Abstract
Spinal cord injury (SCI) is one major cause of death and results in long-term disability even in the most productive periods of human lives with few efficacious drugs. Autophagy is a potential therapeutic target for SCI. In the present study, we examined the role of lithium in functional recovery in the rat model of SCI and explored the related mechanism. Locomotion tests were employed to assess the functional recovery after SCI, Western blotting and RT-PCT to determine the level of p-ERK and LC3-II as well as p62, immunofluorescence imaging to localize LC3 and p62. Here, we found that both the expression of LC3-II and p62 were increased after SCI. However, lithium chloride enhanced the level of LC3-II while abrogated the abundance of p62. Furthermore, lithium treatment facilitated ERK activation in vivo, and inhibition of MEK/ERK signaling pathway suppressed lithium-evoked autophagy flux. Taken together, our results illustrated that lithium facilitated functional recovery by enhancing autophagy flux.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord injury (SCI) is one important cause of death and results in long-term disability even in the most productive periods of human lives. Depending on the severity of the injury, SCI may cause dysfunction of organs and cognitive impairments. SCI causes both primary injury including direct mechanical tissue damage and secondary injury induced by the initial injury (Beattie et al. 2002). Secondary injury processes including inflammation and excitotoxicity (Casella et al. 2006) may occur over hours and days after initial trauma, further intensify tissue damages. Due to the irreversibility and severity of the primary injury, the secondary injury offers a therapeutic opportunity.
Autophagy is a lysosome-dependent intracellular catabolic process for the degradation of cytoplasmic constituents (Levine and Klionsky 2004). Lines of evidence suggest that autophagy is involved in SCI. Due to the dysfunction of autophagy flux (Lipinski et al. 2015), increased markers of autophagy are observed in the early stage of SCI, which represents the accumulation of dysfunctional autophagosomes (C. L. Liu et al. 2008; Clark et al. 2008; Lai et al. 2008). Aggregates of toxic protein and defective organelles contribute to cell death (Erlich et al. 2007). Liu et al. (2015) found that SCI causes lysosomal dysfunction which contributes to autophagy disruption and neuronal apoptosis (S. Liu et al. 2015).
Lithium has various neuroprotective properties including autophagy regulation (Sarkar et al. 2005). However, the role of lithium in autophagy has long remained a controversy, with both positive and negative functions proposed. Some evidence shows that lithium enhanced autophagy (Sarkar et al. 2005; Heiseke et al. 2009; O'Donovan et al. 2015; Chang et al. 2011; Del Grosso et al. 2016; Kim et al. 2013; Shimada et al. 2012; Hou et al. 2015), but some vivo studies did not show a consistent result (Fabrizi et al. 2017; Li et al. 2010). Li et al. (2010) demonstrates that lithium reduces apoptosis and autophagy after neonatal hypoxia-ischemia. This discrepancy could be due to the lithium concentration and lithium-mediated tissue protection. Thus, the role of lithium in autophagy regulation should be examined thoroughly.In the present study, we investigated the effect of lithium on acute SCI in vivo and in vitro. We found improved function recovery after lithium treatment in rat models of traumatic SCI. Moreover, lithium administration restored the dysfunctional autophagy-lysosomal pathway. We also showed that ERK signaling pathway was involved in the therapeutic effect of lithium on acute SCI. These results suggested that lithium contributes to the recovery of acute SCI.
Material and Method
Animal Model of Spinal Cord Injury
Sprague-Dawley rats were purchased from the Animal Center of the Chinese Academy of Sciences of Shanghai, China. Procedures with experimental animals were approved by the Animal Care and Use Committee of Henan University of Chinese Medicine. Adult male Sprague-Dawley rats (8–10 weeks of age) weighing approximately 255 g were anesthetized. Along the midline of the dorsum to expose the vertebral column, a transection of the spinal cord at T4 level was performed. Sham group rats underwent the same surgical procedure but not transected. After surgery, lithium chloride was injected intraperitoneally at a dose of 50 mg/kg/day (mean serum level of 0.7~0.8 mM) until 10 days. At the same time, another group was treated with 50-mg/kg/day lithium chloride and 50-mg/kg/day chloroquine. Equivalent normal saline injections were treated as vehicle control. The experimental groups were divided into sham group, SCI group, and lithium-treated SCI group (lithium-treated group).
