Abstract
Purpose of review
The purpose of this study is to summarize the latest findings regarding the impact of obesity and inflammation on breast cancer recurrence risk.
Recent Findings
Obesity is a risk factor for breast cancer recurrence and cancer-specific mortality. Biologic mechanisms that drive this association vary by tumor subtype and include a dysfunctional tumor microenvironment and systemic inflammation. We discuss the impact of obesity on systemic therapy resistance and review current evidence supporting pharmacological, surgical, and lifestyle modifications for addressing obesity in the context of improving breast cancer survivorship.
Summary
Obesity is associated with poorer survival in breast cancer. Risk stratification by tumor and host-specific characteristics can help identify adjunctive interventions to improve breast cancer outcomes in patients with obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the most common non-cutaneous cancer type in women and comprises 15% of all new cancer cases with an estimated 290,000 new cases diagnosed in the United States in 2023 [1]. Obesity is an established risk factor for the incidence and recurrence of several cancers, though the effects of obesity vary by tumor subtype and other metabolic factors. Lifestyle and pharmacologic interventions targeting the tumor-promoting effects of obesity may be leveraged to reduce resistance and/or improve efficacy of curative-intent adjuvant therapies. In this article, we briefly review mechanisms linking obesity to BC recurrence and focus on updates in the understanding of obesity as a mediator of treatment response and recent advances in anti-obesity interventions that could impact cancer outcomes.
Overview of Mechanisms Linking Obesity to Breast Cancer Recurrence
Obesity has reached pandemic proportions with a prevalence of approximately 14% worldwide, affecting over 1 billion people. In the United States, approximately 50% of women are obese and a further 28% are overweight [2•, 3]. Overweight and obesity are classically defined by a body mass index (BMI) of 25.0 to < 30 kg/m2 and ≥30.0 kg/m2, respectively, while severe obesity refers to a BMI ≥40.0 kg/m2 [3]. Abdominal obesity by waist circumference (defined as > 88 cm in women by the World Health Organization [4]) is one of the criteria for the diagnosis of metabolic syndrome (MetS), which also includes hypertension, hyperglycemia, and hyperlipidemia. Large prospective studies and metanalyses have shown that obesity is associated with an increased risk of all-cause mortality [5]. Obesity is associated with an increased risk of incident BC, BC recurrence, and BC-specific mortality [6•].
In the post BC diagnosis setting, obesity is associated with increased risk of recurrence, worse cancer-specific survival, and worse overall survival (OS), which may be driven by the strong association in hormone receptor-positive BC in both premenopausal and postmenopausal patients [7, 8]. Obese women with hormone receptor-positive BC have increased BC mortality (hazard ratio [HR] 1.78; 95% confidence interval [CI] 1.28–2.48) compared to human epidermal growth factor receptor 2 positive (HER2 +) BC (HR 1.09; 95% CI 0.14–8.84) and triple negative BC (HR 1.18; 95% CI 0.54–2.57) [8]. However, obesity in premenopausal women has not been associated with increased BC mortality (HR = 2.1; 95% CI 0.8–5.1) compared to postmenopausal obese women (HR = 1.4; 95% CI 1.0–2.1) [8].
Additionally, obese BC survivors have an increased risk of developing metastatic recurrence at multiple sites [9•]. However, there are mixed findings on the impact of obesity on metastatic BC survival, with conflicting data depending on subtype and lines of therapy received [10, 11•, 12]. One study found obesity to be an independent predictor of poorer OS in metastatic BC (HR = 7.1; 95% CI 4.4–8.7) [10], while others noted no impact of obesity on OS in metastatic BC [11•, 12].
Adipose tissue dysfunction is a central mediator of obesity-driven cancer growth. White adipose tissue is an active endocrine organ that is responsible for energy homeostasis, and excess adiposity promotes chronic inflammation which establishes a pro-neoplastic tumor microenvironment (TME) [13]. The TME plays an important role in the development, growth, and progression of cancer. Adipose inflammation disrupts adipocytokine balance to favor the production of leptin and cytokines such as tumor necrosis factor-α (TNF-α) which are known to promote tumor growth via angiogenesis, enhanced cell migratory capacity, and genomic instability [14•]. Inflammasome activation drives interleukin-1β signaling in the tumor which stimulates vascular endothelial growth factor A expression, ultimately promoting tumor angiogenesis. Increased myofibroblast content leads to stiffened extracellular matrices and enhanced cancer cell growth, while increased epithelial to mesenchymal transition and promotion of neutrophil expansion enhances the ability for metastatic spread [15]. The microbiome also plays a role in controlling the immune system and mediating inflammation [16]. The microbiota of breast tissue in BC patients have been shown to contain increased cohorts of bacteria with known procarcinogenic effects compared to healthy controls, although further research is required to ascertain whether the differences in the bacterial profiles are truly pathogenic [17]. Molecular features of BC have also been found to differ according to BMI, with differentially prevalent genomic driver alterations in overweight/obese patients compared to lean patients (e.g., higher prevalence of PIK3CA in obese patients compared to lean patients 22.2% vs 9.8%, p = 0.011) [18]. Genomic and transcriptomic data indicate that obesity promotes an inflammatory-like phenotype in BC, whereby chronic low-grade inflammation contributes to disease pathogenesis [18, 19••].
Other key mechanisms that drive BC recurrence include increases in bioavailable estrogens and insulin resistance [20]. More active aromatization of androgens in peripheral adipose tissue increases local estrogen production, and decreases in sex hormone-binding globulin further increase circulating bioavailable estrogens [21]. Adipose inflammation, specifically the production of cytokines interleukin-6 and TNF-α, also increases aromatase production via autocrine and paracrine signaling [22]. Cancer cells often overexpress the insulin receptor which promotes growth and survival [23]. Insulin, insulin-like growth factor-1 (IGF-1), and several IGF-binding proteins act as growth factors, thereby promoting cancer cell proliferation [24•]. Binding of insulin to the insulin receptor causes tissue-specific metabolic effects, which include increased cellular glucose uptake, cell proliferation, and inhibition of apoptosis [25]. Additionally, hyperinsulinemia affects sex hormone levels which can directly stimulate hormonally driven cancers like hormone receptor-positive BC [26]. Insulin resistance is associated with significant increases in BC-specific mortality, with women in the highest versus those in the lowest insulin resistance quartile having a greater risk of death after BC (HR = 1.78; 95% CI 1.32–2.39; p < 0.001) [27, 28]. Interestingly, insulin promotes genomic instability, via DNA damage [29•] which may provide a target for cancer prevention and treatment interventions.
Impact of Obesity on Response to Systemic Therapy
In addition to fostering a pro-tumorigenic state, obesity also confers diminished response to several standard anti-cancer treatments including chemotherapy and endocrine therapies. BC survivors with obesity have higher risks of recurrence even in those who have attained a pathologic complete response (pCR) after neoadjuvant therapy, where obese patients who achieved pCR had a shorter invasive disease-free survival (IDFS) compared to non-obese patients (HR = 2.46; 95% CI 1.13–5.35) [30•]. However, no significant associations have been observed between BC subtype and OS in obese patients who achieved pCR [30•].
Endocrine Therapy
Increased levels of estrogen and secretion of cytokines and adipokines are linked to the promotion of therapeutic resistance [31]. Patients with MetS were found to have a 1.4-fold greater risk of endocrine resistance, where post-treatment Ki67 was used as a surrogate marker of endocrine therapy response [32••]. A retrospective analysis of 53,816 women who received therapy for early BC in accordance with Danish Breast Cancer Cooperative Group protocols between 1977 and 2006 indicated that endocrine therapy was less effective after 10 years in obese patients, with an increased risk of death from all causes (HR = 1.57; 95% CI 1.09–2.26). The primary endocrine therapy used in this study was tamoxifen with a duration ranging from 1 to 5 years, with aromatase inhibitor (AI) as the second most frequently used endocrine therapy [33].
