Abstract
Feeding a growing population is a big challenge for agriculture, being necessary for new and ecological alternatives to reduce chemical fertilizers and pesticides. Scientists have found that micro- and macroalgae are essential reservoirs of chemical compounds with a high potential role as biopesticides. Some of these molecules can act as elicitors, activating systemic and local defensive responses even without biotic stress. Among elicitors from macroalgae, there are ulvans, laminarin, alginate, carrageenan, glucuronan, fucans and tannins, which can activate plant defenses against viruses, bacteria, fungi, oomycetes, nematodes, and insects. The induction of defense mechanisms on crops by microalgae is related to their application as biomass, polysaccharides, exopolysaccharide or other elicitors, such as lactic acid or glucosamine. Unlike macroalgae, the biopesticide effect by microalgae has only been described against bacteria, fungi, and oomycetes, being necessary more studies to elucidate and discover their role as elicitors. In general, both macro- and microalgae are sources of compounds with great potential as biopesticides following the current needs for the development of sustainable agriculture.
Highlights
-
Macro and microalgae are new sources of elicitors with agricultural potential.
-
These algae elicitors activate plant defenses by different mechanisms.
-
Macroalgae elicitors reduce crop losses caused by pathogens and pests.
-
Microalgae elicitors reduce crop losses caused by pathogens.
-
Algae elicitors can be used in a sustainable agriculture development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The world population continues to overgrow. In 2020 we reached 7.8 billion people, projected to reach 10 billion in 2050 and 11 billion in 2100 (Gu et al., 2021). Therefore, world food security is one of the biggest problems to be addressed, which requires an increase in sustainable agricultural production (Boyd et al., 2013). The five main crops worldwide that have the greatest importance in food security are wheat, rice, maize, potato and soybean. There are different biotic agents associated with agricultural losses in these crops, specifically, 137 pathogens and pests have been identified. These biotic stresses cause high global productivity losses: wheat (21.5%), rice (30.0%), maize (22.5%), potato (17.2%) and soybean (21.4%) (Savary et al., 2019). Furthermore, globally, biotic stress causes a loss of between 20 and 40% of global agricultural productivity, of which between 10 and 15% are direct losses caused by pathogens (Mohammad-Razdari et al., 2022). Therefore, plant diseases are widely considered to be one of the most formidable obstacles to achieving global food security in the face of the rising human population in the twenty-first century (Velásquez et al., 2018).
At present, the use of chemical pesticides in agriculture is essential to be able to maintain food production. For example, without the use of chemical pesticides, losses due to pests and diseases in fruit trees would exceed 80% of world production (Tudi et al., 2021). Economically, the use of agrochemical pesticides worldwide represents an expense of > 40 billion USD, with the application of > 3 billion kg (Sharma et al., 2020). However, this strategy causes serious environmental and health problems, accumulating in ecosystems and causing serious diseases, such as cancer to humans (Sharma et al., 2020; Tudi et al., 2021). Therefore, it is necessary to look for new alternatives that respect the environment and health, such as biopesticides or biological control agents (BCAs), including those organisms, chemical compounds or genes derived from them, which are capable of significantly reducing the detrimental effect caused by biotic stresses in crops (Regnault-Roger, 2020). Within these, microorganisms are the main source of BCAs, followed by macro-organisms and natural products. In 2023, the biopesticide market will account for 9.86% of the global agricultural pesticide market, with a constant annual increase, being 6.4% (3.7 billion USD) in 2018 (Regnault-Roger, 2020).
On the other hand, algae constituted a vast source of resources with agricultural applications as in many other areas of interest. Algae are aquatic uni- or multicellular organisms with photosynthetic capacity (Pereira, 2021), which differ from seagrass and macrophytes because they do not have a vascular system (Poveda, 2022), and from cyanobacteria, because they are eukaryotic organisms (Poveda, 2021). In turn, algae are divided into microalgae, not visible to the naked eye, and macroalgae, perfectly visible. Microalgae constitute the phytoplankton, restricted to the euphotic zone of surface waters. Macroalgae or seaweeds are macroscopic marine organisms that can reach 65 m in length (Pereira, 2021).
In recent years, biotechnological research in micro-and macroalgae has accumulated many publications and results of transfer to industry (Garrido-Cardenas et al., 2018). In this sense, national and international laws must be continually renewed to allow all uses and products derived from these organisms to enter the market (Lähteenmäki-Uutela et al., 2021).
The industrial cultivation of microalgae is similar to other microorganisms, although they require specific aspects, such as water, nutrients, oxygen and light. Microalgae are cultivated in open ponds or lakes and closed photo-bioreactors, providing highly controlled environments (Rizwan et al., 2018). Today, microalgae and their products are used in many industries and for very different uses (Rizwan et al., 2018). Highlights, the microalgae are used in different industries as in wastewater as biotreatments (Chai et al., 2021), the production of biogas (Zabed et al., 2020), bioethanol (Simas-Rodrigues et al., 2015) and biodiesel (Chen et al., 2018), or in obtaining high value-added products, such as triacylglycerides, polyunsaturated fatty acids and carotenoids (Liang et al., 2019) for food (Torres-Tiji et al., 2020), cosmeceutical and pharmaceutical use (Mehariya et al., 2021).
In the case of macroalgae, obtaining quantities of industrial biomass is easier than for microalgae. They are mainly obtained from the direct collection in the marine environment, the collection of dead algae on the coast, and their cultivation, mainly in the sea using anchored ropes (Sudhakar et al., 2018). Like microalgae, macroalgae are present in more and more different industries (Leandro et al., 2020). Highlights, their use to obtain biodiesel (Abomohra et al., 2018) and bioethanol (Ramachandra, & Hebbale, 2020), as biomass for livestock feed (Øverland et al., 2019), aquaculture feed (Wan et al., 2019), human food (Afonso et al., 2019), as a source of antimicrobial (Silva et al., 2020) and other bioactive compounds for food (Biris-Dorhoi et al., 2020), cosmetics (Pimentel et al., 2018) or medicine (Biris-Dorhoi et al., 2020).
Macroalgae and microalgae in agriculture
The use of algae as an agricultural resource is established in numerous agricultural systems on the planet, having been widely reviewed by numerous authors, and their use as biofertilizers stands out (Baweja et al., 2019).
In the case of macroalgae, the main way in which they are applied in the field, and found in the market, is through aqueous extracts obtained from biomass, as biostimulants. In the first place, the macroalgal tissues are subjected to cryo-processing or cell rupture with high-pressure treatment to release all the cellular bioactive components. Then these components are separated from the rest of the cellular structures using aqueous (only water-soluble fractions), acidic (with sulfuric acid, extracting phenolic compounds) and/or alkaline (with potassium carbonate solution, extracting small units of polysaccharides) extraction (Sharma et al., 2014). However, macroalgal biomass can be used directly as a biofertilizer (Nabti et al., 2017), and even biomass resulting from other processes, such as obtaining biogas (Akila et al., 2019).
Both in the form of extracts and as solid biomass, macroalgae contribute to crops with different nutrients applied (mainly, tracing, iron, magnesium, potassium and zinc), polysaccharides, amino acids and phytohormones (Nabti et al., 2017). This has numerous benefits for the agricultural system, such as foliar and soil application, promoting plant growth, increasing tolerance to abiotic stresses, acting directly as biopesticides or/and by activating plant defenses or improving plant-bacteria symbiotic relationships (Khan et al., 2009). The promotion of plant growth by macroalgal extracts has been reported in different crops and systems, such as spinach (Fan et al., 2013) and Comanthera mucugensis in vitro (Carmo et al., 2020), pepper in greenhouse (Melo, Abreu, et al., 2020), or Solanaceae crops in greenhouse and field (Pohl et al., 2019). Under salinity, the extracts of different macroalgae increased the tolerance of rapeseed and wheat plants to abiotic stress due to their content of indole acetic acid, indole butyric acid, gibberellic acid, cytokinins, total carbohydrates and phenolic compounds (Hashem et al., 2019; Zou et al., 2019). Lastly, macroalgae can produce and accumulate a wide spectrum of chemically active metabolites with antimicrobial capacity in their tissues, such as alkaloids, polyketides, cyclic peptides, diterpenoids, phlorotannins, polysaccharides, sterols, quinones, lipids, and glycerols. Consequently, the direct application of extracts in crops has been supposed to significantly reduce harmful organisms through their antibacterial, antifungal, antiviral, nematicidal and insecticidal activity (Hamed et al., 2018; Machado et al., 2019).
Concerning microalgae, a disagreement is about their agricultural use from both points of view, scientific and business. However, some authors include cyanobacteria within microalgae (Gonçalves, 2021), while others do not (Alvarez et al., 2021). In this work, the concept of microalgae has been described and, therefore, cyanobacteria, which are agricultural resources widely reviewed by other authors (Chittora et al., 2020; Poveda, 2021) will not be included. In agriculture, microalgae are used directly as solid biomass or as extracts, highlighting the latter way, as macroalgae (Gonçalves, 2021). Obtaining extracts begins with cell wall elimination, using mechanical/physical (microwaving, sonication, ultra-freezing, etc.), chemical (sodium hydroxide, sulfuric acid, etc.) or enzymatic methods (cellulases, proteases, etc.) (Chiaiese et al., 2018). Subsequently, different bioactive compounds (polysaccharides, lipids, amino acids, pigments, phytohormones, antioxidants or vitamins) are obtained using organic solvents (Behera et al., 2021; Chiaiese et al., 2018).
Microalgae can be applied both in soil on the leaves, reporting important benefits for crops, highlighting the promotion of plant growth and increased tolerance to abiotic stresses, but also having direct action as biopesticides and activating plant defenses (Behera et al., 2021; Gonçalves, 2021). The promotion of plant growth has been reported in crops, such as wheat and maize, through root application (Uysal et al., 2015), or petunia, through the foliar application (Plaza et al., 2018). In the case of biotic stresses, microalgae can produce a wide variety of bioactive compounds with antibacterial, antifungal, nematicidal, insecticidal and herbicidal activity, such as phenolic compounds, polyphenols, tocopherols, proteins, oils, carbohydrates, saponins, nitrogen-rich peptides, allelochemicals or sesquiterpenes (Costa et al., 2019). Finally, it is important to note that the microalgae market in agriculture is growing exponentially. A sign of this can be found in the number of registered patents. Between 1982 and 2014, only 10 patents were registered worldwide, however, between 2015 and 2019, a total of 132 current patents were accumulated (Murata et al., 2021).
Elicitors and plant defensive responses in agriculture
Plants are constantly exposed to biotic stresses, which cause structural and metabolic damage, affecting the physiology of the entire plant and reducing its agronomic field. Plants have a sophisticated evolutionary defense strategy to protect themselves from biotic stress. These mechanisms have aroused scientific interest in the search for new compounds for agricultural sustainability (Gimenez et al., 2018). When a plant is attacked by a pest or pathogen, it must recognize chemical components of biotic stress (herbivore-associated molecular patterns [HAMPs] or pathogen-associated molecular patterns [PAMPs]) or the damage they cause to plant (damage-associated molecular patterns [DAMPs]) to activate its defenses in a specific and targeted way (Gimenez et al., 2018; Poveda, 2020). All these chemical structures recognized by plant receptors are called elicitors and lead to plant activation of called pattern-triggered immunity (PTI) (Bigeard et al., 2015). In addition to local plant defense responses against biotic stresses, PTI leads to the activation of a systemic plant defense response in which the hormones salicylic acid (SA), jasmonic acid (JA) and ethylene (ET) are mainly involved. In general, against biotrophic pathogens the so-called systemic acquired resistance (SAR) controlled by SA is activated, while against necrotrophic pathogens and pests the so-called induced systemic resistance (ISR) is activated through JA/ET (Gimenez et al., 2018; Poveda, 2020).
Therefore, elicitors are signal-inducing compounds recognized by the plant that induce and/or prime defensive responses (Wiesel et al., 2014). Elicitors used in agriculture usually have a natural origin, but there are several widely used synthetic products (Bektas & Eulgem, 2015; Wiesel et al., 2014). Among these agrochemicals, the use of SA and JA (and their derivatives and analogs), benzothiadiazole (BTH) and β-amino butyric acid (BABA) stand out, being able to activate the defensive responses of crops against broad-spectrum biotic stresses (Bektas & Eulgem, 2015; Burketova et al., 2015; Poveda, 2020).
