Abstract
The effect of extracts of the brown algae Cystoseira myriophylloides, Laminaria digitata, and Fucus spiralis against the tomato pathogens Verticillium dahliae and Agrobacterium tumefaciens was evaluated in vitro and in the greenhouse. A significant inhibition of growth was observed only with methanolic seaweed extracts (MSE). Disease resistance was assessed in the greenhouse against Verticillium wilt using spray application of aqueous seaweed extracts (ASE) on the whole plant or using seed imbibition. Both methods significantly reduced disease severity whatever the algal species, though protection observed after seed treatments was higher than that observed after spray treatment. Spray application of ASE from C. myriophylloides and F. spiralis also resulted in significant reduction of Crown gall disease caused by the bacterial pathogen A. tumefaciens. ASE-treated plants had significantly higher levels of activity of defense enzymes polyphenol oxidase and peroxidase compared to the control. ASE did not inhibit mycelium growth of V. dahliae or development of A. tumefaciens in vitro; it is therefore suggested that induced resistance is probably the main mechanism of protection afforded by ASE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato (Solanum lycopersicum) is considered among the world’s largest vegetable crops. However, it is targeted by several diseases and pests. Among them Verticillium dahliae, the causal agent of wilting, affect severely the growth and the yield of tomato plants. It persists on the soil as microsclerotia and can infect plants through wounds in the roots. Symptoms become visible on leaves, their edges, and areas between the veins turn yellow and then brown. The infected plants often show a V-shaped lesion at the edge of the leaf, which can expand, resulting in complete browning and death of the leaves. The systemic infection spread and usually stems result in wilting. The fungus can be also revealed by the presence of vascular streaking in stems (Fradin and Thomma 2006; Klosterman et al. 2009).
Agrobacterium tumefaciens is another pathogen that affects tomato. It causes crown gall disease by introducing its T-DNA into the plant genome causing the induction of tumors and associated changes in plant metabolism (Sigee 1993). Crown gall disease can be fatal if infection occurs in young plants, as reduction in crop yield and vigor can be significant. The decreased productivity may be caused by the decreased water and nutrient flow due to damaged vessels at the site of gall development, and to significant deviation of water and nutrients to the rapidly growing gall (Escobar and Dandekar 2003). In general, as for most of bacterial diseases of plants, crown gall is very difficult to control because of the lack of effective chemical treatments. The most effective chemical method is the use of copper; however, it is highly phytotoxic (Chase 1989). Biological control using the agrocin-producing strain K84 of Agrobacterium radiobacter is a good alternative. However, the possible transfer of the agrocin plasmid to pathogenic strains may threaten the success of this method (Farrand 1990; Ryder and Jones 1991).
Soil fumigation and spraying fungicides have been the main recommended measures for controlling Verticillium wilt disease (Talboys 1984; Tian et al. 1998; Rekanovic et al. 2007). However, their negative effect on the environment and human health has intensified the development of new alternative control methods. Within this context, alternative diseases management using natural compounds are highly needed.
Seaweeds represent an important source of a wide variety of natural products and could be a promising source of novel bioactive compounds that can offer protection against such plant diseases. Several studies have reported the in vitro antimicrobial effect of seaweed extracts on phytopathogens. For instance, extracts from Sargassum wightii showed an elevated in vitro activity against Pseudomonas syringae, the causal agent of leaf spot of the medicinal plant Gymnema sylvestre (Kumar et al. 2008) and against Xanthomonas oryzae pv. oryzae, the causal agent of bacterial rice blight (Arunkumar et al. 2005). Indira et al. (2013) revealed the antifungal activity of extracts of the green alga Halimeda tuna against several fungi, including Aspergillus niger, Aspergillus flavus, Alternaria alternaria, Penicillium sp., and Rhizopus sp. Ara et al. (2005) found that fractions containing fatty acid esters from an extract of Spatoglossum asperum inhibited the growth of the soil-borne pathogens Macrophomina phaseolina, Rhizoctonia solani, and Fusarium solani. Chanthini et al. (2012) correlated the direct antifungal activity of some red and green algae against Alternaria solani with their phenolic content.
In addition to the direct antimicrobial effect of seaweed extracts, evidence that they can suppress disease or reduce the incidence of disease on commercially important pathogens in vivo has been reported. Cotton seedlings inoculated with Xanthomonas campestris pv. malvacearum developed considerable resistance to the bacterial blight if the seeds were soaked prior to germination in Dravya, an aqueous formulation of S. wightii extract (Raghavendra et al. 2007). This resistance was drastically enhanced if developing cotton seedlings were sprayed with the seaweed extract, with the greatest reduction in blight. Liquid seaweed extracts of Ascophyllum nodosum have been reported to reduce disease severity caused by the foliar pathogens Alternaria cucumerinium, Alternaria radicina, Didymella applanata, and Botrytis cinerea in carrot (Jayaraj et al. 2008) and cucumber (Jayaraj et al. 2011). Mattner et al. (2014) found that a commercially available seaweed extract (Seasol) made with combination of extracts from Durvillaea potatorum and A. nodosum reduces the ability of Sclerotinia minor to cause disease. The same commercial seaweed extract suppress primary and secondary clubroot infection caused by Plasmodiophora brassicae (Wite et al. 2015) and significantly reduces the incidence of white blister disease, caused by Albugo candida on broccoli leaves (Mattner et al. 2013).
