Abstract
Purpose
Breast cancer patients with metabolic syndrome (MetS) and its components show worse treatment responses to chemotherapy. Metformin is a widely used antidiabetic drug which also shows potential anticancer effect. This study aims to evaluate the efficacy, safety, and metabolic parameters change of metformin combined with docetaxel, epirubicin, and cyclophosphamide (TEC) in neoadjuvant treatment (NAT) for breast cancer patients with metabolic abnormality.
Methods
Eligible breast cancer patients were randomized to receive six cycles of TEC (docetaxel 75 mg/m2, epirubicin 75 mg/m2, and cyclophosphamide 500 mg/m2, d1, q3w) or TEC with metformin (TECM, TEC with oral metformin 850 mg once daily for the first cycle, then 850 mg twice daily for the following cycles). The primary end point was total pathological complete response (tpCR, ypTis/0N0) rate.
Results
Ninety-two patients were enrolled and randomized from October 2013 to December 2019: 88 patients were available for response and safety assessment. The tpCR rates were 12.5% (5/40) and 14.6% (7/48) in the TEC and TECM groups, respectively (P = 0.777). There was no difference in Ki67 decrease after NAT between two groups (P = 0.456). Toxicity profile were similar between two groups. No grade 3 or higher diarrhea were recorded. Total cholesterol (TC) and high-density lipoprotein cholesterol worsened after NAT in the TEC arm but remained stable in the TECM arm. The absolute increase of TC and low-density lipoprotein cholesterol (LDL-C) was significantly lower in the TECM group compared with the TEC group. After a median follow-up of 40.8 (4.7–70.8) months, no survival difference was observed between TEC and TECM groups (all P > 0.05).
Conclusion
Adding metformin to TEC didn’t increase pCR rate and disease outcome in breast cancer patients with metabolic abnormality. However, additional metformin treatment with chemotherapy would prevent TC and LDL-C increase after NAT.
Trial Registration ClinicalTrials.gov Identifier: NCT01929811.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Breast cancer (BC) is the most frequent malignancy and the second leading cause of cancer death among women worldwide [1]. Neoadjuvant therapy (NAT) is an increasingly used therapeutic strategy for BC. Pathologic complete response (pCR), is a powerful predictor of long-term survival [2, 3]. There is no standard neoadjuvant chemotherapy regimen. The NSABP-B27 study demonstrated that preoperative administration of docetaxel following AC increased the pathological complete response (pCR) rate from 13.7% to 26.1% [4]. An M.D.Anderson study showed adding paclitaxel prior to preoperative fluorouracil, doxorubicin and cyclophosphamide regimen increased the pCR rate from 15.7% to 28.2% [5]. Compared to sequential treatment, concurrent use of taxane with anthracycline provide a similar response but with shorten treatment duration. Concurrent docetaxel, epirubicin and cyclophosphamide (TEC) regimen is a well-tolerated and effective neoadjuvant chemotherapy regimen for locally advanced BC that results in a pathologic complete response rate of 17.6% ~ 25% [6, 7].
Metabolic syndrome (MetS), a group of metabolic risk factors including central obesity, insulin resistance, dyslipidemia, and elevated blood pressure, has been shown to upregulate inflammatory, adipose-derived cytokines and select proteases inhibitors, leading to increased BC risk and BC mortality [8, 9]. Compared with BC patients who do not have metabolic abnormalities, those with MetS and its components show worse treatment responses to chemotherapy [10]. Metformin is a widely prescribed oral medication used as first-line therapy for type 2 diabetes. Several case–control and cohort studies identified that treatment with metformin appears to substantially reduce the risk for the development of cancer in individuals diagnosed with diabetes, including a lower risk for BC [11, 12]. For patients with BC diagnosis, some evidence also suggests a role for metformin in prolonging survival [13]. A landmark retrospective study revealed that patients with type 2 diabetes and BC who received metformin and neoadjuvant chemotherapy appeared to have a higher pCR rate than those not receiving metformin [14]. Several prospective randomized trials have been conducted to evaluate the clinical efficacy of metformin in non-diabetic BC patients but had unsatisfactory results. Two small prospective randomized study assessing the efficacy of adding metformin to neoadjuvant chemotherapy in non-diabetic BC patients both showed a numerically but not statistically significant increase of pCR in the metformin arm [15, 16]. Also, in the adjuvant setting, the large Phase III randomized trial (NCIC CTG MA.32) in non-diabetic high risk early-stage BC patients, has also failed to detect improvement of invasive disease–free survival by adding metformin to standard adjuvant therapy [17].