Knowing the plant defensive responses derived from PTI, elicitors can be used in a targeted way to pre-activate plant defenses in the absence of pests/pathogens, causing a faster and more effective response when biotic stresses are present, a physiological status called priming (Wiesel et al., 2014). In this sense, there are very diverse bio-based elicitors used in agriculture as priming activators and/or resistance inducers (when biotic stress has attacked) (Boubakri, 2020; Burketova et al., 2015; Wiesel et al., 2014). The main group of elicitors is found in the structural and biochemical components of pathogens. Among bacteria elictiors are found harpins (translocators related to virulence), flagellin (component of flagella), exopolysaccharides (involved in the formation of biofilms), lipopolysaccharides (major cell membrane components) and rhamnolipids (essential for surface motility and biofilm development). In fungi and pests, highlights chitin, the main polysaccharide of the fungal cell walls and arthropod exoskeleton, together with its deacetylated derivative, chitosan. Furthermore, other components of the fungal cell wall are recognized as elicitors of plant defensive responses, including β-1,3-D-glucans, β-1,6-D-glucans and α-1,3-D-glucans. Finally, ergosterol, a component of the plasma membrane of fungi, is another compound (Boubakri, 2020; Burketova et al., 2015; Wiesel et al., 2014). Plant components are also a good source of agricultural elicitors. All cell wall components that can be released by the action of pathogenic enzymes are elicitors within the DAMPs, including cellulose fragments, hemicellulose and other glucans (such as oligogalacturonides), peptides, lignin and lipids. In addition, defense hormones (SA and JA) and their different derivatives are elicitors that are very widespread in agriculture (Wiesel et al., 2014; Boubakri, 2020; Poveda, 2020).
In recent years, the use of elicitors in agriculture is being combined with nanotechnology, improving plant responses directed towards defense and/or the production of secondary metabolites of industrial interest (Rivero-Montejo et al., 2021). In this sense, the use of elicitors in cell cultures and plant tissues to obtain secondary metabolites of interest has been widely developed during the last decades at an industrial level, being an important economic and resource sector for the manufacture of biopesticides, agrochemicals, flavoring agents, essential oils, food additives and, most importantly, for medicine and cosmetics (Alvarado et al., 2019; Bhaskar et al., 2021).
Elicitors from macroalgae
For many years it has been thought that macroalgae defend themselves against their pathogens by constitutive mechanisms. However, in the last two decades, it has been possible to describe the pathogen-induced defense in macroalgae (Weinberger, 2007). The defensive strategies mainly used by macroalgae against biotic stresses include bioactive chemical compounds (Cosse et al., 2007), which can be used, like extracts, directly in agriculture as pesticides (Pan et al., 2019). Furthermore, these extracts and different structural components of macroalgae can be recognized by plant cell receptors and act as powerful elicitors for agricultural use. Table 1 compiles many studies on macroalgae elicitors and their activity in different crops and model plants. Moreover, Fig. 1 summarizes as an infographic the main mechanisms of action involved in the role of macroalgae elicitors in protecting crops against biotic stresses.
Extracts
The discovery of new elicitors from macroalgae for agricultural use begins with the use of different extracts as inducers of plant defenses. In this sense, less systematic effects are reported, but the mechanisms involved in plant defensive strategies are later studied to describe the implicated macroalgae-elicitors has been carried out. For example, the extracts obtained from the chlorophytes Caulerpa cylindracea and Cladophora crispate reduce incidence and disease severity caused by various viruses in tomato and Nicotiana benthamiana by up to 40% when applied to their seeds or leaves, ignoring the plant defense mechanisms involved (Alburquerque et al., 2019; Salim, 2020). Furthermore, different extracts from macroalgae have been used as inducers of plant defenses in the absence of biotic stress, to describe the possible priming effect. In blackberry cell suspension cultures, the addition of Ascophyllum nodosum-extracts to the culture medium increased the enzymatic activity β, 1–3 glucanase (GLU) (Patier et al., 1993). In whole tomato and pepper plants, extracts from the same Ochrophyta macroalgae activate the defensive SA- and JA/ET-mediated responses, increasing the activity of various defensive enzymes, such as phenylalanine ammonia-lyase (PAL) or chitinase (CHI) (Ali et al., 2019).
Against bacteria, a reduction in Xanthomonas campestris pv. vesicatoria disease severity in tomato and sweet pepper by foliar application of Sargassum vulgare extracts has been reported, due to local induction of SA- and JA/ET-related genes expression (Ochrophyta) (Ali et al., 2021). Induction of gene expression is also described systemically after applying extracts from A. nodosum in Arabidopsis thaliana roots and leaves infection by Pseudomonas syringae pv. tomato, P. aeruginosa or X. campestris (Cook et al., 2018; Subramanian et al., 2011). These transcriptomic changes increase the activity of defensive enzymes, such as peroxidase (POD) and polyphenol oxidase (PPO), being reported after foliar and seed application of Cystoseira myriophylloides (Ochrophyta) extracts against Agrobacterium tumefaciens and P. syringae pv. tabaci (Esserti et al., 2017, 2018). Moreover, plants accumulate defensive secondary metabolites against pathogenic bacteria. Foliar applications with extracts from Gelidium serrulatum (Rhodophyta) and Sargassum spp., the affectation by X. campestris in tomato and cotton is reduced by up to 75%, due to the accumulation of phenolic compounds (Ali et al., 2016; Raghavendra et al., 2007; Ramkissoon et al., 2017).
As far as fungi are concerned, the application of A. nodosum extracts in wheat induces the expression of SA-related genes both locally and systemically, significantly reducing the disease intensity caused by Fusarium graminearum or Zymoseptoria tritici (Gunupuru et al., 2019; Somai-Jemmali et al., 2020). This leads to an increase in defensive enzymatic activity and accumulation of different fungicidal secondary metabolites. For example, root and foliar applications of extracts from A. nodosum reduce the incidence of Alternaria cucumerinum, Didymella applanata, Fusarium oxysporum and Botrytis cinerea in cucumber, through an increase in GLU, CHI, POD, PPO, PAL and lipoxygenase (LOX) activity (Jayaraman et al., 2011). Furthermore, the root, foliar and fruit application of A. nodosum extracts causes the local accumulation of phenolic and flavonoid compounds, reducing the incidence of pathogens such as V. dahliae in pepper by up to 60% (Goicoechea et al., 2004), A. solani in tomato (Ali et al., 2016), Podosphaera aphanis in strawberry (Bajpai et al., 2019), or Monilinia fructicola in plums (Viencz et al., 2020).
In the case of oomycetes, the activation of local defenses by the root application of A. nodosum and Durvillaea potatorum-extracts is related to SA-related gene induction, significantly reducing the disease caused by Phytophthora cinnamomi in A. thaliana (Islam et al., 2020). Similar extracts activate a systemic SA- and JA-mediated resistance against Phytophthora melonis in cucumber (Abkhoo and Sabbagh, 2016), P. infestans in potato (Ahmed et al., 2016) or P. capsici in tomato (Panjehkeh & Abkhoo, 2016), in which the defensive enzymes GLU, POD and PPO, and the accumulation of phenolic compounds are involved. However, activated systemic defensive responses may be macroalgae-extract and plant-dependent. For example, in rubber tree the foliar application of extracts from Sargassum polycystum reduces Phytophthora palmivora disease by 30%, due to an increase in POD, GLU and catalase (CAT) activity, and the accumulation of phytoalexins, such as scopoletin (Khompatara et al., 2019).
Finally, against nematodes and insects, extracts from Cladophora glomerata (C. glomerata) and A. nodosum activate the SA-mediated systemic defensive responses. Similar results are reported in tomato and soybean against Meloidogyne javanica (Ghareeb et al., 2020; Rinaldi et al., 2021), and A. thaliana against Myzus persicae (Weeraddana et al., 2021), through an increase in enzymatic activity such as POD, PPO, PAL and GLU.
Ulvan and derivatives
Among the sulfated polysaccharides of seaweeds, the ulvans are present in chlorophytes and especially within the Ulva genus, where they account for up to 36% of their biomass (Shukla et al., 2021). They are water-soluble polysaccharides, consisting of a central backbone of disaccharide units formed by an L-rhamnose 3-sulphate linked to uronic acids or xylose (Shukla et al., 2021; Vera et al., 2011b). Normally, using hydrochloric acid and high temperatures (100 ºC), or ultrasound, the depolymerization and/or fragmentation of ulvan and the formation of oligoulvans is achieved (Vera et al., 2011b). In the absence of biotic stresses, the foliar application of ulvans from different Ulva species increases systemically and locally plant defenses. In tobacco, alfalfa and blackberry cell suspensions, the addition of ulvan from U. fasciata induces an oxidative burst dependent on NADPH oxidase (Paulert et al., 2021a). In this sense, foliar application of ulvans on alfalfa, basil or parsley induces the systemic expression of SA- and JA-related genes (Jaulneau et al., 2010; Paulert et al., 2021b).
In the presence of fungal pathogens, foliar application of ulvan causes a systemic increase in defensive enzyme activity and accumulation of antifungal compounds. An example is described in bean and apple plants, reducing by 40% the disease caused by Blumeria graminis, Colletotricum lindemuthianum and C. gloeosporoides, due to an increase in GLU and POD enzymatic activity (Borsato et al., 2010; de Freitas and Stadnik, 2012; Araujo & Stadnik, 2013). Similarly, applying ulvan to wounds of papaya fruits causes an increase in CAT, POD and superoxide dismutase (SOD) activity, significantly reducing disease incidence and the lesion diameter of anthracnose (C. gloeosporioides) (Chiquito-Contreras et al., 2019). Regarding the accumulation of defense chemical compounds, the main plant response reported after foliar application of ulvans is the accumulation of hydrogen peroxide, causing a reduction of up to 90% in the severity of Alternaria brassicicola in A. thaliana (from Freitas & Stadnik, 2015) and 80% of B. graminis in wheat and barley (Paulert et al., 2010). Besides, in twigs application of ulvans from U. latuca in olive plants, a systemic increase in the content of polyphenols in the roots was reported, reducing the severity of V. dahliae up to 40% (Ben Salah et al., 2018). Regarding oligoulvans, their application by internodal injection in tomato plants reduces the mortality caused by F. oxysporum f. sp. lycopersici, due to an increase in PAL activity and the accumulation of phenolic compounds in roots (El Modafar et al., 2012). In apple fruits, injection with oligoulvans with a low degree of polymerization causes a significant reduction in the diameter of the lesion formed by the pathogenic fungi Penicillium expansum and Botrytis cinerea, as a consequence of an increase in the activity of various defensive enzymes and the accumulation of hydrogen peroxide, lignin and phenolic compounds (Abouraïcha et al., 2015).
Laminarin
The main component of some brown seaweeds, such as Laminaria digitata, is laminarin, an important reserve polysaccharide and component of the cell wall. In general, laminarin is made up of β-1,3-glucans chains with some 6-O-branching (Shukla et al., 2021; Vera et al., 2011b). In absence of biotic stresses, it has been reported how the application of laminarin from Eisenia bicyclis (Ochrophyta) to alfalfa cotyledons is capable of inducing systemic plant defenses, causing the accumulation of flavonoid content in tissues (Kobayashi et al., 1993).
All the studies carried out to date with laminarin as a plant elicitor against biotic stresses have used the macroalga L. digitata as a source. Against viruses and bacteria, foliar application of laminarin in tobacco and A. thaliana causes a reduction in systemic infection by Tobacco Mosaic Virus (TMV) and Erwinia carotovora subsp. carotovora, as a consequence of an increase in SA- and JA/ET-related genes expression (Klarzynski et al., 2000; Ménard et al., 2004, 2005). In addition to these transcriptomic changes, in grapevine plants infected by the fungus B. cinerea and the oomycete Plasmopara viticola, a reduction of the disease up to 75% has been reported due to the foliar application of laminarin and sulfated laminarin (PS3), which caused an increase in the tissue content of different phytoalexins (resveratrol and epsilon-viniferin), isoprenoids (Adrian et al., 2017) and callose (Aziz et al., 2003; Gauthier et al., 2014; Trouvelot et al., 2008). In the case of pest insects, foliar application of laminarin in tea plants resulted in a lower performance of the Empoasca onukii leafhopper through direct and indirect mechanisms of action. On the one hand, the recognition of laminarin by plant tissues implies an increase in systemic expression of SA-related genes and in the activity of defensive enzymes, such as CHI, PAL, POD, callose and flavonol synthase. On the other hand, laminarin enhanced the attractiveness to the egg parasitoid wasp of E. onukii, Stethynium empoascae (Xin et al., 2019).
