Abstract
Aluminum is a potent neurotoxin, responsible for memory impairment and cognitive dysfunction. The neurotoxic effect of aluminum on cognitive impairment is well documented, however, exposure to aluminum in a time-dependent manner and post-exposure self-recovery still needs to be elaborated. This research aimed to (1) study the time-dependent effect of aluminum exposure by administering a total dose of 5850 mg/kg of Al over two different time periods: 30 and 45 days (130 and 195 mg/kg of AlCl3 respectively), and (2) study 20 days post-exposure self-recovery effect in both aluminum-exposed groups by giving distilled water. Cognitive abilities were investigated through Morris water maze test and hole board test and compared in both exposure and recovery groups. Oxidative stress markers and neurotransmitter levels were measured for both exposure and recovery groups. To understand the mechanism of aluminum exposure and recovery, immunohistochemical analysis of synaptophysin (Syp) and glial fibrillary acidic protein (GFAP) was performed. Results showed cognitive dysfunction, oxidative stress-induced damage, reduced neurotransmitter levels, decreased immunoreactivity of Syp, and increased GFAP. However, these parameters showed a larger improvement in the recovery group where rats were given aluminum for 30 days period in comparison to recovery group followed by 45 days of aluminum exposure. These results suggest that restoration of cognitive ability is affected by the duration of aluminum exposure. The study findings provide us with insight into the adverse effects of aluminum exposure and can be utilized to guide future preventive and therapeutic strategies against aluminum neurotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminum (Al) is one of the most abundant element found in earth’s crust (Saeed et al. 2021) and is a known neurotoxicant (Ahmed et al. 2020). It primarily enters the body through dietary intake. An estimated dose of 10 mg/day is consumed from fruits and vegetables 95 mg/day from commercially processed food (Walton 2007) and <0.2 mg/l from drinking water (World Health Organization 2003). Utensils, pharmaceutical products, and antiperspirants are also a source of Al exposure (Yokel 2000). Al through drinking water contributes to 1% of daily intake (World Health Organisation 2004), but due to its uncomplexed nature it is highly bioavailable (Ferreira et al. 2008) Once present in circulation, due to its chemical similarity with iron, Al can easily cross blood–brain barrier (BBB) by binding to transport protein; transferrin (Cirovic et al. 2023) and, additionally by binding to citrate (Nagasawa et al. 2005). Once Al crosses the BBB it deposits in brain and ultimately contributes to the etiopathogenesis of neurodegenerative disorders (Flaten 2001; Yasui et al. 1992). Nonetheless, the mechanism by which Al causes various pathological conditions remains unclear. Moreover, how time-dependent exposure to Al and post-exposure self-recovery influenced the Al induced pathological changes have not till date been investigated.
Oxidative stress is a well-known mechanism involved in pathophysiology of neurodegenerative disorders (Lee et al. 2015). Al can generate free radicals (Gilani et al. 2022) which trigger DNA damage, nitration of proteins, and lipid peroxidation (LPO) (Haider et al. 2015). It has been shown that that cortex and hippocampus are more prone to oxidative injury, hence, impairing neuronal function (Karim et al. 2017; Memudu and Adanike 2022). Astrocytes are crucial in neuronal survival by providing protection against oxidative stress (Suryavanshi et al. 2022). Reactive astrogliosis occurs in many pathological states and metal-induced toxicity, and the increase in glial fibrillary acidic protein (GFAP) is attributed to astrocytes activation (Pekny and Pekna 2014). Studies showed that Al-induced toxicity activates the astrocytes (Ekong et al. 2017; Laabbar et al. 2021). Al is reported to alter neuronal function by causing neurotransmitters imbalance (El-Shetry et al. 2021). Synaptophysin (Syp), a presynaptic protein, is responsible for carrying neurotransmitters that are crucial for their role in the exocytosis of synaptic vesicles (Disdier et al. 2017); thus, any change in Syp would modify neurotransmitters release, ultimately leading towards cognitive dysfunction (Üçel et al. 2020; Zhang et al. 2014).
There is substantial evidence linking Al-induced neurotoxicity to memory impairment (Chen et al. 2021; Li et al. 2020; Mehpara Farhat et al. 2019). It also has been well-documented that Al impairs cognitive functions and induces anxiety like symptoms by disrupting hippocampus, cortex, and amygdala dependent functions through multiple mechanisms (Liaquat et al. 2019; Verma et al. 2020).
Earlier reports of chronic exposure to Al have been thoroughly investigated through administration of high doses (Farhat et al. 2017b; Liu et al. 2022). Though, no earlier study has reported detailed effects of the same dose given over different time periods. Hence, this study was designed to evaluate the neurotoxicity of Al following different periods of same amount exposure, and how different parameters show improvement at the end of post-exposure recovery period. By keeping total Al exposure same for 30 and 45 days, neurotoxicity was studied through biochemical estimation of oxidative stress markers, neurotransmitter levels, immunohistochemical changes in Syp and GFAP, and behavioral testing. After completion of both exposure periods, 20 days self-recovery period was given. Then same parameters were studied, and comparison was made with Al exposure groups to understand the degree of recovery.
Materials and methods
Chemicals and reagents
Aluminum chloride hexahydrate (AlCl3·6H2O) was purchased from Scharlau (AL0770), Spain. AlCl3·6H2O with ≤0.005% impurities was stored in an air-tight bottle in ventilated area (mentioned in the chemical fact sheet). Hydrogen peroxide (H2O2) stock solution (35%), nitro blue tetrazolium (NBT), thiobarbituric acid (TBA), hydroxylamine hydrochloride (NH2OH·HCl), 5,5-dithiobis 2-nitrobenzoic acid (DTNB), and other reagents of analytical grade were procured from Sigma (USA).
Study design and grouping of animals
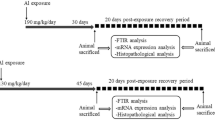
Experiments were conducted as per Institute of Laboratory Animal Research, NIH, USA guidelines. The protocols were permitted by Internal Review Board (ASAB, NUST, IRB Letter # 135). Male Wistar rats 3–4 months of age (145–165 g of weight) were acquired from Animal House, NUST. Rats were kept in animal houses at 24 ± 2 °C temperature, and the natural light and dark cycle consisting of 14 h of light and 10 h of dark. Rats were given water and a standard diet consisting of raw components (proteins 30%, fats 9% and fibers 4%), and moisture (10%) (Farhat et al. 2017a). Water intake was monitored regularly during the study (Supplementary Fig. 1a). The animals’ body weights were also monitored, and there were no observed signs of acute toxicity (Supplementary Fig. 1b). The rats were divided into five groups. Ten rats from every group were allocated for behavioral assessment, six were used in biochemical and neurotransmitters analysis and four animals were tested for histological analysis.
-
1.
Control group was given distilled water
-
2.
Thirty days Al exposure group were given 195 mg/kg AlCl3 through drinking water
-
3.
Recovery group followed by 30 days of Al exposure were given 195 mg/kg AlCl3 for 30 days. After 30 days, rats received distilled water for 20 days self-recovery
-
4.
Forty-five days Al exposure group were given 130 mg/kg AlCl3 through drinking water
-
5.
Recovery group followed by 45 days of Al exposure were given 130 mg/kg AlCl3 for 45 days. After 45 days, rats received distilled water for 20 days self-recovery
The study was designed with the aim to understand the effect of Al exposure in time-dependent manner. For this purpose, Al was given to rats in a time-dependent manner, i.e., by exposing to total dose of 5850 mg/kg in two time-points 30 and 45 days (short- and long-term exposure respectively; Fig. 1). Due to its omnipresence in life through daily food items, humans get exposed to Al, making it difficult to establish safe intake levels for humans. The WHO’s limits are regularly exceeded across different regions and circumstances (Wong et al. 2010). Therefore, this dose was chosen to investigate the neurotoxicity of Al at higher end of the human exposure.
Biochemical estimations of antioxidant enzymes
Measurement of catalase (CAT) activity
CAT activity was assessed as explained earlier (Sinha 1972). For this purpose, the whole brain from each group was taken and then the whole cortex and whole hippocampus were isolated and weighed. Isolated cortex and hippocampus tissues (100 mg) were homogenized by centrifugation at 2000 × g for 15 min (4 °C). After centrifugation 10% (100 μl) of supernatant was used in the mixture having 0.2 M of H2O2 (400 µl) and 0.01 M of phosphate buffer (1000 µl, pH 7.4). The reaction mixture was then incubated for 90 s at 37 °C. The reaction was then terminated by adding 5% dichromate reagent (2000 μl) and was incubated (15 min) in water bath. Then absorbance was recorded at 570 nm to measure the consumption of H2O2. The control was run with all batches of samples without H2O2. The activity was shown as H2O2 expenditure (μmol/min/g of tissue).