Locomotion Tests
According to the well-known open field BBB locomotor scale, motor performance was scored in an open field scale at 1, 3, 10, 20, and 25 days postoperation (Lenzi et al. 2016). Briefly, the BBB locomotion rating scale scores range from 0 points (complete paralysis) to 21 points (normal locomotion). The scale was developed using the natural progression of locomotion recovery in rats with SCI. The paw placement, joint movements, weight bearing, and coordination among the limbs were used to evaluate the BBB locomotion scale. The inclined plane test was performed as described (Seibenhener et al. 2004. In brief, rats were placed horizontally on a smooth tilted board. From the horizontal position (0°), the increment of the angle was 5°. The maximum angle at which a rat could maintain its position for 10 s without falling was recorded.
Cell Culture and Treatments
VSC4.1 motoneurons were formed by fusion of dissociated embryonic rat ventral spinal cord neuron with mouse N18TG2 neuroblastoma cells (Smith et al. 1994). VSC4.1 cells were maintained on culture dishes coated with poly-L-ornithine (PLO) (Sigma) in DMEM:F12 (1:1) containing Glutamax supplemented with 2% FBS, 1% N1 (Sigma), and 1% NEAA (referred to as VSC4.1 complete medium).
Primary septal cultures were prepared from the newborn Sprague-Dawley (SD) rats (provided by laboratory animal center in Henan Province license SCXK 2010-0002) as described previously (Mattson et al. 1992). Briefly, collected neurons were seeded in a concentration of 1 × 106 cells/cm2. Cells were cultured for 6 days in medium, consisting of Eagle’s minimum essential medium containing 10-mm sodium bicarbonate, 1% glucose, 1-mm L-glutamine, 20-mm KCl, 1-mm sodium pyruvate, and 10% (v/v) heat-inactivated fetal bovine serum (Sigma). After 24 h in culture, the culture medium was replaced with neurobasal medium containing B27 supplements (Invitrogen) in a humidified atmosphere (6% CO2, 94% room air) at 37 °C. Approximately 12 h later, 5-μM cytosine arabinoside was added to the dishes to prevent the growth of non-neuronal cells. Cells were treated with 2-mM lithium chloride (Sigma-Aldrich, USA), 1-μM PD184352 (MEK inhibitor; Mattingly et al. 2006) (MedChem Express, USA), and 1-μM SCH772984 (ERK1/2 inhibitor; D. J. L. Wong et al. 2014) (MedChem Express, USA) for 2 h.
Immunofluorescence Imaging
Following the manufacturer’s protocol, the green fluorescent protein (GFP)-light chain 3 (LC3) expression vector (Cell Biolabs, USA) was transfected with Lipofectamine 2000 (Lifescience, USA) for 24 h; cells were then treated with 2-mM lithium chloride in the absence or presence of 1-μM SCH772984 for 2 h. The localization of GFP was directly observed with a laser scanning confocal microscope (Carl Zeiss Microscopy). The number of GFP-LC3 punta per cell was counted.
Cells were treated with 2-mM lithium chloride in the absence or presence of 1-μM SCH772984 for 2 h, and then washed and fixed with 4% paraformaldehyde for 20 min at room temperature. For blocking unspecific binding, cells were incubated with 5% normal goat serum (Yeason, China) and 0.5% Triton in PBS (Biyuntian, China) for 30 min. Cells then were exposed to antibody p62 (Abcam, USA) at 4 °C for. 24 h. After rinsing four times with PBS, cells were incubated with DyLight® 488-conjugated goat anti-rabbit antibody (1:3000, Biomart, USA) for 1 h. After washing, the nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole) ProLong® Gold Mountant (Molecular Probes, Thermo Fisher Scientific, USA) for 30 min at room temperature. The slides and coverslips were mounted with FluorSaveTM Reagent (Calbiochem, USA). Images were taken on a LSM 5 EXCITER confocal laser-scanning microscope (Zeiss, Germany) with a water-immersion Plan-Neofluar 40×/1.3 NA differential interference contrast and analyzed with the instrument’s software.