Alginate and derivates
Another polysaccharide present in the cell wall of brown seaweeds is alginate, consisting of a central backbone of (poly)glucuronic and (poly)mannuronic acid. The hydrolysis of these chemical components causes the formation of oligoalginates (Shukla et al., 2021; Vera et al., 2011b). To study the possible priming effect derived from the use of alginates and oligoalginates as plant elicitors, several studies have been carried out in absence of biotic stresses. Using various brown seaweeds as a source, it has been reported how foliar, root and seed application of alginates and oligoalginates (mannuronic and guluronic acids) systemically increases the activity of defensive enzymes (POD and PAL) and the accumulation of polyphenols and phytoalexins in very different crops (Aitouguinane et al., 2020; An et al., 2009; Bouissil et al., 2020; Chandía et al., 2004).
In presence of pathogens, oligoalginates have only been used in foliar application as plant elicitors with efficient results in disease reduction. In A. thaliana, these elicitors systemically increase the expression of SA-related genes, such as PR-1, significantly decreasing disease index and bacteria colonies of P. syringae pv. tomato (Zhang et al., 2019). Against TMV in tobacco, oligoalginates obtained from Lessonia trabeculata and L. vadosa reduced the number of necrotic lesions up to 75%, due to an increase in PAL and plant ascorbate peroxidase (APX) activity (Laporte et al., 2007).
Carrageenan and derivates
In the case of red macroalgae, the main components of their cell wall is carrageenans, assuming up to 75% of the algal dry weight. Carrageenans are water-soluble sulphated polysaccharides, made up of a central chain of sulphated D-galactose, linked to anhydrogalactose units. Depending on the carbon where the sulfate group is located, we find different carrageenans: iota(Ι)-, kappa(K)-, lambda(λ)-, mu(μ)-, nu (Nv)- and theta(Ɵ)-carrageenans. Oligocarragenans have been obtained by acid hydrolysis of K-, λ—and I-carrageenans (Shukla et al., 2016; Vera et al., 2011b). The priming effect of different carrageenans as plant elicitors has been described under different conditions. In blackberry cell suspensions, the addition of kappa-carrageenan to the culture medium supposes an increase in cellular GLU activity (Patier et al., 1995). In whole plants, foliar application of I-, K- or λ-carrageenan in Eucalyptus globulus plants triggers a systemic increase in tissue accumulation of polyphenolic and terpenoid compounds (González et al., 2013, 2014).
Against viruses and viroids, foliar application of K- or γ-carrageenan increases the systemic expression of SA- and JA/ET-related genes, reducing the appearance of the disease caused by TMV in tobacco or Tomato Chlorotic Dwarf Viroid (TCDVd) in tomato up to 75% (Ghannam et al., 2013; Sangha et al., 2015). More specifically, the foliar application of kappa- and beta-carrageenan from Tichocarpus crinitus in Datura stramonium increases the formation of cellular-defense structures, such as laminar structures, able to bind viral particles and prevent their intracellular translocation and reproduction (Nagorskaya et al., 2010). Diseases caused by different bacteria, fungi and oomycetes are reduced up to 70% after the application of λ-carrageenan. This is due to an increase in the systemic expression of SA- and JA-related genes (Le Mire et al., 2019; Mercier et al., 2001), an increase in PAL and POD activity, and/or a greater accumulation of phytoalexins (scopoletin) and phenylpropanoid compounds in plant-tissues (Pettongkhao et al., 2019; Vera et al., 2012). In the case of kappa- and gamma-carrageenans, the reduction in disease caused by the fungi C. gloeosporioides in pepper and Sclerotinia sclerotiorum in A. thaliana is a consequence of the increase in the expression of defense-related genes, such as PR-1, PR-3, PR-5, PDF1.2, AOS and NPR1 (Mani & Nagarathnam, 2018; Sangha et al., 2010). The synthesis and hydrolysis of glucosinolates compounds are triggered under the pathogen's attack with similar mechanisms to those described in A. thaliana in response to foliar application of I-carrageenan against the attack of cabbage looper (Trichoplusia ni) (Sangha et al., 2011).
Other elicitors
In addition to all the macroalgae-polysaccharides previously described as elicitors of plant defenses, there are many other examples that have caused significant reductions in the disease. Algal glucuronans (β-(1,4)-d-polyglucuronic acids) are present in the cell wall of different green macroalgae (Redouan et al., 2009). In apple fruits, the wound application of glucuronan and oligoglucuronans from U. lactuca reduces the severity of blue and gray mold (P. expansum and B. cinerea), through an increase in CAT, SOD, PAL, POD and PPO tissue activity (Abouraïcha et al., 2017). Fucans (or fucoidans) are polysaccharides present in the cell wall of brown macroalgae and constituted sulfated fucoses (Berteau & Mulloy, 2003). After the extraction of fucans present in Pelvetia canaliculata, they were subjected to an enzymatic hydrolysis process that resulted in the formation of mono- and di-sulfated fucose units (oligofucans). In tobacco plants, the foliar application of these oligofulcans provokes a systemic defensive response that reduces the incidence of TMV (Klarzynski et al., 2003).
Other chemical compounds from macroalgae that can act as elicitors are tannins. In brown macroalgae, such as Ecklonia species and Ishige okamurae, the tannins mainly present are phlorotannins. Specifically, in Ecklonia genus the most common phlorotannin is eckol, mainly made up of phloroglucinols (Manandhar et al., 2019). Eckol from Ecklonia maxima applied foliarly to cabbage plants eliminates the infestation by cabbage aphid (Brevicoryne brassicae), because of a systemic increase in myrosinase activity (an enzyme involved in glucosinolates hydrolysis) (Rengasamy et al., 2016).
Elicitors from microalgae
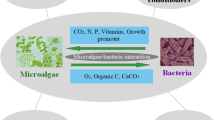
As macroalgae, microalgae can be infected by different pathogens and need to develop efficient defensive strategies to survive (Lin et al., 2021). Some of these defensive mechanisms include the synthesis of antimicrobial compounds, which can be used directly as agricultural pesticides (Jena & Subudhi, 2019). On the other hand, both chemical compounds and structural components of microalgae can be used as elicitors of plant defenses in agriculture. Table 2 compiles many existing studies on the use of microalgae as plant elicitor resources, whose mechanisms of action are summarized in an infographic in Fig. 2.
As with different beneficial microorganisms, the direct application of microalgae on crops roots involves the systemic activation of plant defenses. This is due to the microalgae-plant interaction are reciprocal. Several studies have reported priming-type responses in different crops, even though the exact microalgae-molecule involved is unknown. In broccoli and guar plants, root inoculation with Chlorella vulgaris (Chlorophyta) systemically increased APX, CAT, SOD and glutathione reductase (GR) activity, along with increased tissue accumulation of flavonoid and phenolic compounds (Kusvuran, 2021; Kusvuran & Can, 2020). This elicitation technique with microalgae can be used industrially to produce compounds of industrial interest by plant cells. In this sense, the inoculation of Capsicum frutescens calli with Botryococcus braunii (Chlorophyta) increased the synthesis and accumulation of vanillylamine and capsaicin (Sharma et al., 2010). These priming-type defensive responses prepare plants for attack by different pathogens. The foliar inoculation of cucumber plants with Chlorella fusca (Chlorophyta) reduced the disease caused by Colletotrichum orbiculare, through the activation of SAR and the production of various cytological changes, such as accumulation of vesicles, formation of sheath around penetration hyphae, and thickness of cell wells adjoining with intracellular hyphae (Kim et al., 2018).
In all microalgae formulations and extracts used in agriculture, polysaccharides are usually one of the major components, about 50% of dry weight. In general, microalgae-polysaccharides are heteropolymers of galactose, xylose and glucose (Chanda et al., 2019). So far, the activation of plant systemic defensive responses by microalgae-polysaccharides has been described, although the exact molecules involved are still unknown. Polysaccharides from different Chlorella, Dunaliella (Chlorophyta) and Porphorydium (Rhodophyta) species act as powerful plant elicitors in tomato, increasing the activity of various defense-related enzymes (Farid et al., 2019) and the accumulation of defensive compounds, such as polyphenols (Rachidi et al., 2021) or steroidal glycoalkaloids (Rachidi et al., 2020). In addition to structural polysaccharides, microalgae can produce and release exopolysaccharides with eliciting capacity. Different exopolysaccharides produced by Dunaliella salina induce CAT, POD and SOD activity, and accumulation of phenolic compounds in tomato leaves (Arroussi et al., 2018). Similarly, exopolysaccharides produced by Porphyridium sordidum induce the expression of SA-related genes and PAL activity in A. thaliana leaves, reducing diseases caused by F. oxysporum (Drira et al., 2021).
Besides polysaccharides, microalgae can produce other molecules and chemical compounds recognized by plants as elicitors. Lactic acid is a chiral organic acid that can be produced by some green microalgae (Augustiniene et al., 2021). The D-lactic acid isoform produced by C. fusca is recognized by cellular receptors of A. thaliana, inducing the systemic expression of SA- and JA-related genes, and reducing the disease caused by P. syringae pv. tomato (Lee et al., 2020).
This elicitation of plant defenses can be used at the industrial level to increase the production of secondary metabolites of interest, in systems such as hairy roots. Water extracts obtained from Haematococcus pluvialis (Chlorophyta) and applied in a culture medium of beet and marigold hairy roots increase the production and secretion of betalains and thiophene (Rao et al., 2001). More specifically, glucosamine produced by Scenedesmus obliquus (Chlorophyta) has been described as the elicitor involved in increasing the synthesis of spiroketal enol ether diacetylenes in Tanacetum parthenium hairy roots (Stojakowska et al., 2008).
An aspect recently studied and of great interest for future lines of research is that the activation of plant defenses by microalgae-elicitors can be inherited in future generations. Tomato, pepper and eggplant plants were grown in vermicompost together with the microalgae Ulothrix spp. (Chlorophyta) and Navicula spp. (Ochrophyta). The harvested seeds were germinated in presence of pathogenic oomycete Pythium sp., the results showed a seedling survival increased by 90%, a sign of inherited resistance (Alshehrei et al., 2021).
Conclusions and future perspectives
The scientific evidence of the negative impacts caused by chemical pesticides on human and environmental health has propelled the search for sustainable alternatives to feed an exponentially growing population. Thus, changes in conventional agriculture practices are a big challenge and a necessity. Algae standout as a new and innovative bio-tool to develop agriculture according to the needs of the twenty-first century, being a sustainable way despite the requirements for their cultivation. Additionally, macro- and microalgae are catalogued as biopesticides due to their ability to reduce pest and pathogens diseases. Moreover, it plays an essential role as a preventive tool activating plants' defense mechanisms under no biotic stress. Thus, it will help reduce diseases caused by pests or pathogens and the economic cost.
Regarding their role as biopesticides, macro and microalgae act as elicitors and can activate the plants' defenses in roots and leaves. In the case of macroalgae, the most common elicitors of plant defenses are ulvan, laminarin, alginate, carrageenan, fucans, glucoran, and tannins with a great range of action against bacteria, fungi, viruses, oomycetes, nematodes and insects. Microalgae biopesticides activity is obtained directly by biomass application or using extracts such as polysaccharides, exopolysaccharides, lactic acid, and glucosamine as elicitors. However, unlike macroalgae, the microalgae-biopesticides role is effective against bacteria, fungi and oomycetes, and the exact molecule involved is unknown. Thus, discovering the target molecules is a challenge for future research. Furthermore, the vast majority of studies carried out with micro- and macroalgae elicitors have been developed in in vitro systems, growth chambers or greenhouses, therefore, there is a need for extensive and rigorous field tests.