Measurement of superoxide dismutase (SOD) activity
The test was performed as reported earlier (Liaquat et al. 2019). To assess the activity, 10% (500 µl) brain homogenate from the cortex and hippocampus were used in a mixture of 50 mM (1000 µl) sodium carbonate, 24 µM (400 µl) NBT and 0.1 mM (200 µl) ethylenediaminetetraacetic acid. The reaction was commenced by adding 1 mM of NH2OH·HCl (400 µl). Absorbance was observed at 560 nm at 0- and 5-min intervals. For each batch of samples, a control with no brain homogenate was used. The activity was measured in terms of U/g of the tissue, and 1 unit was defined as enzyme quantity that inhibited NBT decrease by 50%.
Measurement of glutathione peroxidase (GPx) activity
GPx activity was estimated as explained earlier (Flohé and Günzler 1984) with slight modifications. Brain homogenate from the cortex and hippocampus 10% (300 µl) was mixed with solution containing 2 mM (200 μl) reduced glutathione, 1 mM (100 μl) H2O2, 10 mM (100 μl) sodium azide and 0.1 M (300 μl, pH 7.4) phosphate buffer. The solution was incubated (37 °C, 15 min). The reaction was then stopped by adding 5% of TCA (500 μl) solution. The mixture was then centrifuged (4 °C) to collect supernatant at 1000 × g for 10 min. From this, (100 μl) was taken and added to a solution having 0.4 mg/ml DTNB (0.7 ml) and 0.1 M (2 ml, pH 7.4) phosphate buffer. The absorbance was measured (420 nm) and expressed as μmol/min/g of the brain tissue.
Measurement of LPO
LPO was estimated as explained earlier (Haider et al. 2015). Brain homogenates from the cortex and hippocampus 10% (100 μl) were added to a mixture of 15% TCA and 0.37% TBA. The solution was heated for 20 min. After heating, the mixture was allowed to cool and centrifuged at 2000 × g (10 min). The light pink color supernatant was used for measuring absorbance (532 nm). The data was given as μmoles MDA/g of the brain tissue.
Determination of neurotransmitter levels
Neurochemical analysis was performed as explained earlier (Haider et al. 2015) for assessment of noradrenaline (NA), dopamine (DA), dihydroxyphenyl acetic acid (DOPAC), 5-hydroxytrytamine (5-HT) or serotonin and metabolite 5-hydroxyindoleacetic acid (5-HIAA) through reverse-phase HPLC detector (Schimadzu LEC 6-A). For HPLC, the detector was run at +0.8 V. For separation, the stationary phase employed was 5 μ Shim-pack ODS (4 mm diameter and 150 mm length). The column was eluted with a mobile phase consisting of octyl sodium sulfate (0.023%) in phosphate buffer (0.1 M, pH 2.9) at a pressure of 2000–3000 psi.
Immunohistochemical detection of Syp and GFAP
Immunohistochemical examination was performed accordingly (Habib et al. 2019). Ketamine (300 μl/50 g) was administered intraperitoneally to the rats. The rat brain was fixed with 4% paraformaldehyde via transcardial perfusion. For further fixation, the tissues were soaked (24–48 h, 4 °C) in 4% paraformaldehyde. After fixation, the tissue was molded in paraffin embedding, and horizontal sections of 3 μm thickness were prepared through microtome CUT 6062-SLEE GmbH. The 3 µm thick sections were made deparaffinized with xylene. Sections were hydrated with different alcohol concentrations and washed with distilled water. The slides were then incubated for 30 min with H2O2 (3%) to block the activity of peroxidases. After washing from phosphate-buffered saline, the slides were incubated (4 °C) with a primary antibody against Syp (anti-Syp in rabbit, SAB4502906, 1:200 dilution) and GFAP (anti-GFAP rabbit, ab7260, 1:5000 dilution). After washing with PBS, slides were treated with secondary antibody (biotin-conjugated goat anti-rabbit IgG antiserum, ab205718) for 1 h and again incubated (30 min) with conjugated streptavidin peroxidase and avidin–biotin peroxidase (ABC) complex allowed to react for 10 min with DAPA. Then slides were counterstained with Mayer’s hematoxylin.
Quantitative morphometric analysis
Immunohistochemical slides from tested groups were viewed through microscope (Optika B-150, Italy). Image analysis software (Optika Vision Lite 2.1) was used to capture images. The immunostaining intensities were quantified through Java based image processing package, Image J (NIH, USA) as explained previously (Crowe and Yue 2019). The observed parameters were analyzed in three non-overlapping fields per four sections per four animals from each group through 40× resolution. The sections were quantified for the percent area of Syp and GFAP in cortex and hippocampal regions DG, CA1, CA2, and CA3.
Behavioral tests
Behavioral tests were conducted as previously described (Iqbal and Ahmed 2019) during 9 a.m. and 5 p.m. at the end of both exposure and recovery groups. Animals were habituated in a separate room regulated at a temperature of 24 ± 2 °C to ensure human interference and other environmental disturbances were minimal. The sequence and duration of the behavioral tests were designed carefully after a thorough literature review (Eltokhi et al. 2020; Hånell and Marklund 2014; McIlwain et al. 2001; Wolf et al. 2016) to avoid overlap between behaviors. Each test was done in all groups on the same day and same duration to avoid any further time-dependent influences on the results.
Hole board test (HBT)
This behavioral test was performed as explained earlier (Kuc et al. 2006) with few modification to assess the rat’s exploratory ability. The apparatus of a hole board was like an open field box but with the addition of 16 holes floor. Only four (same) holes were baited during all the trials and rats were made to learn these holes. The test was conducted in a box like an open field box with a hole board floor inserted at room temperature. Habituation is done in two trials i.e., the first trial which was performed for 15 min after an hour of removing the feed from the hole board and the second trial which was performed after an inter-trial interval of 3 h. The training period started from the next day (day 1) and lasted for 4 days. Same 4 holes out of total 16 were baited with 300 mg feed pellets and the session was considered completed if 3 min had passed or rats had eaten all the pellets in holes within the 3 min. The parameters to be calculated in this test were latency to enter the first hole, number of nose pokes per minute, working memory errors (WME) (entries to already visited holes during the trial), and reference memory errors (RME) (entries to holes that are non-baited).
Morris water maze test (MWM test)
MWM test was carried out according to earlier explained method (Iqbal and Ahmed 2019) and was conducted to estimate spatial memory of rats. The test was carried out after completion of both exposure and recovery period. For the test a circular swimming tank was used containing a hidden platform. The temperature of the tank water was kept at 23 ± 2 °C. The test was done for five consecutive days, only changing the release positions of rats each trial, by keeping the position of platform constant. To perform the probe trial, the platform was removed on the last day. The number of entries and platform crossings and time spent in target quadrant (TQ) were recorded in the probe trial.
Statistical analysis
All behavioral, biochemical, and morphological data were represented as standard error of mean. For data analysis Graph Pad Prism V8.0 (San Diego, USA) was used. For normality Kolmogorov–Smirnov test was applied on the data, and it suggest that data was normal. One-way ANOVA was used for analysis of oxidative stress markers, neurotransmitter levels, immunohistochemical changes and for behavior test for number of entries, platform crossings and time spent in TQ assessed in MWM test, followed by Bonferroni’s test for multiple comparisons. Two-way ANOVA was performed for the behavior test in HBT for assessing number of nose pokes per minute, latency, RME and WME, and in MWM test for escape latency over different number of days for all the tested groups. Correlation analyses were performed for cognitive performance in HBT and MWM test with Syp, GFAP and neurotransmitters and were analyzed through linear regression and Pearson test and corrected for multiple comparisons through Bonferroni test. The results at p < 0.05 were considered significant.
Results
Effect of exposure to Al and self-recovery on antioxidant enzymes
Effect of exposure to Al and self-recovery on CAT activity
The CAT activity in the cortex decreased significantly in 30 days (27.65 ± 0.91, p < 0.001) as well as 45 days (21.01 ± 3.21, p < 0.001) of exposure in comparison to control (55.44 ± 0.57). After recovery period of 20 days for both 30 (41.76 ± 1.86, p < 0.001) and 45 days (35.25 ± 1.07, p < 0.001; Fig. 2a) exposure, there was increase in CAT activity when compared to Al exposure groups.