In post hoc exploratory analyses of randomized trials, tamoxifen had similar efficacy regardless of BMI, while obesity has been associated with reduced efficacy of AIs. In an exploratory analysis of the NSABP B-14 trial, patients with hormone receptor-positive BC and node negative disease derived benefit from tamoxifen regardless of BMI with reductions in BC recurrence and overall mortality. Patients receiving tamoxifen had a 40% reduction in BC recurrence and 23% reduction in overall mortality compared to placebo which did not vary across BMI groups (p = 0.34 and 0.43, respectively) [34]. Exploratory analyses of the ABCSG-12 and ATAC trials also indicate that tamoxifen efficacy is not modified by BMI, whereas the efficacy of AI for reducing BC recurrence in postmenopausal women is compromised by obesity. Specifically, in the ABCSG-12 trial, patients who received tamoxifen had no significant difference in disease-free survival (DFS) (HR = 0.94; 95% CI 0.60–1.64; p = 0.76) and OS (HR = 0.83; 95% CI 0.35–1.93; p = 0.65) according to BMI. However, patients who were overweight or obese and received anastrozole had increased risk of recurrence (HR = 1.53; 95% CI 1.01–2.31; p = 0.04 and death (HR = 1.93; 95% CI 1.04–3.58; p = 0.03) compared to patients with normal weight [35]. In the ATAC trial, women who received tamoxifen had similar recurrence rates across all BMI groups when compared to the lowest quintile, while postmenopausal women with a BMI > 30 kg/m2 who received anastrozole had increased risk of recurrence (HR = 1.60; 95% CI 1.06–2.14) [36]. Notably, in the Breast International Group 1–98 trial, the treatment effect of letrozole did not differ according to BMI (treatment by BMI interaction p = 0.74) [37]. For now, aromatase inhibitors remain the standard endocrine therapy in postmenopausal women; however, prospective trials are needed to test other endocrine approaches such as novel oral selective estrogen receptor degraders/downregulators in the setting of obesity.
The reduced efficacy of AIs observed in overweight and obese populations is thought to arise from various mechanisms, including adipokine imbalance favoring leptin overproduction which in turn promotes acquired resistance to AI via the leptin signaling pathway [38]. Other contributing mechanisms include increased adipose stromal cell production of aromatase which could overwhelm the potency of pharmacologic inhibition [39]. Consistently, suppression of serum estradiol levels is less efficient in obese patients treated with AI versus normal weight patients, although suppression of estradiol levels is greater with letrozole than anastrozole across BMI ranges [40, 41]. Whether AI dose intensification or modification can overcome these effects is currently unclear, and clinical trials are needed to test this hypothesis.
Chemotherapy
Standard chemotherapy dosing is based on estimated body surface area (BSA). Due to safety concerns, chemotherapy doses were historically capped at a BSA of 2.0 m2 or adjusted to ideal body weight; however, several studies have demonstrated inferior outcomes when doses are capped for obese patients. In the CALBG 8541 trial, obese patients treated with adjuvant cyclophosphamide, doxorubicin, and fluorouracil doses according to ideal body weight had inferior outcomes with increased recurrence rates compared to those doses according to actual body weight (risk ratio (RR) = 0.73; 95% CI 0.53–1.00) [42]. The PANTHER phase III trial compared tailored dosing of adjuvant epirubicin, cyclophosphamide, and docetaxel according to hematological nadirs vs. standard interval dosing based on BSA in early BC. Exploratory analyses showed a trend of improved recurrence-free survival for obese patients with the tailored approach (HR = 0.49; 95% CI 0.26–0.90), but this was not statistically significant when compared to the non-obese cohort (HR = 0.79; 95% CI 0.60–1.04; p = 0.175). No significant difference in toxicity was observed between different BMI groups [43]. Based on these and other similar data, current guidelines from the American Society of Clinical Oncology (ASCO) recommend full weight-based dosing and have noted that there is no evidence of increased toxicity among obese patients who received full weight-based chemotherapy [44].
Even with appropriate dosing, obese patients have worse BC outcomes after treatment. BSA-based formulations do not take body composition into account and thus may not accurately reflect drug distribution and pharmacokinetics in obese patients [45]. An exploratory analysis of the adjuvant BIG 2–98 trial found increased risk of recurrence (HR = 1.12; 95% CI 0.98–1.50; p = 0.21) and all-cause mortality (HR = 1.32; 95% CI 1.08–1.62; p = 0.007) with increasing BMI in the docetaxel containing arm, whereas outcomes were not impacted by BMI in the non-docetaxel arm. This differential effect of BMI may be related to the lipophilic properties of taxanes, which promotes higher affinity to adipose tissue leading to sequestration and decreased tumor delivery [46••]. Accordingly, future risk stratification paradigms could account for obesity-mediated response to specific cancer therapies.
Anti-HER2 Targeted Therapy
Preclinical data indicate that obesity affects the pharmacokinetic availability of anti-HER2 monoclonal antibodies like trastuzumab, including reduced plasma concentrations and potentially less clinical benefit [47]. However, since anti-HER2 targeted therapies are conventionally given alongside chemotherapy or endocrine therapy in the treatment of BC, it is difficult to separate the possible clinical impact of obesity specific to anti-HER2 therapy in humans. The impact of obesity on tyrosine kinase inhibitor efficacy in HER2 + BC is unclear, though it is notable that an exploratory analysis of the neoALTTO trial of neoadjuvant paclitaxel/trastuzumab + / − lapatinib demonstrated that obese patients with hormone receptor-positive tumors were less likely to achieve pCR after neoadjuvant therapy compared to normal weight patients (odds ratio 0.56; 95%CI 0.31–1.01; p = 0.054) [48•]. In an exploratory analysis of the ALTTO BIG 2–06 trial (where some treatment arms included lapatinib in the adjuvant setting), obese patients had significantly increased risk of recurrence (HR 1.25; 95% CI 1.04–1.50) and all-cause mortality (HR 1.25; 95% CI 1.18–2.84). Weight loss of ≥ 5% was also noted to be associated with decreased survival, although diarrhea is a known adverse effect of lapatinib and weight loss may have been an indicator of increased treatment toxicity leading to treatment discontinuation [49•, 50].
In the metastatic setting, some observational studies have suggested a favorable effect of obesity in HER2 + BC. In this potential “obesity paradox,” elevated BMI is associated with greater risk of BC diagnosis and recurrence, whereas after the development of metastatic disease, obesity is paradoxically associated with improved survival compared to normal BMI in some studies. For example, in a pooled analysis of patients with metastatic HER2 + BC, BMI in the obese range was significantly associated with improved OS compared to normal BMI (HR 0.82; 95% CI 0.72–0.95); this observation was independent of hormone receptor status [51•]. One potential explanation for this paradox may be that in advanced metastatic BC, low nutritional reserve and cancer cachexia contribute to poor outcomes. However, adjustment for performance status and albumin level did not modify the association between elevated BMI and improved survival in this metastatic population. Further research and careful disease and host phenotyping are needed to delineate the influence of BC and TME biology, effects of BC treatment, and impact of body composition and other nutritional indicators.
Lifestyle Interventions and Breast Cancer Recurrence
Several clinical trials have investigated lifestyle interventions for weight loss among BC survivors, mostly among women with early BC who have completed initial cancer treatment (surgery, radiation therapy, adjuvant chemotherapy). Modalities of intervention encompass in-person individual or group sessions [52,53,54], print-based personalized recommendations [55], phone-based counseling [56], or combinations of the above methods [57,58,59,60,61].
