Abstract
Aluminum (Al) is a significant environmental contaminant. While a good deal of research has been conducted on the acute neurotoxic effects of Al, little is known about the effects of longer-term exposure at human dietary Al levels. Therefore, the purpose of this study was to investigate the effects of 60-day Al exposure at low doses for comparison with a model of exposure known to produce neurotoxicity in rats. Three-month-old male Wistar rats were divided into two major groups: (1) low aluminum levels, and (2) a high aluminum level. Group 1 rats were treated orally by drinking water for 60 days as follows: (a) control—received ultrapure drinking water; (b) aluminum at 1.5 mg/kg b.w., and (c) aluminum at 8.3 mg/kg b.w. Group 2 rats were treated through oral gavages for 42 days as follows: (a) control—received ultrapure water; (b) aluminum at 100 mg/kg b.w. We analyzed cognitive parameters, biomarkers of oxidative stress and acetylcholinesterase (AChE) activity in hippocampus and prefrontal cortex. Al treatment even at low doses promoted recognition memory impairment seen in object recognition memory testing. Moreover, Al increased hippocampal reactive oxygen species and lipid peroxidation, reduced antioxidant capacity, and decreased AChE activity. Our data demonstrate that 60-day subchronic exposure to low doses of Al from feed and added to the water, which reflect human dietary Al intake, reaches a threshold sufficient to promote memory impairment and neurotoxicity. The elevation of oxidative stress and cholinergic dysfunction highlight pathways of toxic actions for this metal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminum (Al), a metal which accounts for about 8 % of the earth´s crust, is currently a significant environmental contaminant (Exley 2012). Industrialized societies, the burning of fossil fuels, and the versatile properties of Al compounds have all contributed to increased biological availability of this nonessential metal (Exley 2013).
Oral uptake is an important route by which humans are exposed to Al. Al salts are added to commercially prepared foods for numerous reasons, such as food coloring, as anticaking agents, for pH adjusting, as emulsifiers, as a stabilizing agent, to thicken gravies and sauces, as meat-binders, as a rising agent, for pickling vegetables and candying fruits, as buffers, and as neutralizing agents. Al is also added to urban water supplies and some bottled waters as a clarifying agent (Walton 2014). Nondietary sources of Al include vaccines, where Al salts are used as adjuvants to promote immune activation, and some topical applications, such as sunscreens and deodorants. Considerable amounts of Al are also contained in buffered aspirins and antacids (Bondy 2015).
The Joint FAO/WHO Expert Committee on Food Additives (Food and Agriculture Organization of the United Nations/World Health Organization) adjusted a tolerable weekly intake of Al for humans to 1 mg Al/Kg body weight (b.w.) (FAO/WHO 2007), which is routinely exceeded by humans. Greger (1993) estimates that Americans consume from 1 to 95 mg/Al/day in the form of Al additives and up to 10 mg/Al/day from natural sources. Moreover, the use of pharmaceutical elements containing Al considerably increases the human Al exposure. Antacids may provide doses of 50–1000 mg/day (Reinke et al. 2003).
The rate of Al absorption of the gastrointestinal tract is around 0.2 % (Priest et al. 1998). Upon reaching the blood, most of the metal is bound to transferrin which facilitates Al transport across the blood–brain barrier and into neurons and glia bearing transferrin receptors (Roskams and Connor 1990; Shirley and Lote 2005). Al can accumulate in different brain regions and its neurotoxicity has been demonstrated in cell culture, animal models, and humans (Bondy 2015; Walton 2014). Al accumulation in neurons has been related to learning and memory impairments, as well as motor incoordination and decrease in locomotor activity (Kasbe et al. 2015; Lakshmi et al. 2015). Altered cholinergic functions in pups and adulthood have also been reported (Ravi et al. 2000; Yellamma et al. 2010). Most Al studies have entailed doses of Al higher than the ones commonly found among human populations. However, human studies suggest that Al is a potential contributor to the onset, progression, or aggressiveness of neurodegenerative diseases (Walton 2014; Bondy 2015). Most epidemiological studies have shown a positive relationship between Al exposure from drinking water or occupational exposure and Alzheimer Disease (AD) (Wang et al. 2016; Walton 2014; Flaten 2001). Moreover, AD patients showed an increasing Al concentration in cerebral arteries that supply blood to the hippocampus (Bhattacharjee et al. 2013). Experimental evidence has also shown that Al can cause neurochemical and neuropathological changes as observed in AD patients (Walton 2014). However, there are still many inconsistencies in the epidemiological and toxicological data concerning Al and neurodegenerative diseases (Chin-Chan et al. 2015).