Agriculture biotechnology based on ecological alternatives described by macro and micro-algae has different benefits. First, it directly impacts the natural balance of the whole plant health and triggers greater productivity in the present and future campaigns. In addition, their role as biostimulants opens a new path to study and develop compounds that can be used as ecological alternatives to current chemical fertilizers, improving people's health. Thus, algae are postulated as plant growth promoters for their synergic effect between their role as biopesticide and their capacity as biostimulants.
References
Abkhoo, J., & Sabbagh, S. K. (2016). Control of Phytophthora melonis damping-off, induction of defense responses, and gene expression of cucumber treated with commercial extract from Ascophyllum nodosum. Journal of Applied Phycology, 28(2), 1333–1342. https://doi.org/10.1007/s10811-015-0693-3
Abomohra, A. E. F., El-Naggar, A. H., & Baeshen, A. A. (2018). Potential of macroalgae for biodiesel production: Screening and evaluation studies. Journal of Bioscience and Bioengineering, 125(2), 231–237. https://doi.org/10.1016/j.jbiosc.2017.08.020
Abouraïcha, E., El Alaoui-Talibi, Z., El Boutachfaiti, R., Petit, E., Courtois, B., Courtois, J., & El Modafar, C. (2015). Induction of natural defense and protection against Penicillium expansum and Botrytis cinerea in apple fruit in response to bioelicitors isolated from green algae. Scientia Horticulturae, 181, 121–128. https://doi.org/10.1016/j.scienta.2014.11.002
Abouraïcha, E., El Alaoui-Talibi, Z., Tadlaoui-Ouafi, A., El Boutachfaiti, R., Petit, E., Douira, A., Courtois, B., Courtois, J., & El Modafar, C. (2017). Glucuronan and oligoglucuronans isolated from green algae activate natural defense responses in apple fruit and reduce postharvest blue and gray mold decay. Journal of Applied Phycology, 29(1), 471–480. https://doi.org/10.1007/s10811-016-0926-0
Abreu, G. F. D., Talamini, V., & Stadnik, M. J. (2008). Bioprospecção de macroalgas marinhas e plantas aquáticas para o controle da antracnose do feijoeiro. Summa Phytopathologica, 34, 78–82. https://doi.org/10.1590/S0100-54052008000100017
Adrian, M., Lucio, M., Roullier-Gall, C., Héloir, M. C., Trouvelot, S., Daire, X., Kanawati, B., Lemaître-Guillier, C., Poinsoot, B., Gougeon, R., Schmitt-Kopplin, P., & Schmitt-Kopplin, P. (2017). Metabolic fingerprint of PS3-induced resistance of grapevine leaves against Plasmopara viticola revealed differences in elicitor-triggered defenses. Frontiers in Plant Science, 8, 101. https://doi.org/10.3389/fpls.2017.00101
Afonso, N. C., Catarino, M. D., Silva, A., & Cardoso, S. M. (2019). Brown macroalgae as valuable food ingredients. Antioxidants, 8(9), 365. https://doi.org/10.3390/antiox8090365
Agarwal, P., Patel, K., Das, A. K., Ghosh, A., & Agarwal, P. K. (2016). Insights into the role of seaweed Kappaphycus alvarezii sap towards phytohormone signalling and regulating defence responsive genes in Lycopersicon esculentum. Journal of Applied Phycology, 28(4), 2529–2537. https://doi.org/10.1007/s10811-015-0784-1
Ahmed, S. M., El-Zemity, S. R., Selim, R. E., & Kassem, F. A. (2016). A potential elicitor of green alga (Ulva lactuca) and commercial algae products against late blight disease of Solanum tuberosum L. Asian Journal of Agriculture and Food Sciences, 4(2), 86–95.
Aitouguinane, M., Bouissil, S., Mouhoub, A., Rchid, H., Fendri, I., Abdelkafi, S., El-Hadj, M., Boual, Z., Dubessay, P., Gardarin, C., Michaud, P., El Alaoui-Talibi, Z., El Modafar, C., Pierre, G., & Delattre, C. (2020). Induction of natural defenses in tomato seedlings by using alginate and oligoalginates derivatives extracted from Moroccan brown algae. Marine Drugs, 18(10), 521. https://doi.org/10.3390/md18100521
Akila, V., Manikandan, A., Sukeetha, D. S., Balakrishnan, S., Ayyasamy, P. M., & Rajakumar, S. (2019). Biogas and biofertilizer production of marine macroalgae: An effective anaerobic digestion of Ulva sp. Biocatalysis and Agricultural Biotechnology, 18, 101035. https://doi.org/10.1016/j.bcab.2019.101035
Alburquerque, N., Faize, L., Faize, M., Nortes, M. D., Bernardeau, J., Fernandez, J. M. R., & Burgos, L. (2019). Towards the Valorization of the invasive seaweeds Caulerpa cylindracea and Asparagopsis taxiformis in the Mediterranean Sea: Applications for in vitro Plant Regeneration and Crop Protection. Journal of Applied Phycology, 31(2), 1403–1413. https://doi.org/10.1007/s10811-018-1640-x
Ali, N., Ramkissoon, A., Ramsubhag, A., & Jayaraj, J. (2016). Ascophyllum extract application causes reduction of disease levels in field tomatoes grown in a tropical environment. Crop Protection, 83, 67–75. https://doi.org/10.1016/j.cropro.2016.01.016
Ali, O., Ramsubhag, A., & Jayaraman, J. (2019). Biostimulatory activities of Ascophyllum nodosum extract in tomato and sweet pepper crops in a tropical environment. PLoS ONE, 14(5), e0216710. https://doi.org/10.1371/journal.pone.0216710
Ali, O., Ramsubhag, A., & Jayaraman, J. (2021). Phytoelicitor activity of Sargassum vulgare and Acanthophora spicifera extracts and their prospects for use in vegetable crops for sustainable crop production. Journal of Applied Phycology, 33(1), 639–651. https://doi.org/10.1007/s10811-020-02309-8
Alshehrei, F., Al-Enazi, N. M., & Ameen, F. (2021). Vermicomposting amended with microalgal biomass and biochar produce phytopathogen-resistant seedbeds for vegetables. Biomass Conversion and Biorefinery, 1-8. https://doi.org/10.1007/s13399-021-01770-w
Alvarado, A. M., Aguirre-Becerra, H., Vázquez-Hernández, M. C., Magaña-Lopez, E., Parola-Contreras, I., Caicedo-Lopez, L. H., Conteras-Medina, L. M., & Feregrino-Perez, A. A. (2019). Influence of Elicitors and Eustressors on the Production of Plant Secondary Metabolites. In M. S. Akhtar, M. K. Swamy, & U. R. Sinniah (Eds.), Natural Bio-active Compounds (pp. 333–388). Springer Singapore.
Alvarez, A. L., Weyers, S. L., Goemann, H. M., Peyton, B. M., & Gardner, R. D. (2021). Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Research, 54, 102200. https://doi.org/10.1016/j.algal.2021.102200
An, Q. D., Zhang, G. L., Wu, H. T., Zhang, Z. C., Zheng, G. S., Luan, L., Murata, L., & Li, X. (2009). Alginate-deriving oligosaccharide production by alginase from newly isolated Flavobacterium sp. LXA and its potential application in protection against pathogens. Journal of Applied Microbiology, 106(1), 161–170. https://doi.org/10.1111/j.1365-2672.2008.03988.x
Araujo, L., & Stadnik, M. J. (2013). Cultivar-specific and ulvan-induced resistance of apple plants to Glomerella leaf spot are associated with enhanced activity of peroxidases. Acta Scientiarum. Agronomy, 35(3), 287–293. https://doi.org/10.4025/actasciagron.v35i3.16174
Arroussi, H. E., Benhima, R., Elbaouchi, A., Sijilmassi, B., Mernissi, N. E., Aafsar, A., Meftah-Kadmiri, I., Bandaou, N., & Smouni, A. (2018). Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). Journal of Applied Phycology, 30(5), 2929–2941. https://doi.org/10.1007/s10811-017-1382-1
Augustiniene, E., Valanciene, E., Matulis, P., Syrpas, M., Jonuskiene, I., & Malys, N. (2021). Bioproduction of l-and d-lactic acids: advances and trends in microbial strain application and engineering. Critical Reviews in Biotechnology, 1-19. https://doi.org/10.1080/07388551.2021.1940088
Aziz, A., Poinssot, B., Daire, X., Adrian, M., Bézier, A., Lambert, B., Joubert, J. M., & Pugin, A. (2003). Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Molecular Plant-Microbe Interactions, 16(12), 1118–1128. https://doi.org/10.1094/MPMI.2003.16.12.1118
Bajpai, S., Shukla, P. S., Asiedu, S., Pruski, K., & Prithiviraj, B. (2019). A biostimulant preparation of brown seaweed Ascophyllum nodosum suppresses powdery mildew of strawberry. The Plant Pathology Journal, 35(5), 406. https://doi.org/10.5423/PPJ.OA.03.2019.0066
Baweja, P., Kumar, S., & Kumar, G. (2019). Organic Fertilizer from Algae: A Novel Approach Towards Sustainable Agriculture. In B. Giri, R. Prasad, Q.-S. Wu, & A. Varma (Eds.), Biofertilizers for Sustainable Agriculture and Environment (pp. 353–370). Springer International Publishing.
Behera, B., Supraja, K. V., & Paramasivan, B. (2021). Integrated microalgal biorefinery for the production and application of biostimulants in circular bioeconomy. Bioresource Technology, 339, 125588. https://doi.org/10.1016/j.biortech.2021.125588
Bektas, Y., & Eulgem, T. (2015). Synthetic plant defense elicitors. Frontiers in Plant Science, 5, 804. https://doi.org/10.3389/fpls.2014.00804
Ben Salah, I., Aghrouss, S., Douira, A., Aissam, S., El Alaoui-Talibi, Z., Filali-Maltouf, A., & El Modafar, C. (2018). Seaweed polysaccharides as bio-elicitors of natural defenses in olive trees against verticillium wilt of olive. Journal of Plant Interactions, 13(1), 248–255. https://doi.org/10.1080/17429145.2018.1471528
Berteau, O., & Mulloy, B. (2003). Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of Polysaccharide. Glycobiology, 13(6), 29R-40R. https://doi.org/10.1093/glycob/cwg058
Bhaskar, R., Xavier, L. S. E., Udayakumaran, G., Kumar, D. S., Venkatesh, R., & Nagella, P. (2021). Biotic elicitors: A boon for the in-vitro production of plant secondary metabolites. Plant Cell, Tissue and Organ Culture (PCTOC), 1–18. https://doi.org/10.1007/s11240-021-02131-1
Bigeard, J., Colcombet, J., & Hirt, H. (2015). Signaling mechanisms in pattern-triggered immunity (PTI). Molecular Plant, 8(4), 521–539. https://doi.org/10.1016/j.molp.2014.12.022
Biris-Dorhoi, E. S., Michiu, D., Pop, C. R., Rotar, A. M., Tofana, M., Pop, O. L., ... & Farcas, A. C. (2020). Macroalgae—A sustainable source of chemical compounds with biological activities. Nutrients, 12(10), 3085. https://doi.org/10.3390/nu12103085
Borsato, L. C., Di Piero, R. M., & Stadnik, M. J. (2010). Mecanismos de defesa eliciados por ulvana contra Uromyces appendiculatus em três cultivares de feijoeiro. Tropical Plant Pathology, 35(5), 318–322.