While most of these studies showed promising results in achieving significant weight loss and BMI reduction, only a subset examined the effect of such interventions on BC recurrence and survival outcomes with mixed results (Table 1). In the Women’s Intervention Nutrition Study (WINS), which tested dietary fat reduction via telephone-based counseling in a cohort of 2437 women with resected early BC, mean body weight was significantly lower in the low-fat diet group compared to control group, with a difference of 2.7 kg (95% CI 0.9–4.5; p = 0.005), and relapse-free survival (RFS) was improved (HR 0.76; 95% CI 0.60–0.98; p = 0.034) at 5 years [62]. Interestingly, the dietary fat reduction intervention appeared to have a greater effect on RFS among women with hormone receptor negative BC (HR 0.58; 95% CI 0.37–0.91) than those with hormone receptor-positive disease (HR 0.85; 95% CI 0.63–1.14), though the interaction was not statistically significant [62]. In the Lifestyle Intervention in Adjuvant Treatment of Early Breast Cancer (LISA) study, which included 338 postmenopausal women with hormone receptor-positive BC, telephone-based behavioral counseling did not improve DFS (HR 0.71; 95% CI 0.41–1.23; p = 0.23) or OS (HR 0.86; 95% CI 0.35–2.14; p = 0.74) at a median follow-up of 8 years despite a mean weight loss of 3.1 kg (3.6%) in the intervention group vs 0.3 kg (0.4%) in the control group at 24 months (p < 0.001), although this trial was closed early and may have been underpowered for survival endpoints [56, 63]. In the Women’s Healthy Eating and Living (WHEL) trial, which assessed a telephone-based counseling program with cooking classes in a cohort of 3088 women with stage I–III BC, there were no significant differences in change in body weight, event-free survival (HR 0.96; 95% CI 0.80–1.14; p = 0.63), or OS (HR 0.91; 95% CI 0.72–1.15, p = 0.43) between the intervention and control arms [59].
The inconsistent findings from these trials raise the possibility that dietary interventions may affect subgroups of BC survivors differently, and their impact on recurrence and survival outcomes may be mediated by effective weight loss. It is possible the lack of effect on survival outcomes in the WHEL trial may be attributable to the lack of significant weight loss when compared to WINS, a study with a comparable sample size in which dietary intervention led to significant weight loss and improvement in RFS. Differences in cohort baseline characteristics between WINS and WHEL, such as age and menopausal status, time between diagnosis and enrollment, and severity of disease may also account for the discrepant findings [70]. The WINS cohort included women aged 48–79 years, within 1 year of diagnosis, and over half had stage 1 disease; the WHEL trial enrolled women aged 18–70 years, within 4 years of diagnosis, and only about one-third had stage 1 disease. These differences suggest that dietary interventions may impact BC survivors differently based on age at diagnosis, timing of intervention, and disease severity. Further research is needed to elucidate the possible differential effects of lifestyle interventions on various subgroups of BC survivors.
More recently, the ongoing Breast Cancer Weight Loss (BWEL) trial reported the effects of a telephone-based intervention promoting caloric restriction and increased physical activity on weight loss among 2393 women with stage 2–3 HER2 negative BC. Interim analysis demonstrated a significant difference in percent weight change in the intervention group compared to control (− 4.8% vs + 0.8%, p < 0.0001) at 12 months [68••]. Increased weight reduction was observed in the intervention group compared to control across demographics and tumor characteristics [68••]. The intervention led to greater weight loss among post-menopausal compared to pre-menopausal women (− 6.39% vs − 4.68%, interaction p = 0.004) and among non-Black and non-Hispanic individuals (− 6.05%) compared to Black (− 3.74%) and Hispanic (− 4.13%) groups (interaction p = 0.018) [68••]. Whether the intervention will lead to a significant difference in IDFS, the study’s primary endpoint remains to be seen.
These and other trials have established the feasibility of lifestyle and weight loss interventions in BC survivors. However, further investigation is needed to elucidate the impact of lifestyle modification on BC outcomes. Our group has previously recommended a precision medicine approach to testing diet and lifestyle strategies, including highly controlled interventions using metrics adapted from drug development starting with early phase dose-finding trials to phase 3 efficacy trials with classical oncologic endpoints [71]. The use of precision lifestyle interventions, such as personalized exercise prescriptions and pre-prepared meal delivery, provides rigorous assessment of impact on key biologic pathways contributing to treatment resistance and tumor growth. For example, in an ongoing study, our group is testing the impact of an individualized energy-restricted plant-based diet plus exercise prescription during adjuvant AI therapy on aromatase levels, inflammation, and other key biologic pathways within breast tissue [72]. These findings will be used to identify predictors of response, effective dosing of caloric restriction and exercise, and inform the design of phase 3 trials with survival endpoints. Highly controlled lifestyle interventions, if successful, could ultimately be adapted into implementable behavioral interventions, such as telephone counseling, that incorporate relevant biomarkers of response. By inverting the current paradigm of testing broad behavioral interventions, a stepwise approach starting with high fidelity diet and exercise therapies could provide new data and insights for prescribing lifestyle intervention with similar precision as anti-cancer therapies. Multiple ongoing randomized controlled trials will seek to further elucidate the impact of lifestyle interventions on recurrence and survival outcomes which are summarized in Table 1.
Pharmacological and Surgical Interventions for Obesity and Breast Cancer
Type 2 diabetes mellitus (T2DM) and other metabolic syndrome disorders have been associated with increased risk of cancer recurrence through similar pathways as in obesity which includes inflammation, insulin resistance, and pro-tumorigenic changes in the TME. These observations have led to investigations of metformin and other anti-diabetic agents as well as bariatric surgery in patients with BC [73].
Drug Therapy
Metformin is one of the most widely studied anti-diabetic agents in BC. Metformin is a biguanide which acts by increasing insulin sensitivity and decreasing hepatic glucose output; it can induce modest weight loss and has been associated with potential beneficial effects against BC in observational and preclinical studies [74•, 75]. Small prospective trials in BC survivors noted improved levels of biomarkers associated with BC outcomes such as serum estradiol, leptin, and serum insulin [76•, 77]. An exploratory analysis of the ALTTO trial found that metformin improved DFS (HR 1.40; 95% CI 1.01–1.94; p = 0.043) and OS (HR 1.87; 95% CI 1.23 to 2.85; p = 0.004) in patients with hormone receptor and HER2 + disease and diabetes. Patients with diabetes were more likely to have a BMI ≥ 30 kg/m2 and larger primary tumors (p < 0.001) [78].
On the basis of promising observational and pilot data, the randomized phase III MA.32 trial was designed to test the effects of adjuvant metformin versus placebo on IDFS in patients with early BC and no diagnosis of diabetes. After enrollment of 3643 patients, futility was declared for patients with hormone receptor negative BC, and the primary analysis was conducted for 2533 patients with hormone receptor-positive BC. The median BMI was 27 kg/m2 with an interquartile range of 24–32 kg/m2. The incidence rates for IDFS were 2.78 per 100 patient-years in the metformin group vs 2.74 per 100 patient-years in the placebo group (HR 1.01; 95% CI 0.84–1.21; p = 0.93). OS as a secondary endpoint was also not significantly different between groups with an event rate of 1.46 per 100 patient-years in the metformin group vs 1.32 per 100 patient-years in the placebo group (HR 1.10; 95% CI 0.86–1.41; p = 0.47) [79••]. These negative findings suggest that the metabolic changes and any potential direct antitumor effects of metformin were insufficient to significantly affect BC outcomes. Other potential contributors to the lack of significant impact on IDFS include advances in BC treatments over the past two decades that may have outweighed potential benefits of adding metformin, exclusion of patients with known diabetes or fasting glucose of > 126 mg/dL, and low enrollment of Hispanic or African American populations who have higher rates of hyperinsulinemia and worse BC outcomes [80].