Biochemical estimation of antioxidant enzymes and lipid peroxidation in the cortex (a–d) and hippocampus (e–h) of Al exposed and recovery groups. *p < 0.05, **p < 0.01, ***p < 0.001, refer to significant difference compared with the control group while #p < 0.05, ##p < 0.01, ###p < 0.001 refer to significant difference between Al exposed and recovery groups. n number of animals, 30DAl Al exposure for 30 days, 30DR 20 days recovery period after 30 days of Al exposure, 45DAl Al exposure for 45 days, 45DR 20 days recovery period after 45 days of Al exposure, CAT catalase, SOD superoxide dismutase, GPx glutathione peroxidase, MDA malondialdehyde
In the hippocampus, Al exposure for 30 (29.35 ± 1.14, p < 0.001) and 45 days (23.93 ± 2.92, p < 0.001) showed significant decrease in the CAT activity as compared to control (46.59 ± 1.14). Recovery group of 30 days (43.25 ± 1.12, p < 0.001) and 45 days (37.50 ± 2.17, p < 0.001; Fig. 2e) showed increase in activity when compared to respective Al exposure groups.
Effect of exposure to Al and self-recovery on SOD activity
The SOD activity in the cortex in 30 days of Al exposure showed a slight decrease in activity (7.42 ± 0.31, p ˃ 0.05), whereas 45 days (5.12 ± 0.50, p < 0.001) exposure resulted in decreased activity as compared to control (9.54 ± 0.43). There was no difference (p < 0.05; Fig. 2b) in the recovery groups followed by 30 and 45 days of exposure.
SOD activity in the hippocampus, showed significant decrease in activity after 45 days of Al exposure (4.40 ± 0.25, p < 0.001), whereas no difference was observed after 30 days of Al exposure (5.78 ± 0.18, p > 0.05) as compared to control (6.79 ± 0.51). Recovery after 45 days exposure, showed increased activity (6.32 ± 0.14, p < 0.001; Fig. 2f).
Effect of exposure to Al and self-recovery on GPx activity
GPx activity in cortex decreased in 30 days (15.64 ± 0.23, p < 0.001) and 45 days (12.84 ± 0.39, p < 0.001) of exposure compared to control (18.98 ± 0.49). The recovery group of 30 days exposure had shown no effect (p > 0.05) on GPx activity meanwhile, recovery followed by 45 days exposure demonstrated increase in activity (15.18 ± 0.58, p < 0.01; Fig. 2c) as compared to exposure groups.
In the hippocampus, Al exposure after 30 days (17.80 ± 0.83, p < 0.001) as well as 45 days (15.18 ± 0.72, p < 0.001) resulted in decreased GPx activity relative to control (22.23 ± 0.36). Recovery was observed followed by 45 days of exposure (18.31 ± 0.31, p < 0.01) whereas there was no difference (p > 0.05; Fig. 2g) in recovery to 30 days Al exposure.
Effect of exposure to Al and self-recovery on LPO
MDA levels in the cortex, increased significantly following both 30 days (38.82 ± 1.05, p < 0.01) and 45 days (44.01 ± 0.76, p < 0.001) of exposure as compared to control (32.92 ± 1.27). In recovery groups of both 30 days (32.92 ± 1.27, p < 0.01) and 45 days (36.15 ± 0.66, p < 0.001; Fig. 2d) MDA levels were significantly decreased as compared to exposure groups, showing an improved recovery.
In the hippocampus, MDA levels increased in both 30 days (32.15 ± 1.02, p < 0.01) and 45 days (36.34 ± 0.96, p < 0.001) of exposure groups as compared to control (26.42 ± 1.24). Recovery after 30 days (27.92 ± 0.88, p < 0.05) exposure showed decrease in MDA levels when compared to exposure group. While there was no observed recovery (p > 0.05; Fig. 2h) in the recovery after 45 days exposure.
Effect of exposure to Al and self-recovery on neurotransmitter levels
In the cortex, levels of NA decreased in 30 days (9.83 ± 0.99, p < 0.05) and 45 days (6.27 ± 1.58, p < 0.001) of exposure. The 30 day exposure recovery showed improvement (15.92 ± 1.45, p < 0.05; Fig. 3a) in recovery while no improvement (p > 0.05) was observed in 45 days exposure recovery group in comparison to their respective exposure groups. The levels of DA were significantly decreased in both 30 days (10.14 ± 0.59, p < 0.001) as well as in 45 days (9.12 ± 0.25, p < 0.001) exposure group, compared to control (18.60 ± 0.84; Fig. 3b). Recovery group followed by 30 days exposure showed improved recovery (14.49 ± 0.59, p < 0.05) whereas there was no improvement in DA levels in recovery group followed by 45 days exposure group. The levels of DOPAC were significantly decreased in both 30 days (7.86 ± 1.41, p < 0.001) as well as in 45 days (2.83 ± 0.60, p < 0.001) exposure, as compared to control (15.49 ± 1.03; Fig. 3c). Recovery group followed by 30 days exposure showed improved recovery (12.63 ± 1.04, p < 0.05) whilst there was no improvement in DOPAC levels in the recovery group followed by 45 days exposure. The levels of 5-HT remained unaffected (p > 0.05; Fig. 3d) in exposure and recovery groups. The 5-HIAA levels were reduced in 30 days (5.17 ± 0.11, p < 0.001) as well as in 45 days (4.47 ± 0.14, p < 0.001) exposure as compared to control (12.46 ± 1.15; Fig. 3e). Recovery group of 30 days (11.73 ± 1.16, p < 0.001) as well as 45 days (10.23 ± 0.51, p < 0.001) of exposure showed improved recovery.
Neurotransmitter levels in the cortex (a–g), hippocampus (h–n), thalamus (k–o) and amygdala (p–t) of Al exposed and recovery groups. *p < 0.05, **p < 0.01, ***p < 0.001, refer to significant difference compared with the control group while #p < 0.05, ##p < 0.01, ###p < 0.001 refer to significant difference between Al exposed and recovery groups. n number of animals, 30DAl Al exposure for 30 days, 30DR 20 days recovery period after 30 days of Al exposure, 45DAl Al exposure for 45 days, 45DR 20 days recovery period after 45 days of Al exposure, NA noradrenaline, DA dopamine, DOPAC dihydroxyphenyl acetic acid, 5-HT 5-hydroxytryptamine, 5-HIAA 5-hydroxyindoleacetic acid
In the hippocampus, NA levels were significantly decreased following both 30 days (6.26 ± 0.69, p < 0.001) as well as in 45 days (2.10 ± 0.13, p < 0.001) exposure, compared to control (37.23 ± 1.54). Recovery group of 30 days (14.98 ± 1.47, p < 0.001) as well as 45 days (9.72 ± 0.87, p < 0.001; Fig. 3f) showed improved recovery. The DA levels were decreased in 30 days (13.50 ± 1.37, p < 0.001) as well as in 45 days (2.16 ± 0.35, p < 0.001) of exposure as compared to control (28.44 ± 2.31; Fig. 3g). Recovery group of only 30 days Al exposure showed improved recovery (23.16 ± 1.26, p < 0.01). Levels of DOPAC decreased in 30 days (3.95 ± 0.18, p < 0.001) as well as in 45 days (2.16 ± 0.62, p < 0.001) exposure as compared to control (18.39 ± 1.46; Fig. 3h). Recovery group of 30 (10.73 ± 0.56, p < 0.01) as well as 45 days (9.22 ± 1.28, p < 0.01) of exposure showed improved recovery as compared to exposure groups. In hippocampus, 5-HT levels were decreased in 30 days (3.27 ± 0.33, p < 0.001) as well as in 45 days (2.79 ± 0.15, p < 0.001) of exposure as compared to control (11.45 ± 0.29). Recovery group of only 30 days (6.93 ± 1.02, p < 0.01; Fig. 3i) showed improvement. 5-HIAA levels were decreased in 30 days (5.82 ± 0.65, p < 0.001) as well as in 45 days (1.13 ± 0.13, p < 0.001) of Al, as compared to control (12.79 ± 0.70; Fig. 3j). While there was improvement in recovery group followed by 30 days of exposure (11.76 ± 0.90, p < 0.001). The effects of Al exposure and subsequent post-exposure self-recovery of neurotransmitter levels in the thalamus and amygdala have been presented in the supplementary section.
Effect of exposure to Al and self-recovery on Syp and GFAP
Immunostaining of the cortex with Syp showed decrease in the percent positive area of Syp in 45 days exposure (14.30 ± 1.49, p < 0.001) as compared to control (26.50 ± 0.64), while no difference (p > 0.05) was observed in 30 days exposure group. Recovery groups followed by both exposure periods showed no difference (p > 0.05; Fig. 4b) in Syp immunostaining.