The full scale of mechanisms underlying Al neurotoxicity remains to be elucidated. For instance, Al3+ is a pro-oxidant acting on its own by binding to superoxide ion and promoting the formation of an aluminum superoxide radical ion, a species which is potentially more reactive than the superoxide radical (Exley 2004). The pro-oxidant activity of Al might also be explained by its synergistic action with iron promoting the Fenton reaction by reducing Fe(III) to Fe(II) (Ruipérez et al. 2012).
While a good deal of research has been conducted on the neurotoxic effects of Al, little is known about the effects of long-term exposure at human dietary Al levels. Moreover, with Al being a widespread environmental contaminant, it is crucial to explore the human Al intake threshold sufficient to promote adverse effects. Therefore, the purpose of this study was to investigate the effects of a 60-day Al exposure at low doses similar to human dietary levels on long-term object recognition memory, prefrontal cortex, and hippocampal reactive oxygen species levels, lipid peroxidation, total antioxidant capacity, and then compare these results with a model of exposure known to produce neurotoxicity in rats.
Materials and Methods
Animals
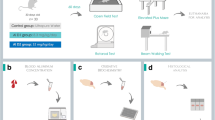
Three-month-old male Wistar rats (252–300 g) were obtained from the Central Animal Laboratory of the Federal University of Santa Maria, Rio Grande do Sul, Brazil. During treatment, rats were housed at a constant room temperature, humidity, and light cycle (12:12 h light–dark), giving free access to water and fed with a standard chow ad libitum. All experiments were conducted in compliance with the guidelines for biomedical research stated by the Brazilian Societies of Experimental Biology and approved by the Ethics Committee on Animal Use Experimentation of the Federal University of Pampa, Uruguaiana, Rio Grande do Sul, Brazil (Process Number: 028/2014).
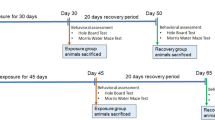
Rats were divided into two major groups: (1) low aluminum levels, and (2) high aluminum level. For group 1, low aluminum levels, 18 rats were subdivided (N = 6/each) and treated for 60 days as follows: (a) control—received ultrapure drinking water (Milli-Q, Merck Millipore Corporation. © 2012 EMD Millipore, Billerica, MA, USA); (b) aluminum at 1.5 mg/kg b.w./day based on human dietary levels according to a published protocol described by Walton (2007), at the reduced Al exposure for 60 days, and (c) aluminum at 8.3 mg/kg b.w./day, which corresponds to the same human dietary aluminum levels (1.5 mg/kg b.w.) when translated to an animal dose, based on a body surface area normalization method (Reagan-Shaw et al. 2008). For group 2, high aluminum level, 12 rats were subdivided (N = 6/each) and treated for 42 days as follows: (a) control—received ultrapure water through oral gavages; (b) aluminum at 100 mg/kg b.w./day, a protocol known to promote cognitive impairment in rats (Prakash and Kumar 2009).
Rat body weights were measured weekly. After 60 or 42 days of treatment, the animals were submitted to behavioral tests, as follows: control behavioral experiments (open field, plus maze, and hot plate (day 01) and object recognition test (days 02–06). At the end of the treatments and tests, animals were euthanized by decapitation, the brain was removed, and bilateral hippocampi and prefrontal cortex were quickly dissected out and homogenized in 50 mM Tris HCl, pH 7.4, (1/10, w/v). Afterward, samples were centrifuged at 2400g for 10 min at 4 °C and the resulting supernatant fraction was frozen at −80 °C for further assay. These brain structures were investigated because of their importance for consolidation of object recognition memory. They also seem to be a target for Al deposition in AD patients (Izquierdo and Medina 1997; Andrási et al. 2005; Rusina et al. 2011).
AlCl3· 6 H2O was purchased from Sigma-Aldrich (St Louis, MO, USA) and dissolved in ultrapure water (Milli-Q © 2012 EMD Millipore, Billerica, MA). Salts and reagents were of analytical grade obtained from Sigma and Merck (Darmstadt, Germany).
Aluminum Content in the Rats’ Feed
The Al content of rat chow was determined using an established method (House et al. 2012). Briefly, approximately 0.2 g of chow was digested in a 1:1 mixture of 15.8 M HNO3 and 30 % w/v H2O2 in a microwave oven, and the Al content of digests was measured by TH GFAAS (Transversely Heated Graphite Furnace Atomic Absorption Spectrometry).