The MA.32 trial and other key randomized trials are summarized in Table 2 and have not shown definitive improvement in pCR or IDFS with the use of metformin in early BC. Glucagon-like peptide-1 receptor agonists (GLP-1RA) are a novel class of medications for weight management that delay gastric emptying, promote satiety, and improve insulin sensitivity [81]. The mean weight reduction with use of these agents is 8–15% of baseline body weight, with 63–86% of patients achieving ≥ 5% reduction in body weight and 33–69% achieving ≥ 10% [82,83,84]. Preclinical studies of GLP-1RA have shown promising anti-tumor activity in breast cancer cell lines via cancer cell apoptosis and inhibition of proliferation [85, 86]. Prospective clinical trials testing GLP-1RAs in patients with breast cancer powered for breast cancer outcomes are needed.
Bariatric Surgery
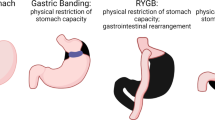
There are limited data available on the role and impact of bariatric surgery in reducing BC recurrence. Observational data from the primary risk reduction setting support the hypothesis that bariatric surgery may be an effective strategy for reducing BC recurrence. Bariatric surgery offers sustained weight loss in patients with obesity, leading to improvements in insulin resistance and inflammation. The four main procedures of bariatric surgery include adjustable gastric banding, sleeve gastrectomy, Roux-en-Y gastric bypass, and bilio-pancreatic diversion. The type of procedure chosen is determined by patient characteristics (e.g., BMI and co-morbidities) and potential procedural risks [90]. Bariatric surgery recommendation criteria include having a BMI ≥ 35 kg/m2, the presence of both T2DM and BMI ≥ 30 kg/m2, or BMI 30–34.9 kg/m2 refractory to medical interventions [91].
In observational studies, bariatric surgery is associated with a decreased risk of developing hormone receptor-positive BC in obese patients (HR 1.38; 95% CI 1.21–1.58) [92, 93•]. The SPLENDID trial was an observational, matched cohort study which reported that the incidence of any obesity-associated cancer (including postmenopausal BC) at 10 years among obese adults was 2.9% in the bariatric surgery group versus 4.9% in the nonsurgical control group (HR 0.68; 95% CI 0.53–0.87; p = 0.002) [94••]. In a recent meta-analysis, bariatric surgery was associated with a significant reduction in the risk of developing BC of all subtypes (pooled RR 0.56; 95% CI 0.44–0.71; p < 0.01) and improved cancer-specific mortality (RR 0.51; 95% CI 0.42–0.62; p < 0.01) [95]. Limited data suggest a protective effect of bariatric surgery in the post-diagnosis setting as well. In a case series including 13 women who completed definitive treatment for early BC, bariatric procedures were well tolerated and induced an average weight loss of 28.2% at 2 years [96]. Only one patient experienced BC recurrence at a median follow-up of 11.7 years and 5.3 years after bariatric surgery. Clinical trials in this setting are now being planned, such as the randomized phase II BariaTric Surgery After Breast Cancer Treatment (BATS) trial [97]. The initial observational findings support the rationale for clinical trials testing bariatric surgery as a strategy to reduce BC recurrence in the setting of severe or refractory obesity.
Beyond BMI: Adiposity as an Indicator of Metabolic Risk
Although BMI is currently the standard metric used to diagnose obesity, its limitations as a predictor of individual cardiometabolic risk are well documented [98]. As an anthropometric index based on height and weight, BMI does not account for the effects of age, menopausal status, sex, fat distribution, or muscle mass, but rather provides an indirect estimate of body fat that is neither sensitive nor specific [99]. Recent research has identified subgroups of individuals with normal BMI who experience adverse health consequences similar to those with obese-range BMI, including excess body fat, dyslipidemia, insulin resistance, elevated blood pressure, and low-grade inflammation [100,101,102]. This condition, known as metabolic obesity in normal weight, has been associated with increased risk of numerous cancers typically associated with classical obesity [103•, 104, 105], including post-menopausal BC [106].
Recent investigations have shown that body composition parameters, particularly central adiposity, are associated with elevated risk of post-menopausal BC among non-obese individuals across ethnicities [103•, 107,108,109,110]. In studies using direct quantification methods for body composition, such as bioelectrical impedance analysis or dual-energy X-ray absorptiometry, total body and trunk fat mass are associated with increased risk of postmenopausal BC among non-obese women [104, 107]. Increased trunk fat is also associated with altered metabolic biomarkers such as elevated insulin, triglycerides, and lower high-density lipoprotein cholesterol, consistent with metabolic dysfunction in this subgroup [107]. These findings raise the concern that reliance on BMI as a risk stratification tool may provide false reassurance by excluding normal-weight individuals with excess body fat who would benefit from risk reduction interventions. Further investigation is needed to better characterize the effect of body composition and increased adiposity on BC recurrence, and prospective clinical trials should consider inclusion of this population.
Conclusion
Obesity has reached pandemic proportions and increases the risk of recurrent BC. The pro-tumorigenic effects of obesity occur at both the local level via adipose dysfunction and alterations in the TME, as well as systematically via circulating inflammatory and metabolic mediators. Concerted efforts are underway globally to improve patient education regarding healthy lifestyle choices to reduce obesity rates; however, precise guidelines and prescribed lifestyle optimization plans are likely to be more successful than generic counseling in terms of adherence and anti-cancer efficacy. Incorporation of metabolic status using more precise metrics than BMI may also improve risk stratification for BC recurrence, and future research is needed to test whether this approach should modify BC treatment selection. The development of lifestyle interventions using a precision medicine paradigm may help to more efficiently and accurately select effective dietary and exercise interventions, which could be further augmented by pharmacologic approaches with metabolic targets that are relevant to cancer growth factor pathways. Ultimately, it is clear that obesity and metabolic dysfunction need to be clinically addressed in the setting of a cancer diagnosis to improve cancer-specific outcomes and overall mortality in cancer survivors.
Data Availability
No datasets were generated or analyzed during the current study.
Abbreviations
- AI:
-
Aromatase inhibitors
- BC:
-
Breast cancer
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- DFS:
-
Disease-free survival
- HR:
-
Hazard ratio
- HER2:
-
Human epidermal growth factor receptor 2
- IGF-1:
-
Insulin-like growth factor-1
- IDFS:
-
Invasive disease–free survival
- MetS:
-
Metabolic syndrome
- OS:
-
Overall survival
- pCR:
-
Pathological complete response
- EFS:
-
Relapse-free survival
- RR:
-
Risk ratio
- TME:
-
Tumor microenvironment
- TNF-α:
-
Tumor necrosis factor-α
- T2DM:
-
Type 2 diabetes mellitus
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cancer of the Breast (Female) – Cancer Stat Facts SEER. [cited 2023 Sept 20]. Available from: https://seer.cancer.gov/statfacts/html/breast.html
• Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. 2022;133:155217. https://doi.org/10.1016/j.metabol.2022.155217. This article highlights the updated incidence and prevalence of obesity worldwide.
Overweight & Obesity Statistics - NIDDK [cited 2023 Sept 20]. Available from: https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity
Nishida C, Ko GT, Kumanyika S. Body fat distribution and noncommunicable diseases in populations: overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist-Hip Ratio. Eur J Clin Nutr. 2010;64(1):2–5. https://doi.org/10.1038/ejcn.2009.139.
Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. https://doi.org/10.1136/bmj.i2156.
• Petrelli F, Cortellini A, Indini A, Tomasello G, Ghidini M, Nigro O, et al. Association of obesity with survival outcomes in patients with cancer: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(3):e213520. https://doi.org/10.1001/jamanetworkopen.2021.3520. This meta-analysis provides an overview of cancer outcomes in obese patients and shows an association of obesity with increased mortality rate.
Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–14. https://doi.org/10.1093/annonc/mdu042.