Immunohistochemical staining of cortical layers 2 and 3 at 40x magnification with Syp antibody (a). White arrows show Syp positive cells. b Histogram shows the percent positive area of Syp in cortex. The image shows representative slides of hippocampus sections at 40× magnification with Syp immunohistochemistry (c). White arrows show Syp positive cells in hippocampus regions. Histogram shows the percent positive area of Syp in hippocampus regions d DG, e CA1, f CA2 and g CA3. *p < 0.05, **p < 0.01, ***p < 0.001 refer to significant difference compared with the control group while #p < 0.05, ##p < 0.01, ###p < 0.001 refer to significant difference between Al exposed and recovery groups. n number of animals, 30DAl Al exposure for 30 days, 30DR 20 days recovery period after 30 days of Al exposure, 45DAl Al exposure for 45 days, 45DR 20 days recovery period after 45 days of Al exposure
In hippocampal region, percentage positive area of Syp decreased significantly in 30 as well as 45 days of exposure in DG (27.81 ± 0.94, p < 0.01 and 22.31 ± 0.85, p < 0.001, respectively), in CA1 (37.30 ± 1.11, p < 0.01 and 15.01 ± 1.08, p < 0.001, respectively), CA2 (26.30 ± 1.55 and 26.31 ± 1.75, p < 0.01, respectively) and in CA3 (46.08 ± 1.49, p < 0.01 and 37.31 ± 1.11, p < 0.001, respectively) compared to control of DG (34.51 ± 1.32; Fig. 4d), CA1 (45.50 ± 1.55; Fig. 4e), CA2 (35.80 ± 0.85; Fig. 4f) and CA3 (55.3 ± 1.25; Fig. 4g). Recovery group followed by 30 days exposure showed increase in percentage of positive area of Syp in DG (33.51 ± 1.32, p < 0.01), CA1 (45.51 ± 1.44, p < 0.001), CA2 (34.30 ± 0.85, p < 0.05) and in CA3 (53.5 ± 1.44, p < 0.05) compared to exposure group.
Immunostaining of the cortex with GFAP showed significant increase in percentage of positive area of GFAP in both 30 days (14.01 ± 1.08, p < 0.01) as well as in 45 days of exposure (19.50 ± 0.64, p < 0.001) groups, as compared to control (7.25 ± 1.11). In recovery groups of both 30 (8.75 ± 0.85, p < 0.05) as well as in 45 days (14.51 ± 1.04, p < 0.05; Fig. 5b) of exposure there was decrease in percentage of positive area of GFAP as compared to respective exposure groups, suggesting an improved recovery.
Immunohistochemical staining of cortical layers 2 and 3 at 40× magnification with GFAP antibody (a). White arrows show GFAP positive cells. b Histogram shows the percent positive area of GFAP in the cortex. The image shows representative slides of hippocampus sections at 40× magnification with GFAP immunostaining (c). White arrows show GFAP positive cells in hippocampus regions. Histogram shows the percent positive area of GFAP in hippocampus regions d DG, e CA1, f CA2 and g CA3. *p < 0.05, **p < 0.01, ***p < 0.001 refer to significant difference compared with the control group while #p < 0.05, ##p < 0.01, ###p < 0.001 refer to significant difference between Al exposed and recovery groups. n number of animals, 30DAl Al exposure for 30 days, 30DR 20 days recovery period after 30 days of Al exposure, 45DAl Al exposure for 45 days, 45DR 20 days recovery period after 45 days of Al exposure
In the hippocampal region, percentage of positive area of GFAP increased significantly after 30 days as well 45 days of exposure in DG (19.31 ± 0.85, p < 0.01 and 27.10 ± 0.91, p < 0.001, respectively), in CA1 (14.01 ± 0.91, p < 0.01 and 17.51 ± 0.64, p < 0.001, respectively), CA2 (9.50 ± 0.64, p < 0.01 and 13.50 ± 0.64, p < 0.001, respectively) and in CA3 (14.51 ± 0.64, p < 0.05 and 18.31 ± 0.47, p < 0.001, respectively) as compared to control in DG (11.01 ± 1.47; Fig. 5d), CA1 (9.50 ± 0.64; Fig. 5e), CA2 (5.50 ± 0.64; Fig. 5f) and CA3 (10.31 ± 0.85; Fig. 5g). Recovery group followed by 30 days of exposure showed decreased percentage of positive area of GFAP in DG (12.51 ± 1.44, p < 0.01), CA1 (9.51 ± 0.64, p < 0.01), CA2 (5.75 ± 0.85, p < 0.05) and CA3 (9.50 ± 0.64, p < 0.01) as compared to exposure group. Whereas there was no observed improvement in the recovery group followed by 45 days exposure group.
Effect of exposure to Al and self-recovery on memory and exploratory behaviour
On day 1, 45 days Al exposure group (4.09 ± 1.70, p < 0.05) showed reduced number of nose pokes while there was no observed difference in the 30 days exposure (p > 0.05; Fig. 6a) than control (5.84 ± 0.56). On day 4, a similar trend was observed but overall number of nose pokes decreased in the control group (2.58 ± 0.59), 30 days (2.54 ± 0.90) and 45 days (2.28 ± 0.93) exposure groups. Recovery group followed by 45 days exposure (5.63 ± 2.35, p < 0.05; Fig. 6b) showed increased number of nose pokes on day 1 than the respective exposure group while there was no observed improvement in recovery group followed by 30 days exposure group.
Hole board test for assessing anxiety and memory in terms of number of nose pokes per minute (a, b), latency (s) for visit to first hole (c, d), reference memory errors (e, f), and working memory errors (g, h) of Al exposed and recovery groups. *p < 0.05, **p < 0.01, ***p < 0.001, refer to significant difference compared with control group while #p < 0.05, ##p < 0.01, ###p < 0.001 refer to significant difference between Al exposed and recovery groups. n number of animals, 30DAl Al exposure for 30 days, 30DR 20 days recovery period after 30 days of Al exposure, 45DAl Al exposure for 45 days, 45DR 20 days recovery period after 45 days of Al exposure
Latency to visit the first hole (baited or un-baited) was calculated to evaluate anxiety level. Only in day 2, latency was increased in 45 days (10.75 ± 1.30, p < 0.05) exposure group while there was no change (p > 0.05) in 30 days exposure group as compared to control (7.47 ± 1.05; Fig. 6c). In recovery groups, latency was increased in 30 days (9.02 ± 1.55, p < 0.05) as well as in 45 days (10.40 ± 1.23, p < 0.01) of exposure on day 2 as compared to control (5.56 ± 1.25; Fig. 6d), however no change was observed compared to exposure groups, thus showing no recovery.
On day 1, 30 days (16.26 ± 1.64, p < 0.01) and 45 days (23.25 ± 1.40, p < 0.01) exposure group showed impairment in referential memory, compared to control group (7.07 ± 0.43; Fig. 6e). RMEs gradually decreased in both groups from day 1 to day 4. In recovery group of 30 days (13.11 ± 1.98, p > 0.05) and 45 days (21.90 ± 1.21, p > 0.05; Fig. 6f), RMEs were decreased from day 1 to 4, however, this difference was not large when compared to exposure groups.
On day 1, in 30 days (7.42 ± 0.91, p < 0.01) as well as in 45 days (9.45 ± 0.41, p < 0.01) of exposure WME were significantly increased as compared to control (2.37 ± 0.19; Fig. 6g). WMEs gradually decreased from day 2 to day 4, however, significant increase was observed in WMEs in exposure groups. In the recovery group significant decrease in WME was observed in 30 days exposure (5.62 ± 0.71, p < 0.05; Fig. 6h) on day 1 as compared to respective Al exposure groups, showing improved recovery. Like RME, WMEs were decreased from day 2 to 4 but this difference was not significant as compared to the exposure groups.
Effect of exposure to Al and self-recovery on spatial memory
From day 1–5, 45 days (p < 0.01; Fig. 7a) exposure, escape latency was increased in MWM test as compared to control. Recovery group followed by 45 days exposure showed decrease (p < 0.01; Fig. 7b) in escape latency suggesting an improved recovery.
Morris water maze memory was assessed in terms of escape latency time (a, b), number of entries in target quadrant (c), number of platform crossings (d), and time spent in target quadrant (e) of Al exposed and recovery groups. *p < 0.05, **p < 0.01, ***p < 0.001, refer to significant difference compared with control group while #p < 0.05, ##p < 0.01, ###p < 0.001 refer to significant difference between Al exposed and recovery groups. n number of animals, 30DAl Al exposure for 30 days, 30DR 20 days recovery period after 30 days of Al exposure, 45DAl Al exposure for 45 days, 45DR 20 days recovery period after 45 days of Al exposure
To assess reference memory, a probe trial was carried out by removing platform, after a 5 day training period. Data was analyzed to observe differences in number of entries in TQ, between all groups. In 45 days (6.5 ± 0.68, p < 0.001; Fig. 7c) exposure group decreased number of entries were observed and no difference was observed in 30 days exposure group (p > 0.05) as compared to control (9.2 ± 0.33). In the recovery groups, no improvement in memory was observed (p > 0.05) as compared to exposure groups. The number of platform crossings were recorded and analyzed during the probe trial. In the 30 days (6.00 ± 0.61, p < 0.001) as well as in 45 days (2.5 ± 0.52, p < 0.001) exposure groups, significant decrease in platform crossing was observed as compared to control (9.2 ± 0.59). Recovery group followed by 45 days exposure showed improvement in recovery (6.4 ± 0.47, p < 0.001; Fig. 7d).
