Abstract
Exercise exerts helpful effects in Parkinson’s disease. In this study, the 6-hydroxydopamine (6-OHDA) injection was used to investigate the effect of exercise on apomorphine-induced rotation and neurorestoration. Rats (n = 32) were divided into four groups: (1) Saline+Noexercise (Sham); (2) 6-OHDA+Noexercise (6-OHDA); (3) Saline+Exercise (S+EXE), and (4) 6-OHDA+Exercise (6-OHDA+EXE). The rats were administered 8 μg 6-OHDA by injection into the right medial forebrain bundle. After 2 weeks, the exercise group was run (14 consecutive days, 30 min per day). One month after the surgery, following the injection of apomorphine, the 6-OHDA group displayed a significant increase in rotation and the 6-OHDA+EXE group showed a significant reduction of rotational asymmetry (P < 0.001). 6-OHDA injection reduced the mRNA and protein expression of the AMP-activated protein kinase, brain-derived neurotropic factor, and tyrosine hydroxylase in relation to the Sham group and exercise increased these levels. Expression of the silent information regulator 2 homolog 1 and peroxisome proliferator-activated receptor gamma coactivator 1-alpha was unexpectedly enhanced in the 6-OHDA groups in relation to the Sham group. These findings suggest that the 6-OHDA injection increased the neurodegeneration and mitochondrial and behavioral dysfunctions and the treadmill running attenuated these disorders in the ipsilateral striatum of the 6-OHDA+EXE group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder that causes suffering of millions of people, especially in the growing aging population. Patients with PD are typically debilitated with movement disorder symptoms, with increased reactive oxygen species production and mitochondrial apoptotic susceptibility, as well as decreased transcriptional drive for mitochondrial biogenesis (Oliveira et al. 2014; Petzinger et al. 2015; Corona and Duchen 2015). However, the precise underlying mechanisms of these processes remain unclear (Oliveira et al. 2014) and currently, there is no cure for PD (Petzinger et al. 2015). The major pathology of PD involves the deletion or downregulation of mitochondrial genes that are essential for supporting mitochondrial biogenesis, leading to mitochondrial dysfunction and contributing to progressive degeneration of the dopaminergic neuron (Gerecke et al. 2010; Patki and Lau 2011). A common neurotoxin substance in animal models of PD is 6-hydroxydopamine (6-OHDA) that destructs dopamine neurons (Aguiar Jr et al. 2016; Garcia et al. 2017; Rezaee et al. 2019a; b).
A set of enzymes fine-tunes the mitochondrial function in a cell. AMP-activated protein kinase (Ampk) is an enzyme that rapidly regulates metabolic and mitochondrial enzymes by their direct phosphorylation, and also affects the transcription of specific genes to adapt gene expression to cellular energy demands (Cantó and Auwerx 2009). The silent information regulator 2 homolog 1 (Sirt1) may influence the aging process and many age-associated diseases. It is downregulated in aging cells, suggesting that Sirt1 may function to extend the life span (Oliveira et al. 2014), and it plays an intricate role in the pathology of multiple diseases (Chong et al. 2012). Ampk and Sirt1, through phosphorylation and deacetylation, respectively (Kang et al. 2013), modulate the activity of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Pgc1α), which is a key factor activating mitochondrial biogenesis and its expression can be used as a marker of this process (Steiner et al. 2011; Oliveira et al. 2014). Sirt1 appears to contribute to the regulation of metabolism by acting in a pathway whereby it deacetylates and activates Pgc1α (Cantó and Auwerx 2009; Kang et al. 2013). Brain-derived neurotrophic factor (Bdnf) is an essential vital protein in learning and memory, neuronal plasticity, neuroprotection, and mitochondrial function (Miranda et al. 2019; Rezaee et al. 2019b). The trophic effect of Bdnf on dopamine neurons is evaluated as potential neuroprotective (Razgado-Hernandez et al. 2015). Finally, tyrosine hydroxylase (Th) is a rate-limiting enzyme involved in the synthesis of catecholamine neurotransmitters, such as dopamine, and it is used as a marker of neuronal degeneration (Yoon et al. 2007; Tuon et al. 2014). One of the important ways to evaluate the efficacy of therapeutic methods of neurodegenerative disease is an assessment of the Th level (Tuon et al. 2014; Aguiar Jr et al. 2016). According to previous studies, Th is positively regulated by Bdnf, and Pgc1α is effective in regulating Bdnf (Hsueh et al. 2018; Rezaee et al. 2019b).