To that end, we initiate this phase II study focused on pre-surgical BC patients with baseline metabolic abnormality, to evaluate the efficacy and safety of the addition of metformin to TEC chemotherapy, as well as the metabolic parameters change during NAT.
Methods
Study design and patients
The NeoMET trial (NCT01929811) is a phase II, randomized, single-center, open-label study, conducted in Ruijin Hospital, Shanghai Jiaotong University School of Medicine. Eligible patients had histologically confirmed invasive BC, tumor ≥ 2 cm or stage IIB or III disease according to the American Joint Committee on Cancer staging system, were aged between 18 and 70 years, and had not received any anti-cancer therapy. Included patients must have at least one component of MetS [18, 19]: (1) Overweight measured by body mass index (BMI ≥ 25 kg/m2), (2) Elevated fasting blood-glucose (FBG ≥ 5.6 mmol/L), (3) Elevated blood pressure (≥ 135 mmHg systolic blood pressure or ≥ 85 mmHg diastolic blood pressure) or on antihypertensive drug treatment in a patient with a history of hypertension, (4) Elevated triglycerides (≥ 1.7 mmol/L) or on drug treatment for elevated triglycerides, (5) Reduced high-density lipoprotein cholesterol (HDL-C) < 1.3 mmol/L or on drug treatment for reduced HDL-C. Patients who have been diagnosed with diabetes and treated without metformin are allowed to be enrolled. Those who received metformin were excluded. Key exclusion criteria were stage IV disease, history of non-breast malignancies, previous use of metformin, inadequate organ function, uncontrolled hypertension, pregnancy.
The protocol was approved by the institutional review boards of Ruijin Hospital. All patients provided written informed consent.
Randomization and treatment procedures
Patients were randomly assigned at a 1:1 ratio to receive TEC, or TEC plus metformin (TECM). Randomization was stratified according to primary tumor stage (LABC or non-LABC). LABC was defined as TanyN2-3M0, or T4NanyM0. TEC (docetaxel 75 mg/m2, epirubicin 75 mg/m2, and cyclophosphamide 500 mg/m2) was given intravenously on day 1 every 21 days. All patients were premedicated with oral dexamethasone 8 mg twice daily for 3 days starting 1 day prior to docetaxel injection administration based on the label of docetaxel. Primary prophylaxis with granulocyte colony-stimulating factor was required in each TEC cycle. Metformin was given orally 850 mg once daily for the first cycle, then 850 mg twice daily for the following cycles. After completion of NAT with 6 intravenous cycles, eligible patients underwent surgery within 6 weeks after the last scheduled chemotherapy cycle. In case of confirmed disease progression at any time during NAT, the patient was asked to withdraw from study treatment and receive treatment according to physician’s choice. Additional adjuvant chemotherapy was not allowed. Patients with HER2 + disease received adjuvant trastuzumab every 3 weeks for 1 year after surgery. Adjuvant hormonal therapy and radiotherapy was prescribed as per local guidelines.
Data collection and study outcomes
Baseline physical examination, breast ultrasound, and mammography were done within 2 weeks before study treatment. Physical examination and ultrasound were repeated every two cycles and performed before surgery. Core needle biopsy and surgical specimens were assessed in the Department of Pathology, Ruijin Hospital. Estrogen receptor (ER), progesterone receptor (PR) and HER2 status were evaluated by immunohistochemical (IHC) in paraffin-embedded tumor samples before and after NAT. The cutoff value for ER or PR positivity was no less than 1% tumor cells with positive nuclear staining. For IHC HER2 2 + patients, fluorescence in-situ hybridization (FISH) was then done to confirm the HER2 status. HER2 positive (HER2 +) BC was considered as HER2 3 + by IHC or positive by FISH. Triple negative breast cancer (TNBC) was defined as ER-, PR-, and HER2-.