The time spent in TQ was analyzed to evaluate differential deficit between all tested groups. Exposure groups of 30 days (44.30 ± 1.26, p < 0.001) as well as 45 days (38.9 ± 3.49, p < 0.001; Fig. 7e) spent less time in TQ as compared to control (64.20 ± 2.64). In recovery groups of both 30 and 45 days no improvement (p > 0.05) was noted when compared to their respective exposure groups.
Correlation analysis was performed between behavioral parameter; time spent in TQ and neurotransmitter levels. The Pearson test showed positive correlation of NA, DA, and its metabolite DOPAC, 5-HT and metabolite 5-HIAA in the hippocampus (Fig. 8f–j), thalamus (Fig. 8k–o) and amygdala (Fig. 8p–t), and time spent in TQ. Correlation analysis was also performed for behavioral parameters; WME in HBT and neurotransmitter levels. The Pearson test (Supplementary Tables 1–4) showed a strong association between neurotransmitter levels and WME in cortex (Supplementary Fig. 2a–e), hippocampus (Supplementary Fig. 2f–j), thalamus (Supplementary Fig. 2k–o) and amygdala (Supplementary Fig. 2p–t).
Correlation between neurotransmitter levels and time spent in target quadrant assessed through Morris water maze test in cortex (a–e), hippocampus (f–j), thalamus (k–o) and amygdala (p–t) of Al exposed and recovery groups. *p < 0.05, **p < 0.01, refer to significant differences. 30DAl Al exposure for 30 days, 30DR 20 days recovery period after 30 days of Al exposure, 45DAl Al exposure for 45 days, 45DR 20 days recovery period after 45 days of Al exposure, NA noradrenaline, DA dopamine, DOPAC dihydroxyphenyl acetic acid, 5-HT 5-hydroxytryptamine, 5-HIAA 5-hydroxyindoleacetic acid
Correlation analyses were performed between percent positive area of Syp, GFAP, time spent in TQ assessed through MWM, WME made in HBT in the cortex and the hippocampus regions DG, CA1, CA2, and CA3 to investigate the association between Syp, GFAP and spatial memory. Positive correlation was observed between Syp and time spent in TQ, suggesting that time spent increases with increase in Syp positive area in cortex (Supplementary Fig. 3a1) and hippocampus (Supplementary Fig. 3b1–e1). Furthermore, correlation analysis showed a negative correlation between time spent in TQ and GFAP (Supplementary Fig. 3a2–e2). A significant negative correlation was noted between WME and Syp suggesting that decreased immunoreactivity of Syp results in an increase of WME in the cortex (Supplementary Fig. 3a3) and hippocampus (Supplementary Fig. 3b3–e3). Meanwhile there was a positive association between GFAP and WME in the cortex and hippocampus (Supplementary Fig. 3a4–b4). Correlation analysis with R2 and p-values have been added in Supplementary Tables 1–4.
Discussion
Research on the neurotoxic effects of Al dates back almost a century (Tomljenovic 2011). Humans get exposed to this toxic metal by various sources, and once acquired through diet, it is absorbed via GIT and eliminated in urine (Krewski et al. 2007). Under normal physiological conditions very little Al accumulates in the body, as almost all is expelled via renal functions. However, when accumulated, Al contributes to cognitive deficits (Zhong et al. 2020). Animal model studies suggest that Al interferes with signals involved in the learning process (Yan et al. 2017). Although it is well documented that Al affects cognitive abilities (Farhat et al. 2017b; Haider et al. 2020) the effects of duration of exposure of Al on cognitive functions is still unclear and required investigation.
The present study focused on evaluating the effects of time-dependent Al exposure on the cortex and hippocampus associated learning and memory impairment. This was done by measuring antioxidant enzyme markers, neurotransmitter levels and conducting behavioral tests, and immunohistochemical studies. This study also aimed to determine if Al-induced neurotoxicity was permanent or temporary.
Relatively higher Al doses were chosen to ensure Al accumulation in the brain tissues, vital for the next study objective which was investigating the effects a post-exposure recovery period would have on Al induced neurotoxicity. Daily dose of Al administered to rats in this study did not go beyond the doses ingested by individuals frequently taking buffered aspirin or antacids, which contributes to hundreds mg/kg of Al (Krewski et al. 2007). A research conducted in China also revealed that people consume a high amount of Al through daily food items (Ma et al. 2019). A study also suggested that American ingested >95 mg/kg daily through different food items (Greger 1993). For example, per serving of a pancakes provide 180 mg of Al (Saiyed and Yokel 2005). Earlier studies have also stated that the Al administration (260 mg/kg) for 35 days is equivalent to the maximum estimated intake by humans (Commissaris et al. 1982; Golub et al. 1989). Additionally, the LD50 of AlCl3 in rats ranges from 200 to 1000 mg/kg through oral route (World Health Organization 1997) which is quite high as compared to the doses used in our study. Based on these earlier studies and studies conducted by our research members (Farhat et al. 2017a, b), the mentioned doses of Al were chosen.
Al accumulation damages the natural antioxidant defense mechanism which ultimately induces oxidative stress (Abu-Taweel and Al-Mutary 2021b). Our study showed that exposure to Al causes decrease in activity of CAT, GPx, and SOD whereas increased MDA activity in cortical and hippocampal tissues, with only recovery in CAT activity. While SOD and GPx activity recovered only in 45 days exposure group. Our study’s findings coincided with those of previous reports (Abu-Taweel and Al-Mutary 2021b; Jadhav and Kulkarni 2023; Saeed et al. 2021), where Al causes oxidative stress that initiates LPO, thus interfering with processes that could result in cognitive dysfunction (Zhang et al. 2020). Moreover, the cortex and hippocampus, due to their involvement in cognitive functions, consume more oxygen and are thus more sensitive to oxidative stress (Karim et al. 2017; Magalingam et al. 2018) and due to the low levels of antioxidant enzymes in these areas, complete recovery may be prevented. However, observed recovery might be directed to the redox enzyme system and endogenous release of glutathione, that halts the production of free radicals (Elizabeth et al. 2020) and repairs neurons, highlighting the ability of self-recovery after the removal of toxins.
Neurotransmission, a dynamic activity that involves continuous release of neurotransmitters. Levels of neurotransmitters NA, DA, and its metabolite DOPAC, 5-HT and its metabolite 5-HIAA were measured. All studied neurotransmitters were significantly reduced in brain tissues from both exposure groups. In the cortex and the hippocampus, recovery of NA, DA, DOPAC, and 5-HIAA was observed in 30 days exposure recovery group, whilst recovery of 5-HIAA levels in the cortex and NA and DOPAC levels in the hippocampus was observed in the 45 day exposure recovery group. In the thalamus and amygdala, recovery of DA, 5-HT, and its metabolite 5-HIAA levels was noted in 30 days exposure group, whilst in the amygdala recovery of DOPAC and 5-HT levels was observed in 45 day exposure group. Study results coincided with previous reports where Al was exposed in a dose-dependent manner (Abu-Taweel and Al-Mutary 2021a; Haider et al. 2020; Liaquat et al. 2019). Moreover, these studies did not address the address the recovery factor. Furthermore, studies are mostly limited to whole brain tissue or cortex and hippocampal regions. Studies have suggested that serotonin and dopamine decreases may be due to oxidative stress that triggers the apoptotic mechanism ultimately leading to neuronal damage. Key role of these neurotransmitters is to signal event relevance, movement, and anxiety (Kumar 2002), that can be linked to memory performance and regulation of emotions (Hauser et al. 2019); hence, it can be suggested that the cognitive dysfunction reported in our studies may be due to the decreased levels of neurotransmitters in the cortex and hippocampus, as both are innervated by serotonin and dopamine afferent proceedings from the raphe complex and nigrostriatal system (Gonzalez-Burgos and Feria-Velasco 2008).
In our study we have studied the toxic effects of Al on Syp and GFAP levels using immunohistochemistry. Decreased immunoreactivity of Syp was observed in the cortex in the 45 days exposure group with no noticeable recovery. In the hippocampus regions DG, CA1, CA2 and CA3, Syp percentage positive cells were significantly decreased in Al exposure groups. This was recovered only in the 30 days exposure group. Our study results coincide with previous reports where Syp decreased in an amyloid beta induced model of Alzheimer’s disease (Kincheski et al. 2017; Qiang et al. 2018; Sanchez-Ramos et al. 2009) ultimately leading to cognitive impairment. Syp is a vesicle protein, an important index of synaptic integrity, and is largely associated with spatial memory (Xu et al. 2019). Loss of Syp is generally considered an early event in initial cognitive impairment, as decreased expression of Syp may result in suppressed neurotransmitter release (Abdelzaher et al. 2021), while restoration of Syp contributes to improved memory function (Tampellini et al. 2010).