Severe motor, mental, functional, and mitochondrial disability following the progressive neuronal degeneration in PD suggests that a therapeutic approach preventing neurodegeneration and promoting neuroprotection would be a valuable strategy to control the disease. Exercise is one intervention that has been shown to reduce the production of free radicals and mitochondrial dysfunction (Gerecke et al. 2010). Implications for an exercise-induced enhancement of neuroprotective effects and cognitive functions are vast and contradictory (Steiner et al. 2011; Chen et al. 2017; Costa et al. 2017). The data differ depending on the exercise program, age of animals, and type and amount of injection of neurotoxin (Choe et al. 2012; Aguiar Jr et al. 2016; Real et al. 2017).
Accordingly, the present study investigates the therapeutic effect of exercise on neuroprotection after the unilateral injection of 6-OHDA.
Materials and Methods
Animals
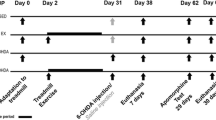
For the study, 32 male Wister rats (weighing 270 ± 20 g, 6 months old, from the Pasteur Institute of Iran) were used. The animals were housed (n = 4 per cage) in a temperature-controlled room (22 ± 2 °C) with a 12-h light/12-h dark cycle, and free access to food and water. One week before the surgery, to familiarize the rats with the experimental conditions, the animals were placed on a treadmill, for 10 min/day at 5 m/min, to ensure that all animals performed similarly prior to 6-OHDA lesioning. After the familiarization, the rats were randomly assigned to four groups (n = 8 animals/group), as follows: (1) Saline+Noexercise (Sham); (2) 6-OHDA+Noexercise (6-OHDA); (3) Saline+Exercise (S+EXE); and (4) 6-OHDA+Exercise (6-OHDA+EXE) (as explained below).
Ethical Standards
All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and have been approved by the Ethic Committee for Animal Experiments at the University of Isfahan (IR.UI.REC.1396,008).
Surgical Procedures
Rats were anesthetized using xylazine (0.67 mg/kg, intraperitoneal injection, i.p.) and ketamine (0.33 mg/kg, i.p.), and then received unilateral stereotaxic injections of 8 μg of 6-OHDA (Sigma-Aldrich; 4 μL of 2 μg/μL solution prepared in 0.2% ascorbic acid and 0.9% NaCl) or saline (4 μL of 0.2% ascorbic acid and 0.9% NaCl) (Tuon et al. 2015), into the right medial forebrain bundle anteroposterior (AP), − 1.8 mm; lateral (LAT), 4.7 mm from the bregma; and vertical (DV), − 8.2 mm from the skull surface (Mabandla et al. 2004; Yoon et al. 2007; Carvalho et al. 2013), by a 5-μL Hamilton syringe attached to an infusion pump (BI Insight 2000), at a rate of 0.5 μL/min for 8 min for each rat. The cannula was left in place for an additional 5 min after the injection before being slowly retracted. Briefly, 5 days following the 6-OHDA or saline administration, a time point to complete the cell death process, the rotations following the intraperitoneal injection of apomorphine were calculated (with a similar way to Behavioral test section). Finally, 6-OHDA-induced rats that showed significant rotations were selected to continue the study and the others were excluded for further investigation (Tuon et al. 2014).
Exercise Protocols
Two weeks after the induction of lesions, exercise groups began following a light treadmill exercise protocol for 30 min, once a day, for 14 consecutive days (Cho et al. 2013). The exercise load was started at a velocity of 5 m/min for the first 5 min, 8 m/min for the next 5 min, and 15 m/min for the last 20 min, without electrical shock and at 0° of inclination. The noexercise groups were placed on an unmoving treadmill for the same period of time.
Behavioral Test (Asymmetry Rotational)
Forty-eight hours after the last training session, rats are placed in a plastic container (25-cm high and 28-cm diameter) for 10 min to habituate. This was followed by a challenge with the dopamine receptor agonist R(−)-apomorphine (0.5 mg/kg, i.p.). The rotation number under apomorphine (Sigma) is one test that used to quantify the degree of lesion in the PD model and is related to the extent of dopamine depletion, after the unilateral 6-hydroxydopamine lesion. The contralateral rotations (opposite to the lesioned right side) induced by apomorphine are related to the unbalance in the nigrostriatal dopaminergic pathways between the right (lesioned) and left (unlesioned) brain hemispheres (Tuon et al. 2012; 2014; Aguiar Jr et al. 2016; Costa et al. 2017; Real et al. 2017). This test was monitored for 60 min.
Euthanasia and Tissue Collection
One day after the behavioral test, the rats were anesthetized with ketamine (5 mg/100 g of body weight, i.p.) and xylazine (1 mg/100 g of body weight, i.p.), and the right striatum was surgically removed. The extracted tissues were deep-frozen in liquid nitrogen, and then stored at − 80 °C. Tissue homogenates were centrifuged at 11,000×g (40 min, 4 °C) for 40 min to remove insoluble material (Tuon et al. 2014; 2015).