Boubakri, H. (2020). Induced resistance to biotic stress in plants by natural compounds: Possible mechanisms. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants (pp. 79–99). Academic Press. https://doi.org/10.1016/B978-0-12-817892-8.00005-2
Bouissil, S., Alaoui-Talibi, E., Pierre, G., Michaud, P., El Modafar, C., & Delattre, C. (2020). Use of alginate extracted from Moroccan brown algae to stimulate natural defense in date palm roots. Molecules, 25(3), 720. https://doi.org/10.3390/molecules25030720
Burketova, L., Trda, L., Ott, P. G., & Valentova, O. (2015). Bio-based resistance inducers for sustainable plant protection against pathogens. Biotechnology Advances, 33(6), 994–1004. https://doi.org/10.1016/j.biotechadv.2015.01.004
Boyd, L. A., Ridout, C., O’Sullivan, D. M., Leach, J. E., & Leung, H. (2013). Plant–pathogen interactions: Disease resistance in modern agriculture. Trends in Genetics, 29(4), 233–240. https://doi.org/10.1016/j.tig.2012.10.011
Carmo, L. P., Moura, C. W. N., & Lima-Brito, A. (2020). Red macroalgae extracts affect in vitro growth and bud formation in Comanthera mucugensis (Giul.) LR Parra & Giul., an endemic dry flower species from the Chapada Diamantina (Brazil). South African Journal of Botany, 135, 29–34. https://doi.org/10.1016/j.sajb.2020.07.033
Chai, W. S., Tan, W. G., Munawaroh, H. S. H., Gupta, V. K., Ho, S. H., & Show, P. L. (2021). Multifaceted roles of microalgae in the application of wastewater biotreatment: A review. Environmental Pollution, 269, 116236. https://doi.org/10.1016/j.envpol.2020.116236
Chanda, M. J., Merghoub, N., & Arroussi, H. E. (2019). Microalgae polysaccharides: The new sustainable bioactive products for the development of plant bio-stimulants? World Journal of Microbiology and Biotechnology, 35(11), 1–10. https://doi.org/10.1007/s11274-019-2745-3
Chandía, N. P., Matsuhiro, B., Mejías, E., & Moenne, A. (2004). Alginic acids in Lessonia vadosa: Partial hydrolysis and elicitor properties of the polymannuronic acid fraction. Journal of Applied Phycology, 16(2), 127–133. https://doi.org/10.1023/B:JAPH.0000044778.44193.a8
Chandía, N. P., & Matsuhiro, B. (2008). Characterization of a fucoidan from Lessonia vadosa (Phaeophyta) and its anticoagulant and elicitor properties. International Journal of Biological Macromolecules, 42(3), 235–240. https://doi.org/10.1016/j.ijbiomac.2007.10.023
Chen, J., Li, J., Dong, W., Zhang, X., Tyagi, R. D., Drogui, P., & Surampalli, R. Y. (2018). The potential of microalgae in biodiesel production. Renewable and Sustainable Energy Reviews, 90, 336–346. https://doi.org/10.1016/j.rser.2018.03.073
Chiaiese, P., Corrado, G., Colla, G., Kyriacou, M. C., & Rouphael, Y. (2018). Renewable sources of plant biostimulation: Microalgae as a sustainable means to improve crop performance. Frontiers in Plant Science, 9, 1782. https://doi.org/10.3389/fpls.2018.01782
Chiquito-Contreras, R. G., Murillo-Amador, B., Carmona-Hernandez, S., Chiquito-Contreras, C. J., & Hernandez-Montiel, L. G. (2019). Effect of marine bacteria and ulvan on the activity of antioxidant defense enzymes and the bio-protection of papaya fruit against Colletotrichum gloeosporioides. Antioxidants, 8(12), 580. https://doi.org/10.3390/antiox8120580
Chittora, D., Meena, M., Barupal, T., Swapnil, P., & Sharma, K. (2020). Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochemistry and Biophysics Reports, 22, 100737. https://doi.org/10.1016/j.bbrep.2020.100737
Cluzet, S., Torregrosa, C., Jacquet, C., Lafitte, C., Fournier, J., Mercier, L., Salamagne, S., Briand, X., Esquerré-Tugayé, M. T., & Dumas, B. (2004). Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from green algae Ulva spp. Plant, Cell & Environment, 27(7), 917–928. https://doi.org/10.1111/j.1365-3040.2004.01197.x
Cook, J., Zhang, J., Norrie, J., Blal, B., & Cheng, Z. (2018). Seaweed extract (Stella Maris®) activates innate immune responses in Arabidopsis thaliana and protects host against bacterial pathogens. Marine Drugs, 16(7), 221. https://doi.org/10.3390/md16070221
Cosse, A., Leblanc, C., & Potin, P. (2007). Dynamic defense of marine macroalgae against pathogens: From early activated to gene-regulated responses. Advances in Botanical Research, 46, 221–266. https://doi.org/10.1016/S0065-2296(07)46006-2
Costa, J. A. V., Freitas, B. C. B., Cruz, C. G., Silveira, J., & Morais, M. G. (2019). Potential of microalgae as biopesticides to contribute to sustainable agriculture and environmental development. Journal of Environmental Science and Health, Part B, 54(5), 366–375. https://doi.org/10.1080/03601234.2019.1571366
de Borba, M. C., de Freitas, M. B., & Stadnik, M. J. (2019). Ulvan enhances seedling emergence and reduces Fusarium wilt severity in common bean (Phaseolus vulgaris L.). Crop Protection, 118, 66–71. https://doi.org/10.1016/j.cropro.2018.12.014
de Freitas, M. B., & Stadnik, M. J. (2012). Race-specific and ulvan-induced defense responses in bean (Phaseolus vulgaris) against Colletotrichum lindemuthianum. Physiological and Molecular Plant Pathology, 78, 8–13. https://doi.org/10.1016/j.pmpp.2011.12.004
de Freitas, M. B., Ferreira, L. G., Hawerroth, C., Duarte, M. E. R., Noseda, M. D., & Stadnik, M. J. (2015). Ulvans induce resistance against plant pathogenic fungi independently of their sulfation degree. Carbohydrate Polymers, 133, 384–390. https://doi.org/10.1016/j.carbpol.2015.07.055
de Freitas, M. B., & Stadnik, M. J. (2015). Ulvan-induced resistance in Arabidopsis thaliana against Alternaria brassicicola requires reactive oxygen species derived from NADPH oxidase. Physiological and Molecular Plant Pathology, 90, 49–56. https://doi.org/10.1016/j.pmpp.2015.03.002
Delgado, D. Z., de Freitas, M. B., & Stadnik, M. J. (2013). Effectiveness of saccharin and ulvan as resistance inducers against rust and angular leaf spot in bean plants (Phaseolus vulgaris). Crop Protection, 47, 67–73. https://doi.org/10.1016/j.cropro.2013.01.003
Dey, P., Ramanujam, R., Venkatesan, G., & Nagarathnam, R. (2019). Sodium alginate potentiates antioxidant defense and PR proteins against early blight disease caused by Alternaria solani in Solanum lycopersicum Linn. PLoS ONE, 14(9), e0223216. https://doi.org/10.1371/journal.pone.0223216x
Drira, M., Elleuch, J., Ben Hlima, H., Hentati, F., Gardarin, C., Rihouey, C., Le Cerf, D., Michaud, P., Abdelkafi, S., & Fendri, I. (2021). Optimization of exopolysaccharides production by Porphyridium sordidum and their potential to induce defense responses in Arabidopsis thaliana against Fusarium oxysporum. Biomolecules, 11(2), 282. https://doi.org/10.3390/biom11020282
El Modafar, C., Elgadda, M., El Boutachfaiti, R., Abouraicha, E., Zehhar, N., Petit, E., Alaoui-Talibi, Z., Courtois, B., & Courtois, J. (2012). Induction of natural defence accompanied by salicylic acid-dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Scientia Horticulturae, 138, 55–63. https://doi.org/10.1016/j.scienta.2012.02.011
El-Sheekh, M. M., Mousa, A. S. H., & Farghl, A. A. (2020). Biological control of fusarium wilt disease of tomato plants using seaweed extracts. Arabian Journal for Science and Engineering, 45(6), 4557–4570. https://doi.org/10.1007/s13369-020-04518-2
Esserti, S., Smaili, A., Rifai, L. A., Koussa, T., Makroum, K., Belfaiza, M., Kabil, E. M., Burgos, L., Alburquerque, N., & Faize, M. (2017). Protective effect of three brown seaweed extracts against fungal and bacterial diseases of tomato. Journal of Applied Phycology, 29(2), 1081–1093. https://doi.org/10.1007/s10811-016-0996-z
Esserti, S., Smaili, A., Makroum, K., Belfaiza, M., Rifai, L. A., Koussa, T., Kasmi, I., & Faize, M. (2018). Priming of Nicotiana benthamiana antioxidant defences using brown seaweed extracts. Journal of Phytopathology, 166(2), 86–94. https://doi.org/10.1111/jph.12664
Fan, D., Hodges, D. M., Critchley, A. T., & Prithiviraj, B. (2013). A commercial extract of brown macroalga (Ascophyllum nodosum) affects yield and the nutritional quality of spinach in vitro. Communications in Soil Science and Plant Analysis, 44(12), 1873–1884. https://doi.org/10.1080/00103624.2013.790404
Farid, R., Mutale-Joan, C., Redouane, B., Najib, E. M., Abderahime, A., Laila, S., & Hicham, E. A. (2019). Effect of microalgae polysaccharides on biochemical and metabolomics pathways related to plant defense in Solanum lycopersicum. Applied Biochemistry and Biotechnology, 188(1), 225–240. https://doi.org/10.1007/s12010-018-2916-y
Featonby-Smith, B. C., & Van Staden, J. (1983). The effect of seaweed concentrate on the growth of tomato plants in nematode-infested soil. Scientia Horticulturae, 20(2), 137–146. https://doi.org/10.1016/0304-4238(83)90134-6
Fiallos, F. R. G., Wordell Filho, J. A., & Stadnik, M. J. (2020). Efecto del extracto de alga Ulva fasciata sobre Pseudocercospora griseola en el cultivo de frijol. Ciencia & Tecnología Agropecuaria, 21(3), 1–14. https://doi.org/10.1016/0304-4238(83)90134-6
Garrido-Cardenas, J. A., Manzano-Agugliaro, F., Acien-Fernandez, F. G., & Molina-Grima, E. (2018). Microalgae research worldwide. Algal Research, 35, 50–60. https://doi.org/10.1016/j.algal.2018.08.005
Gauthier, A., Trouvelot, S., Kelloniemi, J., Frettinger, P., Wendehenne, D., Daire, X., Joubert, J. M., Ferrarini, A., Delledonne, M., Flors, V., & Poinssot, B. (2014). The sulfated laminarin triggers a stress transcriptome before priming the SA-and ROS-dependent defenses during grapevine’s induced resistance against Plasmopara viticola. PLoS ONE, 9(2), e88145. https://doi.org/10.1371/journal.pone.0088145
Gemin, L. G., de Lara, G. B., Mógor, Á. F., Mógor, G., & de Queiroz, C. (2021). Organic onion biofortification using microalgae and humic acid. Research, Society and Development, 10(13), e320101321432–e320101321432. https://doi.org/10.33448/rsd-v10i13.21432
Ghannam, A., Abbas, A., Alek, H., Al-Waari, Z., & Al-Ktaifani, M. (2013). Enhancement of local plant immunity against tobacco mosaic virus infection after treatment with sulphated-carrageenan from red alga (Hypnea musciformis). Physiological and Molecular Plant Pathology, 84, 19–27. https://doi.org/10.1016/j.pmpp.2013.07.001