Weight, BMI, fasting serum insulin, C-peptide, glucose, triglyceride, cholesterol, HDL-C and low-density lipoprotein cholesterol (LDL-C) were assessed before and after NAT. Homeostasis model assessment of insulin resistance (HOMA-IR) and homeostasis model assessment of β-cell function (HOMA-β) was calculated by fasting glucose and insulin measured on the same day. HOMA-IR = glucose (mmol/L) × insulin (μIU/mL)/22.5. HOMA-β = {20 × insulin (μIU/mL)/[glucose (mmol/L)-3.5]}%.
The primary endpoint was total pathological complete response (tpCR) rate, defined as the absence of invasive tumor in the breast and axilla, remaining in-situ lesions were allowed (ypT0/Tis ypN0). Pathological complete response in the breast (bpCR) was defined as the absence of invasive tumor in the breast, with or without residual positive lymph nodes. Secondary endpoints included clinical response rate (derived clinical response according to RECIST version 1.0), event-free survival (EFS), disease-free survival (DFS), overall survival (OS) and safety. Events for calculation of EFS included disease progression during neoadjuvant chemotherapy, all local and regional recurrences, distant metastasis, second malignant carcinoma, contralateral BC, and deaths of any cause. EFS interval was measured from the date of initial diagnosis to the date of last follow-up or above events. DFS definition was similar with EFS exclusive of disease progression. OS was defined as the time interval from the date of initial diagnosis to the date of last follow-up or death. We assessed laboratory parameters, performance status, and vital signs at every cycle and monitored adverse events continuously until 28 days after the last treatment. We graded the intensity of these events according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
Statistical analysis
Based on a two-group binomial design, to detect an absolute increase of 15% in the rate of pCR in the TECM arm, compared with the assumed rate of 20% in the TEC arm at a significance level of 0.2 with more than 80% power, the estimated sample size was 200 eligible patients (100 in each group) with an assumed dropout rate of 20%. After 92 of 200 planned patients were randomized, the trial was prematurely closed due to slow recruitment, which left the study underpowered relative to its primary endpoint (i.e., pCR). Statistical analysis was done based on actual enrollment with exploratory purpose. P values should not be used for drawing conclusions about the impact on pCR when adding neoadjuvant metformin to chemotherapy.
Response rate and toxicities were compared between arms using two-tailed fisher’s exact or χ2 test. Survival data were analyzed for all patients and subgroups with Kaplan–Meier method, and the resulting curves were compared between groups with log-rank tests. Summary statistics for weight and metabolic variables are presented as median, interquartile change (IQR). The statistical significance of difference between baseline and post-NAT for each patient group was tested by Wilcoxon rank sum test. The statistical significance of differences between groups for changes between baseline and post-NAT (defined as median of [post-NAT value – baseline value]) were tested using the Mann Whitney U test. All statistical tests were two-sided and carried out at significance level of 0.05 using the SPSS statistical software package (version 23.0; IBM Company, Chicago, IL).
Results
Patient characteristics and treatment
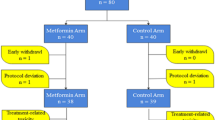
Enrollment detail is demonstrated in Fig. 1. A total of 92 patients underwent randomization between October 2013 and December 2019, of whom 50 were randomized to TECM and 42 to TEC. Two patients in the TECM arm and two in the TEC arm refused treatment and were not included in response assessment. Overall, 88 patients (48 in the TECM arm and 40 in the TEC arm) were eligible as intention-to-treat and safety population. Among the 88 patients, 41 patients in the TECM arm and 35 patients in the TEC arm received 6 full cycles of NAT and had surgery, and the rest 7 patients in the TECM arm and 5 in the TEC arm discontinued due to disease progression, adverse events or patient's decision. The mean cycles of NAT were 5.84 (TECM) and 5.87 (TEC). Dose intensity of docetaxel was 99.6% in both arms. Dose intensity of metformin was 96.4%. Two patients in the TECM arm discontinued to take metformin since the second treatment cycle. One was due to grade 2 diarrhea and the other was due to patient's will.