Western Blotting
Spinal cord tissue samples and cells were collected after the scarification of rats. The spinal cord segment at the contusion epicenter was dissected and frozen at −80 °C (D. Zhang et al. 2016a). Tissues were lysed in RIPA buffer (Beyotime, Shanghai, China) with 1% phenylmethylsulfonyl fluoride (Beyotime) and 1% protein phosphatase inhibitor (Beyotime) on ice for 30 min. The samples were centrifuged at 14,000 rpm and 4 °C for 20 min. The supernatant was removed and used for Western blotting. Total protein (40–60 μg) was loaded onto SDS-PAGE, and then transferred to PVDF membranes and blocked in 5% non-fat milk/Tris-buffered saline/Tween-20 (TBST) at room temperature for 2 h. Membranes were probed overnight at 4 °C with anti-LC3B (1:500, Abcam), p62 (1:1000, Abcam), ERK1/2 (1:1000, Abcam), p-ERK1/2 (ERK1 phospho T202, ERK2 phospho T185) (1:1000, Abcam), and GAPDH (1:500, Biyuntian, Shanghai). After incubation with horseradish peroxidase-conjugated anti-rabbit secondary antibody (1:2000, Sigma, USA) for 1 h at room temperature, the bands were visualized with enhanced chemiluminescence reagents (Sigma, USA). Densitometric quantification of the membranes was performed using ImageJ.
Quantification of mRNA Expression
Total RNA was extracted in TriFast (Peqlab, USA) according to the manufacturer’s instructions. After DNAse digestion, reverse transcription of total RNA was performed using Transcriptor High Fidelity cDNA Synthesis Kit (Roche Diagnostics, USA). Real-time polymerase chain reaction (RT-PCR) of the respective genes were set up in a total volume of 20 μl using 40 ng of cDNA, 500-nM forward and reverse primer, and 2× GoTaq® qPCR Master Mix (Biyuntian, China) according to the manufacturer’s protocol. Cycling conditions were as follows: initial denaturation at 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s, 58 °C for 15 s, and 68 °C for 20 s. For amplification, the following primers were used (5′ > 3′ orientation):
Tbp (TATA box-binding protein) (Z. Zhang et al. 2016b):
forward (5′–3′): ACTCCTGCCACACCAGCC
reverse (5′–3′): GGTCAAGTTTACAGCCAAGATTCA
LC3 (Gong et al. 2016)
forward (5′–3′): GGAAGAATGACAGATGAC
reverse (5′–3′): CTTTCAATCTGTTGGCTG
p62
forward (5′–3′): GGAAGCTGAAACATGGGCAC
reverse (5′–3′): TGGGATCCTCTGATGGAGCA
Specificity of PCR products was confirmed by analysis of a melting curve. Real-time PCR amplifications were performed on a CFX96 Real-time System (Bio-Rad, USA), and all experiments were done in duplicate. The housekeeping gene Tbp (TATA binding protein) was amplified to standardize the amount of sample RNA. Relative quantification of gene expression was achieved using the ΔCT method as described (Matsuzaki et al. 2015).
Gene Silencing
For silencing, 1 × 105 cells (12-well plate) were seeded 24 h before the experiment in antibiotic-free medium. Cells were transfected with 5-μl/1000 μl ONTARGETplus Cdk5 siRNA (10 μM, Santa Cruz Biotechnology, USA) and ON-TARGETplus non-targeting siRNA (10 μM, Santa Cruz Biotechnology, USA) using the cationic lipid DharmaFECT 1 transfection reagent (0.5 μl/1000 μl, Thermo Fisher Scientific) according to the manufacturer’s protocol. Twenty-four hours after transfection, experiments were performed.
Statistics
Data are provided as means ± SEM, n represents the number of independent experiments. All data were tested for significance using unpaired Student t test or ANOVA followed by post hoc Bonferroni test which was applied when multiple comparisons between different groups were made. Only results with p < 0.05 were considered statistically significant.
Results
Lithium Facilitates Locomotor Recovery After SCI
To evaluate the therapeutic role of lithium in locomotor recovery after SCI, functional recovery was assessed after SCI by BBB scores and inclined plane test. As shown in Fig. 1A, the BBB scores of SCI group and lithium-treated SCI group were lower than the sham group. Furthermore, there was no significant difference in BBB scores between the SCI group and lithium-treated group until 10 days later. BBB scores of lithium-treated group increased compared with the SCI group at 10 days after injury. Likewise, the inclined plane test scores showed the same trend (Fig. 1B). The scores of the inclined plane test were higher in lithium-treated group at 10, 20, and 25 days after injury.
Autophagy Flux Inhibition Abrogates Functional Recovery of Lithium Treatment
To confirm the therapeutic effects of lithium on locomotor recovery which was autophagy-lysosome pathway-dependent, locomotion tests were utilized. As shown in Fig. 2, lithium-induced functional recovery was significantly blunted by an autophagy-lysosome pathway inhibitor chloroquine (CQ).