Ghareeb, R. Y., Alfy, H., Fahmy, A. A., Ali, H. M., & Abdelsalam, N. R. (2020). Utilization of Cladophora glomerata extract nanoparticles as eco-nematicide and enhancing the defense responses of tomato plants infected by Meloidogyne javanica. Scientific Reports, 10(1), 1–15. https://doi.org/10.1038/s41598-020-77005-1
Gimenez, E., Salinas, M., & Manzano-Agugliaro, F. (2018). Worldwide research on plant defense against biotic stresses as improvement for sustainable agriculture. Sustainability, 10(2), 391. https://doi.org/10.3390/su10020391
Goicoechea, N., Aguirreolea, J., & Garcıa-Mina, J. M. (2004). Alleviation of verticillium wilt in pepper (Capsicum annuum L.) by using the organic amendment COA H of natural origin. Scientia horticulturae, 101(1–2), 23–37. https://doi.org/10.1016/j.scienta.2003.09.015
Gonçalves, A. L. (2021). The use of microalgae and cyanobacteria in the improvement of agricultural practices: A review on their biofertilising, biostimulating and biopesticide roles. Applied Sciences, 11(2), 871. https://doi.org/10.3390/app11020871
González, A., Contreras, R. A., & Moenne, A. (2013). Oligo-carrageenans enhance growth and contents of cellulose, essential oils and polyphenolic compounds in Eucalyptus globulus trees. Molecules, 18(8), 8740–8751. https://doi.org/10.3390/molecules18088740
González, A., Gutiérrez-Cutiño, M., & Moenne, A. (2014). Oligo-carrageenan kappa-induced reducing redox status and increase in TRR/TRX activities promote activation and reprogramming of terpenoid metabolism in Eucalyptus trees. Molecules, 19(6), 7356–7367. https://doi.org/10.3390/molecules19067356
Gu, D., Andreev, K., & Dupre, M. E. (2021). Major trends in population growth around the world. China CDC Weekly, 3(28), 604. https://doi.org/10.46234/ccdcw2021.160
Gunupuru, L. R., Patel, J. S., Sumarah, M. W., Renaud, J. B., Mantin, E. G., & Prithiviraj, B. (2019). A plant biostimulant made from the marine brown algae Ascophyllum nodosum and chitosan reduce Fusarium head blight and mycotoxin contamination in wheat. PLoS ONE, 14(9), e0220562. https://doi.org/10.1371/journal.pone.0220562
Hamed, S. M., Abd El-Rhman, A. A., Abdel-Raouf, N., & Ibraheem, I. B. (2018). Role of marine macroalgae in plant protection & improvement for sustainable agriculture technology. Beni-Suef University Journal of Basic and Applied Sciences, 7(1), 104–110. https://doi.org/10.1016/j.bjbas.2017.08.002
Hashem, H. A., Mansour, H. A., El-Khawas, S. A., & Hassanein, R. A. (2019). The potentiality of marine macro-algae as bio-fertilizers to improve the productivity and salt stress tolerance of canola (Brassica napus L.) plants. Agronomy, 9(3), 146. https://doi.org/10.3390/agronomy9030146
Hernández-Herrera, R. M., Virgen-Calleros, G., Ruiz-López, M., Zañudo-Hernández, J., Délano-Frier, J. P., & Sánchez-Hernández, C. (2014). Extracts from green and brown seaweeds protect tomato (Solanum lycopersicum) against the necrotrophic fungus Alternaria solani. Journal of Applied Phycology, 26(3), 1607–1614. https://doi.org/10.1007/s10811-013-0193-2
Islam, M. T., Gan, H. M., Ziemann, M., Hussain, H. I., Arioli, T., & Cahill, D. (2020). Phaeophyceaean (brown algal) extracts activate plant defense systems in Arabidopsis thaliana challenged with Phytophthora cinnamomi. Frontiers in Plant Science, 11, 852. https://doi.org/10.3389/fpls.2020.00852
Islam, M. T., Arioli, T., & Cahill, D. M. (2021). Seaweed Extract-Stimulated Priming in Arabidopsis thaliana and Solanum lycopersicum. Plants, 10(11), 2476. https://doi.org/10.3390/plants10112476
Jaulneau, V., Lafitte, C., Jacquet, C., Fournier, S., Salamagne, S., Briand, X., Esquerré-Tugayé, M.T., & Dumas, B. (2010). Ulvan, a sulfated polysaccharide from green algae, activates plant immunity through the jasmonic acid signaling pathway. Journal of Biomedicine and Biotechnology, 2010. https://doi.org/10.1155/2010/525291
Jaulneau, V., Lafitte, C., Corio-Costet, M. F., Stadnik, M. J., Salamagne, S., Briand, X., Esquerré-Tugayé, M. T., & Dumas, B. (2011). An Ulva armoricana extract protects plants against three powdery mildew pathogens. European Journal of Plant Pathology, 131(3), 393–401. https://doi.org/10.1007/s10658-011-9816-0
Jayaraj, J., Wan, A., Rahman, M., & Punja, Z. K. (2008). Seaweed extract reduces foliar fungal diseases on carrot. Crop Protection, 27(10), 1360–1366. https://doi.org/10.1016/j.cropro.2008.05.005
Jayaraman, J., Norrie, J., & Punja, Z. K. (2011). Commercial extract from the brown seaweed Ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. Journal of Applied Phycology, 23(3), 353–361. https://doi.org/10.1007/s10811-010-9547-1
Jena J., Subudhi E. (2019) Microalgae: An Untapped Resource for Natural Antimicrobials. In: Sukla L., Subudhi E., Pradhan D. (eds) The Role of Microalgae in Wastewater Treatment. Springer, Singapore. https://doi.org/10.1007/978-981-13-1586-2_8
Khan, W., Rayirath, U. P., Subramanian, S., Jithesh, M. N., Rayorath, P., Hodges, D. M., Critchley, A. T., Craigie, J. S., Norrie, J., & Prithiviraj, B. (2009). Seaweed extracts as biostimulants of plant growth and development. Journal of Plant Growth Regulation, 28(4), 386–399. https://doi.org/10.1007/s00344-009-9103-x
Khedia, J., Dangariya, M., Nakum, A. K., Agarwal, P., Panda, A., Parida, A. K., Gangapur, D. R., Meena, R., & Agarwal, P. K. (2020). Sargassum seaweed extract enhances Macrophomina phaseolina resistance in tomato by regulating phytohormones and antioxidative activity. Journal of Applied Phycology, 32(6), 4373–4384. https://doi.org/10.1007/s10811-020-02263-5
Khompatara, K., Pettongkhao, S., Kuyyogsuy, A., Deenamo, N., & Churngchow, N. (2019). Enhanced resistance to leaf fall disease caused by Phytophthora palmivora in rubber tree seedling by Sargassum polycystum extract. Plants, 8(6), 168. https://doi.org/10.3390/plants8060168
Kim, S. J., Ko, E. J., Hong, J. K., & Jeun, Y. C. (2018). Ultrastructures of Colletotrichum orbiculare in cucumber leaves expressing systemic acquired resistance mediated by Chlorella fusca. The Plant Pathology Journal, 34(2), 113. https://doi.org/10.5423/PPJ.OA.09.2017.0204
Klarzynski, O., Plesse, B., Joubert, J. M., Yvin, J. C., Kopp, M., Kloareg, B., & Fritig, B. (2000). Linear β-1, 3 glucans are elicitors of defense responses in tobacco. Plant Physiology, 124(3), 1027–1038. https://doi.org/10.1104/pp.124.3.1027
Klarzynski, O., Descamps, V., Plesse, B., Yvin, J. C., Kloareg, B., & Fritig, B. (2003). Sulfated fucan oligosaccharides elicit defense responses in tobacco and local and systemic resistance against tobacco mosaic virus. Molecular Plant-Microbe Interactions, 16(2), 115–122. https://doi.org/10.1094/MPMI.2003.16.2.115
Kobayashi, A., Tai, A., Kanzaki, H., & Kawazu, K. (1993). Elicitor-active oligosaccharides from algal laminaran stimulate the production of antifungal compounds in alfalfa. Zeitschrift Für Naturforschung C, 48(7–8), 575–579. https://doi.org/10.1515/znc-1993-7-808
Kocira, S., Szparaga, A., Kuboń, M., Czerwińska, E., & Piskier, T. (2019). Morphological and biochemical responses of Glycine max (L.) Merr. to the use of seaweed extract. Agronomy, 9(2), 93. https://doi.org/10.3390/agronomy9020093
Kusvuran, S. (2021). Microalgae (Chlorella vulgaris Beijerinck) alleviates drought stress of broccoli plants by improving nutrient uptake, secondary metabolites, and antioxidative defense system. Horticultural Plant Journal, 7(3), 221–231. https://doi.org/10.1016/j.hpj.2021.03.007
Kusvuran, A., & Can, A. G. (2020). Effects of Microalga (Chlorella vulgaris Beijerinck) on Seconder Metabolites and Antioxidative Defense System Improve Plant Growth and Salt Tolerance in Guar [Cyamopsis tetragonoloba (L.) Taub.]. Legume Research-An International Journal, 43(1), 56–60. https://doi.org/10.18805/LR-492
Lähteenmäki-Uutela, A., Rahikainen, M., Camarena-Gómez, M. T., Piiparinen, J., Spilling, K., & Yang, B. (2021). European Union legislation on macroalgae products. Aquaculture International, 29(2), 487–509. https://doi.org/10.1007/s10499-020-00633-x
Laporte, D., Vera, J., Chandía, N. P., Zúñiga, E. A., Matsuhiro, B., & Moenne, A. (2007). Structurally unrelated algal oligosaccharides differentially stimulate growth and defense against tobacco mosaic virus in tobacco plants. Journal of Applied Phycology, 19(1), 79–88. https://doi.org/10.1007/s10811-006-9114-y
Leandro, A., Pereira, L., & Gonçalves, A. M. (2020). Diverse applications of marine macroalgae. Marine Drugs, 18(1), 17. https://doi.org/10.1007/s10811-006-9114-y
Lee, S. M., Kim, S. K., Lee, N., Ahn, C. Y., & Ryu, C. M. (2020). d-Lactic acid secreted by Chlorella fusca primes pattern-triggered immunity against Pseudomonas syringae in Arabidopsis. The Plant Journal, 102(4), 761–778. https://doi.org/10.1111/tpj.14661
Le Mire, G., Siah, A., Marolleau, B., Gaucher, M., Maumené, C., Brostaux, Y., Massart, S., Brisset, M. N., & Jijakli, M. H. (2019). Evaluation of λ-carrageenan, CpG-ODN, Glycine betaine, spirulina platensis, and ergosterol as elicitors for control of Zymoseptoria tritici in wheat. Phytopathology, 109(3), 409–417. https://doi.org/10.1094/PHYTO-11-17-0367-R
Liang, M. H., Wang, L., Wang, Q., Zhu, J., & Jiang, J. G. (2019). High-value bioproducts from microalgae: Strategies and progress. Critical Reviews in Food Science and Nutrition, 59(15), 2423–2441. https://doi.org/10.1080/10408398.2018.1455030
Lin, J., Yan, H., Zhao, L., Li, Y., Nahidian, B., Zhu, M., Hu, Q., & Han, D. (2021). Interaction between the cell walls of microalgal host and fungal carbohydrate-activate enzymes is essential for the pathogenic parasitism process. Environmental Microbiology. https://doi.org/10.1111/1462-2920.15465
Machado, L. P., de Godoy Gasparoto, M. C., & Alves, N. (2019). Seaweeds in the Control of Plant Diseases and Insects. In Seaweeds as Plant Fertilizer, Agricultural Biostimulants and Animal Fodder (pp. 100–127). CRC Press.