Isolation of Total RNA and Real-Time Quantitative PCR
Total RNA was isolated from tissues (n = 8 per group) using the RNeasy mini kit (Qiagen Inc., Valencia, CA, USA), as recommended by the manufacturer. The concentration and purity of RNA were assessed using NanoDrop spectrophotometer (NanoDrop Technologies Wilmington, DE, USA) by reading sample absorbance at 230 nm, 260 nm, and 280 nm. Then, 1 μg of total RNA was used for the synthesis of cDNA (cDNA synthesis kit, Fermentas, Lithuania) by utilizing oligo dT primers. RT-qPCR was performed using SYBR Green PCR master mix (TaKaRa, Japan) and a Step One Plus thermocycler (ABI Applied Biosystems, USA). The forward and reverse primer sequences are provided in Table 1. The Ct value is the fractional cycle number at which sample fluorescence exceeds a fixed threshold. The fold-change in expression was calculated by using the ΔΔCt method, and the data are presented as the percentage of fold-change of expression in treated groups compared with the corresponding control group after normalization to Gapdh endogenous control (Patki and Lau 2011).
Protein Extraction and Western Blotting
Proteins were extracted from the striatum samples (n = 5 per group) in the RIPA buffer (Sigma) according to the manufacturer’s protocol. As previously described by our group (Rezaee et al. 2019a), briefly, 1 mL of RIPA buffer was added to 100 mg of brain tissue in a 1.5-mL tube and homogenized (five times, 5 min each, at 4 °C). Insoluble material was removed by centrifugation (12,000×g for 20 min). Soluble protein concentration in sample supernatants was determined using a protein assay kit (Bio-Rad, Hercules, CA). Equal amounts of protein (0.2 mg per sample) were resolved on 10% SDS-PAGE and transferred on to polyvinylidene difluoride membranes (Bio-Rad Laboratories, USA). The membranes were blocked overnight in 10% (w/v) non-fat dried milk (Merk, Germany) in phosphate-buffered saline, incubated with antibodies, and the signals developed as described elsewhere (Tuon et al. 2012). The antibodies used were antiphospho (Thr172) Ampk (1:200; sc-33524), anti-Sirt1 (1:200; sc-15404), anti-Pgc1α (1:200, sc-55476), anti-brain-derived neurotrophic factor (1:200, sc-65514), anti-Th (1:200; sc-25269) and anti–β-actin antibodies (1:200; sc-47778), from Santa Cruz Biotechnology (Santa Cruz, USA). Chemiluminescent detection was enabled by using a secondary antibody conjugated with a horseradish peroxidase (1:2500; Bio-Rad, 170-6516), and the bands were visualized using an Amersham ECL advanced western blotting detection kit. NIH ImageJ program was used to compare the density of bands on Western blot.
Data Analysis
All data are presented as the mean + standard error of the mean (SEM). Data for the treatment groups, 1 month after the surgery, were compared using GraphPad Prism 8 Software, La Jolla, CA, USA and one-way analysis of variance (ANOVA) (version 23.0), followed by Tukey’s post hoc test. P < 0.05 was considered to be statistically significant.
Results
Apomorphine-Induced Rotations
Apomorphine-induced RT was performed 30 days after 6-OHDA injection. Statistical analysis indicated differences in apomorphine-induced rotation behavior of animals in different groups [F(3,28) = 2174.7, P < 0.001], with post hoc analysis revealing a significantly higher rotational asymmetry in the 6-OHDA group than in other groups. As shown in Fig. 1, the treadmill exercise significantly reduced the rotational asymmetry in the 6-OHDA+EXE group (318.7 ± 6.7 turns/h) compared with the sedentary parkinsonian group (363.6 ± 22.2 turns/h) (P < 0.001). This analysis was complemented by the assessment of Th level (Figs. 2, 3e).