Blair CK, Wiggins CL, Nibbe AM, Storlie CB, Prossnitz ER, Royce M, et al. Obesity and survival among a cohort of breast cancer patients is partially mediated by tumor characteristics. NPJ Breast Cancer. 2019;5:33. https://doi.org/10.1038/s41523-019-0128-4.
• Olsson LT, Walens A, Hamilton AM, Benefield HC, Fleming JM, Carey LA, et al. Obesity and breast cancer metastasis across genomic subtypes. Cancer Epidemiol Biomarkers Prev. 2022;31(10):1944–51. https://doi.org/10.1158/1055-9965.EPI-22-0013. This study reports the incidence of metastasis in obese patients with breast cancer and indicates an increased risk of metastatic recurrence in the setting of obesity.
von Drygalski A, Tran TB, Messer K, Pu M, Corringham S, Nelson C, et al. Obesity is an independent predictor of poor survival in metastatic breast cancer: retrospective analysis of a patient cohort whose treatment included high-dose chemotherapy and autologous stem cell support. Int J Breast Cancer. 2011;2011:523276. https://doi.org/10.4061/2011/523276.
• Saleh K, Carton M, Dieras V, Heudel PE, Brain E, D’Hondt V, et al. Impact of body mass index on overall survival in patients with metastatic breast cancer. Breast. 2021;55:16–24. https://doi.org/10.1016/j.breast.2020.11.014. This observational cohort study indicates that overweight and obesity are not associated with worse outcomes in women with metastatic breast cancer.
Barba M, Pizzuti L, Sperduti I, Natoli C, Gamucci T, Sergi D, et al. Body mass index and treatment outcomes in metastatic breast cancer patients treated with eribulin. J Cell Physiol. 2016;231(5):986–91. https://doi.org/10.1002/jcp.25213.
Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34(35):4270–6. https://doi.org/10.1200/JCO.2016.67.4283.
• Lee-Rueckert M, Canyelles M, Tondo M, Rotllan N, Kovanen PT, Llorente-Cortes et al. Obesity-induced changes in cancer cells and their microenvironment: mechanisms and therapeutic perspectives to manage dysregulated lipid metabolism. Semin Cancer Biol. 2023;6:S1044–579X(23)00076–7. https://doi.org/10.1016/j.semcancer.2023.05.002. This study provides an overview of the progress of research regarding the impact of obesity on the tumor microenvironment and crosstalk with systemic metabolic signaling.
Olson OC, Quail DF, Joyce JA. Obesity and the tumor microenvironment. Science. 2017;358(6367):1130–1. https://doi.org/10.1126/science.aao5801.
Fulbright LE, Ellermann M, Arthur JC. The microbiome and the hallmarks of cancer. PLoS Pathog. 2017;13(9):e1006480. https://doi.org/10.1371/journal.ppat.1006480.
Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G. The microbiota of breast tissue and its association with breast cancer. Appl Environ Microbiol. 2016;82(16):5039–48. https://doi.org/10.1128/AEM.01235-16.
Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–90. https://doi.org/10.1038/s41574-018-0059-4.
•• Nguyen HL, Geukens T, Maetens M, Aparicio S, Bassez A, Borg A, et al. Obesity-associated changes in molecular biology of primary breast cancer. Nat Commun. 2023;14(1):4418. https://doi.org/10.1038/s41467-023-39996-z. This article provides an overview of several genomic alterations that are differentially prevalent in overweight or obese patients compared to lean patients as well as evidence supporting an ageing accelerating effect of obesity at the genetic level.
Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101(1):48–60. https://doi.org/10.1093/jnci/djn415.
Hetemäki N, Savolainen-Peltonen H, Tikkanen MJ, Wang F, Paatela H, Hämäläinen E, et al. Estrogen metabolism in abdominal subcutaneous and visceral adipose tissue in postmenopausal women. J Clin Endocrinol Metab. 2017;102(12):4588–95. https://doi.org/10.1210/jc.2017-01474.
Sun XZ, Zhou D, Chen S. Autocrine and paracrine actions of breast tumor aromatase. A three-dimensional cell culture study involving aromatase transfected MCF-7 and T-47D cells. J Steroid Biochem Mol Biol. 1997;63(1–3):29–36. https://doi.org/10.1016/s0960-0760(97)00068-x.
Shen S, Iyengar NM. Insulin-lowering diets in metastatic cancer. Nutrients. 2022;14(17):3542. https://doi.org/10.3390/nu14173542.
• De Santi M, Annibalini G, Marano G, Biganzoli G, Venturelli E, Pellegrini M, et al. Association between metabolic syndrome, insulin resistance, and IGF-1 in breast cancer survivors of DIANA-5 study. J Cancer Res Clin Oncol. 2023. https://doi.org/10.1007/s00432-023-04755-6. This study describes the association between IGF-1 levels and indices of insulin resistance in breast cancer survivors which could be a potential guide for to test strategies that modulate IGF-1 levels.
Poloz Y, Stambolic V. Obesity and cancer, a case for insulin signaling. Cell Death Dis. 2015;6(12):e2037. https://doi.org/10.1038/cddis.2015.381.
Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Endogenous Hormones Breast Cancer Collaborative, et al. Group Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95(16):1218–26. https://doi.org/10.1093/jnci/djg022.
Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20(1):42–51. https://doi.org/10.1200/JCO.2002.20.1.42.
Pan K, Chlebowski RT, Mortimer JE, Gunter MJ, Rohan T, Vitolins MZ, et al. Insulin resistance and breast cancer incidence and mortality in postmenopausal women in the Women’s Health Initiative. Cancer. 2020;126(16):3638–47. https://doi.org/10.1002/cncr.33002.
• Ciminera AK, Shuck SC, Termini J. Elevated glucose increases genomic instability by inhibiting nucleotide excision repair. Life Sci Alliance. 2021;4(10):e202101159. https://doi.org/10.26508/lsa.202101159. This article describes the role of hyperglycemia and insulin in promoting genomic instability which in turn is a potential mechanism for increasing cancer risk in metabolic disease.
• Acevedo F, Walbaum B, Muñiz S, Petric M, Martínez R, Guerra C, et al. Obesity is associated with early recurrence on breast cancer patients that achieved pathological complete response to neoadjuvant chemotherapy. Sci Rep. 2022;12(1):21145. https://doi.org/10.1038/s41598-022-25043-2. This study describes the association of obesity with breast cancer recurrence in the setting of pathologic complete response.
Hillers-Ziemer LE, Kuziel G, Williams AE, Moore BN, Arendt LM. Breast cancer microenvironment and obesity: challenges for therapy. Cancer Metastasis Rev. 2022;41(3):627–47. https://doi.org/10.1007/s10555-022-10031-9.
•• Bergman R, Berko YA, Sanchez V, Sanders ME, Gonzalez-Ericsson PI, Arteaga CL, et al. Obesity and metabolic syndrome are associated with short-term endocrine therapy resistance in early ER + breast cancer. Breast Cancer Res Treat. 2023;197(2):307–17. https://doi.org/10.1007/s10549-022-06794-y. This study describes the association of obesity with endocrine therapy resistance and reports a 1.4-fold increased risk of treatment resistance in patients with metabolic syndrome.
Ewertz M, Jensen MB, Gunnarsdóttir KÁ, Højris I, Jakobsen EH, Nielsen D, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29(1):25–31. https://doi.org/10.1200/JCO.2010.29.7614.
Dignam JJ, Wieand K, Johnson KA, Fisher B, Xu L, Mamounas EP. Obesity, tamoxifen use, and outcomes in women with estrogen receptor-positive early-stage breast cancer. J Natl Cancer Inst. 2003;95(19):1467–76. https://doi.org/10.1093/jnci/djg060.