Morocco is bordered by Atlantic Ocean and Mediterranean Sea harboring a very high diversity of brown algae, which includes more than 131 Phaeophyceae (Benhissoune et al. 2002). Given this richness, this work is a part of the valorization of algal resources in Morocco. In this context, and trying to reduce pesticides input to control phytopathogens in tomato, the present investigation was carried out to examine of the effects of seaweed extracts derived from three brown algae (Cystoseira myriophylloides, Laminaria digitata, and Fucus spiralis) against Verticillium wilt and crown gall disease in tomato. Most of published studies have considered either the direct in vitro antimicrobial role of seaweed extracts or their activity in planta, but rarely both of them. In this work, seaweeds extracts were tested for in vitro antimicrobial activities and for their ability to protect tomato seedlings in the greenhouse. Analyses of the activities of two defense-related enzymes were carried out to determine whether these extracts are able to stimulate tomato resistance mechanisms.
Materials and methods
Plant, seaweed species, and extraction procedures
Tomato plants (Solanum lycopersicum) var. ‘Pomodoro’ was used for tumorigenicity and Verticillium wilt biocontrol assays in the greenhouse. Tomato seeds were disinfected with 5% commercial bleach for 3 min and rinsed three times and with sterile distilled water (DW). They were divided into batches of 100 seeds. One batch of seeds was placed in petri dishes containing paper filter soaked with 5 mL water and was considered as the control. The remainders of the batches were treated with 5 mL of 0.5 or 1.5% of aqueous seaweed extract for 24 h. Subsequently, all batches were watered daily with 5 mL of water for 6 days. On the seventh day, the percentage of germination was recorded, and the index of germination (GI) was calculated as the ratio of germinated seeds on root length according to Zucconi et al. (1981). Seedlings were transferred to pots containing a sterile mixture of peat and sand (3:1 v/v) and were grown in a greenhouse with a 12-h photoperiod at 26 °C and 70% relative humidity.
Fucus spiralis, Cystoseira myriophylloides, and Laminaria digitata were sampled at low tide, at Sidi Bouzid, located on the Moroccan Atlantic coast south of the city of El Jadida (Lat 32° 15′ to 33° 15′; Long 7° 55′ to 9° 15′). The collected algae were washed thoroughly and packaged in polyethylene bags. They were air dried and then oven dried at 65 °C for 24 h. Dried seaweeds were ground and sieved into a fine powder.
Preparation of aqueous seaweed extracts (ASE) was adapted from the protocol described by Kumari et al. (2011). Six hundred milliliter of distilled water (DW) were added to 60 g algal powder, heated for 45 min at 60 °C and allowed to stand for 48 h at room temperature. The resulting solution was prefiltered and centrifuged at 4000 rpm for 30 min. The supernatant was used as 10% (w/v) from which four concentrations, 0.5, 1, 1.5, and 3% (v/v), were prepared using DW. ASEs were stored at −20 °C before use.
Methanolic seaweed extracts (MSE) were prepared by adding 6 g of algal powder in 30 mL methanol. The resulting mixture was then continuous stirring in dim light for 48 h at room temperature, followed by filtration and evaporation under reduced pressure with a rotary evaporator. The three extracts were kept in a dry place before use.
Bacterial, fungal material, and effect of seaweed extracts on their in vitro growth
Three isolates of Verticillium dahliae isolated previously from olive trees in Morocco were used (Cherrab et al. 2002). The fungi were cultured on potato dextrose agar (PDA) plates at 27 °C in the dark.
ASE were added at concentrations of 0, 0.5, and 1.5% to the PDA medium before being autoclaved and cooled. MSE were added at concentrations of 50, 100, and 500 μg mL−1 to the PDA medium. The amended media was poured into Petri dishes and inoculated using 5-mm plugs of agar and mycelium taken from actively growing cultures of fungus on PDA. Petri plates were incubated in the dark at 27 °C. Mycelial growth was assessed by measuring two orthogonal diameters of each colony after 5 days of incubation. Mycelial growth was compared with growth on non-amended PDA and the percentage of growth inhibition relative to the control was calculated.
Three oncogenic strains (Ach5, A281, and C58) and one non virulent strain (EHA105) of Agrobacterium tumefaciens were used. Bacteria were grown overnight at 28 °C in LB medium, re-suspended in water, and adjusted to a titer of 3 × 108 CFU mL−1.