The effect of treadmill exercise on apomorphine-induced rotation in rats. Values are expressed as the mean + SEM (n = 8 per group); # P < 0.001 in relation to 6-OHDA group, according to one-way ANOVA followed by Tukey’s post hoc test. Sham, Saline+Noexercise; S+EXE, Saline+Exercise; 6-OHDA, 6-OHDA+Noexercise; and 6-OHDA+EXE, 6-OHDA+Exercise
Effects of treadmill exercise on Ampk, Sirt1, Pgc1a, Bdnf, and Th mRNA levels. RT-qPCR was performed to detect changes in the expression of Ampk (a), Sirt1 (b), Pgc1a (c), Bdnf (d), and Th (e) genes in the striatum of animals administered 6-OHDA before the exercise. Values are expressed as the mean + SEM (n = 8 per group); *P < 0.001 and **P < 0.01 compared with the Sham group; #P < 0.001 compared with the 6-OHDA group, according to one-way ANOVA followed by Tukey’s post hoc test. Sham, Saline+Noexercise; S+EXE, Saline+Exercise; 6-OHDA, 6-OHDA+Noexercise; and 6-OHDA+EXE, 6-OHDA+Exercise
Effects of treadmill exercise on Ampk (a), Sirt1 (b), Pgc1a (c), Bdnf (d), and Th (e) levels in the striatum of animals administered 6-OHDA before the exercise, as assessed by western blotting. Values are expressed as the mean + SEM (n = 5 per group); *P < 0.001 and **P < 0.01 compared with the Sham group, #P < 0.001 compared with the 6-OHDA group, according to one-way ANOVA followed by Tukey’s post hoc test. Sham, Saline+Noexercise; S+EXE, Saline+Exercise; 6-OHDA, 6-OHDA+Noexercise; and 6-OHDA+EXE, 6-OHDA+Exercise
Assessment of Ampk/Sirt1/Pgc1a, Bdnf, and Th mRNA Levels
Forty-eight hours after the behavioral test, the effect of exercise on expression of specific genes in animals was investigated. One-way ANOVA analysis revealed that the interaction between groups was significantly different in the case of Ampk [F(3,28) = 26.04, P < 0.001], Bdnf [F(3,28) = 203, P < 0.001], and Th [F(3,28) = 56.42, P < 0.001]. We found that the 6-OHDA administration led to a significant reduction of these mRNA levels (Fig. 2a–e) compared with the Sham group. Exercise resulted in increased mRNA levels in the 6-OHDA+EXE group up to Sham group for every three genes and there were significant increases compared with the 6-OHDA group (respectively 80%, 73%, and 71%). Furthermore, analysis of the interaction between all experimental groups by one-way ANOVA revealed a significant difference in Sirt1 levels [F(3,28) = 13.6, P < 0.001] (Fig. 2b) and Pgc1a expression [F(3,28) = 10.08, P < 0.001] (Fig. 2c). Unexpectedly, and contrary to previous mRNA levels, the striatal expression of Sirt1 (30%) and Pgc1a (27%) in the 6-OHDA group was significantly higher than in the Sham group. The striatal levels for later genes in 6-OHDA and 6-OHDA+EXE groups were similar and there were no significant differences between them (Fig. 2b, c).
Protein Analysis by Western Blotting
The striatal Ampk, Bdnf, and Th protein levels were shown in Fig. 3 a, d, and e, respectively. A significant difference was evident by interaction analysis of all groups in Ampk [F(3,16) = 352.8, P < 0.001], Bdnf [F(3,16) = 231.9, P < 0.001], and Th [F(3,16) = 265.9, P < 0.001] levels. These protein levels in animals administered 6-OHDA were significantly reduced compared with the Sham group. Exercise in the 6-OHDA+EXE group significantly increased the Ampk (60%), Bdnf (72%), and Th (66%) levels compared with sedentary 6-OHDA animals, but these levels significantly were lower than the Sham group (P < 0.001).
Surprisingly, the Sirt1 protein level in all of groups increased significantly (~ 30%) compared with the Sham group [F(3,16) = 269.3, P < 0.001] (Fig. 3b), also Pgc1α protein level in the Sham group was significantly lower (~ 20%) than the other groups [F(3,16) = 38.7, P < 0.001] (Fig. 3c). Here were no statistically significant differences between 6-OHDA and 6-OHDA+EXE groups in Sirt1 and Pgc1α protein levels (Fig. 3b, c).
