes from < 5 to 300 μm depending on the application. Though this equipment is relatively expensive, it will be very valuable for the testing of electro-ceramics which are becoming smaller and more heavily layered day by day. A set of new polymeric candidate reference materials were prepared and characterized by XRF as well as LA-ICP-MS for Br, Pb, Cd, Cr and Hg with detection limits in the range of 2.3–26.8 µg/g to establish sufficient macroscopic and microscopic homogeneities of the candidate reference materials [42]. Though HR-ICP-MS technique is the most sensitive and accurate analytical technique, probably its enormous capital and running cost is preventing it’s use in RoHS applications. In a related study, total chlorine and bromine in solid wastes were determined by sintering and HR-ICP-MS [43]. Coupling ICP-MS with different available chromatography technique like high performance liquid chromatography (HPLC), gives answers to many industrial and regulatory issues in terms of speciation analysis. For example, ICP-MS can be coupled to chromatographic techniques like HPLC, gas chromatography (GC) and capillary electrophoresis for the most sensitive, accurate and selective determination of Cr6+ in corrosion protection coatings after alkaline extraction for a routine laboratory use [44].
3.8 Microwave Plasma Atomic Emission Spectrometry (MP-AES)
In 2011, a new commercial instrument representing yet another analytical technique called the MP-AES was introduced. The principle of this technique is similar to any other emission technique such as flame emission or the well-known ICP-AES. Microwave plasma which is generated from the atmospheric nitrogen using a nitrogen generator, is used here for sample decomposition and excitation of atoms/ions. The intensity of each emitted line will be directly proportional to the concentration of a particular element of interest at a specified wavelength, and computation of the concentration can be done by comparing it with that of a known concentration. The utility of MP-AES has been demonstrated in recent times using several different geological and environmental matrices, including industrial effluents, water, sediments, soils, rocks and ores [45,46,47,48], as well as ethanol and gasoline [49]. MP-AES is a potential analytical technique for the analysis of Hg, Pb, Cd and Cr in electronics and plastics with number of obvious advantages over other techniques such as F-AAS and ICP-OES.
4 Methods for the Determination of Hg
In cold vapor-AAS (CV-AAS) method, mercury in solution (Hg2+) is reduced to metallic mercury vapor and the ground state atoms (Hg0) are then transported to an optical cell and detector for absorption measurement. By comparing the amount of absorption of an unknown sample with that of a standard, mercury concentration in an unknown sample can be computed. Several methods, including CV-AAS [21] and cold vapor atomic fluorescence spectroscopy (CV-AFS) and direct analysis by thermal decomposition [50] are used to detect and determine mercury levels in solids, liquids, or gases for environmental and safety reasons. Most modern CV-AAS and CV-AFS instruments are more sensitive and provide 2 and 0.2 pg/g detection limits respectively for mercury [50]. On the other hand, the direct analysis by thermal decomposition yields a detection limit of 0.005 ng/g for mercury. Analysis by ICP-MS requires addition of small amount of gold to the sample to expedite the baseline recovery. However, the application of ICP-OES technique for mercury determination can be problematic at trace concentration levels due to poor sensitivity. Since the threshold limit of Hg is very high in RoHS applications, even ICP-OES can be applied. CV-AAS method is probably the best method for the accurate determination of Hg in RoHS applications [21]. For mercury determination, microwave digestion proved to be satisfactory as increased temperature and pressure ensure that resistant components are solubilized and the closed system retains the mercury in solution [51].
5 Methods for the Determination of Cr6+
Chromium species may exist in several oxidation states, but the Cr6+ and Cr3+ are the most abundant in the environment. However, the toxicity and bioavailability of chromium depends on its chemical form (species) and concentration. Cr3+ is insoluble, immobile, a micronutrient, essential to human health. On the other hand, Cr6+ is considered toxic and carcinogenic [52]. Reliable determination of Cr3+ in the presence of Cr6+ and vice versa are complicated by the aqueous chemistry of each oxidation state, as Cr3+ exists primarily as a cation in solution, and Cr6+ exists primarily as an anion, although these states are dependent on solution pH. By the hyphenation of a chromatographic technique like HPLC with a powerful element detection technique like ICP-MS can take the detection limit of Cr6+ to sub-ng/ml level. Ion chromatograph (IC) and IC-ICP-MS are also used for Cr6+ analyses. Alkaline extraction coupled with HPLC-ICP-MS is the most sensitive, accurate and selective method for the determination of Cr6+ in corrosion protection coatings for a routine laboratory use. A novel method to determine Cr6+ was developed by the combination of ED-XRF, spot test, alkali digestion and UV–vis spectrophotometric analysis. First, the presence of Cr will be established by ED-XRF screening, then the valency state of Cr will be established using a spot test, then Cr6+ will be extracted and separated without an undesired Cr6+–Cr3+ interconversions because the mixed alkali method could effectively extract Cr6+ from complicated plastic matrices and eliminate the interference of Cr3+, simultaneously [53]. Cr6+ will be complexed with a color reagent 1,5-diphenylcarbazide (DPC), the solution finally quantitatively determined by a UV–vis spectrophotometer at a wavelength of 540 nm [54]. An organic-assisted alkaline extraction method was found to be very effective determination of Cr6+ in plastics by spectrophotometry [55].