Behavioral Testing
Open Field, Plus Maze, and Hot Plate
Exploratory and locomotor activities were analyzed to test whether any of the procedures impaired the rat behaviors, altering the memory tests results. Each rat was placed in the left quadrant of a 50 cm × 50 cm × 39 cm open field made with wood painted white, with a frontal glass wall. Black lines were drawn on the floor to divide it into 12 equal quadrants. Crossing and rearing, as measures for locomotor activity and exploration, respectively, were measured over 5 min (Bonini et al. 2006). To evaluate thereby anxiety state, rats were exposed to an elevated plus maze (Pellow et al. 1985). The maze had a central platform (5 × 5 cm), two open arms (50 cm long × 10 cm wide, with 0.5-cm-high borders), and two enclosed arms (50 cm deep × 10 cm wide, with 10-cm-high walls), elevated 50 cm above the ground. An animal was placed in the center of the apparatus facing the open arm and its locomotion was monitored by infrared sensors for 5 min. The time spent in the open arms was recorded. The pain threshold was measured using a hot plate test (Jacob et al. 1974) to ensure that treatment did not impair nociception. In the hot plate test, the animal is placed on the metal plate heated to 55 °C surrounded by a glass cylinder (13 × 17 cm). The latency (in seconds) of the hind paw-licking or jumping was measured. A cut-off time of 45 s was applied. Data from these tests were compared between the groups to ensure all rats presented any impairment in behavior that could affect the variables of interest in our study (i.e., memory measurements).
Object Recognition Memory Test
Chronic Al intake has been related with AD-related symptoms and disruption of object recognition as one of the earliest signs of AD (Walton 2014); thus, we proposed to investigate object recognition memory. After the treatments were completed, training and testing for the object recognition (OR) task were performed in an open-field arena (50 cm × 50 cm × 50 cm) built with polyvinyl chloride plastic, plywood, and transparent acrylic (Ennaceur and Delacour 1988; Mello-Carpes and Izquierdo 2013). Rats were first habituated to the apparatus during 20 min of free exploration for 4 consecutive days. For training, two different objects (a and b) were placed in the apparatus, and rats were allowed to freely explore the objects for 5 min. The objects were made of metal, glass, or glazed ceramic. Exploration was defined as sniffing or touching the objects with the nose and/or forepaws. Sitting on or turning around the objects were not considered as exploratory behaviors. A video camera was positioned over the OR arena, and the behavior was recorded using a video tracking system for offline analyses. After 24 h, in the test phase, one of the objects was randomly exchanged for a novel object (c), and the rats were reintroduced into the apparatus to freely explore the objects (familiar and new ones) for 5 min. To avoid confounding by lingering olfactory stimuli and preferences, the objects and the arena were cleaned with 70 % ethanol after each animal was tested. The time spent exploring the familiar and the novel object was recorded.
Biochemical Assay
Reactive Oxygen Species Levels
The levels of ROS in hippocampus and prefrontal cortex were determined by a spectrofluorometric method, as described by Loetchutinat et al. (2005). The supernatant fraction of the sample was diluted (1:10) in 50 mM Tris–Hcl (pH 7.4), and 2′, 7′-dichlorofluorescein diacetate (DCFH-DA; 1 mM) was added to the medium. DCFH-DA is enzymatically hydrolyzed by intracellular esterases to form nonfluorescent DCFH, which is then rapidly oxidized to form highly fluorescent 2´,7´-dichlorofluorescein (DCF) in the presence of ROS. DCF fluorescence intensity is proportional to the amount of ROS that is formed. The DCF fluorescence intensity emission was recorded at 520 nm (with 480 nm excitation) (SpectraMax M5 Molecular Devices, CA, USA) for 60 min at 15 min intervals. The ROS levels were expressed as fluorescence unit.
Lipid Peroxidation
The levels of lipid peroxidation in hippocampus and prefrontal cortex were measured as malondialdehyde (MDA) using a colorimetric method, as previously described by Ohkawa et al. (1979), with modifications. An aliquot of each tissue was incubated with thiobarbituric acid 0.8 % (TBA), phosphoric acid buffer 1 % (H3PO4), and sodium dodecyl sulfate 0.8 % (SDS) at 100 °C for 60 min. The color reaction was measured at 532 nm against blanks (SpectraMax M5 Molecular Devices, CA, USA). The results were expressed as nanomoles of MDA per mg of protein.
Ferric Reducing/Antioxidant Power (FRAP) Assay
The total antioxidant capacity was measured in hippocampus and prefrontal cortex by FRAP assay (Benzie and Strain 1996). This method is based on the ability of sample to decrease ferric ion (Fe3+) to ferrous ion (Fe2+) which forms with 2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ) the chelate complex Fe+2-TPTZ. Briefly, 10 μL of the supernatant fraction of each tissue was added to 1 mL freshly prepared and prewarmed (37 °C) FRAP reagent (300 mM acetate buffer (pH 3.6), 10 mM TPTZ in 40 mM HCl, and 20 mM FeCl3 in the ratio of 10:1:1) in a test tube and incubated at 37∘C for 10 min. The absorbance of the blue-colored complex was read against a blank reagent (1 mL FRAP reagent + 10 μL distilled water) at 593 nm (SpectraMax M5 Molecular Devices, CA, USA). A standard dose–response curve of Trolox (50–1000 μM—water soluble analog of vitamin E) was prepared, and the FRAP assay is described. Results are presented with particular reference to Trolox equivalents.