Discussion
The aim of the current study was to examine the effect of exercise on the neuroprotection of dopaminergic neuron, rotational behavior and some mitochondrial factors following a 6-OHDA–induced cell death. Using the rat model of PD, we showed that 6-OHDA injection resulted in behavioral and mitochondrial disorders. Furthermore, treadmill exercise ameliorated the behavioral impairment in rats with a significant effect on Bdnf and Th expression levels (Figs. 2d, e and 3d, e). We observed that regular treadmill exercise resulted in increase of mRNAs and proteins in the striatum of S+EXE rats (Figs. 2, 3). This was different to what has been reported previously by Tillerson et al. (2003). One difference between the current study and that was the age of the experimental animals (aged vs. young). In the present study, the rats were young. Thus, age is an important factor affecting the expression of biochemical factors following exercise, even in the healthy rat. Similar results with our observations, on the heart and liver, were reported by previous studies (Bayod et al. 2012; Oliveira et al. 2014). Furthermore, Bayod et al. (2012) demonstrated that Ampk, Sirt1, and Pgc1a genes are expressed in young human after exercise, indicating that mitochondrial biogenesis-related genes are readily activated in young individuals, and that these responses are attenuated in older individuals. Muscle heat generation during exercise can play a role in the activation of the Ampk–Sirt1–Pgc1a pathway and mitochondrial biogenesis (Bayod et al. 2012). Mitochondrial abnormality, mainly in the brain, plays a vital role in the development of PD (Chong et al. 2012). Many studies reported that exercise is an effective intervention that upregulates important factors of mitochondrial biogenesis and neurogenesis, e.g., Pgc-1α (Patki and Lau 2011; Kang et al. 2013; Oliveira et al. 2014; LaHue et al. 2016). In the present study, the exercise after the 6-OHDA injection resulted in attenuation of behavioral abnormality and an increase of the genes expression, especially Th level, that confirm neuroprotective effects of exercise on the dopaminergic system. This finding is supported by the previous study that assessed preventive exercise before 6-OHDA injection (Carvalho et al. 2013; Rezaee et al. 2019b). In contrast, Real et al. (2017) indicated exercise-induced stress causes neurodegeneration and increases rotations in exercised rats compare with the control group.
It has been also demonstrated that exercise leads to increased expression of neurotrophic factors especially Bdnf, resulting in neuroprotection and increase of Th protein level (Pothakos et al. 2009) that has been confirmed in the present study (Figs. 2d, e and 3d, e). Indeed, the efficiency of every proposed experimental protocol on PD is confirmed by measuring the Th levels (Phillipson 2014; Real et al. 2017).
Consistent with what has been previously reported (Aguiar Jr et al. 2016; Garcia et al. 2017), and contrary to what has been anticipated, we observed 6-OHDA injection leading to increased Sirt1 and Pgc1a mRNA and protein level 30 days after the 6-OHDA injection (Figs. 2b, c and 3b, c). This indicated continuous mitochondrial transcription and activity of metabolic factors, probably to compensate for the cellular damage caused by 6-OHDA (Patki and Lau 2011). However, these observations suggested that increasing expression of these factors were not sufficient to prevent the reduction of Th protein level and behavioral disorder in the 6-OHDA sedentary group (Figs. 1, 2, 3e). Since in this group, regardless of compensatory increase in expression of these genes, disorders of behavioral and neurodegeneration are not attenuated. On the other hand, 14-day light exercise after the lesion reduced these abnormalities (Figs. 1, 2, 3). It is not clear why exercise can alleviate disorders in PD. Probably, exercise increases blood flow for the removal of the neurotoxin from the various brain regions (Mabandla et al. 2004). Furthermore, exercise by enhancing the expression of Ampk, Sirt1, and Pgc1a, as upstream of Bdnf gene, enhances mitochondrial biogenesis, brain plasticity, and neuroprotective effects due to neurotrophins and reduces of behavioral disorders and brain insults (McMurphy et al. 2019; Miranda et al. 2019; Rezaee et al. 2019b).
Several explanations can account for the differences in conclusions of studies involving animal models of PD. These include the induction method used and severity of lesion, the type and intensity of the exercise regimen, and the time of starting the exercise after the induction of lesion (Gerecke et al. 2010; Garcia et al. 2017). Inconsistent with previous studies (Fisher et al. 2004; Yoon et al. 2007; Pothakos et al. 2009; Gerecke et al. 2010), in the current study, we observed that the exercise exerted a protective effect against behavioral disorder (asymmetry rotation) (Fig. 1). The rotational test produces a useful parameter for evaluating behavioral deficits and the imbalances of dopamine in striatum of the unilateral rat model of PD (Tuon et al. 2012). Loss of Th expression and increase of apomorphine-induced turning behavior displayed that our model of 6-OHDA MFB injections caused dopaminergic degeneration. We found that the exercised animals with 6-OHDA injection when compared with the 6-OHDA sedentary group showed a reduction in the number of turns that reflects a protective effect of exercise on dopaminergic neurons. This result is reinforced by larger Th expression in the striatum of this group that occurred during the 14-day training. Bdnf as upstream gene of Th is upregulated also in the 6-OHDA+EXE group (Figs. 2, 3e). One of the few studies published that the exercise exerts neuroprotective effects on the dopaminergic system, partly through the upregulation of Bdnf (Tajiri et al. 2010). Bdnf is an essential vital protein that is involved in learning and memory and can improve brain plasticity. Moreover, the expression of Bdnf is a protective mechanism against toxicity (Wrann Christiane et al. 2013; Sleiman et al. 2016; Xia et al. 2017). On the other hand, regardless of increase of Th level in the 6-OHDA+EXE group compare with the 6-OHDA sedentary group, its level was significantly lower than control rats in the Sham group (Figs. 2, 3e). It is important to note that there was a 14-day delay before the beginning of exercise (after the surgery) in the present study. Since depletion of dopamine in the striatum is reportedly maximal 1 week after 6-OHDA infusion into the medial forebrain bundle (Yoon et al. 2007), in this study, starting training protocol after the short time of surgery might have better results. Garcia et al. (2017) also reported only in the third month after injection that the changes in Th level were similar to control animals and its level in closer assessments to injection was significantly lower than the Sham group. Hence, probably longer times after surgery, more than 1 month, are necessary to the rehabilitation of the deficits up to normal level.