The antibacterial activity of ASE (1.5%) or MSE (100 μg mL−1) was evaluated using cellulose disks of 6-mm diameter soaked with 100 μL of extracts. Disks were placed on LB solid medium previously amended with bacteria. After 24-h incubation at 28 °C, the antibacterial activity is measured as the diameter of zone inhibition appearing around the disk and compared to control disks soaked with 100 μL of water or methanol.
Plant treatment, inoculation, and diseases assessments
DW or ASE, diluted to 0.5 and 1.5%, were applied to run off the aerial part of 4-week-old tomato seedlings two times (7 and 3 days before Verticillium inoculation). Plants were subsequently inoculated by dipping their roots for 10 min in a spore suspension of the strain SH of V. dahliae previously adjusted to 107 conidia mL−1. Controls roots were dipped in sterile DW.
In another set of experiments, disinfected tomato seeds were soaked in water or in ASE (diluted to 0.5 and 1.5%), allowed to germinate and inoculated 1 month later, as described above.
The quantitative and qualitative evaluation of the Verticillium wilt disease was by assessing vegetative parameters such as foliar alteration index, stunting index, and browning index at 21 days after inoculation as previously described (Zine et al. 2016). Reproductive parameters, such as the number of flowers, were recorded at 21 days after inoculation, and the number of fruits and their fresh weight were recorded on day 60.
The experimental design for V. dahliae inoculation was a randomized block design with three blocks. The experimental units were eight plants. Experiments were repeated three times and results from one representative experiment are given.
For tumorigenic assays, plants were sprayed with ASE (diluted to 0.5, 1, 1.5, and 3%) and inoculated 2 or 4 days later with the oncogenic strain C58 of A. tumefaciens as described in Faize et al. (2012). Bacteria was grown overnight at 28 °C in LB medium to a titer of 3 × 108 CFU mL−1, then pelleted and re-suspended in 5 mM sodium citrate pH 5.5 supplemented with 2% sucrose and 0.1 mM acetosyringone. Stems were wounded using a scalpel (1 cm length) and filled with 10 μL of bacterial suspension. Inoculations were performed at four sites in the same stem. Inoculated plants were kept in the greenhouse and scored for tumors every week over 4 weeks. The diameter of lesions was recorded 4 weeks after inoculation.
The experimental design for A. tumefaciens inoculation experiments was a randomized block design with three blocks. The experimental units were four plants. Experiments were repeated three times, and results from one representative experiment are given.
Enzymes and biochemical assays
Four-week-old tomato seedlings were sprayed with DW or 0.5–1.5% ASE, two times, at 7 and 3 days before Verticillium inoculation. Apical leaves were harvested from three plants at 0, 1, 2, 4, 7, 9, 11, and 15 days after the first treatment and were used for H2O2 and for enzymatic activities determination.
Enzymatic activities were also recorded from tomato seedlings derived from seeds soaked in water or in ASE. Apical leaves were harvested from three plants at 21, 30, 32, 35, and 38 days after soaking and used for enzymes extractions.
Four hundred milligram of tissue leaves were ground in an ice bath with 3 mL of 50 mM phosphate buffer, pH 7.5, containing 0.01% (v/v) Triton X-100, 1 mM polyethylene glycol, and 8% polyvinylpyrrolidone phosphate (PVPP). The homogenate was centrifuged at 16,000×g for 20 min at 4 °C, and the supernatant was assayed immediately for enzymatic activities. The total protein concentration was determined using bovine serum albumin (BSA) as a standard, according to Bradford (1976).
Peroxidase (POX, EC 1.11.1.7) activity was determined as described by Moerschbacher et al. (1986). Changes in absorbance at 470 nm were recorded for 2 min, and enzyme activity was expressed as μmol per minute per milligram of protein (ε470 = 26.6 mM−1 cm−1).
Polyphenol oxidase (PPO, EC 1.10.3.1) activity was measured as described by Masia et al. (1998). Changes in absorbance were followed for 3 min at 410 nm. The activity of PPO was expressed as ΔOD per minute per milligram of protein.
H2O2 was measured according to Alexieva et al. (2001). Five hundred milligrams of leaf tissues were homogenized with 5 mL of 0.1% trichloroacetic acid. The homogenate was centrifuged at 16,000×g during 20 min and 250 μL of the supernatant was added to 500 μL of 100 mM potassium phosphate buffer, pH 7.0 and 1 mL of 1 M KI. The reaction was incubated for 1 h and the absorbance measured at 390 nm. H2O2 content was determined using a standard curve with known concentrations of commercial H2O2.
All of these experiments were repeated twice, and results from one representative experiment are given.
Statistical analyses
For determination of parameters of disease severity, enzymatic activities, and H2O2 quantitation, the statistical design was the randomized complete blocks. The effects of seaweed extracts on these parameters as well as on seed germination were tested using ANOVA. Means from plants or seeds pre-treated with seaweeds were compared to the control pre-treated with DW by a Dunnett’s test (P < 0.05).