Baseline characteristics were balanced across treatment groups (Table 1). The median age of 54 years in both groups. 44.0% of patients had LABC in the TECM group compared with 33.3% in the TEC group (P = 0.296). Overall, twenty-one (22.8%) patients had one component of MetS, 30 (32.6%) patients were with two components of MetS and 41 (44.6%) patients had three or more elements of MetS, which was also comparable between two arms (P = 0.528). Among the 11 patients with hyperglycemia, two was diagnosed with diabetes at the time of enrollment and was not treated with metformin for diabetes, both of whom were randomized to the TECM group. Twelve (13.0%) patients were with HER2 positive disease and 19 (20.7%) had TNBC.
Tumor response to NAT
Clinical and pathological response in NAT are summarized in Table 2. A tpCR was noted in 7 of 48 patients (14.6%) in the TECM arm, compared with 5 of 40 (12.5%) in the TEC arm (P = 0.777; Table 2). Another case in the TEC arm achieved bpCR with residual disease in the nodes. The tpCR rate was 17.0% among LABC and 8.6% in patients with non-LABC, and there was no significant difference between treatment groups (Supplementary Table 1). The tpCR rate was 22.2%, 6.9%, 17.4%, 7.1% and 50.0% in patients with 1, 2, 3, 4 and 5 components of MetS, respectively (Supplementary Table 2). Objective response (complete response or partial response) rate was 57.5% in TECM arm and 52.5% in the TEC arm (P = 0.906). Progressive disease was recorded in 6 (12.5%) patients in the TECM group and 3 (7.5%) patients in the TEC arm. Forty-five (93.7%) patients received TECM treatment underwent surgery, while 38 (95.0%) received TEC treatment underwent surgery. The breast conserving rate was 10.4% in the TECM arm and 12.5% in the TEC arm (P = 0.759). In patients with residual disease after NAT, the median percentage change of Ki67 after NAT was −10 (−25, 0) in the TECM arm and −10 (−27.3, 0) in the TEC arm (P = 0.456).
Safety
The most common adverse events occurred in more than 10% of study population were leucopenia, neutropenia, anemia, nausea, vomiting, anorexia, diarrhea, fatigue, hand foot syndrome, alopecia, arthralgia, and elevated liver enzymes (Fig. 2). Most adverse events were grade 1–2. The most common adverse events of grade 3 or higher were neutropenia and leucopenia, which was 42.5% and 55.0% in the TEC arm and 22.9% and 45.8% in the TECM arm, retrospectively. Grade 1–2 diarrhea was reported by 22.9% of patients from the TECM arm and 12.5% of patients from the TEC arm. No grade 3 or higher diarrhea was recorded in both arms. No severe adverse event was recorded. There is no significant increase of adverse events by adding metformin to TEC as NAT.
MetS-related variables change pre- and post-NAT
The changes of metabolic parameters post- and pre-NAT are summarized in Supplementary Table 3 and Supplementary Fig. 1. A significant increase of triglyceride was observed in both TEC [0.39, IQR (−0.10, 1.08), P = 0.005] and TECM [0.28, IQR (−0.20, 0.70), P = 0.008] groups (Supplementary Fig. 1f). The elevation of TC [TEC: 0.26, IQR(−0.29, 1.15), P = 0.037, TECM: −0.18, IQR (−0.67, 0.58), P = 0.458, Supplementary Fig. 1 g) were more frequently reported in the TEC arm but not in the TECM arm, as well as the decrease of HDL-C (TEC: −0.12, IQR (−0.34, 0.09), P = 0.004, TECM: −0.09, IQR (−0.18,0.13), P = 0.167, Supplementary Fig. 1 h). Other MetS-related variables, including weight, BMI, insulin, C-peptide, glucose, HOMA-IR, HOMA-β, were comparable before and after NAT in both arms (all P > 0.05, Supplementary Fig. 1a-e, 1i-k).