Lithium Enhances LC3-II Level While Decreases p62 Expression in Acute SCI
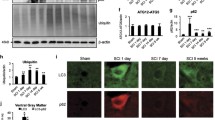
Given that the conversion of LC3-I to LC3-II is pivotal for the formation of autophagosomes, the levels of LC3 and p62 were determined by Western blot and qPCR. As illustrated in Fig. 3, the expression of LC3-II both in protein level and in transcriptional level was significantly higher after lithium treatment. Furthermore, the protein abundance of p62 also decreased after lithium treatment compared with that of the SCI group.
Lithium enhances autophagy in acute SCI. a Original Western blot showing LC3-II and p62 abundance following treatment with lithium in SCI models. b and d Arithmetic means ± SEM (n = 5) showing LC3-II and p62 abundance following treatment with Lithium in SCI models. c and e Arithmetic means ± SEM (n = 6) showing LC3-II and p62 mRNA levels following treatment with lithium in SCI models. *p < 0.05, **p < 0.01, ***p < 0.001 indicate significant difference
Lithium Activates MEK/ERK Pathway in Acute SCI
To better understand the mechanism of the lithium therapy, MEK/ERK pathway involved in lithium-evoked autophagy flux in SCI was explored by Western blot. As illustrated in Fig. 4, lithium administration remarkably increased the level of p-ERK1/2 compared with the SCI group. These findings suggested that lithium may activate ERK1/2 pathway to facilitate autophagy in acute SCI.
Lithium activates MEK/AMPK pathway in acute SCI. a Original Western blot showing p-ERK1/2 abundance following treatment with Lithium in SCI models. b Arithmetic means ± SEM (n = 5) showing p-ERK1/2 abundance following treatment with lithium in SCI models. c Arithmetic means ± SEM (n = 5) showing p-ERK1/2 mRNA level following treatment with lithium in SCI models. *p < 0.05, **p < 0.01, ***p < 0.001 indicate significant difference
Lithium Promoted Autophagy Flux by Activating MEK/AMPK Pathway in Cells
To confirm our findings that lithium activated MEK/ERK pathway in vitro, PD184352 (MEK inhibitor; Mattingly et al. 2006) and SCH772984 (ERK1/2 inhibitor; D. J. L. Wong et al. 2014) were utilized to treat the septal neurons and VSC4.1 cells. As illustrated in Fig. 5, PD184352 and SCH772984 blunted lithium-induced autophagy in cells. Additionally, ERK1/2 silencing also reduced lithium-evoked LC3-II expression while reversed the effect of lithium upon p62 level in both kinds of cells (Fig. 6).
Lithium promoted autophagy flux by activating MEK/ERK pathway in cells. Original Western blot showing LC3-II and p62 abundance following treatment with lithium in the presence of PD184352 and SCH772984 in neurons. a Arithmetic means ± SEM (n = 5) showing LC3-II abundance following treatment with lithium in the presence of PD184352 and SCH772984 in neurons. b Arithmetic means ± SEM (n = 5) showing LC3-II mRNA level following treatment with lithium in the presence of PD184352 and SCH772984 in neurons. (C) Arithmetic means ± SEM (n = 5) showing p62 abundance following treatment with lithium in the presence of PD184352 and SCH772984 in neurons. d Arithmetic means ± SEM (n = 5) showing LC3-II mRNA level following treatment with lithium in the presence of SCH772984 in VSC4.1cells. e Arithmetic means ± SEM (n = 5) showing p62 mRNA level following treatment with lithium in the presence of SCH772984 in VSC4.1 cells. *p < 0.05), **p < 0.01, ***p < 0.001 indicate significant difference
Silencing of ERK1/2 abrogated lithium-induced autophagy flux in cells. a Original Western blot showing LC3-II and p62 abundance with lithium treatment in erk +/+ and erk −/− septal neurons. b Arithmetic means ± SEM (n = 5) showing LC3-II with lithium treatment in erk +/+ and erk −/− septal neurons. c Arithmetic means ± SEM (n = 5) showing p62 abundance with lithium treatment in erk +/+ and erk −/− septal neurons. d Arithmetic means ± SEM (n = 5) showing p62 mRNA level with lithium treatment in erk +/+ and erk −/− VSC4.1 cells. e Arithmetic means ± SEM (n = 5) showing LC3-II mRNA level with Lithium treatment in erk +/+ and erk −/− VSC4.1 cells
*p < 0.05, **p < 0.01, ***p < 0.001 indicate significant difference
The activation of autophagy by lithium was further confirmed by the localization of LC3-II and p62 by fluorescence microscope. As illustrated in Fig. 7A and B, cells were transfected with the GFP-LC3 plasmid transiently. The number of GFP-LC3 puncta in VSC4.1 cells significantly increased following lithium treatment, and SCH772984 resulted in a significant degradation of green fluorescence in cells. Moreover, immunofluorescence of p62 significantly decreased after lithium treatment, which could be blunted by SCH772984 (Fig. 7C and D). Taken together, our results indicated that lithium enhanced autophagy flux via MEK/ERK signaling pathway.