Results
Effect of seaweed extracts on tomato seeds germination
We tested the effect of ASE on germination parameters such as root length, germination rates, and germination index (Table 1). No noticeable differences were observed for germination rates between the control and ASE from the three algae when used at 0.5 or at 1.5%. However, root length was enhanced by all treatments by approximately 2.5-fold. Subsequently, germination index was highly enhanced by all the treatments, whatever the ASE and concentration used.
In vitro antifungal activity of the seaweed extracts
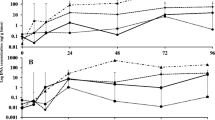
Mycelial growth inhibition was determined at 5 days after culture of three strains of V. dahliae at 27 °C in PDA medium amended with different concentrations of the MSE (Fig. 1). A slight inhibition of mycelial growth was recorded with F. spiralis and C. myriophylloides extracts and only at the highest concentrations. At 500 μg L−1, the maximum inhibition did not reach 50%. No remarkable inhibition was observed with MSE from L. digitata whatever the concentration and strain combinations tested.
Effect of methanolic seaweed extracts (MSE) from a Fucus spiralis (FS), b Laminaria digitata (LD), or from c Cystoseira myriophlloides (CM) at 50, 100, or 500 μg mL−1 on the in vitro mycelial growth of the strains SE, SH, and SJ of Verticillium dahliae. Data are means and confidence intervals (95%) from three replicates
Mycelial growth inhibition was also determined for V. dahliae using ASE at 0.5 and 1.5% (Fig. 2). ASE from F. spiralis did not exhibit any notable effect against the three strains used. However, extracts from L. digitata and C. myriophylloides stimulated the growth of strain SE. This stimulation increased with increasing seaweed extract concentration.
Effect of aqueous seaweed extracts (ASE) from a Fucus spiralis (FS), b Laminaria digitata (LD), or from c Cystoseira myriophlloides (CM) at 0.5 or 1.5% on the in vitro mycelial growth of the strains SE, SH, and SJ of Verticillium dahliae. Data are means and confidence intervals (95%) from three replicates
In vitro antibacterial activity of the seaweed extracts
Bacterial growth inhibition was studied using methanolic or aqueous extracts (Fig. 3). MSE from L. digitata and C. myriophylloides inhibited the growth of the four stains used, when compared to the control. However, no inhibition was recorded from MSE of F. spiralis.
Effect of methanolic seaweed extracts (MSE) at 100 μg mL−1 from a Fucus spiralis (FS), b Laminaria digitata (LD), or from c Cystoseira myriophlloides (CM) on the in vitro growth of the strains C58, A281, Ach5, and EHA105 of Agrobacterium tumefaciens. Methanol was used as negative control. Data are means and confidence intervals (95%) from three replicates
ASE were also tested at 1.5% for the inhibition of A. tumefaciens growth. No inhibition was observed whatever the concentration, the alga, or the bacterial strain used (data not shown).
Protective effects of ASE on Verticillium wilt disease
ASE were evaluated for their ability to protect tomato seedlings against V. dahliae in the greenhouse. Two methods were used: spray treatment and seed soaking. Disease assessment was carried out based on leaf alteration index, stunting index, and browning index of the vessels. Reproductive parameters, such as the number of flowers, the number of fruits, and their fresh weight, were also recorded.
The suppressive effect of ASE was first studied on plants sprayed twice with AES at 0.5 and 1.5% at 7 and 4 days before Verticillium inoculation (Table 2).
The index of foliar alteration reached 0.4 at 21 days after inoculation in the control. This index was significantly reduced in plants pre-treated with ASE, giving rise to a percentage of protection ranging from 41 to 46%.
The stunting index averaged a value of 0.4 at 21 days after inoculation. In plants pre-treated with ASE, however, stunting index was significantly much lower and varied from 0.15 in plants treated with 0.5% of ASE from C. myriophylloides to 0.33 in those treated with 1.5% of L. digitata.
The browning index of vessels caused by V. dahliae was determined at 21 days after inoculation. In plants pre-treated with DW, browning index averaged 0.72 while in ASE this was significantly lower, resulting in percentage of protection of about 96%.
The average number of flowers was significantly higher in inoculated plants previously treated with 0.5% ASE from C. myriophylloides or with 0.5–1% of ASE from F. spiralis, than in the control.
The average number of fruits was also significantly elevated in plants pre-treated with 0.5% of ASE from F. spiralis and C. myriophylloides. Consequently, the fresh weight of fruits was significantly higher in plants pre-treated with these two extracts. With 0.5% ASE from C. myriophylloides, the yield was increased by 2.5-fold the control. An illustration of the enhanced yield by C. myriophylloides extracts is shown in Supplementary Fig. 1S.
The suppressive effect of ASE was also studied on seedlings derived from seeds soaked directly with the extracts (Table 3). Thirty days after germination, plants were inoculated with V. dahliae.