The absolute change of MetS-related variables pre- and post-NAT were also calculated and compared between two arms (Supplementary Table 3). The absolute increases of TC were significantly lower in the TECM group compared with the TEC group [TEC: 0.26, IQR (−0.29, 1.15), TECM: −0.18, IQR (−0.67, 0.58), P = 0.028]. Low-density lipoprotein cholesterol (LDL-C) increased in the TEC arm but decreased in the TECM arm [TEC: 0.42, IQR (−0.33, 0.77), TECM: −0.14, IQR (−0.64, 0.33), P = 0.036]. The change of other metabolic parameters including weight, blood-glucose, etc. during NAT was comparable between TEC and TECM arms (all P > 0.05, Supplementary Table 3).
Survival
After a median follow-up of 40.8 months (range: 4.7 to 70.9 months), 15 cases were recorded to have disease progress, recurrence or death, including 8 in the TECM group and 7 in the TEC group. Only one patient in the TEC group died following recurrent BC. No difference of EFS (3-year estimated EFS 85.9% vs 82.0%, P = 0.987, Supplementary Fig. 2a) or DFS (3-year estimated DFS 90.6% vs 82.3%, P = 0.451, Supplementary Fig. 2d) were observed between two treatment groups. EFS (3-year estimated EFS 90.0% vs 84.2%, P = 0.459 Supplementary Fig. 2b) and DFS (3-year estimated DFS 90.0% vs 84.2%, P = 0.460, Supplementary Fig. 2e) were also similar between patients achieved tpCR or non-tpCR, or among patients stratified combining treatment group and tpCR status (Supplementary Fig. 2c, f). 3-year estimated OS was 100.0% in TECM group and 97.1% in TEC group.
Discussion
Our study demonstrated that the addition of metformin to TEC did not significantly improve tpCR rate or survival rate in BC patients with metabolic abnormality. Ki67 decrease after NAT were observed in both treatment groups but without significant difference. TC and LDL-C increasement after NAC was alleviated by adding metformin to TEC.
Pre-clinical research has identified inhibitory effects of metformin on the proliferation of BC cells in culture and also on tumor growth in mice [20]. In 2009, Jiralerspong et al. showed in a retrospective BC patients cohort receiving NAT, that adding metformin to regular NAT led to a higher pCR rate for diabetic BC patients, which provided the first clinical evidence of the efficacy of metformin as a NAT partner in BC treatment [14]. However, in the NeoMET trial, metformin did not add to the treatment efficacy of chemotherapy backbone TEC in patients with metabolic abnormality, with relatively low pCR rate in both arms, in agreement with previous prospective studies. The METTEN study assessed the efficacy of adding metformin to neoadjuvant chemotherapy plus trastuzumab in early-stage non-diabetic human epidermal growth factor receptor-2 positive (HER2 +) BC but closed prematurely with a reduced sample size after 84 of 244 planned patients were randomly assigned [15]. The pCR rate was numerically higher in the metformin-containing arm (65.5%) than in the control arm (58.6%), without statistical significance. Another Egypt randomized controlled trial including 80 LABC patients regardless of subtypes also showed a numerically but not statistically increase of pCR (22.2% vs 10.5%) by adding metformin to anthracycline/taxane-based NAT [16]. An exploratory analysis from the METTEN study showed that HER2 + patients bearing the rs11212617 C allele, which was found to be associated with the metabolic response to metformin in patients with type 2 diabetes [21], had a significantly higher probability of pCR in the metformin-containing arm [22]. The benefit of metformin might be restricted to selected patients. In the current trial including patients with abnormality component of MetS, only 12% of patients reported hyperglycemia at baseline, and only 13% of patients had HER2 + disease, which might contribute to the negative results of the trial. Moreover, over 60% of patients enrolled in this study were with HR + /HER2- subtype, which was related with lower pCR rate compared with other subtypes treated with NAT.