Manandhar, B., Paudel, P., Seong, S. H., Jung, H. A., & Choi, J. S. (2019). Characterizing eckol as a therapeutic aid: A systematic review. Marine Drugs, 17(6), 361. https://doi.org/10.3390/md17060361
Mani, S. D., & Nagarathnam, R. (2018). Sulfated polysaccharide from Kappaphycus alvarezii (Doty) Doty ex PC Silva primes defense responses against anthracnose disease of Capsicum annuum Linn. Algal Research, 32, 121–130. https://doi.org/10.1016/j.algal.2018.02.025
Mehariya, S., Goswami, R. K., Karthikeysan, O. P., & Verma, P. (2021). Microalgae for high-value products: A way towards green nutraceutical and pharmaceutical compounds. Chemosphere, 280, 130553. https://doi.org/10.1016/j.chemosphere.2021.130553
Melo, P., Abreu, C., Bahcevandziev, K., Araujo, G., & Pereira, L. (2020a). Biostimulant effect of marine macroalgae bioextract on pepper grown in greenhouse. Applied Sciences, 10(11), 4052. https://doi.org/10.3390/app10114052
Melo, P. C. D., Collela, C. F., Sousa, T., Pacheco, D., Cotas, J., Gonçalves, A. M., Bahcevandziev, K., & Pereira, L. (2020b). Seaweed-Based Products and Mushroom β-Glucan as Tomato Plant Immunological Inducers. Vaccines, 8(3), 524. https://doi.org/10.3390/vaccines8030524
Ménard, R., Alban, S., de Ruffray, P., Jamois, F., Franz, G., Fritig, B., Yvin, J. C., & Kauffmanna, S. (2004). b-1, 3 Glucan Sulfate, but Not b-1, 3 Glucan, Induces the Salicylic Acid Signaling Pathway in Tobacco and Arabidopsis. The Plant Cell, 16, 30203032. https://doi.org/10.1105/tpc.104.024968
Ménard, R., de Ruffray, P., Fritig, B., Yvin, J. C., & Kauffmann, S. (2005). Defense and resistance-inducing activities in tobacco of the sulfated β-1, 3 glucan PS3 and its synergistic activities with the unsulfated molecule. Plant and Cell Physiology, 46(12), 1964–1972. https://doi.org/10.1093/pcp/pci212
Mercier, L., Lafitte, C., Borderies, G., Briand, X., Esquerré-Tugayé, M. T., & Fournier, J. (2001). The algal polysaccharide carrageenans can act as an elicitor of plant defence. New Phytologist, 149(1), 43–51. https://doi.org/10.1046/j.1469-8137.2001.00011.x
Mohammad-Razdari, A., Rousseau, D., Bakhshipour A., Taylor S., Poveda, J., Kiani, H. (2022). Recent advances in E-monitoring of Plant Diseases. Biosensors and Bioelectronics (In Press). https://doi.org/10.1016/j.bios.2021.113953
Murata, M. M., Ito Morioka, L. R., Da Silva Marques, J. B., Bosso, A., & Suguimoto, H. H. (2021). What do patents tell us about microalgae in agriculture? AMB Express, 11(1), 1–12. https://doi.org/10.1186/s13568-021-01315-4
Nabti, E., Jha, B., & Hartmann, A. (2017). Impact of seaweeds on agricultural crop production as biofertilizer. International Journal of Environmental Science and Technology, 14(5), 1119–1134. https://doi.org/10.1007/s13762-016-1202-1
Nagorskaya, V. P., Reunov, A. V., Lapshina, L. A., Yermak, I. M., & Barabanova, A. O. (2008). Influence of κ/β-carrageenan from red alga Tichocarpus crinitus on development of local infection induced by tobacco mosaic virus in Xanthi-nc tobacco leaves. Biology Bulletin, 35(3), 310–314. https://doi.org/10.1134/S1062359008030126
Nagorskaya, V. P., Reunov, A. V., Lapshina, L. A., Ermak, I. M., & Barabanova, A. O. (2010). Inhibitory effect of κ/β-carrageenan from red alga Tichocarpus crinitus on the development of a potato virus X infection in leaves of Datura stramonium L. Biology Bulletin, 37(6), 653–658. https://doi.org/10.1134/S1062359010060142
Øverland, M., Mydland, L. T., & Skrede, A. (2019). Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. Journal of the Science of Food and Agriculture, 99(1), 13–24. https://doi.org/10.1002/jsfa.9143
Pan S., Jeevanandam J., Danquah M.K. (2019) Benefits of Algal Extracts in Sustainable Agriculture. In: Hallmann A., Rampelotto P. (eds) Grand Challenges in Algae Biotechnology. Grand Challenges in Biology and Biotechnology. Springer, Cham. https://doi.org/10.1007/978-3-030-25233-5_14.
Panjehkeh, N., & Abkhoo, J. (2016). Influence of marine brown alga extract (Dalgin) on damping-off tolerance of tomato. J Mater Environ Sci, 7, 2369–2374.
Paris, F., Krzyżaniak, Y., Gauvrit, C., Jamois, F., Domergue, F., Joubès, J., Ferriéres, V., Adrian, M., Legentil, L., Daire, X., & Trouvelot, S. (2016). An ethoxylated surfactant enhances the penetration of the sulfated laminarin through leaf cuticle and stomata, leading to increased induced resistance against grapevine downy mildew. Physiologia Plantarum, 156(3), 338–350. https://doi.org/10.1111/ppl.12394
Patel, J. S., Selvaraj, V., Gunupuru, L. R., Rathor, P. K., & Prithiviraj, B. (2020). Combined application of Ascophyllum nodosum extract and chitosan synergistically activates host-defense of peas against powdery mildew. BMC Plant Biology, 20(1), 1–10. https://doi.org/10.1186/s12870-020-2287-8
Patier, P., Yvin, J. C., Kloareg, B., Liénart, Y., & Rochas, C. (1993). Seaweed liquid fertilizer from Ascophyllum nodosum contains elicitors of plant D-glycanases. Journal of Applied Phycology, 5(3), 343–349. https://doi.org/10.1007/BF02186237
Patier, P., Potin, P., Rochas, C., Kloareg, B., Yvin, J. C., & Liénart, Y. (1995). Free or silica-bound oligokappa-carrageenans elicit laminarinase activity in Rubus cells and protoplasts. Plant Science, 110(1), 27–35. https://doi.org/10.1016/0168-9452(95)04182-T
Paulert, R., Talamini, V., Cassolato, J. E. F., Duarte, M. E. R., Noseda, M. D., Smania, A., & Stadnik, M. J. (2009). Effects of sulfated polysaccharide and alcoholic extracts from green seaweed Ulva fasciata on anthracnose severity and growth of common bean (Phaseolus vulgaris L.). Journal of Plant Diseases and Protection, 116(6), 263–270. https://doi.org/10.1007/BF03356321
Paulert, R., Ebbinghaus, D., Urlass, C., & Moerschbacher, B. M. (2010). Priming of the oxidative burst in rice and wheat cell cultures by ulvan, a polysaccharide from green macroalgae, and enhanced resistance against powdery mildew in wheat and barley plants. Plant Pathology, 59(4), 634–642. https://doi.org/10.1111/j.1365-3059.2010.02300.x
Paulert, R., Brunel, F., Melcher, R. L., Cord-Landwehr, S., Niehues, A., Mormann, M., & Moerschbacher, B. M. (2021a). The non-sulfated ulvanobiuronic acid of ulvans is the smallest active unit able to induce oxidative burst in dicot cells. Carbohydrate Polymers, 118338. https://doi.org/10.1016/j.carbpol.2021.118338
Paulert, R., Ascrizzi, R., Malatesta, S., Berni, P., Noseda, M. D., Mazetto de Carvalho, M., et al. (2021). Ulva intestinalis extract acts as biostimulant and modulates metabolites and hormone balance in basil (Ocimum basilicum l.) and parsley (Petroselinum crispum L.). Plants, 10(7), 1391. https://doi.org/10.3390/plants10071391
Pereira, L. (2021). Macroalgae. Encyclopedia, 1(1), 177–188.
Pettongkhao, S., Bilanglod, A., Khompatara, K., & Churngchow, N. (2019). Sulphated polysaccharide from Acanthophora spicifera induced Hevea brasiliensis defense responses against Phytophthora palmivora infection. Plants, 8(3), 73. https://doi.org/10.3390/plants8030073
Pimentel, F. B., Alves, R. C., Rodrigues, F., Oliveira, P. P., & M. B. (2018). Macroalgae-derived ingredients for cosmetic industry—An update. Cosmetics, 5(1), 2. https://doi.org/10.3390/cosmetics5010002
Plaza, B. M., Gómez-Serrano, C., Acién-Fernández, F. G., & Jimenez-Becker, S. (2018). Effect of microalgae hydrolysate foliar application (Arthrospira platensis and Scenedesmus sp.) on Petunia x hybrida growth. Journal of Applied Phycology, 30(4), 2359–2365. https://doi.org/10.1007/s10811-018-1427-0
Pohl, A., Kalisz, A., & Sekara, A. (2019). Seaweed extracts’ multifactorial action: influence on physiological and biochemical status of Solanaceae plants. Acta Agrobotanica, 72(1). https://doi.org/10.5586/aa.1758
Poveda, J. (2020). Use of plant-defense hormones against pathogen-diseases of postharvest fresh produce. Physiological and Molecular Plant Pathology, 111, 101521. https://doi.org/10.1016/j.pmpp.2020.101521
Poveda, J. (2021). Cyanobacteria in plant health: Biological strategy against abiotic and biotic stresses. Crop Protection, 141, 105450. https://doi.org/10.1016/j.cropro.2020.105450
Poveda, J. (2022). The use of freshwater macrophytes as a resource in sustainable agriculture. Journal of Cleaner Production. 367, 18025R2. https://doi.org/10.1016/j.jclepro.2022.18025R2
Rachidi, F., Benhima, R., Sbabou, L., & El Arroussi, H. (2020). Microalgae polysaccharides bio-stimulating effect on tomato plants: Growth and metabolic distribution. Biotechnology Reports, 25, e00426. https://doi.org/10.1016/j.btre.2020.e00426
Rachidi, F., Benhima, R., Kasmi, Y., Sbabou, L., & El Arroussi, H. (2021). Evaluation of microalgae polysaccharides as biostimulants of tomato plant defense using metabolomics and biochemical approaches. Scientific Reports, 11(1), 1–16. https://doi.org/10.1038/s41598-020-78820-2
Raghavendra, V. B., Lokesh, S., & Prakash, H. S. (2007). Dravya, a product of seaweed extract (Sargassum wightii), induces resistance in cotton against Xanthomonas campestris pv. malsvacearum. Phytoparasitica, 35(5), 442–449. https://doi.org/10.1007/BF03020602
Raj, T. S., Muthukumar, A., Muthukumaran, N., Rao, G. S., & Suji, H. A. (2019). Induction of defence enzymes activities in rice plant treated by seaweed algae against Rhizoctonia solani Kuhn causing sheath blight of rice. Journal of Pharmacognosy and Phytochemistry, 8, 210–218.
Ramachandra, T. V., & Hebbale, D. (2020). Bioethanol from macroalgae: Prospects and challenges. Renewable and Sustainable Energy Reviews, 117, 109479. https://doi.org/10.1016/j.rser.2019.109479
Ramkissoon, A., Ramsubhag, A., & Jayaraman, J. (2017). Phytoelicitor activity of three Caribbean seaweed species on suppression of pathogenic infections in tomato plants. Journal of Applied Phycology, 29(6), 3235–3244. https://doi.org/10.1007/s10811-017-1160-0
Rao, S. R., Tripathi, U., Suresh, B., & Ravishankar, G. A. (2001). Enhancement of secondary metabolite production in hairy root cultures of Beta vulgaris and Tagetes patula under the influence of microalgal elicitors. Food Biotechnology, 15(1), 35–46. https://doi.org/10.1081/FBT-100103893
Redouan, E., Cedric, D., Emmanuel, P., Bernard, C., Philippe, M., Cherkaoui, E. M., & Josiane, C. (2009). Improved isolation of glucuronan from algae and the production of glucuronic acid oligosaccharides using a glucuronan lyase. Carbohydrate Research, 344(13), 1670–1675. https://doi.org/10.1016/j.carres.2009.05.031
Regnault-Roger, C. (2020) Trends for Commercialization of Biocontrol Agents (Biopesticide). In: Mérillon JM., Ramawat K.G. (eds) Plant Defence: Biological Control. Progress in Biological Control, vol 22 (pp. 445–471. Springer, Cham. https://doi.org/10.1007/978-3-030-51034-3_17
Rengasamy, K. R., Kulkarni, M. G., Pendota, S. C., & Van Staden, J. (2016). Enhancing growth, phytochemical constituents and aphid resistance capacity in cabbage with foliar application of eckol–a biologically active phenolic molecule from brown seaweed. New Biotechnology, 33(2), 273–279. https://doi.org/10.1016/j.nbt.2015.11.002
Righini, H., Somma, A., Cetrullo, S., D’Adamo, S., Flamigni, F., Quintana, A. M., & Roberti, R. (2020). Inhibitory activity of aqueous extracts from Anabaena minutissima, Ecklonia maxima and Jania adhaerens on the cucumber powdery mildew pathogen in vitro and in vivo. Journal of Applied Phycology, 32(5), 3363–3375. https://doi.org/10.1007/s10811-020-02160-x
Righini, H., Francioso, O., Di Foggia, M., Prodi, A., Quintana, A. M., & Roberti, R. (2021). Tomato seed biopriming with water extracts from Anabaena minutissima, Ecklonia maxima and Jania adhaerens as a new agro-ecological option against Rhizoctonia solani. Scientia Horticulturae, 281, 109921. https://doi.org/10.1016/j.scienta.2021.109921
Rinaldi, L. K., Miamoto, A., Calandrelli, A., e Silva, M. T. R., Chidichima, L. P. S., Pereira, C. B., & Dias-Arieira, C. R. (2021). Control of Meloidogyne javanica and induction of resistance-associated enzymes in soybean by extracts of Ascophyllum nodosum. Journal of Applied Phycology, 1-12. https://doi.org/10.1007/s10811-021-02454-8
Rivero-Montejo, S. D. J., Vargas-Hernandez, M., & Torres-Pacheco, I. (2021). Nanoparticles as Novel Elicitors to Improve Bioactive Compounds in Plants. Agriculture, 11(2), 134. https://doi.org/10.3390/agriculture11020134
Rizwan, M., Mujtaba, G., Memon, S. A., Lee, K., & Rashid, N. (2018). Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renewable and Sustainable Energy Reviews, 92, 394–404. https://doi.org/10.1016/j.rser.2018.04.034
Salim, H. (2020). Inhibition of tomato yellow leaf curl virus (TYLCV) by extract of algae Cladophora crispate. Plant Archives, 20(1), 260–264.