Acetylcholinesterase (AChE) Activity
AChE is a marker of the loss of cholinergic neurons in the forebrain. The AChE activity was assessed by the Ellman et al. (1961). The reaction mixture was composed of 100 mM phosphate buffer pH (7.4) and 1 mM 5,5′-dithiobis-2-nitrobenzoic acid (DTNB). The method is based on the formation of a yellow anion, 4,4′-dithio-bis-acid nitrobenzoic after adding 0.8 mM acetylthiocholine iodide. The change in absorbance was measured for 2 min at 30 s intervals at 412 nm (SpectraMax M5 Molecular Devices, CA, USA). Results were expressed as micromoles of acetylthiocholine iodide hydrolyzed/min/mg of protein. Proteins were measured according to Bradford (1976) using bovine serum albumin as a standard.
Statistical Analysis
Data are expressed as mean ± SEM. The OR task results were converted to a percentage of total exploration time and were analyzed using a one-sample t test based on a theoretical mean of 50 %. Additional data of group 1 were analyzed by ANOVA followed by Bonferroni post hoc tests when appropriate. Data of group 2 were analyzed by Student´s t test. Values of p < 0.05 were considered significant and the significance level is indicated by asterisk (*p < 0.05; **p < 0.01; ***p < 0.001).
Results
Animal Weight, Fluid and Feed Intakes, and Al Content in the Rats’ Feed
Individual weights of rats were similar between groups at the start and end of interventions. Water and feed intakes were also similar between the groups (Table 1).
The total Al content in rats’ feed was 86.03 ± 4.76 μg Al/g/feed, which equated to a daily intake of Al from feed of 1.88 mg/Al/rat.
Behavioral Results
Open Field, Plus Maze, and Hot Plate
Rats were exposed to an open-field arena, a plus maze, and a hot plate after 42 or 60 days of treatment to verify exploratory and locomotor activity, anxiety, and pain threshold, respectively. Subchronic exposure to Al at different doses did not affect the number of crossings and rearings during the 5-min long free exploration session in the open field test (Table 2—open field). Similarly, AlCl3 had no effect on the time spent in the open arms during the plus maze session (Table 2—plus maze) and in time latency to reaction on the hot plate (Table 2—hot plate).
Object Recognition Memory Test
To investigate the effect of AlCl3 on object recognition long-term memory (LTM) consolidation, group 1 rats were treated for 60 days with Al at 1.5 or 8.3 mg/kg b.w./day and group 2 rats were exposed to Al at 100 mg/kg b.w./day for 42 days, as the control rats treated with ultrapure water, were trained in the OR learning task. All rats explored the two new objects (a and b) for a similar percentage of the total exploration time (about 50 % each, Fig. 1a, b) in the training session. 24 h after training, in the LTM testing session, control rats explored the novel object (c) significantly more than 50 % of the total exploration time (p = 0.0028 for control of group 1, Fig. 1a, LTM test; p = 0.0001 for control of group 2, Fig. 1b, LTM test). However, in all the Al-treated groups, animals spent about 50 % of the total exploration time exploring each object (a and c), without differences between the time spent for exploring the familiar (a) and the novel objects (c). (p = 0.55 for Al 1.5 mg/kg and p = 0.76 for Al 8.3 mg/kg of group 1, Fig. 1a, LTM test; p = 0.69 for Al 100 mg/kg of group 2, Fig. 1b, LTM test).
Effect of subchronic aluminum exposure to low (group 1) and high (group 2) doses on object recognition memory at the conclusion of the treatment periods. The two groups of rats were trained on the OR task for 4 days and were tested 24 h later to evaluate their long-term recognition memory (LTM) after 60 or 42 days of treatment for groups 1 and 2, respectively. In the training session, the animals were exposed to objects “a” and “b.” In the test session, the rats were exposed to a familiar object (a) and to a novel object (c). Data are expressed as mean ± SEM of the percentage of total exploration time. ***p < 0.001 in one-sample t-test, considering a theoretical mean of 50 % (N = 6)
Biochemical Results
Reactive Oxygen Species and Lipid Peroxidation Levels
Al treatment at different doses increased ROS levels in hippocampus (p = 0.0251 for control vs. Al 1.5 mg/kg and p = 0.0041 for control vs. Al 8.3 mg/kg, group 1, Fig. 2a; p = 0.0241 for control vs. Al 100 mg/kg, group 2, Fig. 2b). In prefrontal cortex, only Al at 8.3 mg/kg b.w. increased ROS levels (p = 0.0054 for control vs. Al 8.3 mg/kg, group 1, Fig. 2c).