In our study, Ki67 reduction were observed in both treatment groups but without significant difference between groups. Two pre-surgical trials observed a significant but numerically small decrease in Ki67, which was 3.3% to 3.5%, after a median of 14–18 days of metformin use [23, 24]. In other two pre-surgical BC trials using metformin for 23–28 days before surgery, no change in Ki67 was identified [25, 26]. Small sample size and different duration of metformin use might contribute to the difference of results. In addition, metformin was administrated with the backbone of chemotherapy in our trial. The absolute decrease of Ki67 after neoadjuvant chemotherapy has been reported to be larger (13.0% to 14.6%) in previous studies [27, 28]. In our study, the Ki67 decrease may be driven by the use of chemotherapy and the effect of metformin may be weakened and no longer significant.
In our study, TC and HDL-C worsened after NAT in the TEC arm but remained stable in the TECM arm. The absolute increase of TC was significantly lower in the TECM group compared with the TEC group, while the increase of LDL-C was reversed by adding metformin to TEC. These results indicated that the use of metformin can contribute to alleviate the worsening of lipid profile during chemotherapy. In a pre-surgical trial of metformin in 35 overweight and obese BC patients, in which metformin was administered for a median of 23 days between diagnosis and surgery, a significant reduction in BMI, cholesterol, and leptin was also observed [26]. In our study, we also identified the benefit of metformin on preventing the increase of cholesterol. However, the differences of BMI between two arms were not detected. The chemotherapy backbone and the host difference i.e., baseline obese status of patients might contribute to the different results. In the MA.32 study, improved weight, insulin, glucose, leptin and CRP were observed after metformin use in the adjuvant setting [29], while the difference of these markers has not been identified in our study. We need to notice that in MA.32 trial, chemotherapy, if given, was completed at least one month prior to enrollment. The concurrent use of chemotherapy in our study may weaken the benefit of metformin.
Our study didn’t demonstrate survival difference by adding metformin during NAT in patients with at least one component of MetS. The benefit of metformin in long-term survival of BC patients was ambiguous in previous studies. A meta-analysis including five phase II randomized controlled trials showed that additional use of metformin was not associated with improved PFS or OS in non-diabetic BC patients [30]. A retrospective study of patients with surgically resected BC in South Korea showed that metformin administration may be associated with reduced mortality especially in patients receiving hormonal therapy or HER2 targeted therapy [31]. The MA.32 study showed that metformin improved invasive disease–free survival and overall survival only in HER2 + population with any C allele of the rs11212617 SNV [17]. In our study, we enrolled ER + (75%), ER- (25%) and HER2 + (13%) patients, while the trial wasn’t powered enough for subgroup analysis in different subtypes.
One major concern regarding the utility of metformin is its known ability to induce diarrhea, which might limit patient compliance, particularly when combined with cytotoxic chemotherapy [32]. In the NeoMET study, no grade 3 or higher diarrhea were recorded. The most common grade 3 or higher adverse events were neutropenia and leucopenia in both arms. Our study confirmed that adding metformin to neoadjuvant chemotherapy can be well-tolerated.
Limitations of the current trial lay in that, first of all, the premature termination and relatively small enrollment led to the impuissance to draw a convincing conclusion, or to conduct further subgroup analysis. Meantime, we didn’t conduct pathologic biopsy or metabolic measurements during the course of NAT, thus unable to reflect the dynamic influence of metformin on tumor progression or metabolic disturbances in these patients. Further follow-up might offer additional information on the long-term outcomes of metformin-treated patients.
In conclusion, the NeoMET trial showed that adding metformin to TEC had no significant improvement on pCR rate and long-term survival in BC patients with metabolic abnormality. However, the addition of metformin would prevent TC and LDL-C increase after NAT. Our study can be a reference when designing any further trials of this intervention.