The index of foliar alteration in plants derived from seeds that germinated in DW (control) reached a value of 0.6 at 21 days post inoculation (dpi). This index was drastically reduced in plants derived from germinated seeds pre-treated with ASE. It hardly exceeded 0.2 leading to percentage of protection of around 67%.
The stunting index reached 0.5 in the control while in plants derived from seed soaked with ASE, this index was significantly reduced. It reached in the best cases 0.1 with 5% of F. spiralis, where the percentage of protection averaged 80%.
The browning index of vessels caused by V. dahliae was close to 0.6 in the control, whereas in plants derived from ASE-treated seeds, this was much lower, giving rise to a percentage of protection ranging from 87 to 95%.
The average number of flowers was significantly higher in inoculated plants derived from seeds pre-soaked in 0.5% F. spiralis or with 0.5–1% C. myriophylloides, than in the control.
An illustration of the protective effect provided by C. myriophylloides is shown in Supplementary Fig. 2S.
Protective effect of ASE on crown gall disease
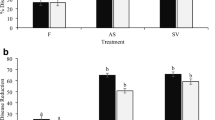
ASEs were evaluated for their ability to protect tomato seedlings against the oncogenic strain C58 of A. tumefaciens in the greenhouse. Plants were sprayed with ASE and then inoculated 2 or 4 days later. The diameter of tumor was recorded 4 weeks after inoculation (Fig. 4).
Effect of aqueous seaweed extracts (ASE) from Fucus spiralis (FS), Laminaria digitata (LD), or from Cystoseira myriophlloides (CM) at 0.5, 1, 1.5, or 3% on the development of tumors induced by the strain C58 of Agrobacterium tumefaciens at 4 weeks after inoculation. Tomato plants were sprayed with ASE a 2 days or b 4 days before inoculation at concentration of 3 × 105 CFU mL−1. Data are means and confidence intervals (95%) of 12 replicates. Asterisks denote significant difference from the control (P < 0.05), according to Dunnett’s test
When plants were elicited 2 days before inoculation, only seaweed extracts from F. spiralis and C. myriophylloides were able to reduce the diameter of lesion. The highest reduction of disease was obtained when 3% C. myriophylloides was applied 2 days (Fig. 4a) or 4 days (Fig. 4b) before inoculation, giving rise to protection percentages of 48 and 57%, respectively. This ranged from 33 to 35% with 0.5% F. spiralis extracts. No significant disease reduction was observed with L. digitata extracts whatever the concentration or the time before pathogen inoculation. An illustration of the induced protection is shown in Supplementary Fig. 3S.
Induction of POX and PPO activities by ASE
We examined if ASE were able to induce plant defenses by analyzing kinetics of activation of two enzymes, POX and PPO, after inoculation with V. dahliae.
Time course analysis of POX activity was first studied on plants sprayed twice with 0.5 and 1.5% AES at 7 and 3 days before Verticillium inoculation (Fig. 5). In non-inoculated plants, a transient and significant increase of POX activity was observed at 1 day after the first spray treatment with 0.5% F. spiralis (Fig. 5a). The second spray treatment performed day with 0.5 and 1.5% F. spiralis at the fourth day significantly stimulated POX activity. This activity decreased from the seventh day. In inoculated plants, V. dahliae enhanced significantly POX activity at the 11th day after the first treatment (4 dpi) with DW. These activities were highly stimulated in plants pre-treated with 0.5 or 1.5% F. spiralis 2, 4, and 8 days after inoculation.
Time course analysis of peroxidase in tomato seedlings after spray treatment of aqueous seaweed extracts (ASE) from a Fucus spiralis (FS), b Laminaria digitata (LD), or from c Cystoseira myriophlloides (CM) at 0.5 or 1.5 %. Four-week-old seedlings were pre-treated twice with ASE or DW (control) at 7 and 3 days before inoculation. Plants were inoculated by dipping their roots during 10 min in a spore suspension of the strain SH of Verticillium dahliae previously adjusted to 107 conidia mL−1. Controls roots were dipped in sterile DW (mock inoculated). Data are means and confidence intervals (95%) from three replicates. Asterisks denote significant difference from the control plants pre-treated with DW and mock inoculated (P < 0.05), according to Dunnett’s test
In non-inoculated plants sprayed with L. digitata, a significant increase of POX activity was observed only with 0.5% at 1 day after treatment (Fig. 5b). However, inoculation of V. dahliae highly enhanced these activities in tomato pre-treated with 0.5 or 1.5% L. digitata.
With C. myriophylloides extracts, a significant POX activity was obtained at the seventh day after the first treatment only with the lowest concentration (Fig. 5c). Similarly to what was observed with other extracts, POX activity was highly enhanced in inoculated plants. These results suggest that ASE primed plants for enhanced POX activity.