Pfeiler G, Königsberg R, Fesl C, Mlineritsch B, Stoeger H, Singer CF, et al. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 trial. J Clin Oncol. 2011;29(19):2653–9. https://doi.org/10.1200/JCO.2010.33.2585.
Sestak I, Distler W, Forbes JF, Dowsett M, Howell A, Cuzick J. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28(21):3411–5. https://doi.org/10.1200/JCO.2009.27.2021.
Ewertz M, Gray KP, Regan MM, Ejlertsen B, Price KN, Thürlimann B, et al. Obesity and risk of recurrence or death after adjuvant endocrine therapy with letrozole or tamoxifen in the breast international group 1–98 trial. J Clin Oncol. 2012;30(32):3967–75. https://doi.org/10.1200/JCO.2011.40.8666.
Gelsomino L, Giordano C, Camera G, Sisci D, Marsico S, Campana A, et al. Leptin signaling contributes to aromatase inhibitor resistant breast cancer cell growth and activation of macrophages. Biomolecules. 2020;10(4):543. https://doi.org/10.3390/biom10040543.
Bhardwaj P, Brown KA. Obese adipose tissue as a driver of breast cancer growth and development: update and emerging evidence. Front Oncol. 2021;30(11):638918. https://doi.org/10.3389/fonc.2021.638918.
Pfeiler G, Königsberg R, Hadji P, Fitzal F, Maroske M, Dressel-Ban G, et al. Impact of body mass index on estradiol depletion by aromatase inhibitors in postmenopausal women with early breast cancer. Br J Cancer. 2013;109(6):1522–7. https://doi.org/10.1038/bjc.2013.499.
Folkerd EJ, Dixon JM, Renshaw L, A’Hern RP, Dowsett M. Suppression of plasma estrogen levels by letrozole and anastrozole is related to body mass index in patients with breast cancer. J Clin Oncol. 2012;30(24):2977–80. https://doi.org/10.1200/JCO.2012.42.0273.
Rosner GL, Hargis JB, Hollis DR, Budman DR, Weiss RB, Henderson IC, et al. Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: results from cancer and leukemia group B study 8541. J Clin Oncol. 1996;14(11):3000–8. https://doi.org/10.1200/JCO.1996.14.11.3000.
Matikas A, Foukakis T, Moebus V, Greil R, Bengtsson NO, Steger GG, et al. Dose tailoring of adjuvant chemotherapy for breast cancer based on hematologic toxicities: further results from the prospective PANTHER study with focus on obese patients. Ann Oncol. 2019;30(1):109–14. https://doi.org/10.1093/annonc/mdy475.
Griggs JJ, Mangu PB, Anderson H, Balaban EP, Dignam JJ, Hryniuk WM, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30(13):1553–61. https://doi.org/10.1200/JCO.2011.39.9436.
LeVee A, Mortimer J. The challenges of treating patients with breast cancer and obesity. Cancers (Basel). 2023;15(9):2526. https://doi.org/10.3390/cancers15092526.
•• Desmedt C, Fornili M, Clatot F, Demicheli R, De Bortoli D, Di Leo A, et al. Differential benefit of adjuvant docetaxel-based chemotherapy in patients with early breast cancer according to baseline body mass index. J Clin Oncol. 2020;38(25):2883–91. https://doi.org/10.1200/JCO.19.01771. This post hoc analysis of the adjuvant BIG 2-98 trial investigates the efficacy of docetaxel stratified by BMI, which demonstrated reduced DFS and OS in obese patients.
Ligorio F, Zambelli L, Fucà G, Lobefaro R, Santamaria M, Zattarin E, et al. Prognostic impact of body mass index (BMI) in HER2+ breast cancer treated with anti-HER2 therapies: from preclinical rationale to clinical implications. Ther Adv Med Oncol. 2022;8(14):17588359221079124. https://doi.org/10.1177/17588359221079123.
• Di Cosimo S, Porcu L, Agbor-Tarh D, Cinieri S, Franzoi MA, De Santis MC, et al. Effect of body mass index on response to neo-adjuvant therapy in HER2-positive breast cancer: an exploratory analysis of the NeoALTTO trial. Breast Cancer Res. 2020;22(1):115. https://doi.org/10.1186/s13058-020-01356-w. This post hoc analysis of the neoadjuvant NeoALTTO trial indicates an association between obesity and reduced pathologic complete response in patients with HER2+ BC.
• Martel S, Lambertini M, Agbor-Tarh D, Ponde NF, Gombos A, Paterson V, et al. Body mass index and weight change in patients with HER2-positive early breast cancer: exploratory analysis of the ALTTO BIG 2–06 trial. J Natl Compr Canc Netw. 2021;19(2):181–9. https://doi.org/10.6004/jnccn.2020.7606. This post hoc analysis of the adjuvant ALTTO BIG2-06 trial examined the impact of BMI at BC diagnosis and weight change after 2 years on outcomes in patients with HER2+ early BC. Obesity and weight loss ≥5% was associated with significantly worse DFS.
Iyengar NM, Ligibel JA. Letter to the editor: lapatinib confounds post-hoc weight loss analysis in the ALTTO trial. J Natl Compr Canc Netw. 2022;20(1):xliv-xlv. https://doi.org/10.6004/jnccn.2021.7082.
• Modi ND, Tan JQE, Rowland A, Koczwara B, Abuhelwa AY, Kichenadasse G, et al. The obesity paradox in early and advanced HER2 positive breast cancer: pooled analysis of clinical trial data. NPJ Breast Cancer. 2021;7(1):30. https://doi.org/10.1038/s41523-021-00241-9. This pooled analysis indicates an obesity paradox in patients in metastatic HER2+ breast cancer where higher BMI was associated with improved survival.
Rock CL, Flatt SW, Byers TE, Colditz GA, Demark-Wahnefried W, Ganz PA, et al. Results of the exercise and nutrition to enhance recovery and good health for you (ENERGY) trial: a behavioral weight loss intervention in overweight or obese breast cancer survivors. J Clin Oncol. 2015;33(28):3169–76. https://doi.org/10.1200/JCO.2015.61.1095.
Rock CL, Pande C, Flatt SW, Ying C, Pakiz B, Parker BA, et al. Favorable changes in serum estrogens and other biologic factors after weight loss in breast cancer survivors who are overweight or obese. Clin Breast Cancer. 2013;13(3):188–95. https://doi.org/10.1016/j.clbc.2012.12.002.
Swisher AK, Abraham J, Bonner D, Gilleland D, Hobbs G, Kurian S, et al. Exercise and dietary advice intervention for survivors of triple-negative breast cancer: effects on body fat, physical function, quality of life, and adipokine profile. Support Care Cancer. 2015;23(10):2995–3003. https://doi.org/10.1007/s00520-015-2667-z.
Demark-Wahnefried W, Jones LW, Snyder DC, Sloane RJ, Kimmick GG, Hughes DC, et al. Daughters and mothers against breast cancer (DAMES): main outcomes of a randomized controlled trial of weight loss in overweight mothers with breast cancer and their overweight daughters. Cancer. 2014;120(16):2522–34. https://doi.org/10.1002/cncr.28761.
Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux A, et al. The LISA randomized trial of a weight loss intervention in postmenopausal breast cancer. NPJ Breast Cancer. 2020;21(6):6. https://doi.org/10.1038/s41523-020-0149-z.
Demark-Wahnefried W, Morey MC, Sloane R, Snyder DC, Miller PE, Hartman TJ, et al. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J Clin Oncol. 2012;30(19):2354–61. https://doi.org/10.1200/JCO.2011.40.0895.
Harrigan M, Cartmel B, Loftfield E, Sanft T, Chagpar AB, Zhou Y, et al. Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: the lifestyle, exercise, and nutrition (LEAN) study. J Clin Oncol. 2016;34(7):669–76. https://doi.org/10.1200/JCO.2015.61.6375.
Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the women’s healthy eating and living (WHEL) randomized trial. JAMA. 2007;298(3):289–98. https://doi.org/10.1001/jama.298.3.289.
Pakiz B, Flatt SW, Bardwell WA, Rock CL, Mills PJ. Effects of a weight loss intervention on body mass, fitness, and inflammatory biomarkers in overweight or obese breast cancer survivors. Int J Behav Med. 2011;18(4):333–41. https://doi.org/10.1007/s12529-010-9079-8.
Sheppard VB, Hicks J, Makambi K, Hurtado-de-Mendoza A, Demark-Wahnefried W, Adams-Campbell L. The feasibility and acceptability of a diet and exercise trial in overweight and obese black breast cancer survivors: The stepping STONE study. Contemp Clin Trials. 2016;46:106–13. https://doi.org/10.1016/j.cct.2015.12.005.
Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the women’s intervention nutrition study. J Natl Cancer Inst. 2006;98(24):1767–76. https://doi.org/10.1093/jnci/djj494.
Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux A, et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J Clin Oncol. 2014;32(21):2231–9.
Villarini A, Pasanisi P, Traina A, Mano MP, Bonanni B, Panico S, et al. Lifestyle and breast cancer recurrences: the DIANA-5 trial. Tumori J. 2012;98(1):1–18.
Bruno E, Krogh V, Gargano G, Grioni S, Bellegotti M, Venturelli E, et al. Adherence to dietary recommendations after one year of intervention in breast cancer women: the DIANA-5 trial. Nutrients. 2021;13(9):2990. https://doi.org/10.3390/nu13092990.
Prevention of breast cancer recurrence through weight control, diet, and physical activity intervention (PREDICOP) [cited 2023 Sept 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT02035631
Rack B, Andergassen U, Neugebauer J, Salmen J, Hepp P, Sommer H, et al. The German SUCCESS C study – the first European lifestyle study on breast cancer. Breast Care (Basel). 2010;5(6):395–400. https://doi.org/10.1159/000322677.
•• Ligibel JA, Ballman KV, McCall LM, Goodwin PJ, Weiss A, Delahanty L, et al. Effect of a telephone-based weight loss intervention (WLI) on weight at 12-months in women with early breast cancer: results from the breast cancer weight loss (BWEL) trial. J Clin Oncol. 2023;41(16_suppl):12001–12001. This study abstract presented at ASCO 2023 reports a telephone-based weight-loss intervention that induced significant, clinically meaningful weight loss in patients with stage II or III breast cancer with overweight or obesity. Longer follow-up is required to evaluate whether the intervention improves disease outcomes in this patient population.
Ligibel JA, Barry WT, Alfano CM, Hershman DL, Irwin ML, Neuhouser M, et al. The breast cancer weight loss (BWEL) trial: randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early-stage breast cancer (Alliance A011401). J Clin Oncol. 2018;36(15_suppl):TPS598–TPS598.
Pierce JP. Diet and breast cancer prognosis: making sense of the Women’s Healthy Eating and Living and women’s intervention nutrition study trials. Curr Opin Obstet Gynecol. 2009;21(1):86–91. https://doi.org/10.1097/gco.0b013e32831da7f2.
Iyengar NM, Jones LW. Development of exercise as interception therapy for cancer: a review. JAMA Oncol. 2019;5(11):1620–7.
A study of the body’s response to exercise and a plant-based diet in overweight postmenopausal women with breast cancer [cited 2023 Sept 20]. Available from: https://classic.clinicaltrials.gov/ct2/show/NCT04298086
Cejuela M, Martin-Castillo B, Menendez JA, Pernas S. Metformin and breast cancer: where are we now? Int J Mol Sci. 2022;23(5):2705. https://doi.org/10.3390/ijms23052705.
• Bellerba F, Chatziioannou AC, Jasbi P, Robinot N, Keski-Rahkonen P, Trolat A, et al. Metabolomic profiles of metformin in breast cancer survivors: a pooled analysis of plasmas from two randomized placebo-controlled trials. J Transl Med. 2022;20(1):629. https://doi.org/10.1186/s12967-022-03809-6. This study investigated the metabolomic effects of metformin therapy in patients with BC and indicates changes in biochemical pathways implicated in cancer cell growth.
Coyle C, Cafferty FH, Vale C, Langley RE. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27(12):2184–95. https://doi.org/10.1093/annonc/mdw410.
• El-Attar AA, Ibrahim OM, Alhassanin SA, Essa ES, Mostafa TM. Effect of metformin as an adjuvant therapy to letrozole on estradiol and other biomarkers involved in the pathogenesis of breast cancer in overweight and obese postmenopausal women: a pilot study. Eur J Clin Pharmacol. 2023;79(2):299–309. https://doi.org/10.1007/s00228-022-03444-6. This study investigated the effect of metformin on biomarkers associated with breast cancer recurrence and found improvement in prognostic biomarkers.
Patterson RE, Marinac CR, Sears DD, Kerr J, Hartman SJ, Cadmus-Bertram L, et al. The effects of metformin and weight loss on biomarkers associated with breast cancer outcomes. J Natl Cancer Inst. 2018Nov 1;110(11):1239–47. https://doi.org/10.1093/jnci/djy040.
Sonnenblick A, Agbor-Tarh D, Bradbury I, Di Cosimo S, Azim HA Jr, Fumagalli D, et al. Impact of diabetes, insulin, and metformin use on the outcome of patients with human epidermal growth factor receptor 2-positive primary breast cancer: analysis from the ALTTO phase III randomized trial. J Clin Oncol. 2017;35(13):1421–9. https://doi.org/10.1200/JCO.2016.69.7722.
•• Goodwin PJ, Chen BE, Gelmon KA, Whelan TJ, Ennis M, Lemieux J, et al. Effect of metformin vs placebo on invasive disease-free survival in patients with breast cancer: the MA.32 randomized clinical trial. JAMA. 2022;327(20):1963–73. https://doi.org/10.1001/jama.2022.6147. This definitive trial investigated the efficacy of adjuvant metformin versus placebo in patients with primary breast cancer. Addition of metformin to standard breast cancer treatment did not improve invasive disease-free survival.
Gallagher EJ, Kase NG, Bickell NA, LeRoith D. Metformin and cancer: is this the end? Endocr Pract. 2022;28(8):832–4. https://doi.org/10.1016/j.eprac.2022.06.005.
Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740–56. https://doi.org/10.1016/j.cmet.2018.03.001.
Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. https://doi.org/10.1056/NEJMoa1411892.
Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. https://doi.org/10.1056/NEJMoa2032183.
Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–16. https://doi.org/10.1056/NEJMoa2206038.
Ligumsky H, Wolf I, Israeli S, et al. The peptide-hormone glucagon-like peptide-1 activates cAMP and inhibits growth of breast cancer cells. Breast Cancer Res Treat. 2012;132(2):449–61. https://doi.org/10.1007/s10549-011-1585-0.
Iwaya C, Nomiyama T, Komatsu S, et al. Exendin-4, a glucagonlike peptide-1 receptor agonist, attenuates breast cancer growth by inhibiting NF-κB activation. Endocrinology. 2017;158(12):4218–32. https://doi.org/10.1210/en.2017-00461.
Martin-Castillo B, Pernas S, Dorca J, Álvarez I, Martínez S, Pérez-Garcia JM, et al. A phase 2 trial of neoadjuvant metformin in combination with trastuzumab and chemotherapy in women with early HER2-positive breast cancer: the METTEN study. Oncotarget. 2018;9(86):35687–704. https://doi.org/10.18632/oncotarget.26286.