Effect of subchronic aluminum exposure to low (group 1) and high (group 2) doses on levels of reactive oxygen species (ROS). Values of ROS on hippocampus (a, b) and prefrontal cortex (c, d). Data are expressed as mean ± SEM (N = 6). *p < 0.05, ***p < 0.001 (ANOVA followed by Bonferroni or Student’s t test). UF units of fluorescence
There was a significant increase on lipid peroxidation in hippocampus of Al-treated rats at major doses when compared with the controls groups (p = 0.0307 for control vs. Al 8.3 mg/kg, group 1, Fig. 3a; p = 0.0002 for control vs. Al 100 mg/kg, group 2, Fig. 3b) and no differences in MDA levels after Al exposure at 1.5 mg/kg b.w. (Figure 3a). No alteration on prefrontal cortex lipid peroxidation was observed in any group (Figs. 3c, d).
Effect of subchronic aluminum exposure to low (group 1) and high (group 2) doses on lipid peroxidation measurements. Values of MDA (malondialdehyde) on hippocampus (a, b) and prefrontal cortex (c, d). Data are expressed as mean ± SEM (n = 6). *p < 0.05, ***p < 0.001 (ANOVA followed by Bonferroni or Student’s t test)
Total Antioxidant Capacity—Ferric Reducing/Antioxidant Power (FRAP)
Al decreased the hippocampal total antioxidant capacity in all exposed groups, even at low levels (p = 0.0193 for control vs. Al 1.5 mg/kg and p = 0.0035 for control vs. Al 8.3 mg/kg, group 1, Fig. 4a; p = 0.0495 for control vs. Al 100 mg/kg, group 2, Fig. 4b). The antioxidant capacity of prefrontal cortex was decreased only in rats exposed to 100 mg/kg b.w. Al for 42 days (p = 0.0339 for control vs. Al 100 mg/kg, group 2, Fig. 4d).
Effect of subchronic aluminum exposure to low (group 1) and high (group 2) doses on total antioxidant capacity. Values of ferric reducing/antioxidant power (FRAP) on hippocampus (a, b) and prefrontal cortex (c, d). Data are expressed as mean ± SEM (n = 6). *p < 0.05, ***p < 0.001 (ANOVA followed by Bonferroni or Student’s t test)
Acetylcholinesterase (AChE) Activity
Aluminum exposure for 60 days at low levels or for 42 days at high levels decreased the enzymatic activity of AChE in hippocampus (p = 0.0116 for control vs. Al at 1.5 mg/kg and p = 0.0006 for control vs. Al at 8.3 mg/kg, group 1, Fig. 5a; p = 0.0467 for control vs. Al at 100 mg/kg, group 2, Fig. 5b). No change was observed in AChE activity in prefrontal cortex of aluminum-treated rats (Fig. 5c, d).
Effect of subchronic aluminum exposure to low (group 1) and high (group 2) doses on acetylcholinesterase (AChE) activity. Values of AChE activity on hippocampus (a, b) and prefrontal cortex (c, d). Data are expressed as mean ± SEM (n = 6). *p < 0.05, ***p < 0.001 (ANOVA followed by Bonferroni or Student’s t test)
Discussion
Our study aimed to investigate if Al exposure at human dietary levels could promote similar toxic effects found in a model for neurotoxicity induced by Al. Our results suggest that this metal reaches a threshold sufficient to promote neurotoxicity even at low doses. Here, we show that rats exposed to low doses of Al for 60 days could not recognize familiar objects as control rats do; this memory impairment was the same observed in rats treated with Al at a dose 66 times higher. This cognitive dysfunction in Al-treated rats came together with a marked hippocampal oxidative stress condition, with increased ROS production, lipid peroxidation, and decreased antioxidant capacity, as well as decreased AChE activity.
Human exposure to Al is practically inevitable and, due to the omnipresence of this metal in our daily life, it is difficult to maintain a “safe” Al intake level. The tolerable weekly intake of Al for humans has been set at 1 mg Al/Kg b.w. dose (FAO/World Health, Organization 2007), which is routinely exceeded by humans. Total human Al intake is difficult to determine due to its high bioavailability and ubiquity. Greger (1993) estimates that Americans consume 1–10 mg/Al/day from fresh food. In addition, 50 % of Americans consume up to 24 mg/day, 45 % between 24 and 95 mg, and about 5 % ingest more than 95 mg/Al/day in the form of Al additives. The above-mentioned study is one of the most accurate, taking into account the Al amounts in manufactured food. Moreover, the Al intake through the gastrointestinal tract can reach 126–5000 mg/day from ingested pharmaceuticals, antacids in particular (Reinke et al. 2003; Shaw and Tomljenovic 2013).