In the current study, our lesion protocol was successful in causing a rat model of PD. As shown, the 6-OHDA injection increased apomorphine-induced rotation behavior and dopamine degeneration that is confirmed by loss of Th expression in response to 6-OHDA. On the other hand, the exercise following the surgery could reduce behavioral (apomorphine-induced rotation) and non-behavioral impairments and increase neuroprotection. However, a compensatory increase of Sirt1 and Pgc1a expression in the sedentary 6-OHDA group were not lead to the attenuation of behavioral disorder and dopamine loss. Therefore, based on the findings, the compensatory regulation of Sirt1 and Pgc-1α is not a useful strategy for treating PD. It seems that exercise triggers mechanisms on CNS resulted in neuroprotective effects and mitochondrial recovery in the 6-OHDA animal models. Hence, light treadmill exercise provides a therapeutic response for the treatment of PD.
References
Aguiar AS Jr, Duzzioni M, Remor AP, Tristão FSM, Matheus FC, Raisman-Vozari R, Latini A, Prediger RD (2016) Moderate-intensity physical exercise protects against experimental 6-hydroxydopamine-induced hemiparkinsonism through Nrf2-antioxidant response element pathway. Neurochem Res 41(1–2):1–9. https://doi.org/10.1007/s11064-015-1709-8
Bayod S, Del Valle J, Lalanza J, Sanchez-Roige S, de Luxan-Delgado B, Coto-Montes A, Canudas A, Camins A, Escorihuela RM, Pallas M (2012) Long-term physical exercise induces changes in sirtuin 1 pathway and oxidative parameters in adult rat tissues. Exp Gerontol 47(12):925–935. https://doi.org/10.1016/j.exger.2012.08.004
Cantó C, Auwerx J (2009) PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 20(2):98–105. https://doi.org/10.1097/MOL.0b013e328328d0a4
Carvalho MM, Campos FL, Coimbra B, Pêgo JM, Rodrigues C, Lima R, Rodrigues AJ, Sousa N, Salgado AJ (2013) Behavioral characterization of the 6-hydroxidopamine model of Parkinson’s disease and pharmacological rescuing of non-motor deficits. Mol Neurodegener 8:14. https://doi.org/10.1186/1750-1326-8-14
Chen W, Qiao D, Liu X, Shi K (2017) Treadmill exercise improves motor dysfunction and hyperactivity of the corticostriatal glutamatergic pathway in rats with 6-OHDA-induced Parkinson’s disease. Neural Plast 2017. https://doi.org/10.1155/2017/2583910
Cho H-S, Shin M-S, Song W, Jun T-W, Lim B-V, Kim Y-P, Kim C-J (2013) Treadmill exercise alleviates short-term memory impairment in 6-hydroxydopamine-induced Parkinson’s rats. J Exerc Rehabil 9(3):354–361. https://doi.org/10.12965/jer.130048
Choe M, Koo B-S, An GJ, Jeon S (2012) Effects of treadmill exercise on the recovery of dopaminergic neuron loss and muscle atrophy in the 6-OHDA lesioned Parkinson’s disease rat model. Korean J Physiol Pharmacol 16(5):305–312. https://doi.org/10.4196/kjpp.2012.16.5.305
Chong ZZ, Shang YC, Wang S, Maiese K (2012) SIRT1: new avenues of discovery for disorders of oxidative stress. Expert Opin Ther Targets 16(2):167–178. https://doi.org/10.1517/14728222.2012.648926
Corona J, Duchen M (2015) PPARγ and PGC-1α as therapeutic targets in Parkinson’s. Neurochem Res 40(2):308–316. https://doi.org/10.1007/s11064-014-1377-0
Costa ROD, Gadelha-Filho CVJ, Costa AEMD, Feitosa ML, Jo DPD, Lucena JDD, Aquino PEAD, Lima FAV, Neves KRT, Barros Viana GSD (2017) The treadmill exercise protects against dopaminergic neuron loss and brain oxidative stress in parkinsonian rats. Oxid Med Cell Longev:2017. https://doi.org/10.1155/2017/2138169
Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, Jakowec MW (2004) Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res 77(3):378–390. https://doi.org/10.1002/jnr.20162