Time course analysis of POX activity was also determined in plants derived from seeds that germinated in 0.5 or 1.5% of ASE and inoculated 1 month later (Fig. 6). In non-inoculated plants, derived from seeds treated with 0.5 or 1.5% F. spiralis, POX activity was significantly higher than in the control (plants that germinated in DW) at 21, 30, 32, and 35 days after seed soaking (Fig. 6a). In inoculated plants, POX activity was significantly enhanced by the pathogen in DW at 2 dpi (32 day after seed treatment) and it reached the same level in plants derived from 0.5 and 1.5% F. spiralis whether inoculated or not. This activity decreased at 5 dpi (35 day after seed treatment), in inoculated control and in plants derived from 1.5% F. spiralis, while in plants derived from 0.5% F. spiralis it remained significantly higher. At 8 dpi (38 day after seed treatment), no significant differences were observed between treatments except for inoculated plants pre-treated with 0.5 % F. spiralis, in which POX remained significantly higher.
Time course analysis of peroxidase in tomato seedlings derived from seeds soaked in aqueous seaweed extracts (ASE) from a Fucus spiralis (FS), b Laminaria digitata (LD), or from c Cystoseira myriophlloides (CM) at 0.5 or 1.5%. Seedlings derived from seeds soaked with the extracts or DW were inoculated 30 days after germination by dipping their roots during 10 min in a spore suspension of the strain SH of Verticillium dahliae previously adjusted to 107 conidia mL−1. Controls roots were dipped in sterile DW (mock inoculated). Data are means and confidence intervals (95%) from three replicates. Asterisks denote significant difference from the control pre-treated with DW and mock inoculated (P < 0.05), according to Dunnett’s test
In non-inoculated plants derived from seeds pre-treated with L. digitata, POX activity was significantly higher than in the control at 21, 30, 32, and 35 days after seed treatment. In inoculated plants, activities higher than in the inoculated control were recorded at 2, 5, and 8 dpi, whatever the concentration used (Fig. 6b).
In mock-inoculated plants, significantly higher POX activities were also recorded from plants derived from C. myriophylloides at 5 and 8 dpi (Fig. 6c). However, highest POX activities were recorded from inoculated plants pre-treated with C. myriophylloides at 2 dpi, 5 dpi, and 8 dpi at both concentrations.
PPO activity was used as a second plant defense response marker. It was first studied in plants sprayed twice with AES at 0.5 and 1.5% at 7 and 3 days before Verticillium inoculation (Fig. 7).
Time course analysis of polyphenol oxidase in tomato seedlings after spray treatment of aqueous seaweed extracts (ASE) from a Fucus spiralis (FS), b Laminaria digitata (LD), or from c Cystoseira myriophlloides (CM) at 0.5 or 1.5%. Four-week-old seedlings were pre-treated twice with ASE or DW (control) at 7 and 3 days before inoculation. Plants were inoculated by dipping their roots during 10 min in a spore suspension of the strain SH of Verticillium dahliae previously adjusted to 107 conidia mL−1. Controls roots were dipped in sterile DW (mock inoculated). Data are means and confidence intervals (95%) from three replicates. Asterisks denote significant difference from the control plants pre-treated with DW and mock inoculated (P < 0.05), according to Dunnett’s test
In non-inoculated plants, PPO activity was significantly enhanced at 1 day after the first spray of 0.5% F. spiralis (Fig. 7a). This activity was 3.5-times higher than in the control, but it decreased at the second day. The second spray treatment with 0.5 and 1.5% F. spiralis at the fourth day enhanced significantly PPO activity, which decreased just after. In inoculated plants, V. dahliae induced a slight but significant increase of PPO activity in plants pre-treated with DW when compared to the non-inoculated control at 4 dpi. These activities were highly enhanced in plants pre-treated with 0.5 and 1.5% F. spiralis at 4 and 8 dpi.
Although elevated PPO activities were recorded from non-inoculated plants sprayed with L. digitata, they were significant only at 9, 11, and 15 days after treatment (Fig. 7b). These activities were enhanced further more after inoculation of V. dahliae.
With C. myriophylloides extracts, PPO activities were higher, but significant differences with DW were observed at the fourth day after treatment and with 0.5% (Fig. 7c). After inoculation, PPO was enhanced more at 11 and 15 days after treatment (4 and 8 dpi).
Time course analysis of PPO activity was also determined in plants derived from seeds that germinated in ASE (Fig. 8). Higher PPO activities were recorded from non-inoculated plants pre-treated with 0.5 and 1.5% at 21, 30, 35, and 38 days after soaking (Fig. 8a). In inoculated plants, the pathogen caused an increase in PPO activity in the control at 5 dpi. However, highest and significant activities were observed in plants pre-treated with 1.5% of F. spiralis at the same day. At 8 dpi, significant higher differences were observed with F. spiralis at both concentrations.