Data availability
The datasets analyzed in the present study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Fuchs HE et al (2021) Cancer statistics. CA Cancer J Clin 71(1):7–33. https://doi.org/10.3322/caac.21654
Kaufmann M, von Minckwitz G, Mamounas EP et al (2012) Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol 19(5):1508–1516. https://doi.org/10.1245/s10434-011-2108-2
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172. https://doi.org/10.1016/S0140-6736(13)62422-8
Rastogi P, Anderson SJ, Bear HD et al (2008) Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol 26(5):778–785. https://doi.org/10.1200/JCO.2007.15.0235
Green MC, Buzdar AU, Smith T et al (2005) Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol 23(25):5983–5992. https://doi.org/10.1200/JCO.2005.06.232
Yao X, Hosenpud J, Chitambar CR et al (2012) A phase II study of concurrent docetaxel, epirubicin and cyclophosphamide as a neoadjuvant chemotherapy regimen in patients with locally advanced breast cancer. J Cancer 3:145–151. https://doi.org/10.7150/jca.3980
Chen X, Ye G, Zhang C et al (2013) Superior outcome after neoadjuvant chemotherapy with docetaxel, anthracycline, and cyclophosphamide versus docetaxel plus cyclophosphamide: results from the NATT trial in triple negative or HER2 positive breast cancer. Breast Cancer Res Treat 142(3):549–558. https://doi.org/10.1007/s10549-013-2790-910.1007/s10549-013-2761-1
Bjorge T, Lukanova A, Jonsson H et al (2010) Metabolic syndrome and breast cancer in the me-can (metabolic syndrome and cancer) project. Cancer Epidemiol Biomarkers Prev 19(7):1737–1745. https://doi.org/10.1158/1055-9965.EPI-10-0230
Bhandari R, Kelley GA, Hartley TA et al (2014) Metabolic syndrome is associated with increased breast cancer risk: a systematic review with meta-analysis. Int J Breast Cancer 2014:189384. https://doi.org/10.1155/2014/189384
Stebbing J, Sharma A, North B et al (2012) A metabolic phenotyping approach to understanding relationships between metabolic syndrome and breast tumour responses to chemotherapy. Ann Oncol 23(4):860–866. https://doi.org/10.1093/annonc/mdr347
Decensi A, Puntoni M, Goodwin P et al (2010) Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 3(11):1451–1461. https://doi.org/10.1158/1940-6207.CAPR-10-0157
Dankner R, Agay N, Olmer L et al (2019) Metformin treatment and cancer risk: cox regression analysis, with time-dependent covariates, of 320,000 persons with incident diabetes mellitus. Am J Epidemiol 188(10):1794–1800. https://doi.org/10.1093/aje/kwz157
Tang GH, Satkunam M, Pond GR et al (2018) Association of metformin with breast cancer incidence and mortality in patients with type II diabetes: a grade-assessed systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 27(6):627–635. https://doi.org/10.1158/1055-9965.EPI-17-0936
Jiralerspong S, Palla SL, Giordano SH et al (2009) Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 27(20):3297–3302. https://doi.org/10.1200/JCO.2009.19.6410
Martin-Castillo B, Pernas S, Dorca J et al (2018) A phase 2 trial of neoadjuvant metformin in combination with trastuzumab and chemotherapy in women with early HER2-positive breast cancer: the METTEN study. Oncotarget 9(86):35687–35704. https://doi.org/10.18632/oncotarget.26286
Barakat HE, Hussein RRS, Elberry AA et al (2022) The impact of metformin use on the outcomes of locally advanced breast cancer patients receiving neoadjuvant chemotherapy: an open-labelled randomized controlled trial. Sci Rep 12(1):7656. https://doi.org/10.1038/s41598-022-11138-3
Goodwin PJ, Chen BE, Gelmon KA et al (2022) Effect of metformin vs placebo on invasive disease-free survival in patients with breast cancer: the MA.32 randomized clinical trial. JAMA 327(20):1963–1973. https://doi.org/10.1001/jama.2022.6147
Liu J, Grundy SM, Wang W et al (2006) Ethnic-specific criteria for the metabolic syndrome: evidence from China. Diabetes Care 29(6):1414–1416. https://doi.org/10.2337/dc06-0481
Group., C.D.S.M.S.R (2004) Metabolic syndrome: suggestion from Chinese Diabetes Society. Chin J Diabetes 12(3):156–161