Sangha, J. S., Ravichandran, S., Prithiviraj, K., Critchley, A. T., & Prithiviraj, B. (2010). Sulfated macroalgal polysaccharides λ-carrageenan and ι-carrageenan differentially alter Arabidopsis thaliana resistance to Sclerotinia sclerotiorum. Physiological and Molecular Plant Pathology, 75(1–2), 38–45. https://doi.org/10.1016/j.pmpp.2010.08.003
Sangha, J. S., Khan, W., Ji, X., Zhang, J., Mills, A. A., Critchley, A. T., & Prithiviraj, B. (2011). Carrageenans, sulphated polysaccharides of red seaweeds, differentially affect Arabidopsis thaliana resistance to Trichoplusia ni (Cabbage Looper). PLoS ONE, 6(10), e26834. https://doi.org/10.1371/journal.pone.0026834
Sangha, J. S., Kandasamy, S., Khan, W., Bahia, N. S., Singh, R. P., Critchley, A. T., & Prithiviraj, B. (2015). λ-carrageenan suppresses tomato chlorotic dwarf viroid (TCDVd) replication and symptom expression in tomatoes. Marine Drugs, 13(5), 2875–2889. https://doi.org/10.3390/md13052875
Savary, S., Willocquet, L., Pethybridge, S. J., Esker, P., McRoberts, N., & Nelson, A. (2019). The global burden of pathogens and pests on major food crops. Nature Ecology & Evolution, 3(3), 430–439. https://doi.org/10.1038/s41559-018-0793-y
Sbaihat, L., Takeyama, K., Koga, T., Takemoto, D., & Kawakita, K. (2015). Induced resistance in Solanum lycopersicum by algal elicitor extracted from Sargassum fusiforme. The Scientific World Journal, 2015. https://doi.org/10.1155/2015/870520
Sharma, A., Ambati, R. R., Chandrappa, D., Ravi, S., & Aswathanarayana, R. G. (2010). Botryococcus braunii, a new elicitor for secondary metabolite production in Capsicum frutescens. Functional Plant Science and Biotechnology, 5, 9–13.
Sharma, H. S., Fleming, C., Selby, C., Rao, J. R., & Martin, T. (2014). Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. Journal of Applied Phycology, 26(1), 465–490. https://doi.org/10.1007/s10811-013-0101-9
Sharma, A., Shukla, A., Attri, K., Kumar, M., Kumar, P., Suttee, A., Singh, G., Barnwal, R. P., & Singla, N. (2020). Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicology and Environmental Safety, 201, 110812. https://doi.org/10.1016/j.ecoenv.2020.110812
Shukla, P. S., Borza, T., Critchley, A. T., & Prithiviraj, B. (2016). Carrageenans from red seaweeds as promoters of growth and elicitors of defense response in plants. Frontiers in Marine Science, 3, 81. https://doi.org/10.3389/fmars.2016.00081
Shukla, P. S., Borza, T., Critchley, A. T., & Prithiviraj, B. (2021). Seaweed-based compounds and Products for sustainable protection against plant pathogens. Marine Drugs, 19(2), 59. https://doi.org/10.3390/md19020059
Silva, A., Silva, S. A., Carpena, M., Garcia-Oliveira, P., Gullón, P., Barroso, M. F., Prieto, M. A., & Simal-Gandara, J. (2020). Macroalgae as a source of valuable antimicrobial compounds: Extraction and applications. Antibiotics, 9(10), 642. https://doi.org/10.3390/antibiotics9100642
Simas-Rodrigues, C., Villela, H. D., Martins, A. P., Marques, L. G., Colepicolo, P., & Tonon, A. P. (2015). Microalgae for economic applications: Advantages and perspectives for bioethanol. Journal of Experimental Botany, 66(14), 4097–4108. https://doi.org/10.1093/jxb/erv130
Somai-Jemmali, L., Siah, A., Randoux, B., Magnin-Robert, M., Halama, P., Hamada, W., & Reignault, P. (2020). Brown alga Ascophyllum nodosum extract-based product, Dalgin Active®, triggers defense mechanisms and confers protection in both bread and durum wheat against Zymoseptoria tritici. Journal of Applied Phycology, 32(5), 3387–3399. https://doi.org/10.1007/s10811-020-02200-6
Stojakowska, A., Burczyk, J., Kisiel, W., Zych, M., Banas, A., & Duda, T. (2008). Effects of various elicitors on the accumulation and secretion of spiroketal enol ether diacetylenes in feverfew hairy root culture. Acta Societatis Botanicorum Poloniae, 77(1), 17–21.
Subramanian, S., Sangha, J. S., Gray, B. A., Singh, R. P., Hiltz, D., Critchley, A. T., & Prithiviraj, B. (2011). Extracts of the marine brown macroalga, Ascophyllum nodosum, induce jasmonic acid dependent systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato DC3000 and Sclerotinia sclerotiorum. European Journal of Plant Pathology, 131(2), 237–248. https://doi.org/10.1007/s10658-011-9802-6
Sudhakar, K., Mamat, R., Samykano, M., Azmi, W. H., Ishak, W. F. W., & Yusaf, T. (2018). An overview of marine macroalgae as bioresource. Renewable and Sustainable Energy Reviews, 91, 165–179. https://doi.org/10.1016/j.rser.2018.03.100
Torres-Tiji, Y., Fields, F. J., & Mayfield, S. P. (2020). Microalgae as a future food source. Biotechnology Advances, 41, 107536. https://doi.org/10.1016/j.biotechadv.2020.107536
Trouvelot, S., Varnier, A. L., Allegre, M., Mercier, L., Baillieul, F., Arnould, C., et al. (2008). A β-1, 3 glucan sulfate induces resistance in grapevine against Plasmopara viticola through priming of defense responses, including HR-like cell death. Molecular Plant-Microbe Interactions, 21(2), 232–243. https://doi.org/10.1094/MPMI-21-2-0232
Tudi, M., Daniel Ruan, H., Wang, L., Lyu, J., Sadler, R., Connell, D., Chu, C., & Phung, D. T. (2021). Agriculture development, pesticide application and its impact on the environment. International Journal of Environmental Research and Public Health, 18(3), 1112. https://doi.org/10.3390/ijerph18031112
Uysal, O., Uysal, F. O., & Ekinci, K. (2015). Evaluation of microalgae as microbial fertilizer. European Journal of Sustainable Development, 4(2), 77–77. https://doi.org/10.14207/ejsd.2015.v4n2p77
Velásquez, A. C., Castroverde, C. D. M., & He, S. Y. (2018). Plant–pathogen warfare under changing climate conditions. Current Biology, 28(10), R619–R634. https://doi.org/10.1016/j.cub.2018.03.054
Vera, J., Castro, J., Gonzalez, A., Barrientos, H., Matsuhiro, B., Arce, P., et al. (2011a). Long-term protection against tobacco mosaic virus induced by the marine alga oligo-sulphated-galactan Poly-Ga in tobacco plants. Molecular Plant Pathology, 12(5), 437–447. https://doi.org/10.1111/j.1364-3703.2010.00685.x
Vera, J., Castro, J., Gonzalez, A., & Moenne, A. (2011b). Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Marine Drugs, 9(12), 2514–2525. https://doi.org/10.3390/md9122514
Vera, J., Castro, J., Contreras, R. A., González, A., & Moenne, A. (2012). Oligo-carrageenans induce a long-term and broad-range protection against pathogens in tobacco plants (var. Xanthi). Physiological and Molecular Plant Pathology, 79, 31–39. https://doi.org/10.1016/j.pmpp.2012.03.005
Viencz, T., Oliari, I. C. R., Ayub, R. A., Faria, C. M. D. R., & Botelho, R. V. (2020). Postharvest quality and brown rot incidence in plums treated with Ascophyllum nodosum extract. Semina: Ciências Agrárias, 41(3), 753–766. https://doi.org/10.5433/1679-0359.2020v41n3p753
Wan, A. H., Davies, S. J., Soler-Vila, A., Fitzgerald, R., & Johnson, M. P. (2019). Macroalgae as a sustainable aquafeed ingredient. Reviews in Aquaculture, 11(3), 458–492. https://doi.org/10.1111/raq.12241
Weeraddana, C. D. S., Kandasamy, S., Cutler, G. C., Shukla, P. S., Critchley, A. T., & Prithiviraj, B. (2021). An alkali-extracted biostimulant prepared from Ascophyllum nodosum alters the susceptibility of Arabidopsis thaliana to the green peach aphid. Journal of Applied Phycology, 33(5), 3319–3329. https://doi.org/10.1007/s10811-021-02534-9
Weinberger, F. (2007). Pathogen-induced defense and innate immunity in macroalgae. The Biological Bulletin, 213(3), 290–302.
Wiesel, L., Newton, A. C., Elliott, I., Booty, D., Gilroy, E. M., Birch, P. R., & Hein, I. (2014). Molecular effects of resistance elicitors from biological origin and their potential for crop protection. Frontiers in Plant Science, 5, 655. https://doi.org/10.3389/fpls.2014.00655
Wite, D., Mattner, S. W., Porter, I. J., & Arioli, T. (2015). The suppressive effect of a commercial extract from Durvillaea potatorum and Ascophyllum nodosum on infection of broccoli by Plasmodiophora brassicae. Journal of Applied Phycology, 27(5), 2157–2161. https://doi.org/10.1007/s10811-015-0564-y
Xin, Z., Cai, X., Chen, S., Luo, Z., Bian, L., Li, Z., et al. (2019). A disease resistance elicitor laminarin enhances tea defense against a piercing herbivore Empoasca (Matsumurasca) onukii Matsuda. Scientific Reports, 9(1), 1–13. https://doi.org/10.1038/s41598-018-37424-7
Zabed, H. M., Akter, S., Yun, J., Zhang, G., Zhang, Y., & Qi, X. (2020). Biogas from microalgae: Technologies, challenges and opportunities. Renewable and Sustainable Energy Reviews, 117, 109503. https://doi.org/10.1016/j.rser.2019.109503
Zhang, C., Howlader, P., Liu, T., Sun, X., Jia, X., Zhao, X., Shen, P., Qin, Y., Wang, W., & Yin, H. (2019). Alginate Oligosaccharide (AOS) induced resistance to Pst DC3000 via salicylic acid-mediated signaling pathway in Arabidopsis thaliana. Carbohydrate Polymers, 225, 115221. https://doi.org/10.1016/j.carbpol.2019.115221
Zou, P., Lu, X., Zhao, H., Yuan, Y., Meng, L., Zhang, C., & Li, Y. (2019). Polysaccharides derived from the brown algae Lessonia nigrescens enhance salt stress tolerance to wheat seedlings by enhancing the antioxidant system and modulating intracellular ion concentration. Frontiers in Plant Science, 10, 48. https://doi.org/10.3389/fpls.2019.00048
Funding
Grants for the Recualification of the Spanish University System for 2021–2023, Public University of Navarra; Recualification Modality; Funded by the European Union – NextGenerationEU.
Author information
Authors and Affiliations
Contributions
J.P. conceptualized and designed the manuscript. J.P. performed the bibliographic search and analyzed the information. J.P. wrote the first version of the manuscript. A.D.M. contributed to the manuscript writing, correction and critical reading. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Poveda, J., Díez-Méndez, A. Use of elicitors from macroalgae and microalgae in the management of pests and diseases in agriculture. Phytoparasitica 51, 667–701 (2023). https://doi.org/10.1007/s12600-022-01009-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-022-01009-y