Time course analysis of polyphenol oxidase in tomato seedlings derived from seeds soaked in aqueous seaweed extracts (ASE) from a Fucus spiralis (FS), b Laminaria digitata (LD), or from c Cystoseira myriophlloides (CM) at 0.5 or 1.5%. Seedlings derived from seeds soaked with the extracts or DW were inoculated 30 days after germination by dipping their roots during 10 min in a spore suspension of the strain SH of Verticillium dahliae previously adjusted to 107 conidia mL−1. Controls roots were dipped in sterile DW (mock inoculated). Data are means and confidence intervals (95%) from three replicates. Asterisks denote significant difference from the control pre-treated with DW and mock inoculated (P < 0.05), according to Dunnett’s test
In non-inoculated plants pre-treated with L. digitata, PPO activity was higher than in the control whatever the concentration and during the whole duration of the experiment. In inoculated plants, significantly higher activities were obtained with 1.5% L. digitata at 2 dpi, and 0.5% L. digitata at 5 dpi and with both concentrations at 8 dpi (Fig. 8b).
PPO activity was significantly elevated in either non-inoculated or inoculated plants pre-treated with 0.5 or 1.5% C. myriophylloides extracts at 2 and 5 dpi (Fig. 8c).
Effect of ASE on H2O2 content
The effect of ASE on H2O2 accumulation is shown in Supplementary Fig. 4S. In non-inoculated plants, a significantly transient increase was observed 2 days after the first spray application of 0.5% F. spiralis. After a second treatment with 0.5 or 1.5% F. spiralis, another significant increase was observed at 7, 11, and 15 days. Verticillium dahliae enhanced slightly H2O2 content in the control at 11 and 15 days (4 and 8 dpi). However, significantly higher content was recorded from inoculated plants pre-treated with 0.5 or 1.5% ASE (Fig. 4S A).
In non-inoculated plants, significant increase in H2O2 content was observed at the end of the experiment after treatment with 1.5% L. digitata. Verticillium dahliae inoculation enhanced its accumulation in plants pre-treated with 0.5% L. digitata at 9 days and in plants pre-treated with the two concentrations at the 15th day (Fig. 4S B).
In non-inoculated plants, a significant increase was recorded at 15 days after treatment with 0.5 or 1.5% C. myriophylloides. However, in inoculated plants, increases were earliest as they started at 9 day after treatment with 0.5% C. myriophylloides (Fig. 4S C).
Discussion
With the exception of the study of Jiménez et al. (2011), the activity of algal extracts on pathogens was carried out only in vitro or in vivo. In the present study, we showed the effectiveness of aqueous seaweed treatment from C. myriophylloides and F. spiralis in the reduction of Verticillium wilt and crown gall diseases. To our knowledge, this is the first published report of natural seaweed extract directly suppressing these diseases on tomato. This effect seems to be independent from the direct antimicrobial activity as the ASE used for greenhouse protection assays did not inhibit the growth of V. dahliae and A. tumefaciens. Direct inhibition of pathogen growth was observed for A. tumefaciens but only using methanolic extracts (MSE) while for V. dahliae, they did not exhibit significant inhibition against any of the three strains used. This result is not surprising since methanol was found to be more effective for the extraction of active compounds from seaweeds than other solvents (Kumar et al. 2008; Varahalarao and Chandrasekhar 2009; Cox et al. 2010; Alghazeer et al. 2013). Commonly, the brown seaweeds are well known for their higher concentrations of phenolic compounds mainly phlorotannin, consisting of phloroglucinol, eckol, and dieckol that possess antimicrobial activities (Suleria et al. 2015). In addition, terpenes and phenolic lipids readily extractable using methanol may contribute to the observed activity as reported with Kappaphycus alvarezii extracts (Prabha et al. 2013).
The in vivo activity of ASE against Verticillium was assessed on seedlings derived from seeds previously soaked in AES or on plants sprayed twice with ASE before challenge inoculation. Both application methods provided significant protection for tomato against V. dahliae and superior reproductive performances were observed in plants pre-treated with ASE from F. spiralis and C. myriophylloides, under high disease pressure. However, seed soaking was more effective than foliar spray since the percentage of protection increased from 46 to 67% for leaf alteration and from 63 to 80% for stunting index. Seed treatment with various elicitors such as chitosan, acibenzolar-S-methyl, jasmonate, and BABA has been reported to be a suitable method for crop protection against several phytopathogens (Buzi et al. 2004; Orzali et al. 2014; Paudel et al. 2014). This method can be particularly useful, since it can provide protection to young plants during vulnerable stages of their development. In addition, protection afforded by such elicitors was long-lasting, with enhanced resistance sustained for 8 weeks (Worrall et al. 2012).