6 Test Methods for Brominated Flame Retardants and Phthalates
Organic polymers like plastics and rubber are used in many consumer electronic products, which are indispensable to our comfort in life. Flame retardants, PBDEs and PBBs are added as additives in plastics of electrical and electronic equipments to improve fire retardancy properties [56]. The impact of brominated fire retardants on the environment and their potential risk on human health is an actual concern. When burnt, e.g., as content of the polymer input of incineration plants, halogenated fire retardants can form halogenated dibenzo-dioxins and-furans, which are highly toxic and potential carcinogens. In addition, fire retardants can accumulate in the environment and thus, in the food chain [57, 58]. Moreover, PBB, PBDE were proven to be physiologically harmful when assimilated [59]. Several methods are available for the determination of PBDEs and PBBs in plastics, considering the trace content and the complexity of the samples. Reversed phase high performance liquid chromatography (RP-HPLC) supported by ultrasonic solvent extraction, provides good opportunities for the necessary analytical separation. In addition, low limits of detection can also be reached by ultraviolet (UV) detection [60]. The specific phthalates are substances mainly used as plasticizers for softening and giving adequate plasticity to stiff and brittle organic polymeric materials like poly (vinyl chloride) (PVC) products and also use them in numerous industrial and consumer products [58, 61]. For screening and quantitative determination of phthalates, typically 1–5 g of the material is extracted with a solvent [62]. The extracted solvent is then analyzed for the presence of phthalates by GC/MS or flame ionization detector (GC/FID). These methods which are used to separate complex mixtures of polymer additives also provide identification of the individual phthalates and their concentrations. If a RoHS listed phthalate is found to be present in the extract, then its concentration is determined by using a standard calibration curve for a given phthalate [63]. Following are the instrumental methods which are most frequently utilized for the accurate determination of PBDE, PBB and phthalates in different materials during RoHS compliance operations.
6.1 Gas Chromatography Combined with Mass Spectrometry (GC–MS)
GC works on the principle based on the separation of components of sample under the flow of gas as a mobile phase (such as helium) over a liquid stationary phase [64]. As the separated substances emerge from the column opening, they flow into the MS which identifies compounds by the mass of the analyte molecule. For the determination of PBDE and PBB, gas and liquid chromatographic techniques were predominantly applied [59]. Several of these methods include extraction and preparation procedures applicable to actual requirements [65]. GC–MS was utilized in the determination of several RoHS related brominated fire retardants (BFRs) [66]. The first step is to extract the substances from the plastic which require solvents such as acetone, tetrahydrofuran, toluene, hexane, methylene chloride, chloroform and methanol. After grinding the polymer material to 1000 μm maximum size, Soxhlet extraction is carried out with a suitable solvent and analyzed on GC–MS. Soxhlet extraction is, until now, the most applied sample preparation method for the GC–MS measurement of extractable substances from polymers. Its advantages are technical maturity, reproducibility, and good coverage of high boiling substances [67]. This method, however, takes a longer time (about 50 h) and is destructive. Shao et al. [68] presented a review of developments on the analysis of PBDE and PBB in plastics. There is an ever-increasing demand for new analytical methods suitable for monitoring different phthalates in various environmental, biological, and other matrices. Qureshi et al. [69] outlines possibilities and limitations of various analytical methods for the precise determination of several phthalates including the present four.
6.2 Fourier-Transform Infrared Spectroscopy (FT-IR)
Infrared spectroscopy is an important technique in organic chemistry. It is an easy way to identify the presence of certain functional groups in a molecule. FTIR offers a number of advantages over conventional infrared systems, including sensitivity, speed and improved data processing. FT-IR is a very fast and easy method for the qualitative identification and accurate determination of brominated flame retardants in polymers. Since the lowest limit of detection is only a few per cent, this method is not suitable for analyzing at µg/g levels. Hence, it can be more suitable for scanning applications. Portable FTIR can be used for a very rapid screening of plastic products and components to determining the presence and the levels of phthalate plasticizers in plastics as each phthalate has a distinct FTIR spectrum. The calibrated method can be used for quantification.
6.3 Raman Spectroscopy
Raman spectroscopy is a technique used to observe vibrational, rotational, and other low-frequency modes in a system. This technique is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified. It can distinguish the differences between analogous compounds and offers several advantages for microscopic analysis [70]. Since it is a scattering technique, specimens need not be fixed or sectioned. Raman spectra can be collected from a very small volume (< 1 µm in diameter) and these spectra allow the identification of species present in that volume. In the case of PBDE in matrix polymers, the IR bands of PBDE overlap those of matrix polymers. Therefore, Raman spectroscopy (both table-top and hand-held instruments) has often been used for quantitative analyses of bromine in matrix polymers [71]. Kikuchi et al. [72] have carried out non-destructive rapid analysis of brominated flame retardants in electrical and electronic equipment using Raman Spectroscopy with a detection limit of about 100 µg/g for PBDE with analysis time of only 1 min. compared to a couple of days for the usual method of solvent extraction and GC–MS. Kolarik et al. [61] used Raman Spectroscopy to determine the concentration of different phthalates in household dust samples collected from the children’s bedrooms from 177 Bulgarian homes and analyzed for the content of DEHP, BBP, DBP and DIBP in addition to other phthalates, and found highest concentration of DEHP, in order to understand the building characteristic and cleaning habits in the family.
7 Analytical Methodology
In order to decide on the course of analytical procedure to be adopted during RoHS compliance testing, the analytical instruments available must be considered first. After the initial screening of the substances to be analyzed, the choice of analytical technique and the decomposition methods have to be finalized basing on the information obtained from screening (Fig. 1).