The immunohistochemistry of GFAP showed that the percentage of cells in the cortex with GFAP significantly increased in both exposure groups, with improved recovery for both groups. Whereas in the hippocampus regions increase was observed in both exposure groups with recovery only in the 30 days of exposure group. Previous studies of Al induced neurotoxicity showed similar results where increased GFAP cells were observed in the cortex (Erazi et al. 2010) and hippocampus of the rat brain (El-Shetry et al. 2021). The same observation was made in cadmium induced toxicity (Yang et al. 2023). Increased GFAP is associated with astrogliosis that initiates events involved in inflammatory response and neuronal death (Ekong et al. 2017). Several reports have also suggested that oxidative damage is characterized by gliosis (Cabezas et al. 2014). Studies also show that mitochondria and astrocyte interplay is requisite for normal neuronal function and any mitochondrial damage may lead to increased gliosis due to release of reactive oxygen species (Memudu and Adanike 2022). Thus, Al exposure in our study may contribute to oxidative stress mediated astrogliosis. Moreover, recovery can be attributed to endogenous glutathione that can prevent possible gliosis.
In this study, exploratory activity, anxiety levels, RME, and WME were assessed through HBT. Exposure to Al for 45 days decreased locomotor activity but increased other parameters. Conversely, 30 days of exposure resulted in increased RME and WME. In the recovery of 45 days exposure, recovery was ascertained in locomotor activity while recovery was observed only in WME for the 30 days exposure. Results of this study are consistent with previously reported studies, where exposure to Al decreased the number of nose pokes, which may indicate the anxiogenic potential of Al (Amari et al. 2020; Minigalieva et al. 2018). Studies also suggested that other toxins such as deltamethrin (Souza et al. 2022), lead (Flores-Montoya and Sobin 2015), arsenic (Kumar and Reddy 2018; Saritha et al. 2019), and ethanol (Cullen et al. 2013) decreased the exploratory ability of animals by reducing the head dips, thus leading to high levels of anxiety. These high levels of anxiety resulted in spatial memory deficit through increased RME and WME in HBT. Earlier studies have documented a decline in RME and WME in response to repeated mobility tests due to Al exposure (Douichene et al. 2016; Justin Thenmozhi et al. 2017). Moreover, toxic effect of Al exposure on RME and WME were also reported through radial arm maze test (Abd el-Rady et al. 2021; Abdelmeguid et al. 2021; Yin et al. 2020). Here we have employed HBT to assess the spatial deficit through RME and WME. Furthermore, all these reported studies were followed by treatment of Al induced toxicity either through synthetic or natural drugs, but no studies have reported the post-exposure recovery effect.
In the MWM test, Al exposure for 45 days resulted in substantial increase in escape latency. Whereas decrease in entries and time spent in TQ and platform crossings, with recovery in escape latency and platform crossings following the 20 day recovery period. Meanwhile the 30 days exposure group showed decrease in time spent in TQ and platform crossings with no recovery in both parameters, Comparable outcomes from cognition analysis also supported our results (Abu-Taweel and Al-Mutary 2021b; Mehpara Farhat et al. 2019; Verma et al. 2020; Zhang et al. 2020) whereas in previously reported study no improvement in recovery was observed in spatial memory maybe because 2 weeks of recovery period was provided after giving Al intraperitonially (Verma et al. 2020). Report from previous study showed that spatial memory performance (investigated through novel object recognition) was positively correlated with the number of Syp in DG and CA1 (Xu et al. 2019). Findings from separate study suggested that protein levels of Syp positively correlated to time spent in TQ and inversely associated with latency reversal assessed through MWM test (Portero-Tresserra et al. 2018). Syp is a crucial component of synaptogenesis, a process involved in learning. Neurotransmitters in the brain are also responsible for cognitive functions and contribute to memory consolidation (White and Stowell 2021). It is suggested that Syp may be involved in the release of neurotransmitters, for example acetylcholine (Ach), glutamate, and DA, through its functional role in both exo- and endocytosis (Zhang et al. 2014). Studies showed that astrocyte signaling on synapse level is associated to cognitive performance (Wilhelmsson et al. 2019) and mice that showed poor performance in object recognition tests also showed reduction in astrocytic exocytosis (Lee et al. 2014). Supporting this notion, in this study, spatial memory parameters were associated with Syp, GFAP and neurotransmitter levels.
Findings of our studies indicate that duration of Al exposure has differential effects on cognitive abilities of rats, despite administration of the same dose of Al. This implies that neurotoxic effects of Al exposure are both dose- and time-dependent. Consequently, this provides insight into why similar recovery periods have different effects. The plausible explanation for this difference in recovery is the time-dependent differences in neurotoxicity observed. Due to the time-dependent toxicity, Al removal would also be dependent on time, hence, explaining the better recovery observed following short-term exposure compared to prolonged exposure.
The limitations of this research are that only one recovery period was studied, i.e., 20 day recovery period. Further study with multiple time-points can help in better understanding of recovery phenomenon. Moreover, Al accumulation and removal can be studied by measuring Al levels both in the brain and plasma at multiple time-points. This will give detailed understanding about Al accumulation and removal pattern. Furthermore, along with Syp and GFAP IHC, mRNA expressions and protein level studies can be carried out in the future to understand the mechanism of Al-induced neurotoxicity.
Conclusion
Exposure to Al in a time-dependent manner can cause cognitive impairment by exerting neurotoxic effects on the cortex and hippocampus. Mechanisms of neurotoxicity include a decrease in antioxidant enzymes and neurotransmitter levels, immune reactivity of Syp, and increase in LPO and astrogliosis. These parameters were however improved following a 20 day recovery period in rats exposed to Al short-term.
Abbreviations
- Al:
-
Aluminum
- CAT:
-
Catalase
- SOD:
-
Superoxide dismutase
- GPx:
-
Glutathione peroxidase
- MDA:
-
Malondialdehyde
- LPO:
-
Lipid peroxidation
- NA:
-
Noradrenaline
- DA:
-
Dopamine
- DOPAC:
-
Dihydroxyphenyl acetic acid
- 5-HT:
-
5-Hydroxytryptamine
- 5-HIAA:
-
5-Hydroxyindoleacetic acid
- DG:
-
Dentate gyrus
- Syp:
-
Synaptophysin
- GFAP:
-
Glial fibrillary protein
- HBT:
-
Hole board test
- TQ:
-
Target quadrant
- MWM:
-
Morris water maze test
- RME:
-
Reference memory error
- WME:
-
Working memory error
References
Abd el-Rady NM, Ahmed A, Abdel-Rady MM, Ismail OI (2021) Glucagon-like peptide-1 analog improves neuronal and behavioral impairment and promotes neuroprotection in a rat model of aluminum-induced dementia. Physiol Rep 8(24):e14651
Abdelmeguid NE, Khalil MIM, Elhabet R, Sultan AS, Salam SA (2021) Combination of docosahexaenoic acid and Ginko biloba extract improves cognitive function and hippocampal tissue damages in a mouse model of Alzheimer’s disease. J Chem Neuroanat 116:101995
Abdelzaher LA, Hussein OA, Ashry IEM (2021) The novel potential therapeutic utility of montelukast in alleviating autistic behavior induced by early postnatal administration of thimerosal in mice. Cell Mol Neurobiol 41:129–150
Abu-Taweel GM, Al-Mutary MG (2021a) Pomegranate juice rescues developmental, neurobehavioral and biochemical disorders in aluminum chloride-treated male mice. J Trace Elem Med Biol 63:126655
Abu-Taweel GM, Al-Mutary MG (2021b) Pomegranate juice reverses AlCl3-induced neurotoxicity and improves learning and memory in female mice. Environ Res 199:111270