In our study, all rats including controls received 1.88 mg/Al/day from their standard feed; considering the rats mean body weights of 300 g, this amount represents 6.26 mg/Al/kg/b.w./day from feed. For the experimental groups, taking into account their mean body weights of 300 g, the total amount of Al exposure was for group 1, low aluminum levels: (a) 1.5 mg/Al/kg b.w.–2.33 mg/Al/day (0.45 mg/Al from water plus 1.88 mg/Al from feed); (b) 8.3 mg/Al/kg b.w.–4.37 mg/Al/day (2.49 mg/Al from water plus 1.88 mg/Al from feed), and for group 2, high aluminum level: (c) 100 mg/Al/kg b.w–31.88 mg/Al/day (30 mg/Al from gavage plus 1.88 mg/Al from feed). Considering that all rats consumed approximately the same amount of Al in their feed, the presence of Al in their drinking water points to this additional Al via a different exposure route as a critical issue for achieving the threshold responsible for the development of the behavioral dysfunction in Al-treated rats.
Aluminum chloride (100 mg/kg/day, p.o., 6 weeks) is a well-known model for dementia resulting in progressive deterioration of spatial memory in Morris water maze associated with oxidative damage and mitochondria impairment (Prakash and Kumar 2009, 2013). Here, we showed that this dose level of Al also promotes recognition memory dysfunction and oxidative stress in rats. It was not surprising, considering that this high dose of Al is usually used as a dementia model (Prakash and Kumar 2009, 2013) and previous studies demonstrated that it promotes cognitive deficits (Kasbe et al. 2015; Lakshmi et al. 2015). However, according to our best knowledge, the present study has been the only one to evaluate the effects of 60-day low Al exposure, similar to human dietary Al exposure, on memory. We developed the same behavioral evaluations in rats exposed to low Al doses and, surprisingly, the neurotoxicity effects are practically the same as those induced by the 100 mg/kg/day dose level. Namely, Al exposure for 60 days at 1.5 or 8.3 mg/kg/day in adult life could cause recognition memory deficits in rats.
Walton (2009) developed a longitudinal study exposing rats to Al at an equivalent-human dietary level. Rats treated from 12 months of age until their deaths developed memory impairment and displayed AD-like behaviors during old age. The present study found that Al at the same dose used by Walton (1.5 mg/kg/day) but, for a much shorter time, 60 days (approximating 70 months for humans or 6 years) (Everitt 1991), was sufficient to start the neurotoxicity process of this metal. Moreover, Al at 8.3 mg/kg/day, the amount equivalent to human Al exposure translated to the animal species used in this study, also impairs memory consolidation.
The neurotoxicity mechanisms induced by Al require intensive investigation. Oxidative stress seems to be one possible pathway by which this nonessential metal acts (Exley 2004). The brain, especially the hippocampus, is very sensitive to oxidative stress, mainly by its low antioxidant capacity and high level of lipid content (Kumar and Gill 2014; Erfani et al. 2015). Our results show that Al exposure caused redox imbalance in the hippocampus, as evident from an increase in ROS generation, independent of the dose. Additionally, elevation of MDA levels and a significant decline of total antioxidant capacity were observed in response to Al exposure at all Al doses investigated. On the other hand, in the prefrontal cortex, only the intermediate Al dose investigated here (8.3 mg/kg b.w.) increased ROS generation and only the high dose (100 mg/kg/b.w.) impaired the total antioxidant capacity. These results suggest that the specific toxic effects of this metal are dependent on the contamination threshold that is achieved, and the duration of exposure, but that a low dose is able to promote hippocampal toxicity and memory impairments.
Another way by which Al impairs memory is by its interference with the cholinergic system (Ravi et al. 2000; Yellamma et al. 2010). Our data reveal hippocampal dysfunction of AChE activity induced by Al at high and low doses of exposure, without altering the prefrontal cortex enzyme activity. AChE activity is a marker for loss of cholinergic neurons, and memory dysfunction is associated mainly with cholinergic neuron loss in several regions of the brain (Whitehouse et al. 1982; Fraser and MacVicar 1996). The current study shows that the AlCl3 exposure, even at low doses, decreases the activity of AChE in rat hippocampus. However, the available literature has shown that Al exposure can both stimulate and inhibit enzymatic function (Prakash and Kumar 2009; Lakshmi et al. 2015; Noremberg et al. 2016). Al has been shown to produce a biphasic effect on AChE, stimulating AChE at low levels or short exposures and inhibiting AChE at high doses and/or long exposures periods. Among the suggested hypothesis, the biphasic effect of Al on the AChE activity may be due to the direct effect of the metal or due to the peroxidation-induced changes in the structure of membrane following Al exposure (Kumar 1999). Therefore, it is likely that peroxidation of membrane structures may be responsible for the inhibited hippocampal AChE found after Al exposure.