7.1 Sampling and Sample Homogeneity
In fact, the RoHS directive places restrictions on concentrations of specific substances as measured only at the homogeneous material level. This means that the sample material collected must be a representative one as non-representative samples will not yield a valid interpretation no matter how good the subsequent chemical analysis is. The IEC 62321 protocol contains sampling guidelines for different materials including metals, polymers and electronic components which require analysis for RoHS hazardous substances. The term “homogeneous” means that the limits do not apply to the total weight of the final product, or even to an individual item in a product, but to any single component that could be mechanically separated out. So, for example, in a printed circuit board, this would refer to the plastic substrate material, the metallic connecting wire, the plastic sheath covering a cable, or any other material that is used in the manufacturing process of the board. Examples of homogeneous materials are individual types of plastic, ceramics, glass, alloys, paper, board, resins and coatings. A plastic cover is a “homogeneous material” and an electric cable that consists of metal wires surrounded by non-metallic insulation materials is an example of a “non-homogeneous material”. Some of the samples tested here in this investigation (Table 6) were clearly homogeneous, such as the back cover and sim slot. Some were not homogeneous, such as the circuit board and ribbon cable. Some materials are plated or coated and yet times, it will be extremely difficult to take a representative sample for testing. In instances where further mechanical disjointing will not yield a homogeneous material, a determination must be made to test the composite material. It is therefore extremely important to understand these sampling–heterogeneity issues. In general, the CRM used in the analysis will be homogenous in terms of their elemental composition. However, this may not always be the case with the samples and hence, it is recommended to analyze multiple sample chips from any given sample in order to establish homogeneity. It must be clearly understood that the RoHS regulation applies to not only to each component but also to the product as whole. For example, the concentrations (µg/g) of different hazardous substances obtained by ICP-OES in different parts of compact fluorescent tube lights (CFL) of various commercial brands available in Indian market currently are presented in Table 5. For Brand-1 out of the four components tested, though mercury pill met the RoHS limit for Hg, high concentrations which are more than the permitted concentration are present in other components, namely, solder pin, PCB solder and Vitritte. Thus, the CFL of this brand failed to meet RoHS specifications and hence the product doesn’t comply with RoHS regulations.
7.2 Sample Dissolution
The electrical and electronic parts should first be ground up to speed up the dissolution process. A typical problem that can occur during grinding is the smearing and adherence of sample material to the walls of the grinding vessel. If one component of the sample preferentially sticks to the vessel walls, this could lead to concentration changes in the sample. In the case of plastics, it may be necessary to use a cryogenic grinding method to turn the soft plastic into powder. It is well-accepted that cryogenic grinding of chilled materials at liquid nitrogen temperatures approaching − 200 °C, has two important benefits for sample preparation. First, it makes flexible samples extremely brittle so they can be pulverized by impact milling relatively quickly, and second, it preserves structural and compositional aspects usually damaged or lost during room temperature pulverizing [73]. Another question is whether the grinding device itself is contributing metal or abrasive particles to the sample. In the case of passive components, the ceramics in the components can be quite abrasive to grinding equipment causing extraction of vessel wall material into the sample. After grinding, the sample powder must be dissolved in a suitable solvent for analysis.
Sample preparation methods typically consist of a digestion (including hot plate or microwave assisted acid digestion), or extraction (including organic solvent via Soxhlet apparatus, hot plate assisted water or alkaline solution) and fusion methods. Since there are a wide variety of sample types which fall under the RoHS directive, there is no single sample preparation method to suite to all type of samples. However, microwave digestion provides the most flexibility and highest probability of complete digestion which is satisfactory for the wide range of materials [74]. Microwave digestions are simple and very effective and can be completed within minutes. Other advantages include yielding of low blank levels, use of relatively small amounts of acids and other reagents apart from allowing fast dissolutions [56, 74]. A set of data exclusively obtained on the samples dissolved using microwave digestions is presented in Table 6 for information.
Microwave-assisted solvent extraction is a procedure using microwave energy to produce elevated temperature and pressure conditions (i.e., 100–115 °C and 50–175 psi) in a closed vessel, containing the sample and organic solvent(s) to achieve analyte recoveries equivalent to those from Soxhlet extraction. Microwave-assisted extraction was utilized for the qualitative and quantitative determination of brominated flame retardants in styrenic plastic fractions from waste electrical and electronic equipment [75].
7.3 Destructive and Non-destructive Analyses
In the RoHS testing of materials, there are essentially two types of analyses: (1) destructive analysis and (2) non-destructive analysis. Techniques such as XRF, LIBS and LA-ICP-MS do nondestructive analysis, on the other hand techniques such as F-AAS, ICP-OES and ICP-MS are considered destructive techniques because these require samples to be in solution form. There are many parameters that affect the results that are obtained from both destructive and non-destructive evaluations. One significant source of potential error is in the sampling technique itself as already described. If the material being evaluated is of sufficient size (mass or volume), it is desirable to select material from various locations on the part, and then combine the materials into a single test material. This technique will help to eliminate local anomalous zones where poor mixing or other manufacturing processes may have allowed a substance of concern to collect in larger-than-expected amounts. Due to its limited sampling area and in situ approach, results obtained from true non-destructive testing (without particle size reduction via milling or grinding) are most at risk for precision and accuracy due to sample non-homogeneity error. Destructive test methods are generally considered to be the most accurate, precise and sensitive type of evaluation; however, they are more labor-intensive and time-consuming compared to non-destructive techniques. Table 7 presents the comparison of the results obtained for RoHS substances in printed circuit board by ED-XRF, ICP-MS, CV-AAS, GC–MS, IC and UV–Visible spectrophotometric methods. Though concentrations of Hg and polybrominated compounds are within limits, the concentrations of Cd, Cr6+ and Pb are above the RoHS regulation limits, and the agreement between different analytical techniques is very good in general.