De A, Kuppusamy G (2020) Metformin in breast cancer: preclinical and clinical evidence. Curr Probl Cancer 44(1):100488. https://doi.org/10.1016/j.currproblcancer.2019.06.003
GoDarts, U.D.P.S. Group, C. Wellcome Trust Case Control et al (2011) Common variants near ATM are associated with glycemic response to metformin in type 2 diabetes. Nat Genet 43(2):117–20. https://doi.org/10.1038/ng.735
Cuyas E, Buxo M, Ferri Iglesias MJ et al (2019) The C Allele of ATM rs11212617 associates with higher pathological complete remission rate in breast cancer patients treated with neoadjuvant metformin. Front Oncol. https://doi.org/10.3389/fonc.2019.00193
Niraula S, Dowling RJ, Ennis M et al (2012) Metformin in early breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res Treat 135(3):821–830. https://doi.org/10.1007/s10549-012-2223-1
Hadad S, Iwamoto T, Jordan L et al (2011) Evidence for biological effects of metformin in operable breast cancer: a pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res Treat 128(3):783–794. https://doi.org/10.1007/s10549-011-1612-1
Bonanni B, Puntoni M, Cazzaniga M et al (2012) Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J Clin Oncol 30(21):2593–2600. https://doi.org/10.1200/JCO.2011.39.3769
Kalinsky K, Crew KD, Refice S et al (2014) Presurgical trial of metformin in overweight and obese patients with newly diagnosed breast cancer. Cancer Invest 32(4):150–157. https://doi.org/10.3109/07357907.2014.889706
Pistelli M, Merloni F, Crocetti S et al (2021) Prognostic impact of Ki-67 change in locally advanced and early breast cancer after neoadjuvant chemotherapy: a single institution experience. J Oncol 2021:5548252. https://doi.org/10.1155/2021/5548252
Luo N, Ji Y, Huang X et al (2019) Changes in apparent diffusion coefficient as surrogate marker for changes in ki-67 index due to neoadjuvant chemotherapy in patients with invasive breast cancer. Acad Radiol 26(10):1352–1357. https://doi.org/10.1016/j.acra.2019.01.007
Goodwin PJ, Parulekar WR, Gelmon KA et al (2015) Effect of metformin vs placebo on and metabolic factors in NCIC CTG MA.32. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv006
Wang Q, Ma X, Long J et al (2022) Metformin and survival of women with breast cancer: a meta-analysis of randomized controlled trials. J Clin Pharm Ther 47(3):263–269. https://doi.org/10.1111/jcpt.13500
Kim BH, Cho MJ, Kwon J (2021) Potential intrinsic subtype dependence on the association between metformin use and survival in surgically resected breast cancer: a Korean national population-based study. Int J Clin Oncol 26(11):2004–2016. https://doi.org/10.1007/s10147-021-02005-8
Chae YK, Arya A, Malecek MK et al (2016) Repurposing metformin for cancer treatment: current clinical studies. Oncotarget 7(26):40767–40780. https://doi.org/10.18632/oncotarget.8194
Acknowledgements
The authors are grateful to the patients, their families, and caregivers for participating in NeoMET trial. The authors also thank the study investigators and site staff for their participation.
Funding
This study was funded by the National Natural Science Foundation of China (82072937, 82072897); Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20172007); Shanghai Jiao Tong University Yi Gong Jiao Cha Funding (YG2019QNA30); and Ruijin Hospital, Shanghai Jiao Tong University School of Medicine- “Guangci Excellent Youth Training Program” (GCQN-2019-B07). All these financial sponsors had no role in the study design, data collection, analysis, or interpretation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The present study was reviewed and approved by independent ethical committees of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (2013-32a/2013–7-31). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, J., Tong, Y., Hong, J. et al. Neoadjuvant docetaxel, epirubicin, and cyclophosphamide with or without metformin in breast cancer patients with metabolic abnormality: results from the randomized Phase II NeoMET trial. Breast Cancer Res Treat 197, 525–533 (2023). https://doi.org/10.1007/s10549-022-06821-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06821-y