•• Huang J, Tong Y, Hong J, Huang O, Wu J, He J, et al. Neoadjuvant docetaxel, epirubicin, and cyclophosphamide with or without metformin in breast cancer patients with metabolic abnormality: results from the randomized phase II NeoMET trial. Breast Cancer Res Treat. 2023;197(3):525–33. https://doi.org/10.1007/s10549-022-06821-y. This trial investigated the efficacy of adding metformin to neoadjuvant chemotherapy in early breast cancer. Addition of metformin did not significantly improve pathological complete response rates.
• Yee D, Isaacs C, Wolf DM, Yau C, Haluska P, Giridhar KV, et al. Ganitumab and metformin plus standard neoadjuvant therapy in stage 2/3 breast cancer. NPJ Breast Cancer. 2021;7(1):131. https://doi.org/10.1038/s41523-021-00337-2. This trial explores the efficacy of adding metformin and ganitumab to neoadjuvant chemotherapy in stage 2/3 breast cancer. Although an improved pathological complete response rate was observed, the trial did not meet the prespecified threshold for advancement to phase 3 testing.
Topart P. Obesity surgery: which procedure should we choose and why? J Visc Surg. 2023;160(2S):S30–7. https://doi.org/10.1016/j.jviscsurg.2022.12.010.
Eisenberg D, Shikora SA, Aarts E, Aminian A, Angrisani L, Cohen RV, et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): indications for metabolic and bariatric surgery. Surg Obes Relat Dis. 2022;18(12):1345–56. https://doi.org/10.1016/j.soard.2022.08.013.
Mackenzie H, Markar SR, Askari A, Faiz O, Hull M, Purkayastha S, et al. Obesity surgery and risk of cancer. Br J Surg. 2018;105(12):1650–7. https://doi.org/10.1002/bjs.10914.
•Doumouras AG, Lovrics O, Paterson JM, Sutradhar R, Paszat L, Sivapathasundaram B et al. Residual Risk of Breast Cancer After Bariatric Surgery. JAMA Surg. 2023;12:e230530. https://doi.org/10.1001/jamasurg.2023.0530. Epub ahead of print. In this matched cohort study, bariatric surgery was associated with a reduction in breast cancer risk.
•• Aminian A, Wilson R, Al-Kurd A, Tu C, Milinovich A, Kroh M, et al. Association of bariatric surgery with cancer risk and mortality in adults with obesity. JAMA. 2022;327(24):2423–33. https://doi.org/10.1001/jama.2022.9009. The SPLENDID matched cohort study investigated time to incidence of obesity induced cancers in patients treated with and without bariatric surgery. Bariatric surgery was associated with a significantly lower incidence of obesity-associated cancers and cancer-related mortality.
Wilson RB, Lathigara D, Kaushal D. Systematic review and meta-analysis of the impact of bariatric surgery on future cancer risk. Int J Mol Sci. 2023;24(7):6192. https://doi.org/10.3390/ijms24076192.
Zhang S, Ikramuddin S, Beckwith HC, Sheka AC, Wirth KM, Blaes AH. The impact of bariatric surgery on breast cancer recurrence: case series and review of literature. Obes Surg. 2020;30(2):780–5. https://doi.org/10.1007/s11695-019-04099-6.
BariaTric Surgery After Breast Cancer Treatment (BATS) [cited 2023 Sept 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT03946423
Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes (Lond). 2008;32(Suppl 3):S56–9. https://doi.org/10.1038/ijo.2008.87.
Deurenberg P, Andreoli A, Borg P, Kukkonen-Harjula K, de Lorenzo A, van Marken Lichtenbelt WD, et al. The validity of predicted body fat percentage from body mass index and from impedance in samples of five European populations. Eur J Clin Nutr. 2001;55(11):973–9. https://doi.org/10.1038/sj.ejcn.1601254.
Ruderman NB, Schneider SH, Berchtold P. The, “metabolically-obese”, normal-weight individual. Am J Clin Nutr. 1981;34(8):1617–21. https://doi.org/10.1093/ajcn/34.8.1617.
Stefan N, Schick F, Häring HU. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab. 2017;26(2):292–300. https://doi.org/10.1016/j.cmet.2017.07.008.
Franco LP, Morais CC, Cominetti C. Normal-weight obesity syndrome: diagnosis, prevalence, and clinical implications. Nutr Rev. 2016;74(9):558–70. https://doi.org/10.1093/nutrit/nuw019.
• Arthur RS, Dannenberg AJ, Kim M, Rohan TE. The association of body fat composition with risk of breast, endometrial, ovarian and colorectal cancers among normal weight participants in the UK Biobank. Br J Cancer. 2021;124(9):1592–605. This study reports an association between body fat levels and risk of several cancers in normal weight individuals; high body fat levels were associated with increased risk of obesity-related cancers in this normal weight population.
Kim JW, Ahn ST, Oh MM, Moon DG, Han K, Park HS. Incidence of prostate cancer according to metabolic health status: a nationwide cohort study. J Korean Med Sci. 2019;34(6):e49–e49.
Kabat GC, Kim MY, Stefanick M, Ho GYF, Lane DS, Odegaard AO, et al. Metabolic obesity phenotypes and risk of colorectal cancer in postmenopausal women. Int J Cancer. 2018;143(3):543–51.
Liu B, Giffney HE, Arthur RS, Rohan TE, Dannenberg AJ. Cancer risk in normal weight individuals with metabolic obesity: a narrative review. Cancer Prev Res (Phila). 2021;14(5):509–20. https://doi.org/10.1158/1940-6207.CAPR-20-0633.
Iyengar NM, Arthur R, Manson JE, Chlebowski RT, Kroenke CH, Peterson L, et al. Association of body fat and risk of breast cancer in postmenopausal women with normal body mass index: a secondary analysis of a randomized clinical trial and observational study. JAMA Oncol. 2019;5(2):155–63. https://doi.org/10.1001/jamaoncol.2018.5327.
Park YM, White AJ, Nichols HB, O’Brien KM, Weinberg CR, Sandler DP. The association between metabolic health, obesity phenotype and the risk of breast cancer. Int J Cancer. 2017;140(12):2657–66. https://doi.org/10.1002/ijc.30684.
Gunter MJ, Xie X, Xue X, Kabat GC, Rohan TE, Wassertheil-Smoller S, Ho GY, Wylie-Rosett J, Greco T, Yu H, Beasley J, Strickler HD. Breast cancer risk in metabolically healthy but overweight postmenopausal women. Cancer Res. 2015;75(2):270–4. https://doi.org/10.1158/0008-5472.CAN-14-2317.
Ogundiran TO, Huo D, Adenipekun A, Campbell O, Oyesegun R, Akang E, Adebamowo C, Olopade OI. Body fat distribution and breast cancer risk: findings from the Nigerian breast cancer study. Cancer Causes Control. 2012;23(4):565–74. https://doi.org/10.1007/s10552-012-9916-y.
Funding
This work was supported in part through the National Institutes of Health/National Cancer Institute (NIH/NCI) Cancer Center Support Grant P30 CA008748. SS is supported by a Young Investigator Award from the Conquer Cancer Foundation of the American Society of Clinical Oncology and by the Clinical and Translational Science Center at Weill Cornell Medical Center and Memorial Sloan Kettering Cancer Center CTSA UL1TR00457 grant. NMI is supported through NIH 1R01CA235711, the Breast Cancer Research Foundation, American Cancer Society Research Scholar Grant, and the Kat’s Ribbon of Hope Foundation. NMI discloses research grants (to institution) from Novartis and SynDevRx and consulting fees from Pfizer, Novartis, Seattle Genetics, Gilead, Astra Zeneca, and BD Life Sciences outside the submitted work.
Ethics declarations
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chew, S.M., Liu, B., Shen, S. et al. The Role of Obesity and Inflammation in Breast Cancer Recurrence. Curr Breast Cancer Rep 16, 237–250 (2024). https://doi.org/10.1007/s12609-024-00550-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-024-00550-5