7.4 Challenges
Implementation of the RoHS presents special challenges to the firms and also to the laboratories which are in this business. The first major challenge is to obtain a representative sample for analysis. For example, a single mobile phone, with an average weight of about 75–100 g, contains about 75 different elements, almost three quarters of the periodic table in its components [75]. Regardless of manufacturer, a mobile phone device typically consists of an electronic circuitry, a printed circuit board (PCB), a liquid crystal display (LCD), a battery (nickel–cadmium, nickel metal hydride or lithium ion/polymer technology), a keyboard, an antenna (which is occasionally an integrated circuit) and a plastic case (which sometimes coexists with metal coating or lining). Table 4 presents the results of our study about the content of RoHS substances in different components of a commercial mobile phone by different analytical techniques. The printed circuit board contained relatively high concentrations of heavy metals like Pb and Cr, while in plastic housing the concentration levels of these metals are much lower. Taking into consideration the RoHS Directive, results revealed that this particular mobile phone tested contain significant amount of Pb that exceeds the permissible concentration limits. On the other hand, Pb and Cr concentration levels exceeded the limit of 1000 µg/g in handset’s printed circuit board. Hence, such mobile phones can pose potential threat to the environment and to human health unless RoHS regulations are followed very strictly. Examples of homogenous materials are described in further detail in a FAQ document [76]. The components present on a PCB, such as resistors and capacitors, are progressively diminishing in size and can have dimensions of 0.2 × 0.1 mm. To be able to screen a PCB on the level of individual components, a 2-dimensional mapping is performed. The mappings of the different elements have been combined into a single image and can subsequently be displayed on top of the photo to have a direct correlation between the element distribution and the PCB lay-out. Testing at the assembly level for extremely low levels of the RoHS elements/compounds at the homogenous constituent layer level is problematic for many tiny electronic components. Particularly in solution analysis, it’s very hard to convert extremely heterogeneous solid electronic components into a liquid that can be injected into chemical analysis equipment. As many electronic components are smaller than the minimum sample size for wet chemical methods, multiple components must be used to make up one sample for analysis. Adding to these difficulties is the requirement that each “homogeneous constituent” layer (plating layers, sub-component constructions, etc.) of a component must meet the RoHS substance maximum limits. However, there are expensive methods such as LA-ICP-MS that will permit µg/g level chemical analysis of the individual layers of one component.
7.5 Quality Assurance (QA) and Quality Control (QC)
In the manufacturing and supply chain, from the raw material stage to the finished product stage, it is necessary for the supplier to ensure that the restricted substances in the final products are below threshold limits to respond properly to the RoHS directive. Therefore, precise and accurate analytical procedures are needed for verification. It is not easy to objectively demonstrate that one’s own analytical method or a result for a particular component is appropriate and accurate. In addition, quality control protocols involving measurement against appropriate certified reference materials (CRMs) are also required for testing the reliability of each batch of data produced in the laboratory [77]. It’s also important that the CRM used for calibration should closely match to the sample under investigation with respect to its major and minor element composition, for obtaining reliable data [78, 79]. Lot of groups have developed plastic CRMs for the analysis of heavy metals and brominated flame retardants regulated by RoHS directive [22]. For example, cryogenic milling of polymeric materials prior to destructive or non-destructive analysis has been found invaluable in the optimization of restricted substance analyte measurement recovery [73]. The Institute of Reference Materials and Measurements of the European Commission (www.irmm.jrc.be) and other reference material producers such as National Institute of Standards and Technology (NIST), US and BAM, the National Metrology Institute of Germany, are developing specific reference materials suitable for this purpose. Pöhlein et al. [80] have developed several reference materials for the determination of RoHS-relevant flame retardants in styrenic polymers. Earlier to their work, only available CRM with certain suitability for flame retardant analysis was NMIJ CRM 8108-a [81], a polystyrene plastic containing 317 ± 14 mg/kg decaBDE. Ohata and Hioki [82] have developed PVC and PP resin pellet CRMs which are useful for the determination of Cd, Cr, Hg and Pb in plastics with respect to the RoHS directive. Bergh et al. [83] prepared a reference material of house dust containing several organic contaminants including phthalates.
8 Conclusion
This article has focused on the detailed description of the on-going and future high technology test methods for RoHS substances of high concern in manufactured electrical and electronic products. It is very important for the firms to understand the options that are currently available and the latest developments in this area. Ensuring sample homogeneity is important before the testing methods are introduced. Since the detection limits of the banned substances are reasonably higher, XRF methods are very convenient at least for initial screening. Microwave digestion is a good sample digestion technique for RoHS compliance because of sample variability tolerance and the ability to retain volatile elements. Digestion time is predictable and reduced below hotplate digestion time, due to high temperature/pressure. Both ICP-OES and ICP-MS provide several benefits for the analyses of RoHS matrices, including multi-element capabilities to provide rapid information on a variety of elements, suitable detection limits, and wide dynamic range to meet the needs of potentially diverse samples. LA-ICP-MS is set to become a potential and very powerful analytical tool in this area mainly because of the nature of samples. When more CRMs become available, it is hoped that it would promote more consistency among the various test labs across the world, leading to nationally and internationally accepted testing schemes. It is likely that more hazardous elements and compounds will be identified and added to the already existing list in future, and more R&D work is needed in this area. Hand held XRF, LIBS and Raman Spectrometer will have greater roles in RoHS compliance testing in future, as these analyzers can speed up the work-flow very efficiently. When the environmental regulations become increasingly stringent with corresponding decreases in acceptable levels of regulated substances, there will be a great need for newer, faster, and more convenient and accurate high technology analytical methods such as the LA-ICP-MS and HR-ICP-MS in the near future.
References
V. Kuntz, White paper on European Union RoHS directive: understanding exemptions, Assent, Canada, (2015) 1–14.
S. Chatterjee, India’s readiness on ROHS directives: a strategic analysis, Glob. J. Sci. Frontier Res. Interdiscip., 12(1) (2012) 1–13.