The mechanism responsible for the formation and consolidation of long-term memory has been extensively studied (Izquierdo and Medina 1997; Alarcon et al. 2004), while the mechanism of action of Al in promoting memory dysfunction is unclear. However, considering that the hippocampus is the main structure required for memory formation and consolidation, we can affirm, based on our results, that the hippocampal oxidative stress and damage, as well as the AChE impairment induced by Al exposure may be at least partially responsible for the memory impairments.
Conclusions
Our results demonstrate that a 60-day subchronic exposure to low doses of Al from feed and added to the water, which reflect human dietary Al intake, reaches a threshold sufficient to promote memory impairment. These effects are similar to a known model of Al-induced neurotoxicity at high levels. Moreover, the hippocampal oxidative stress and the cholinergic dysfunction found in the three doses investigated highlight a pathway of action of this metal.
References
Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A (2004) Chromatin acetylation, memory, and LTP are impaired in CBP± mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 42:947–959
Andrási E, Páli N, Molnár Z, Kösel S (2005) Brain aluminum, magnesium and phosphorus contents of control and Alzheimer-diseased patients. J Alzheimers Dis 7(4):273–284
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: the FRAP assay. Anal Biochem 239:70–76
Bhattacharjee S, Zhao Y, Hill JM, Culicchia F, Kruck TP, Percy ME, Pogue AI, Walton JR, Lukiw WJ (2013) Selective accumulation of aluminum in cerebral arteries in Alzheimer’s disease (AD). J Inorg Biochem 126:35–37
Bondy SC (2015) Low levels of aluminum can lead to behavioral and morphological changes associated with Alzheimer’s disease and age-related neurodegeneration. Neurotoxicology 52:222–229
Bonini JS, Bevilaqua LR, Zinn CG, Kerr DS, Medina JH, Izquierdo I et al (2006) Angiotensin II disrupts inhibitory avoidance memory retrieval. Horm Behav 50:308–313
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein–dye binding. Anal Biochem 72:248–254
Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B (2015) Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci 10(9):124. doi:10.3389/fncel.2015.00124
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Ennaceur A, Delacour J (1988) A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res 31:47–59
Erfani S, Khaksari M, Oryan S, Shamsaei N, Aboutaleb N, Nikbakht F, Jamali-Raeufy N, Gorjipour F (2015) Visfatin reduces hippocampal CA1 cells death and improves learning and memory deficits after transient global ischemia/reperfusion. Neuropeptides 49:63–68. doi:10.1016/j.npep.2014.12.004
Everitt AV (1991) Ageing rat colonies at the University of Sydney. Proc Aust Assoc Gerontol 26:79–82
Exley C (2004) The pro-oxidant activity of aluminum. Free Radic Biol Med 3:380–387
Exley C (2012) Elucidating aluminium´s exposome. Curr Inorg Chem 2:3–7
Exley C (2013) Human exposure to aluminium. Environ Sci Process Impacts 10:1807–1816
Flaten TP (2001) Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res Bull 2:187–196
Fraser DD, MacVicar BA (1996) Cholinergic-dependent plateau potential in hippocampal CA1 pyramidal neurons. J Neurosci 16:4113–4128
Greger JL (1993) Aluminum metabolism. Annu Rev Nutr 13:42–63
House E, Esiri M, Forster G, Ince PG, Exley C (2012) Aluminium, iron and copper in human brain tissues donated to the medical research council’s cognitive function and ageing study. Metallomics 4:56–65
Izquierdo I, Medina JH (1997) Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem 68:285–316
Jacob JJ, Tremblay EC, Colombel MC (1974) Enhancement of nociceptive reactions by naloxone in mice and rats. Psychopharmacologia 37:217–223
Kasbe P, Jangra A, Lahkar M (2015) Mangiferin ameliorates aluminium chloride-induced cognitive dysfunction via alleviation of hippocampal oxido-nitrosative stress, proinflammatory cytokines and acetylcholinesterase level. J Trace Elem Med Biol 31:107–112
Kumar S (1999) Aluminium-induced biphasic effect. Med Hypotheses 52:557–559
Kumar V, Gill KD (2014) Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology 41:154–166
Lakshmi BV, Sudhakar M, Prakash KS (2015) Protective effect of selenium against aluminum chloride-induced Alzheimer’s disease: behavioral and biochemical alterations in rats. Biol Trace Elem Res 1:67–74