Ahmed GAR, Khalil SKH, El Hotaby W et al (2020) ATR-IR and EPR spectroscopy for following the membrane restoration of isolated cortical synaptosomes in aluminium-induced Alzheimer’s disease—like rat model. Chem Phys Lipids 231:104931
Amari NO, Djebli N, Le Mai Huong TTH, Ha NDL, Bich VT (2020) Neuroprotective effect of 1,3-β-glucan-curcumin mixing (Bioglucur) on Alzheimer disease induced in mice by aluminium toxicity. Indian J Pharm Educ Res 54(4):1089–1097
Cabezas IL, Batista AH, Rol GP (2014) The role of glial cells in Alzheimer disease: potential therapeutic implications. Neurología (English Edition) 29(5):305–309
Chen X, Zhang M, Ahmed M, Surapaneni KM, Veeraraghavan VP, Arulselvan P (2021) Neuroprotective effects of ononin against the aluminium chloride-induced Alzheimer’s disease in rats. Saudi J Biol Sci 28(8):4232–4239
Cirovic A, Cirovic A, Orisakwe OE, Lima RR (2023) Local and systemic hypoxia as inductors of increased aluminum and iron brain accumulation promoting the onset of Alzheimer’s Disease. Biol Trace Elem Res. https://doi.org/10.1007/s12011-023-03599-y
Commissaris RL, Cordon JJ, Sprague S, Keiser J, Mayor GH, Rech RH (1982) Behavioral changes in rats after chronic aluminum and parathyroid hormone administration. Neurobehav Toxicol Teratol 4(3):403–410
Crowe AR, Yue W (2019) Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis: an integrated protocol. Bio Protoc 9(24):e3465
Cullen CL, Burne THJ, Lavidis NA, Moritz KM (2013) Low dose prenatal ethanol exposure induces anxiety-like behaviour and alters dendritic morphology in the basolateral amygdala of rat offspring. PLoS ONE 8(1):e54924
Disdier C, Chalansonnet M, Gagnaire F et al (2017) Brain inflammation, blood brain barrier dysfunction and neuronal synaptophysin decrease after inhalation exposure to titanium dioxide nano-aerosol in aging rats. Sci Rep 7(1):1–13
Douichene S, Hammadi K, Djebli N (2016) Neuroprotective effect of Hypericum thymopsis against chronic exposure to aluminum chloride and Alzheimer’s disease. J Pharm Pharmacol 3(3):20–28
Ekong MB, Ekpo MM, Akpanyung EO, Nwaokonko DU (2017) Neuroprotective effect of Moringa oleifera leaf extract on aluminium-induced temporal cortical degeneration. Metab Brain Dis 32:1437–1447
Elizabeth MA, Samson P, Itohan OR (2020) Histomorphological evaluations on the frontal cortex extrapyramidal cell layer following administration of N-Acetyl cysteine in aluminum induced neurodegeneration rat model. Metab Brain Dis 35:829–839
El-Shetry ES, Mohamed AA-R, Khater SI et al (2021) Synergistically enhanced apoptotic and oxidative DNA damaging pathways in the rat brain with lead and/or aluminum metals toxicity: expression pattern of genes OGG1 and P53. J Trace Elem Med Biol 68:126860
Eltokhi A, Kurpiers B, Pitzer C (2020) Behavioral tests assessing neuropsychiatric phenotypes in adolescent mice reveal strain- and sex-specific effects. Sci Rep 10(1):11263. https://doi.org/10.1038/s41598-020-67758-0
Erazi H, Sansar W, Ahboucha S, Gamrani H (2010) Aluminum affects glial system and behavior of rats. C R Biol 333(1):23–27
Farhat SM, Mahboob A, Ahmed T (2017a) Cortex-and amygdala-dependent learning and nicotinic acetylcholine receptor gene expression is severely impaired in mice orally treated with AlCl3. Biol Trace Elem Res 179(1):91–101
Farhat SM, Mahboob A, Iqbal G, Ahmed T (2017b) Aluminum-induced cholinergic deficits in different brain parts and its implications on sociability and cognitive functions in mouse. Biol Trace Elem Res 177(1):115–121
Ferreira PC, Piai KdA, Takayanagui AMM, Segura-Muñoz SI (2008) Aluminum as a risk factor for Alzheimer’s disease. Rev Lat Am Enfermagem 16:151–157
Flaten TP (2001) Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res Bull 55(2):187–196
Flohé L, Günzler WA (1984) [12] Assays of glutathione peroxidase. Methods Enzymol 105:114–120
Flores-Montoya MG, Sobin C (2015) Early chronic lead exposure reduces exploratory activity in young C57BL/6J mice. J Appl Toxicol 35(7):759–765
Gilani SJ, Bin-Jumah MN, Al-Abbasi FA et al (2022) Antiamnesic potential of malvidin on aluminum chloride activated by the free radical scavenging property. ACS Omega 7(28):24231–24240
Golub MS, Donald JM, Gershwin ME, Keen CL (1989) Effects of aluminum ingestion on spontaneous motor activity of mice. Neurotoxicol Teratol 11(3):231–235
Gonzalez-Burgos I, Feria-Velasco A (2008) Serotonin/dopamine interaction in memory formation. Prog Brain Res 172:603–623
Greger JL (1993) Aluminum metabolism. Annu Rev Nutr 13(1):43–63
Habib R, Wahdan SA, Gad AM, Azab SS (2019) Infliximab abrogates cadmium-induced testicular damage and spermiotoxicity via enhancement of steroidogenesis and suppression of inflammation and apoptosis mediators. Ecotoxicol Environ Saf 182:109398
Haider S, Liaquat L, Shahzad S et al (2015) A high dose of short term exogenous d-galactose administration in young male rats produces symptoms simulating the natural aging process. Life Sci 124:110–119
Haider S, Liaquat L, Ahmad S et al (2020) Naringenin protects AlCl3/d-galactose induced neurotoxicity in rat model of AD via attenuation of acetylcholinesterase levels and inhibition of oxidative stress. PLoS ONE 15(1):e0227631
Hånell A, Marklund N (2014) Structured evaluation of rodent behavioral tests used in drug discovery research. Front Behav Neurosci 8:252–252. https://doi.org/10.3389/fnbeh.2014.00252
Hauser TU, Eldar E, Purg N, Moutoussis M, Dolan RJ (2019) Distinct roles of dopamine and noradrenaline in incidental memory. J Neurosci 39(39):7715–7721
Iqbal G, Ahmed T (2019) Co-exposure of metals and high fat diet causes aging like neuropathological changes in non-aged mice brain. Brain Res Bull 147:148–158
Jadhav R, Kulkarni YA (2023) Neuroprotective effect of quercetin and memantine against AlCl3-induced neurotoxicity in albino Wistar rats. Molecules 28(1):417
Justin Thenmozhi A, William Raja TR, Manivasagam T, Janakiraman U, Essa MM (2017) Hesperidin ameliorates cognitive dysfunction, oxidative stress and apoptosis against aluminium chloride induced rat model of Alzheimer’s disease. Nutr Neurosci 20(6):360–368
Karim N, Khan I, Abdelhalim A, Abdel-Halim H, Hanrahan JR (2017) Molecular docking and antiamnesic effects of nepitrin isolated from Rosmarinus officinalis on scopolamine-induced memory impairment in mice. Biomed Pharmacother 96:700–709
Kincheski GC, Valentim IS, Clarke JR et al (2017) Chronic sleep restriction promotes brain inflammation and synapse loss, and potentiates memory impairment induced by amyloid-β oligomers in mice. Brain Behav Immun 64:140–151
Krewski D, Yokel RA, Nieboer E et al (2007) Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health B 10(S1):1–269
Kuc K, Gregersen B, Gannon K, Dodart JC (2006) Holeboard discrimination learning in mice. Genes Brain Behav 5(4):355–363
Kumar S (2002) Aluminium-induced changes in the rat brain serotonin system. Food Chem Toxicol 40(12):1875–1880
Kumar MR, Reddy GR (2018) Influence of age on arsenic-induced behavioral and cholinergic perturbations: amelioration with zinc and α-tocopherol. Hum Exp Toxicol 37(3):295–308
Laabbar W, Abbaoui A, Elgot A et al (2021) Aluminum induced oxidative stress, astrogliosis and cell death in rat astrocytes, is prevented by curcumin. J Chem Neuroanat 112:101915
Lee HS, Ghetti A, Pinto-Duarte A et al (2014) Astrocytes contribute to gamma oscillations and recognition memory. Proc Natl Acad Sci U S A 111(32):E3343–E3352
Lee J-S, Kim H-G, Lee H-W et al (2015) Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci Rep 5(1):9651
Li S, Zhang Q, Ding Y, Wang X, Liu P (2020) Flavonoids ameliorate aluminum chloride-induced learning and memory impairments via suppression of apoptosis and oxidative stress in rats. J Inorg Biochem 212:111252
Liaquat L, Sadir S, Batool Z et al (2019) Acute aluminum chloride toxicity revisited: study on DNA damage and histopathological, biochemical and neurochemical alterations in rat brain. Life Sci 217:202–211
Liu W, Liu J, Gao J, Duan X, Zhang L (2022) Effects of subchronic aluminum exposure on learning, memory, and neurotrophic factors in rats. Neurotox Res 40(6):2046–2060
Ma J, Jiang G, Zheng W, Zhang M (2019) A longitudinal assessment of aluminum contents in foodstuffs and aluminum intake of residents in Tianjin metropolis. Food Sci Nutr 7(3):997–1003