U. Rambabu, V. Balaram, R. Ratheesh, S. Chatterjee, M.K. Babu and N.R. Munirathnam, Assessment of hazardous substances in electrical cables: implementation of RoHS regulations in India, J. Test. Eval., 46(5) (2018). https://doi.org/10.1520/JTE20160645. ISSN 0090-3973.
https://www.revolvy.com/main/index.php?s=Restriction%20of%20Hazardous%20Substances%20Directive.
T.W. Dahl, M. Ruhl, E.U. Hammarlund, D.E. Canfield, M.T. Rosing and C. J. Bjerrum, Tracing euxinia by molybdenum concentrations in sediments using handheld X-ray fluorescence spectroscopy (HHXRF), Chem. Geol., 360–361 (2013) 241–251.
G.E.M. Hall, M.B. McClenaghan and L. Pagé, Application of portable XRF to the direct analysis of till samples from various deposit types in Canada, Geochem. Explor. Environ. Anal., 16 (2015) 62–84.
V. Balaram, Field-portable instruments in mineral exploration: past, present and future, J. Appl. Geochem., 19(4) (2017) 382–399.
N.H. Nilsson, B. Malmgren-Hansen and I. Christensen, Development and use of screening methods to determine chromium (VI) and brominated flame retardants in electrical and electronic equipment, Danish Ministry of the Environment (2009) pp. 1–35.
K.Ting, M. Gill and O. Garbin, GC/MS screening method for phthalate esters in children’s toys, J. AOAC Int., 92(3) (2009) 951–958.
A. E. Harvey Jr., J.A. Smart and E.S. Amis, Simultaneous spectrophotometric determination of iron(ii) and total iron with 1,10-phenanthroline, Anal. Chem., 27(1) (1955) 26–29.
L. Hua, Y.C. Chan, Y.P. Wu and B.Y. Wu, Determination of hexavalent chromium (Cr6+) in electronic and electrical components and products to comply with RoHS regulations, J Hazard. Mater., 163(2–3) (2008) 1360–1368.
S.K. Pradhan and P.K. Tarafder, Scheme for performance evaluations of UV–visible spectrophotometer by standard procedures including certified reference materials for the analysis of geological samples, MAPAN-J. Metrol. Soc. India, 31(4) (2017) 275–281.
V. Balaram, R. Mathur, M. Satyanarayanan, S.S. Sawant, P. Roy, K.S.V. Subramanyam, C.T. Kamala, K.V. Anjaiah, S.L. Ramesh and B. Dasaram, A rapid method for the determination of gold in rocks, ores and other geological materials by F-AAS and GF-AAS after separation and preconcentration by DIBK extraction for prospecting studies. MAPAN-J. Metrol. Soc. India, 27(2) (2012) 87–95.
A.M.G. Alegria, M.G. Canez-Carrasco, M. Serna-Felix and A. Gomez-Alverez, Estimation of uncertainty in the determination of serum electrolytes (Na, K, Ca, Mg) by flame atomic absorption spectroscopy, MAPAN-J. Metrol. Soc. India, (2018), https://doi.org/10.1007/s12647-017-0244-2.
A. Walsh, The application of atomic absorption spectra to chemical analysis, Spectrochim. Acta 7 (1955) 108–117.
L. Boris, Recent advances in absolute analysis by graphite furnace atomic absorption spectrometry, Spectrochim. Acta Part B At. Spectrosc., 45 (1990) 633–655.
A.T. Duarte, M.B. Dessuy, M.M. Silva, M.G.R Vale and B. Welz, Determination of cadmium and lead in plastic material from waste electronic equipment using solid sampling graphite furnace atomic absorption spectrometry, Microchem. J., 96 (2010) 102–107.
A.T. Duarte, M.B. Dessuy, M.G.R. Vale and B. Welz, Determination of chromium and antimony in polymers from electrical and electronic equipment using high resolution continuum source graphite furnace atomic absorption spectrometry, Anal. Methods, 24 (2013) 6941–6946.
R. Mathur, V. Balaram and S. Babu, Determination of mercury in geological samples by cold vapor atomic absorption spectrometric technique, Indian J. Chem., 44A (2005) 1619–1624.
A. Hioki, M. Ohata, S. Matsuyama and S. Kinugasa, Development of plastic certified reference materials (CRMs) to cope with restrictions on hazardous substances—CRMs for analysis of heavy metals and brominated flame retardants regulated by RoHS directive, Synthesiol. Engl. Ed., 8(1) (2015) 29–42.
R. Glocker and H. Schreiber, Quantitative Röntgenspektralanalyse mit Kalterregung des Spektrums, Ann. Physik., 85 (1928) 1089–1102.
P.J. Potts and C. Webb, X-ray fluorescence spectrometry, J. Geochem. Explor., 44(1–3) (1992) 251–296.
E.L. Hoffman, Instrumental neutron activation in geoanalysis, J. Geochem. Explor., 44(1–3) (1992) 297–319.
A. El-Taher, Elemental analysis of granite by instrumental neutron activation analysis (INAA) and X-ray fluorescence analysis (XRF), Appl. Radiat. Isot., 70(1) (2012) 350–354.
R.A. Nadkarni and G.H. Morrison, Multielement analysis of sludge samples by instrumental neutron activation analysis, Environ. Lett., 6(4) (1974) 273–285.
K. Park and N. Kang, Instrumental neutron activation analysis of mass fractions of toxic metals in plastic, Talanta, 73 (2007) 791–794.
M. Tsutomu, O. Ryo, I. Yuto, S. Shun, T. Koichi and O. Masaki, Precise determination of bromine in PP resin pellet by instrumental neutron activation analysis using internal standardization, J. Radioanal. Nucl. Chem., 303(2) (2015) 1417–1420.
N. Ismail, and J. Yoo, Determination of Lead (Pb) concentration level in solder finished product using Laser Induced Breakdown Spectroscopy (LIBS), Proceedings on 12th electronics packaging technology conference, Singapore, (2010) pp. 456–461.