Garcia PC, Real CC, Britto LR (2017) The impact of short and long-term exercise on the expression of arc and AMPARs during evolution of the 6-hydroxy-dopamine animal model of Parkinson’s disease. J Mol Neurosci 61(4):542–552. https://doi.org/10.1007/s12031-017-0896-y
Gerecke KM, Jiao Y, Pani A, Pagala V, Smeyne RJ (2010) Exercise protects against MPTP-induced neurotoxicity in mice. Brain Res 1341:72–83. https://doi.org/10.1016/j.brainres.2010.01.053
Hsueh S-C, Chen K-Y, Lai J-H, Wu C-C, Yu Y-W, Luo Y, Hsieh T-H, Chiang Y-H (2018) Voluntary physical exercise improves subsequent motor and cognitive impairments in a rat model of Parkinson’s disease. Int J Mol Sci 19(2):508–521. https://doi.org/10.3390/ijms19020508
Kang C, Chung E, Diffee G, Ji LL (2013) Exercise training attenuates aging-associated mitochondrial dysfunction in rat skeletal muscle: role of PGC-1α. Exp Gerontol 48(11):1343–1350. https://doi.org/10.1016/j.exger.2013.08.004
LaHue SC, Comella CL, Tanner CM (2016) The best medicine? The influence of physical activity and inactivity on Parkinson’s disease. Mov Disord 31(10):1444–1454. https://doi.org/10.1002/mds.26728
Mabandla M, Kellaway L, Gibson ASC, Russell VA (2004) Voluntary running provides neuroprotection in rats after 6-hydroxydopamine injection into the medial forebrain bundle. Metab Brain Dis 19(1–2):43–50. https://doi.org/10.1023/B:MEBR.0000027416.13070.c3
McMurphy T, Huang W, Liu X, Siu JJ, Queen NJ, Xiao R, Cao L (2019) Hypothalamic gene transfer of BDNF promotes healthy aging in mice. Aging Cell 18:e12846–e12846. https://doi.org/10.1111/acel.12846
Miranda M, Morici JF, Zanoni MB, Bekinschtein P (2019) Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci 13(363). https://doi.org/10.3389/fncel.2019.00363
Oliveira NR, Marques SO, Luciano TF, Pauli JR, Moura LP, Caperuto E, Pieri BL, Engelmann J, Scaini G, Streck EL (2014) Treadmill training increases SIRT-1 and PGC-1α protein levels and AMPK phosphorylation in quadriceps of middle-aged rats in an intensity-dependent manner. Mediat Inflamm 2014. https://doi.org/10.1155/2014/987017
Patki G, Lau Y-S (2011) Impact of exercise on mitochondrial transcription factor expression and damage in the striatum of a chronic mouse model of Parkinson’s disease. Neurosci Lett 505(3):268–272. https://doi.org/10.1016/j.neulet.2011.10.036
Petzinger G, Holschneider D, Fisher B, McEwen S, Kintz N, Halliday M, Toy W, Walsh J, Beeler J, Jakowec M (2015) The effects of exercise on dopamine neurotransmission in Parkinson’s disease: targeting neuroplasticity to modulate basal ganglia circuitry. Brain Plast 1(1):29–39. https://doi.org/10.3233/BPL-150021
Phillipson OT (2014) Management of the aging risk factor for Parkinson’s disease. Neurobiol Aging 35(4):847–857. https://doi.org/10.1016/j.neurobiolaging.2013.10.073
Pothakos K, Kurz MJ, Lau Y-S (2009) Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson’s disease with severe neurodegeneration. BMC Neurosci 10(1):6–20. https://doi.org/10.1186/1471-2202-10-6
Razgado-Hernandez LF, Espadas-Alvarez AJ, Reyna-Velazquez P, Sierra-Sanchez A, Anaya-Martinez V, Jimenez-Estrada I, Bannon MJ, Martinez-Fong D, Aceves-Ruiz J (2015) The transfection of BDNF to dopamine neurons potentiates the effect of dopamine D3 receptor agonist recovering the striatal innervation, dendritic spines and motor behavior in an aged rat model of Parkinson’s disease. PLoS One 10(2):e0117391. https://doi.org/10.1371/journal.pone.0117391
Real CC, Garcia PC, Britto LRG (2017) Treadmill exercise prevents increase of neuroinflammation markers involved in the dopaminergic damage of the 6-OHDA Parkinson’s disease model. J Mol Neurosci 63(1):36–49. https://doi.org/10.1007/s12031-017-0955-4