Localization of LC3 and p62 in VSC4.1 cells. a Images acquired by fluorescence microscopy, green fluorescent protein (GFP)-light chain 3 (LC3)-transfected cells treated with lithium treatment in the presence of SCH772984 in VSC4.1 cells. b Quantitative analysis of GFP-LC3 positive puncta per cell. c Immunofluorescence of p62 in VSC4.1 cells with lithium treatment in the presence of SCH772984. d Nuclei were counter stained with DAPI and graph represents average p62 dot numbers per cell after the indicated treatments (n = 30 cells for each group in three independent experiments). **p < 0.01, ***p < 0.001 indicate significant difference
Discussion
Our observations in a rat model of acute SCI support the notion that lithium enhances autophagy via activating ERK signaling pathway and facilitates locomotor recovery after SCI. Increased markers of autophagy after SCI (Kim et al. 2013; Chang et al. 2011; Hou et al. 2015; Y. W. Wong et al. 2011) represent the accumulation of autophagosomes, the mechanism however remains controversial. Whether the accumulation of autophagosomes is due to enhanced autophagy flux and autophagosome synthesis or decreased flux is uncertain. Enhancing autophagy-lysosomal function may represent a potential and effective therapeutic target against SCI.
LC3 and p62 are standard markers for autophagy. LC3, a central protein in the autophagy pathway, functions in autophagy substrate selection and autophagosome biogenesis. Conjugated to the head group of the lipid phosphatidylethanolamine through a series of ubiquitin-like reactions, the lipid modified form of LC3 is referred to as LC3-II and involved in autophagosome membrane expansion and fusion events (Weidberg et al. 2011). Once autophagosome containing LC3-II fuses with the lysosome, subsequent degradation of its contents by lysosomal hydrolysis occurs. P62 is an endogenous autophagy substrate and polyubiquinated protein degraded by lysosome (Cohen-Kaplan et al. 2016a). By binding to LC3-II via its LC3-interacting region, P62 is degraded along with its substrate (Y. Wang et al. 2017). In this study, we addressed the issue of flux by assessing the levels of p62 and LC3-II. Enhanced levels of LC3-II and p62 were observed in the model of SCI, suggesting that increased autophagy markers are at least partly induced by suppression of degradation pathway (D. Zhang et al. 2016a; C. Wang et al. 2016).
Lithium has various neuroprotective properties including autophagy regulation (Sarkar et al. 2005; Kim et al. 2013; Chang et al. 2011; Hou et al. 2015; Y. W. Wong et al. 2011). However, both positive and negative functions are proposed. Sets of evidence demonstrate that lithium enhanced autophagy (Sarkar et al. 2005; Heiseke et al. 2009; O'Donovan et al. 2015; Chang et al. 2011; Del Grosso et al. 2016; Kim et al. 2013; Shimada et al. 2012; Hou et al. 2015), but others (Fabrizi et al. 2017; Li et al. 2010) suggest that lithium reduces apoptosis and autophagy after neonatal hypoxia-ischemia. This discrepancy may be explained by the different lithium concentrations and lithium-mediated tissue protection. As our results show, lithium enhanced the protein abundance of LC3-II while decreased the level of p62 compared with the SCI group, suggesting a positive effect of lithium on autophagy-lysosomal pathway. Furthermore, lithium may also result in a selective autophagy activation with a decrease in autophagoproteasome. Autophagoproteasome, a cytosolic membrane-bound compartment containing both LC3 and ubiquitin antigens, is derived from the inclusion of ubiquitin-proteasome structures within autophagosomes containing cytoplasmic material at various stages of degradation (Klionsky 2016). P62 could either target the proteasome for degradation or deliver ubiquitinated substrates for degradation through ubiquitin (Ub)-proteasome system (UPS) (Seibenhener et al. 2004; Cohen-Kaplan et al. 2016b). Therefore, p62 decrease may lead to a decrease in the uptake of proteasome within LC3 autophagy vacuoles (Lenzi et al. 2016), which causes LC3 accumulation.