A.A. Bol’shakov, J.H. Yoo, C. Liu, J.R. Plumer and R.E. Russo, Laser-induced breakdown spectroscopy in industrial and security applications, Appl. Opt., 49(13) (2010) C133–C142.
S. Greenfield, I.L.I. Jones and C.T. Berry, High pressure plasmas as spectroscopic emission sources, Analyst, 89 (1964) 713–720.
R.H. Wendt and V. Fassel, Inductively-coupled plasma spectrometric excitation source, Anal. Chem., 37 (1965) 920–922.
V. Balaram, K.V. Anjaiah and M.R.P. Reddy, A comparative study on the trace and rare earth element analysis of an Indian polymetallic nodule reface sample by inductively coupled plasma atomic emission spectrometry and inductively coupled plasma mass spectrometry, Analyst, 120 (1995) 1401–1406.
H.J. Cho and S.W. Myung, Determination of cadmium, chromium and lead in polymers by ICP-OES using a high pressure asher (HPA), Bull. Korean Chem. Soc., 32(2) (2011) 489–497.
R.S. Houk, V.A. Fassel, G.D. Flesch, H.J. Svec, A.L. Gray and C.E. Taylor, Inductively coupled argon plasma as an ion source for mass spectrometric determination of trace elements, Anal. Chem., 52 (1980) 2283–2289.
V. Balaram, Recent trends in the instrumental analysis of rare earth elements in geological and industrial materials, Trends Anal. Chem., 15 (1996) 475–486.
V. Balaram, M. Satyanarayanan, P.K. Murthy, C. Mohapatra and K.L. Prasad, Quantitative multi-element analysis of cobalt crust from Afanasy-Nikitin seamount in the north central Indian Ocean by inductively coupled plasma time-of-flight mass spectrometry, MAPAN-J. Metrol. Soc. India, 28(2) (2013) 63–77.
M. Satyanarayanan, V. Balaram, S.S. Sawant, K.S.V. Subramanyam, V. Krishna, B. Dasaram, and C. Manikyamba, Rapid determination of REE, PGE and other trace elements in geological and environmental materials by HR-ICP-MS, At. Spectrosc., 39(1) (2018) 1–15.
M.C. Santos, J.A. Nóbregab and S. Cadorea, Determination of Cd, Cr, Hg and Pb in plastics from waste electrical and electronic equipment by inductively coupled plasma mass spectrometry with collision–reaction interface technology, J. Hazard. Mater., 190 (2011) 833–839.
F. Kyoko and C. Atsushi, Determination of trace amounts of mercury, lead, and cadmium in steel and iron ore, JFE GIHO, 13 (2006) 35–41.
C. Mans, D. Alber, M. Radtke, S. Hanning, A. Buhler and M. Kreyenschmidt, New polymeric candidate reference materials for XRF and LA-ICP-MS—development and preliminary characterization, X-ray Spectrom., 38 (2009) 52–57.
H. Osterlund, L. Rodushkin, K. Ylinenjarvi and D.C. Baxter, Determination of total chlorine and bromine in solid wastes by sintering and inductively coupled plasma-sector field mass spectrometry, Waste Manage., 29(4) (2008) 1258–1264.
B. Novotnik, T. Zuliani, J. Scancar and R. Milacic, The determination of Cr(VI) in corrosion protection coatings by speciated isotope dilution, ICP-MS.J. Anal. At. Spectrom., 27 (2012) 1484–1493.
M.R. Hammer, A magnetically excited microwave plasma source for atomic emission spectroscopy with performance approaching that of the inductively coupled plasma, Spectrochim. Acta Part B, 63 (2008) 456–464.
V. Balaram, V. Dharmendra, P. Roy, C. Taylor, P. Kar, A.K. Raju and A. Krishnaiah, Determination of precious metals in rocks and ores by microwave plasma-atomic emission spectrometry (MP-AES) for geochemical prospecting, Curr. Sci., 104(9) (2013) 1207–1215.
C.T. Kamala, V. Balaram, V. Dharmendra, P. Roy, M. Satyanarayanan, K.S.V. Subramanyam, Application of microwave plasma atomic emission spectrometry (MP-AES) for environmental monitoring of industrially contaminated sites in Hyderabad city, Environ. Monit. Assess. 186 (2014) 7097–7113.
V. Sreenivasulu, V. Dharmendra, V. Balaram, C.N. Rao A. Krishnaiah, H. Zhengxu and Z. Zhen, Determination of B, P and Mo in bio-sludge samples by microwave plasma atomic emission spectrometry (MP-AES), Appl. Sci., 7 (2017) 264–273.
L.D. George, S.A. Renata, D. Schiavo, A.N. Joaquim, Determination of Cr, Ni, Pb and V in gasoline and ethanol fuel by microwave plasma optical emission spectrometry, J. Anal. At. Spectrom., 28 (2013) 755–759.
D. Pfeil, Measurement techniques for mercury: which approach is right for you?, Spectroscopy, 26(9) (2011) 40–43.
Z. Grosser, L. Thompson and L. Davidowski, Inorganic analysis for environmental RoHS compliance, American Laboratory, October (2007) pp. 1–4.
G.M.M. Rahman, H.M.S. Kingston, T.G. Towns, R.J. Vitale and K.R. Clay, Determination of hexavalent chromium by using speciated isotope-dilution mass spectrometry after microwave speciated extraction of environmental and other solid materials, Anal. Bioanal. Chem., 382 (2005) 1111–1120.
S. Yue-Feng, W. Feng, B. Li-Ying, X. Bin and C. Shi, Determination and factor analysis of trace hexavalent chromium in plastics for RoHS directive, Acta Chim. Sin., 66(6) (2008) 662–668.