Magalingam KB, Radhakrishnan A, Ping NS, Haleagrahara N (2018) Current concepts of neurodegenerative mechanisms in Alzheimer’s disease. Biomed Res Int 2018:3740461
McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R (2001) The use of behavioral test batteries: effects of training history. Physiol Behav 73(5):705–717
Mehpara Farhat S, Mahboob A, Ahmed T (2019) Oral exposure to aluminum leads to reduced nicotinic acetylcholine receptor gene expression, severe neurodegeneration and impaired hippocampus dependent learning in mice. Drug Chem Toxicol 44(3):310–318. https://doi.org/10.1080/01480545.2019.1587452
Memudu AE, Adanike RP (2022) Alpha lipoic acid reverses scopolamine-induced spatial memory loss and pyramidal cell neurodegeneration in the prefrontal cortex of Wistar rats. IBRO Neurosci Rep 13:1–8
Minigalieva IA, Katsnelson BA, Privalova LI et al (2018) Combined subchronic toxicity of aluminum(III), titanium(IV) and silicon(IV) oxide nanoparticles and its alleviation with a complex of bioprotectors. Int J Mol Sci 19(3):837
Nagasawa K, Ito S, Kakuda T et al (2005) Transport mechanism for aluminum citrate at the blood–brain barrier: kinetic evidence implies involvement of system Xc− in immortalized rat brain endothelial cells. Toxicol Lett 155(2):289–296
Pekny M, Pekna M (2014) Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev 94(4):1077–1098
Portero-Tresserra M, Martí-Nicolovius M, Tarrés-Gatius M, Candalija A, Guillazo-Blanch G, Vale-Martínez A (2018) Intra-hippocampal d-cycloserine rescues decreased social memory, spatial learning reversal, and synaptophysin levels in aged rats. Psychopharmacology 235:1463–1477
Qiang W, Cai W, Yang Q et al (2018) Artemisinin B improves learning and memory impairment in AD dementia mice by suppressing neuroinflammation. Neuroscience 395:1–12
Saeed A, Qusti SY, Almarwani RH et al (2021) Effects of aluminum chloride and coenzyme Q10 on the molecular structure of lipids and the morphology of the brain hippocampus cells. RSC Adv 11(48):29925–29933
Saiyed SM, Yokel RA (2005) Aluminium content of some foods and food products in the USA, with aluminium food additives. Food Addit Contam 22(3):234–244
Sanchez-Ramos J, Song S, Sava V et al (2009) Granulocyte colony stimulating factor decreases brain amyloid burden and reverses cognitive impairment in Alzheimer’s mice. Neuroscience 163(1):55–72
Saritha S, Davuljigari CB, Kumar KP, Reddy GR (2019) Effects of combined arsenic and lead exposure on the brain monoaminergic system and behavioral functions in rats: reversal effect of MiADMSA. Toxicol Ind Health 35(2):89–108
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47(2):389–394. https://doi.org/10.1016/0003-2697(72)90132-7
Souza MF, Medeiros KAAL, Lins LCRF et al (2022) Motor, memory, and anxiety-like behavioral impairments associated with brain-derived neurotrophic factor and dopaminergic imbalance after inhalational exposure to deltamethrin. Brain Res Bull 181:55–64
Suryavanshi J, Prakash C, Sharma D (2022) Asiatic acid attenuates aluminium chloride-induced behavioral changes, neuronal loss and astrocyte activation in rats. Metab Brain Dis 37(6):1773–1785
Tampellini D, Capetillo-Zarate E, Dumont M et al (2010) Effects of synaptic modulation on β-amyloid, synaptophysin, and memory performance in Alzheimer’s disease transgenic mice. J Neurosci 30(43):14299–14304
Tomljenovic L (2011) Aluminum and Alzheimer’s disease: after a century of controversy, is there a plausible link? J Alzheimers Dis 23(4):567–598
Üçel Uİ, Can ÖD, Özkay ÜD, Ulupinar E (2020) Antiamnesic effects of tofisopam against scopolamine-induced cognitive impairments in rats. Pharmacol Biochem Behav 190:172858
Verma S, Ranawat P, Nehru B (2020) Studies on the neuromodulatory effects of Ginkgo biloba on alterations in lipid composition and membrane integrity of rat brain following aluminium neurotoxicity. Neurochem Res 45(9):2143–2160
Walton JR (2007) A longitudinal study of rats chronically exposed to aluminum at human dietary levels. Neurosci Lett 412(1):29–33
White DN, Stowell MHB (2021) Room for two: the synaptophysin/synaptobrevin complex. Front Synaptic Neurosci 13:740318
Wilhelmsson U, Pozo-Rodrigalvarez A, Kalm M et al (2019) The role of GFAP and vimentin in learning and memory. Biol Chem 400(9):1147–1156
Wolf A, Bauer B, Abner EL, Ashkenazy-Frolinger T, Hartz AM (2016) A comprehensive behavioral test battery to assess learning and memory in 129S6/Tg2576 mice. PLoS ONE 11(1):e0147733
Wong WWK, Chung SWC, Kwong KP, Yin Ho Y, Xiao Y (2010) Dietary exposure to aluminium of the Hong Kong population. Food Addit Contam 27(4):457–463
World Health Organisation (2004) Guidelines for drinking-water quality, vol 1. World Health Organization, Geneva
World Health Organization (1997) International programme on chemical safety. Environmental Health Criteria, Geneva, p 194
World Health Organization (2003) Aluminium in drinking-water: background document for development of WHO Guidelines for drinking-water quality. World Health Organization, Geneva
Xu L, Long J, Su Z et al (2019) Restored presynaptic synaptophysin and cholinergic inputs contribute to the protective effects of physical running on spatial memory in aged mice. Neurobiol Dis 132:104586
Yan D, Jin C, Cao Y et al (2017) Effects of aluminium on long-term memory in rats and on SIRT 1 mediating the transcription of CREB-dependent gene in hippocampus. Basic Clin Pharmacol Toxicol 121(4):342–352
Yang J-Y, Wang J, Hu Y et al (2023) Paeoniflorin improves cognitive dysfunction, restores glutamate receptors, attenuates gliosis and maintains synaptic plasticity in cadmium-intoxicated mice. Arab J Chem 16(1):104406
Yasui M, Kihira T, Ota K (1992) Calcium, magnesium and aluminum concentrations in Parkinson’s disease. Neurotoxicology 13(3):593–600
Yin S, Ran Q, Yang J, Zhao Y, Li C (2020) Nootropic effect of neferine on aluminium chloride-induced Alzheimer’s disease in experimental models. J Biochem Mol Toxicol 34(2):e22429
Yokel RA (2000) The toxicology of aluminum in the brain: a review. Neurotoxicology 21(5):813–828
Zhang L, Zhao Q, Chen C-H, Qin Q-Z, Zhou Z, Yu Z-P (2014) Synaptophysin and the dopaminergic system in hippocampus are involved in the protective effect of rutin against trimethyltin-induced learning and memory impairment. Nutr Neurosci 17(5):222–229
Zhang J, Huang W, Xu F, Cao Z, Jia F, Li Y (2020) Iron dyshomeostasis participated in rat hippocampus toxicity caused by aluminum chloride. Biol Trace Elem Res 197:580–590
Zhong W, Wang L, Fang S et al (2020) Two novel colorimetric fluorescent probes: Hg2+ and Al3+ in the visual colorimetric recognition environment. RSC Adv 10(6):3048–3059
Acknowledgements
We like to express our sincere gratitude to Saba Mehak Zahoor (MSc Neurosciences), King’s College London, UK, for her valuable assistance in proofreading and English language editing. We like to acknowledge Atta-ur-Rahman School of Applied Biosciences (ASAB), National University of Sciences and Technology (NUST), Pakistan, and Higher Education Commission (HEC) of Pakistan for providing support and facilities for this study. First author is recipient of Indigenous 5000 PhD Scholarship program, HEC. HEC is not involved in study design, data interpretation, writing of manuscript, and decision to submit article for publication.
Funding
Authors declare no finding source.
Author information
Authors and Affiliations
Contributions
Humna Asghar: Data curation, Writing - original draft, Investigation, Methodology, Data interpretation, Writing - review & editing. Alveena Siddiqui, Laraib Batool, Zehra Batool: Methodology and Data interpretation. Touqeer Ahmed: Conceptualization, Investigation, Supervision, Writing - review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or non-financial interests to disclose.
Ethical approval
All experimental procedures were approved by an Internal Review Board. Approval number: IRB-135.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asghar, H., Siddiqui, A., Batool, L. et al. Post-exposure self-recovery reverses oxidative stress, ameliorates pathology and neurotransmitters imbalance and rescues spatial memory after time-dependent aluminum exposure in rat brain. Biometals 37, 819–838 (2024). https://doi.org/10.1007/s10534-023-00570-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-023-00570-1