Rezaee Z, Marandi S, Alaei H, Esfarjani F, Feizollahzadeh S (2019a) Effects of preventive treadmill exercise on the recovery of metabolic and mitochondrial factors in the 6-hydroxydopamine rat model of Parkinson’s disease. Neurotox Res 35(4):908–917. https://doi.org/10.1007/s12640-019-0004-x
Rezaee Z, Marandi SM, Alaei H, Esfarjani F (2019b) The effect of preventive exercise on the neuroprotection in 6-hydroxydopamine-lesioned rat brain. Appl Physiol Nutr Metab 44(12):1267–1275. https://doi.org/10.1139/apnm-2018-0545
Sleiman SF, Henry J, Al-Haddad R, El Hayek L, Haidar EA, Stringer T, Ulja D, Karuppagounder SS, Holson EB, Ratan RR (2016) Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 5:e15092. https://doi.org/10.7554/eLife.15092
Steiner JL, Murphy EA, McClellan JL, Carmichael MD, Davis JM (2011) Exercise training increases mitochondrial biogenesis in the brain. J Appl Physiol (1985) 111(4):1066–1071. https://doi.org/10.1152/japplphysiol.00343.2011
Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, Wang F, Baba T, Tayra JT, Morimoto T, Jing M, Kikuchi Y, Kuramoto S, Agari T, Miyoshi Y, Fujino H, Obata F, Takeda I, Furuta T, Date I (2010) Exercise exerts neuroprotective effects on Parkinson’s disease model of rats. Brain Res 1310:200–207. https://doi.org/10.1016/j.brainres.2009.10.075
Tillerson JL, Caudle WM, Reverón ME, Miller GW (2003) Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson's disease. Neuroscience 119(3):899–911. https://doi.org/10.1016/S0306-4522(03)00096-4
Tuon T, Valvassori SS, Lopes-Borges J, Luciano T, Trom CB, Silva LA, Quevedo J, Souza CT, Lira FS, Pinho RA (2012) Physical training exerts neuroprotective effects in the regulation of neurochemical factors in an animal model of Parkinson’s disease. Neuroscience 227:305–312. https://doi.org/10.1016/j.neuroscience.2012.09.063
Tuon T, Valvassori SS, Dal Pont GC, Paganini CS, Pozzi BG, Luciano TF, Souza PS, Quevedo J, Souza CT, Pinho RA (2014) Physical training prevents depressive symptoms and a decrease in brain-derived neurotrophic factor in Parkinson’s disease. Brain Res Bull 108:106–112. https://doi.org/10.1016/j.brainresbull.2014.09.006
Tuon T, Souza PS, Santos MF, Pereira FT, Pedroso GS, Luciano TF, De Souza CT, Dutra RC, Silveira PC, Pinho RA (2015) Physical training regulates mitochondrial parameters and neuroinflammatory mechanisms in an experimental model of Parkinson’s disease. Med Cell Longev 2015. https://doi.org/10.1155/2015/261809
Wrann Christiane D, White James P, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin Jiandie D, Greenberg Michael E, Spiegelman Bruce M (2013) Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab 18(5):649–659. https://doi.org/10.1016/j.cmet.2013.09.008
Xia D-Y, Huang X, Bi C-F, Mao L-L, Peng L-J, Qian H-R (2017) PGC-1α or FNDC5 is involved in modulating the effects of aβ1−42 oligomers on suppressing the expression of BDNF, a beneficial factor for inhibiting neuronal apoptosis, aβ deposition and cognitive decline of APP/PS1 Tg mice. Front Cell Neurosci 9(65). https://doi.org/10.3389/fnagi.2017.00065
Yoon M-C, Shin M-S, Kim T-S, Kim B-K, Ko I-G, Sung Y-H, Kim S-E, Lee H-H, Kim Y-P, Kim C-J (2007) Treadmill exercise suppresses nigrostriatal dopaminergic neuronal loss in 6-hydroxydopamine-induced Parkinson’s rats. Neurosci Lett 423:12–17. https://doi.org/10.1016/j.neulet.2007.06.031
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and have been approved by the Ethic Committee for Animal Experiments at the University of Isfahan (IR.UI.REC.1396,008).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rezaee, Z., Marandi, S.M., Alaei, H. et al. Exercise-Induced Neuroprotection in the 6-Hydroxydopamine Parkinson’s Disease Model. Neurotox Res 38, 850–858 (2020). https://doi.org/10.1007/s12640-020-00189-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-020-00189-x