L. Hua, Y.C. Chan, Y.P. Wu and B.Y. Wu, The determination of hexavalent chromium (Cr6+) in electronic and electrical components and products to comply with RoHS regulations, J. Hazard. Mater., 163 (2009) 1360–1368.
J.S. Kim, Y.R. Choi, Y.S. Kim, Y.J. Lee, Y.H. Ko, S.Y. Kwon and S.B. Heo, Determination of hexavalent chromium (Cr(VI)) in plastics using organic-assisted alkaline extraction, Anal. Chim. Acta, 690(2) (2011) 182–189.
E. Hoh, L. Zhu, R.A. Hites, Novel flame retardants, 1,2-bis(2,4,6-tribromophenoxy) ethane and 2,3,4,5,6-pentabromoethylbenzene, in United States’ environmental samples, Environ. Sci. Technol., 39 (2005) 2472–2477.
J.H. Christensen, M. Glasius, M. Pécseli, J. Platz, G. Pritz, Polybrominated diphenyl ethers (PBDEs) in marine fish and blue mussels from southern Greenland, Chemosphere, 47(6) (2002) 631–638.
E. Eljarrat and D. Barceló, Priority lists for persistent organic pollutants and emerging contaminants based on their relative toxic potency in environmental samples, Trends Anal. Chem., 22(10) (2003) 655–665.
L.G. Costa and G. Giordano, Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants, Neurotoxicology, 28(6) (2007)1047–1067.
M. Riess and R. Eldi, Identification of brominated flame retardants in polymeric materials by reversed-phase liquid chromatography with ultraviolet detection, J. Chromatogr. A, 827 (1998) 65–71.
B. Kolarik, C. Bornehag, K. Naydenov, J. Sundell, P. Stavova and O.F. Nielsen, Concentrations of phthalates in settled dust in Bulgarian homes in relation to building characteristic and cleaning habits in the family, Atmos. Environ., 42(37) (2008) 8553–8559.
H. Shen, Simultaneous screening and determination eight phthalates in plastic products for food use by sonication-assisted extraction/GC–MS methods, Talanta, 66(3) (2005) 734–739.
C.K. Wei, L.C. Fung and M. Pang, Determination of six phthalates in polypropylene consumer products by sonification assisted extraction-GC–MS method, Malaysian J. Anal. Sci., 15(2) (2011) 167–174.
S. G. Aggarwal, Recent developments in aerosol measurement techniques and the metrological issues, MAPAN-J. Metrol. Soc. India, 25(3) (2010) 165–189.
M. Schlummer, F. Brandl, A. Mäurer and R. van Eldik, Analysis of flame retardant additives in polymer fractions of waste of electric and electronic equipment (WEEE) by means of HPLC-UV/MS and GPC-HPLC-UV. J Chromatogr. A, 1064(1) (2005) 39–51.
Y. Chen, J. Li, L. Chen, S. Chen and W. Diao, Brominated flame retardants (BFRs) in waste electrical and electronic equipment (WEEE) plastics and printed circuit boards (PCBs), Procedia Environ. Sci., 16 (2012) 552–559.
M. Pöhlein, B. Müller, M. Wolf and R. van Eldik, GIT Labor-Fachzeitschrift, 48 (2004) 754 (in German language).
M. Shao, J. Jiang, M. Li, L. Wu and M. Hu, Recent developments in the analysis of polybrominated diphenyl ethers and polybrominated biphenyls in plastic, Rev. Anal. Chem., 35(3) (2016) 133–143.
M.S. Qureshi, A.R.M. Yusoff, M.D.H. Wirzal, Sirajuddin, J. Barek, H.I. Afridi and Z. Ustundag, Methods for the determination of endocrine-disrupting phthalate esters, Crit. Rev. Anal. Chem., 46(2) (2016) 146–159.
H. Vasakova, A powerful tool for material identification: Raman Spectroscopy, Int. J. Math. Models Methods Appl. Sci., 7(5) (2011) 1205–1212.
R. Taurino, M. Cannio, T. Mafredini and P. Pozzi, An efficient and fast analytical procedure for the bromine determination in waste electrical and electronic equipment plastics, Environ. Technol., 35(21–24) (2014) 3147–3152.
S. Kikuchi, K. Kawauchi, S. Ooki, M. Kurosawa, H. Honjho and T. Yagishita, Non-destructive rapid analysis of brominated flame retardants in electrical and electronic equipment using Raman Spectroscopy, Anal. Sci., 20 (2004) 1111–1112.
J.E. Martin, L.L.A. Smith, G. Adjei-Bekoe and R. Thomas, Comparison of different sample preparation procedures for the determination of RoHS/WEEE-regulated elements in printed circuit boards and electrical components by EDXRF, Spectroscopy 25(4) (2010) 40–47.
V. Balaram, Microwave dissolution techniques for the analysis of geological materials by ICP-MS, Curr. Sci., 73 (1997) 1019–1023.
F. Vilaplana, A. Ribes-Greus and S. Karlsson, Microwave-assisted extraction for qualitative and quantitative determination of brominated flame retardants in styrenic plastic fractions from waste electrical and electronic equipment (WEEE), Talanta, 78(1) (2009) 33–39.
USGS (2017) https://pubs.usgs.gov/gip/0167/gip167.pdf.
RoHS FAQ Guidance document (2016) http://ec.europa.eu/environment/waste/rohs_eee/pdf/faq.pdf.
K.S.V. Subramanyam, V. Balaram, U.V.B. Reddy, M. Satyanarayanan, P. Roy and S.S. Sawant. Problems involved in using improper calibration CRMs in geochemical analyses: a case study on mafic rocks of Boggulakonda Pluton, East of Cuddapah Basin, India, MAPAN-J. Metrol. Soc. India, 28(1) (2013) 1–9.