ERK activity has also been associated with autophagy (J. Wang et al. 2009) and autophagic cell death in many non-neuronal cellular models in response to different stresses (Ogier-Denis et al. 2000). In human ovarian cancer cells, cytoplasmic sequestration of ERK promotes autophagy (Bartholomeusz et al. 2008). Moreover, direct ERK activation by overexpression of constitutively active MEK can promote autophagy without any other stimulus (Corcelle et al. 2006). Reports show that the effect of lithium is dependent on the activation of MEK/ERK signaling pathway (Pardo et al. 2003; Hull et al. 2014; Zassadowski et al. 2015). In the present study, we confirmed the activation of ERK by lithium both in vivo and in vitro. In vivo study, we found that lithium promoted activation of ERK1/2 and improved locomotor recovery after SCI. In vitro study, MEK/ERK inhibitors abrogated the effects of lithium on autophagy in primary septal neurons and VSC 4.1 cells.
Clinical trials indicate the safety and efficacy of lithium in the therapies of traumatic diseases and various neuropsychiatric diseases (Yang et al. 2012; Y. W. Wong et al. 2011; Raja et al. 2015; Guttuso 2016). Our study confirmed that lithium treatment promotes the recovery after SCI. Further research regarding neuroprotective effect of lithium by oral administration would be of great help in the clinical usage of lithium for SCI patients.
In conclusion, our results demonstrate that lithium promotes autophagy after SCI and may provide potential therapeutic interventions for SCI.
References
Bartholomeusz C, Rosen D, Wei C, Kazansky A, Yamasaki F, Takahashi T, Itamochi H, Kondo S, Liu J, Ueno NT (2008) PEA-15 induces autophagy in human ovarian cancer cells and is associated with prolonged overall survival. Cancer res 68(22):9302–9310
Beattie MS, Hermann GE, Rogers RC, Bresnahan JC (2002) Cell death in models of spinal cord injury. Prog Brain res 137:37–47
Casella GT, Bunge MB, Wood PM (2006) Endothelial cell loss is not a major cause of neuronal and glial cell death following contusion injury of the spinal cord. Exp Neurol 202(1):8–20
Chang JW, Choi H, Cotman SL, Jung YK (2011) Lithium rescues the impaired autophagy process in CbCln3(Δex7/8/Δex7/8) cerebellar cells and reduces neuronal vulnerability to cell death via IMPase inhibition. J Neurochem 116(4):659–668
Clark RS, Bayir H, Chu CT, Alber SM, Kochanek PM, Watkins SC (2008) Autophagy is increased in mice after traumatic brain injury and is detectable in human brain after trauma and critical illness. Autophagy 4(1):88–90
Cohen-Kaplan V, Ciechanover A, Livneh I (2016a) p62 at the crossroad of the ubiquitin-proteasome system and autophagy. Oncotarget 7(51):83833–83834
Cohen-Kaplan V, Livneh I, Avni N, Fabre B, Ziv T, Kwon YT, Ciechanover A (2016b) p62- and ubiquitin-dependent stress-induced autophagy of the mammalian 26S proteasome. Proc Natl Acad Sci U S a 113(47):E7490–E7499
Corcelle E, Nebout M, Bekri S, Gauthier N, Hofman P, Poujeol P, Fénichel P, Mograbi B (2006) Disruption of autophagy at the maturation step by the carcinogen lindane is associated with the sustained mitogen-activated protein kinase/extracellular signal-regulated kinase activity. Cancer res 66(13):6861–6870
Del Grosso A, Antonini S, Angella L, Tonazzini I, Signore G, Cecchini M 2016 Lithium improves cell viability in psychosine-treated MO3.13 human oligodendrocyte cell line via autophagy activation. E. R. Bongarzone, ed., J Neurosci Res, 94(11), pp.1246–1260.