Loetchutinat C, Kothan S, Dechsupa S, Meesungnoen J, Jay-Gerin J, Mankhetkorn S (2005) Spectrofluorometric determination of intracellular levels of reactive oxygen species in drug-sensitive and drug-resistant cancer cells using the 2′,7′-dichlorofluorescein diacetate assay. Rad Phys Chem 72:323–331
Mello-Carpes PB, Izquierdo I (2013) The nucleus of the solitary tract → nucleus paragigantocellularis → locus coeruleus → CA1 region of dorsal hippocampus pathway is important for consolidation of object recognition memory. Neurobiol Learn Mem 100:56–63
Noremberg S, Bohrer D, Schetinger MR, Bairros AV, Gutierres J, Gonçalves JF et al (2016) Silicon reverses lipid peroxidation but not acetylcholinesterase activity induced by long-term exposure to low aluminum levels in rat brain regions. Biol Trace Elem Res 1:77–85
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167
Prakash A, Kumar A (2009) Effect of N-acetyl cysteine against aluminium-induced cognitive dysfunction and oxidative damage in rats. Basic Clin Pharmacol Toxicol 2:98–104
Prakash A, Kumar A (2013) Mitoprotective effect of Centella asiatica against aluminum-induced neurotoxicity in rats: possible relevance to its anti-oxidant and anti-apoptosis mechanism. Neurol Sci 8:1403–1409
Priest ND, Talbot RJ, Newton D, Day JP, King SJ, Fifield LK (1998) Uptake by man of aluminium in a public water supply. Hum Exp Toxicol 6:296–301
Ravi SM, Prabhu BM, Raju TR, Bindu PN (2000) Long-term effects of postnatal aluminium exposure on acetylcholinesterase activity and biogenic amine neurotransmitters in rat brain. Indian J Physiol Pharmacol 4:473–478
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 3:659–661
Reinke CM, Breitkreutz J, Leuenberger H (2003) Aluminium in over-the-counter drugs: risks outweigh benefits? Drug Saf 14:1011–1025
Roskams AJ, Connor JR (1990) Aluminum access to the brain: a role for transferrin and its receptor. Proc Natl Acad Sci 87:9024–9027
Ruipérez F, Mujika JI, Ugalde JM, Exley C, Lopez X (2012) Pro-oxidant activity of aluminum: promoting the Fenton reaction by reducing Fe(III) to Fe(II). J Inorg Biochem 117:118–123
Rusina R, Matěj R, Kašparová L, Kukal J, Urban P (2011) Higher aluminum concentration in Alzheimer’s disease after box-cox data transformation. Neurotox Res 4:329–333
Shaw CA, Tomljenovic L (2013) Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res 2–3:304–316
Shirley DG, Lote CJ (2005) Renal handling of aluminium. Nephron Physiol 101:99–103
Walton JR (2007) A longitudinal study of rats chronically exposed to aluminum at human dietary levels. Neurosci Lett 1:29–33
Walton JR (2009) Functional impairment in aged rats chronically exposed to human range dietary aluminum equivalents. Neurotoxicology 30:182–193
Walton JR (2014) Chronic aluminum intake causes Alzheimer’s disease: applying Sir Austin Bradford Hill’s causality criteria. J Alzheimers Dis 4:765–838. doi:10.3233/JAD-132204
Wang Z, Wei X, Yang J, Suo J, Chen J, Liu X, Zhao X (2016) Chronic exposure to aluminum and risk of Alzheimer’s disease: a meta-analysis. Neurosci Lett 610:200–206
Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR (1982) Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215:1237–1239
World Health Organization (2007) Safety evaluation of certain food additives and contaminants. Food Additive Series: 58. http://whqlibdoc.who.int/trs/WHO TRS 940 eng.pdf
Yellamma K, Saraswathamma S, Nirmala Kumari B (2010) Cholinergic system under aluminium toxicity in rat brain. Toxicol Int 2:106–112
Acknowledgments
The study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; Programa Nacional de Cooperação Acadêmica; Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq 406715/2013-0]; Fundação de Amparo a Pesquisa do Espírito Santo; Fundo Estadual de Ciência e Tecnologia [39767531/07]; and Pró-reitoria de Pesquisa - Universidade Federal do Pampa [Nº 10.134.14]. The authors would like to thank Professor Christopher Exley from Keele University, Staffordshire, UK, for the support on GFAAS.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Martinez, C.S., Alterman, C.D.C., Peçanha, F.M. et al. Aluminum Exposure at Human Dietary Levels for 60 Days Reaches a Threshold Sufficient to Promote Memory Impairment in Rats. Neurotox Res 31, 20–30 (2017). https://doi.org/10.1007/s12640-016-9656-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-016-9656-y