V. Balaram, M.L. Patil, A.K. Agrawal, D.V.S. Rao, S.N. Charan, M. Satyanarayanan, R. Mathur, K. Kapilavastu, D.S. Sarma, M.S. Gowda, S.L. Ramesh, P. Sangurmath, K.V. Anjaiah, B. Dasaram, R.K. Saxena and Z. Begum, Preparation and certification of high-grade gold geochemical reference material, Accred. Qual. Assur., 11 (2006) 329–335.
M. Pöhlein, R.U. Bertran, M. Wolf, R. van Eldik, Preparation of reference materials for the determination of RoHS-relevant flame retardants in styrenic polymers, Anal. Bioanal. Chem., 394 (2009) 583–595.
NMIJ 2008 CRM 8108-a http://www.seishinsyoji.co.jp/Standard/2007.11Plastic%20Reference%20Material.pdf.
M. Ohata and A. Hioki, Development of PVC and PP resin pellet certified reference materials for heavy metal analysis with respect to the RoHS directive, Anal. Sci., 29 (2013) 239–246.
C. Bergh, G. Luongo, S. Wise and C. Östman, Organophosphate and phthalate esters in standard reference material 2585 organic contaminants in house dust, Anal. Bioanal. Chem., 402(1) (2012) 51–59.
G. Flora, D. Gupta and A. Tiwari, Toxicity of lead: a review with recent updates, Interdiscip. Toxicol., 5(2) (2012) 47–58.
B.F. Azevedo, L.B. Furieri, F.M. Peçanha, G.A. Wiggers, P.F. Vassallo, M.R. Simões, J. Fiorim, P.R. Batista, M. Fioresi, L. Rossoni, I. Stefanon, M.J. Alonso, M. Salaices and D.V. Vassallo, Toxic effects of mercury on the cardiovascular and central nervous systems, J. Biomed. Biotechnol., Article ID 949048, (2012) 1–11, https://doi.org/10.1155/2012/949048.
U.S. Department of Health and Human Services, Public Health Service, Agency for toxic Substances and Disease Registry (ATSDR), Toxicological Profile for Cadmium (2012).
U.S. Department of Labor, Health effects of hexavalent chromium, Occupational Safety and Health Administration (2006).
M.A. Siddiqi, R.H. Laessig and K.D. Reed, Polybrominated diphenyl ethers (PBDEs): new pollutants-old diseases, Clin. Med. Res., 1(4) (2003) 281–290.
A. Covaci, S. Voorspoels, L. Ramos, H. Neels and R. Blust, Recent developments in the analysis of brominated flame retardants and brominated natural compounds, J. Chromatogr. A, 1153 (2007) 145–171.
C.F. Wilkinson and J.C. Lamb IV, The potential health effects of phthalate esters in children’s toys: a review and risk assessment, Regul. Toxicol. Pharmacol., 30(2) (1999) 140–155.
L. Randall, V. Wal, T.M. Ticich, J.R. West and A. Paul, Trace metal detection by laser-induced breakdown spectroscopy, Appl. Spectrosc., 53(10) (1999) 1226–1236.
V. Balaram, V. Dharmendra, P. Roy, C. Taylor, C.T. Kamala, M. Satyanarayanan, P. Kar, K.S.V. Subramanyam, A.K. Raju, A. Krishnaiah, Analysis of geochemical samples by microwave plasma-AES, At. Spectrosc., 35(2) (2014) 65–78.
B. Welz, Atomic absorption spectroscopy, 2nd edn., VCH, Weinheim and Deerfield Beach, FL (1985) p. 506.
X. Hou and B.T. Jones, Inductively coupled plasma/optical emission spectrometry. In: Encyclopedia of analytical chemistry, R.A. Meyers (Ed.) John Wiley & Sons Ltd, Chichester (2000) pp. 9468–9485
V. Balaram and T.G. Rao, Rapid determination of REE and other trace elements in geological samples by microwave acid digestion and ICP-MS, At. Spectrosc., 24(6) (2003) 206–212.
B. Passariello, M. Barbaro, S. Quaresima, A.A. Marabini, Determination of mercury by inductively coupled plasma-mass spectrometry, Microchemical J., 54(4) (1996) 348–354.
V.R. Bellotto and N. Miekeley, Improvements in calibration procedures for the quantitative determination of trace elements in carbonate material (mussel shells) by laser ablation ICP-MS, Fresenius J. Anal. Chem., 367(2000) 635–640.
R. Van Grieken and J. Injuk, Current applications of XRF and micro-XRF techniques in environmental and industrial fields (1999) (unpublished work).
B. Binici, M. Bilsel, M. Karakas, I. Koyuncu and A.C. Goren, An efficient GC-IDMS method for determination of PBDEs and PBB in plastic materials, Talanta, 116 (2013) 417–426.
N. Boley, Development of a procedure for the determination of selected brominated flame retardants (PBB, PBDE) in plastics by HPLC-ICP-MS. National Measurement Office, Teddington, (2010) pp. 1–41.
Acknowledgements
One of the authors (VB) would like to express sincere thanks to the Ministry of Electronics and Information Technology (MeitY), Government of India, New Delhi and the Director, C-MET, Hyderabad for appointing him as the Chairman of the Monitoring Committee for the RoHS Project at C-MET, Hyderabad during 2012–2017.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balaram, V., Rambabu, U., Reddy, M.R.P. et al. RoHS Regulation: Challenges in the Measurement of Substances of Concern in Industrial Products by Different Analytical Techniques. MAPAN 33, 329–346 (2018). https://doi.org/10.1007/s12647-018-0263-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12647-018-0263-7