Erlich S, Mizrachy L, Segev O, Lindenboim L, Zmira O, Adi-Harel S, Hirsch JA, Stein R, Pinkas-Kramarski R (2007) Differential interactions between Beclin 1 and Bcl-2 family members. Autophagy 3(6):561–568
Fabrizi C, Pompili E, Somma F, De Vito S, Ciraci V, Artico M, Lenzi P, Fornai F, Fumagalli L (2017) Lithium limits trimethyltin-induced cytotoxicity and proinflammatory response in microglia without affecting the concurrent autophagy impairment. Journal of Applied Toxicology: JAT 37(2):207–213
Gong X, Wang H, Ye Y, Shu Y, Deng Y, He X, Lu G, Zhang S (2016) miR-124 regulates cell apoptosis and autophagy in dopaminergic neurons and protects them by regulating AMPK/mTOR pathway in Parkinson's disease. Am J Transl res 8(5):2127–2137
Guttuso T (2016) Low-dose lithium adjunct therapy associated with reduced off-time in Parkinson's disease: a case series. J Neurol Sci 368:221–222
Heiseke A, Aguib Y, Riemer C, Baier M, Schätzl HM (2009) Lithium induces clearance of protease resistant prion protein in prion-infected cells by induction of autophagy. J Neurochem 109(1):25–34
Hou L, Hou L, Xiong N, Liu L, Huang J, Han C, Zhang G, Li J, Xu X, Lin Z, Wang T 2015 Lithium protects dopaminergic cells from rotenone toxicity via autophagy enhancement. BMC Neuroscience, 16(1), p.82
Hull M, Lee E, Lee T, Anand N, LaLone V, Parameswaran N (2014) Lithium chloride induces TNFα in mouse macrophages via MEK-ERK-dependent pathway. J Cell Biochem 115(1):71–80
Kim EC, Meng H, Jun AS (2013) Lithium treatment increases endothelial cell survival and autophagy in a mouse model of Fuchs endothelial corneal dystrophy. Br J Ophthalmol 97(8):1068–1073
Klionsky DJ (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12(1):1–222
Lai Y, Hickey RW, Chen Y, Bayir H, Sullivan ML, Chu CT, Kochanek PM, Dixon CE, Jenkins LW, Graham SH, Watkins SC, Clark RS (2008) Autophagy is increased after traumatic brain injury in mice and is partially inhibited by the antioxidant gamma-glutamylcysteinyl ethyl ester. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism 28(3):540–550
Lenzi P, Lazzeri G, Biagioni F, Busceti CL, Gambardella S, Salvetti A, Fornai F (2016) The autophagoproteasome a novel cell clearing organelle in baseline and stimulated conditions. Front Neuroanat 10:78
Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6(4):463–477
Li Q, Li H, Roughton K, Wang X, Kroemer G, Blomgren K, Zhu C (2010) Lithium reduces apoptosis and autophagy after neonatal hypoxia-ischemia. Cell Death dis 1(7):e56
Lipinski MM, Wu J, Faden AI, Sarkar C (2015) Function and mechanisms of autophagy in brain and spinal cord trauma. Antioxid Redox Signal 23(6):565–577
Liu CL, Chen S, Dietrich D, Hu BR (2008) Changes in autophagy after traumatic brain injury. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism 28(4):674–683
Liu S, Sarkar C, Dinizo M, Faden AI, Koh EY, Lipinski MM, Wu J (2015) Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death. Cell Death dis 6(1):e1582
Matsuzaki T, Iwasa T, Tungalagsuvd A, Munkhzaya M, Kawami T, Yamasaki M, Murakami M, Kato T, Kuwahara A, Yasui T, Irahara M (2015) The responses of hypothalamic NPY and OBRb mRNA expression to food deprivation develop during the neonatal-prepubertal period and exhibit gender differences in rats. International Journal of Developmental Neuroscience: the Official Journal of the International Society for Developmental Neuroscience 41:63–67
Mattingly RR, Kraniak JM, Dilworth JT, Mathieu P, Bealmear B, Nowak JE, Benjamins JA, Tainsky MA, Reiners JJ Jr (2006) The mitogen-activated protein kinase/extracellular signal-regulated kinase kinase inhibitor PD184352 (CI-1040) selectively induces apoptosis in malignant schwannoma cell lines. J Pharmacol Exp Ther 316(1):456–465
Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE (1992) Beta-amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience 12(2):376–389
O'Donovan TR, Rajendran S, O'Reilly S, O'Sullivan GC, McKenna SL 2015 Lithium modulates autophagy in esophageal and colorectal cancer cells and enhances the efficacy of therapeutic agents in vitro and in vivo. I. Ulasov, ed. PloS one, 10(8), p.e0134676
Ogier-Denis E, Pattingre S, El Benna J, Codogno P (2000) Erk1/2-dependent phosphorylation of Galpha-interacting protein stimulates its GTPase accelerating activity and autophagy in human colon cancer cells. J Biol Chem 275(50):39090–39095
Pardo R, Andreolotti AG, Ramos B, Picatoste F, Claro E (2003) Opposed effects of lithium on the MEK-ERK pathway in neural cells: inhibition in astrocytes and stimulation in neurons by GSK3 independent mechanisms. J Neurochem 87(2):417–426
Raja M, Soleti F, Bentivoglio AR (2015) Lithium treatment in patients with Huntington's disease and suicidal behavior. Movement Disorders: Official Journal of the Movement Disorder Society 30(10):1438–1438
Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC (2005) Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol 170(7):1101–1111
Seibenhener ML, Babu JR, Geetha T, Wong HC, Krishna NR, Wooten MW (2004) Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol Cell Biol 24(18):8055–8068
Shimada K, Motoi Y, Ishiguro K, Kambe T, Matsumoto SE, Itaya M, Kunichika M, Mori H, Shinohara A, Chiba M, Mizuno Y, Ueno T, Hattori N (2012) Long-term oral lithium treatment attenuates motor disturbance in tauopathy model mice: implications of autophagy promotion. Neurobiol dis 46(1):101–108
Smith RG, Alexianu ME, Crawford G, Nyormoi O, Stefani E, Appel SH (1994) Cytotoxicity of immunoglobulins from amyotrophic lateral sclerosis patients on a hybrid motoneuron cell line. Proc Natl Acad Sci U S a 91(8):3393–3397
Wang J, Whiteman MW, Lian H, Wang G, Singh A, Huang D, Denmark T (2009) A non-canonical MEK/ERK signaling pathway regulates autophagy via regulating Beclin 1. J Biol Chem 284(32):21412–21424
Wang C, Liu C, Gao K, Zhao H, Zhou Z, Shen Z, Guo Y, Li Z, Yao T, Mei X (2016) Metformin preconditioning provide neuroprotection through enhancement of autophagy and suppression of inflammation and apoptosis after spinal cord injury. Biochem Biophys res Commun 477(4):534–540
Wang Y, Zhu WG, Zhao Y (2017) Autophagy substrate SQSTM1/p62 regulates chromatin ubiquitination during the DNA damage response. Autophagy 13(1):212–213
Weidberg H, Shpilka T, Shvets E, Abada A, Shimron F, Elazar Z (2011) LC3 and GATE-16 N termini mediate membrane fusion processes required for autophagosome biogenesis. Dev Cell 20(4):444–454
Wong YW, Tam S, So KF, Chen JY, Cheng WS, Luk KD, Tang SW, Young W (2011) A three-month, open-label, single-arm trial evaluating the safety and pharmacokinetics of oral lithium in patients with chronic spinal cord injury. Spinal Cord 49(1):94–98
Wong DJ, Robert L, Atefi MS, Lassen A, Avarappatt G, Cerniglia M, Avramis E, Tsoi J, Foulad D, Graeber TG, Comin-Anduix B, Samatar A, Lo RS, Ribas A (2014) Antitumor activity of the ERK inhibitor SCH772984 [corrected] against BRAF mutant, NRAS mutant and wild-type melanoma. Mol Cancer 13(1):194
Yang ML, Li JJ, So KF, Chen JY, Cheng WS, Wu J, Wang ZM, Gao F, Young W (2012) Efficacy and safety of lithium carbonate treatment of chronic spinal cord injuries: a double-blind, randomized, placebo-controlled clinical trial. Spinal Cord 50(2):141–146
Zassadowski F, Pokorna K, Ferre N, Guidez F, Llopis L, Chourbagi O, Chopin M, Poupon J, Fenaux P, Ann Padua R, Pla M, Chomienne C, Cassinat B (2015) Lithium chloride antileukemic activity in acute promyelocytic leukemia is GSK-3 and MEK/ERK dependent. Leukemia 29(12):2277–2284
Zhang Z, Sun S, Du C, Li W, Zhang J, Zhu Y, Liu P, Xing Y (2016a) Effects of leptin on Na+/Ca2+ exchanger in PC12 cells. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology 40(6):1529–1537
Zhang D, Xuan J, Zheng BB, Zhou YL, Lin Y, Wu YS, Zhou YF, Huang YX, Wang Q, Shen LY, Mao C, Wu Y, Wang XY, Tian NF, Xu HZ, Zhang XL 2016b Metformin improves functional recovery after spinal cord injury via autophagy flux stimulation. Molecular neurobiology, pp.1–15
Author information
Authors and Affiliations
Contributions
PL, ZZ, QW, and RG performed experiments and analyzed data. WM designed the project. PL drafted the manuscript. All authors corrected and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Liu, P., Zhang, Z., Wang, Q. et al. Lithium Chloride Facilitates Autophagy Following Spinal Cord Injury via ERK-dependent Pathway. Neurotox Res 32, 535–543 (2017). https://doi.org/10.1007/s12640-017-9758-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-017-9758-1