Seaweed extracts are considered as biostimulants, and their positive effects occur independently of their nutrient content (Calvo et al. 2014). Biostimulation has been often attributed to the presence of plant growth hormones and related low molecular weight compounds in the extracts. At concentrations used, ASE did not show any phytotoxicity as neither seed germination nor plant growth was negatively affected. Furthermore, root lengths and germination index were even enhanced by ASE. This effect may be explained by the presence of growth promoting compounds such as indole acetic acid, gibberillins, and cytokinins (Reitz and Trumble 1996). Cytokinin may allow plants to increase their resistance to the disease. Elevated cytokinin levels have been shown to enhance plant defense against pathogens (Naseem et al. 2014). In addition, the three brown seaweeds used here are known for their richness in water-soluble polysaccharides such as laminarin (Stadnik and de Freitas 2014) and sulfated fucans (Li et al. 2008). They act as elicitors of systemic resistance in plants (Klarzynski et al. 2000; Mercier et al. 2001). In this study, we have shown that ASEs induced the activity of POX and PPO, two enzymes that catalyze the formation of lignin and other phenolic compounds involved in the establishment of physical barriers for reinforcing the cell structure against pathogens (Hiraga et al. 2001; Mayer 2006). Interestingly, ASEs were able to prime tomato plants for enhanced POX activity upon inoculation with V. dahlia. Priming is a phenomenon that switches plants into an alarmed state of defense, allowing them to upgrade their defenses responses (Conrath et al. 2015). Enhancement of the activity of POX, PPO, and several other enzymes involved in plant defenses by seaweed extracts have been well described (Raghavendra et al. 2007; Hernández-Herrera et al. 2014; Abkhoo and Sabbagh 2016). However, to our knowledge, this is the first report of priming effect after spray treatment with seaweed extracts. H2O2 plays a prominent role in plant defense as it is involved in the oxidation of phenolic compounds catalyzed by POX and PPO activities. In addition, it is involved in resistance to pathogens by its direct antimicrobial effect and also in the cellular transduction of signal, which leads to the activation of the expression involved in plant defense production (Lamb and Dixon 1997). Our results showed that tomato plants responded to seaweed extracts by enhancing transiently their H2O2 content, and this was reactivated after pathogen infection. This result is not surprising since several studies have reported the generation of ROS and the antioxidant defenses by algal extracts (Zhang et al. 2003; Jayaraj et al. 2008) and by their derived-elicitors (Aziz et al. 2003; Paulert et al. 2010; Abouraïcha et al. 2016).
In the context of growing public concern to minimize the use of chemical fungicides in agricultural production, the use of eco-friendly approaches is more attractive. Since tomato grown in the greenhouse is a crop of great commercial interest and giving the fact that aqueous seaweed extracts trigger plant defenses and enhance resistance against multiple pathogens, the possibility of using natural seaweed extracts as an alternative strategy for tomato growers in Morocco could be of economic interest. Work is in progress to deepen our understanding on molecular mechanisms involved in plant disease resistance using seaweed extracts as elicitors. Other studies are being conducted to evaluate their effectiveness on other tomato diseases.
References
Abkhoo J, Sabbagh SK (2016) Control of Phytophthora melonis damping-off, induction of defense responses, and gene expression of cucumber treated with commercial extract from Ascophyllum nodosum. J Appl Phycol 28:1333–1342
Abouraïcha E, El Alaoui-Talibi Z, Tadlaoui-Ouafi A, El Boutachfaiti R, Petit E, Douira A, Courtois B, Courtois J, El Modafar C (2016) Glucuronan and oligoglucuronans isolated from green algae activate natural defense responses in apple fruit and reduce postharvest blue and gray mold decay. J Appl Phycol DOI. doi:10.1007/s10811-016-0926-0
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Alghazeer R, Whida F, Abduelrhman E, Gammoudi F, Azwai S (2013) Screening of antibacterial activity in marine green, red and brown macroalgae from the western coast of Libya. Natural Sci 5:7–14
Ara J, Sultana V, Qasim R, Etheshamul-Haque S, Vigar Uddin A (2005) Biological activity of Spatoglossum asperum: a brown alga. Phytother Res 19:618–623
Arunkumar K, Selvapalam N, Rengasamy R (2005) The antibacterial compound sulphoglycerolipid palmitoyl-3-0(6′-sulpho-αquinovopyranosyl)-glycerol from Sargassum wightii Greville (Phaeophyceae). Bot Mar 40:441–445
Aziz A, Poinssot B, Daire X, Adrian M, Bézier A, Lambert B, Joubert JM, Pugin A (2003) Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol Plant Microbe Int 16:1118–1128
Benhissoune S, Boudoiuresque C-F, Verlaque M (2002) A checklist of the seaweeds of the Mediterranean and Atlantic coasts of Morocco. II. Phaeophyceae. Bot Mar 45:217–230
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buzi A, Chilosi G, De Sillo D, Magro P (2004) Induction of resistance in melon to Didymella bryoniae and Sclerotinia sclerotiorum by seed treatments with acibenzolar-S-methyl and jasmonate but not with salicylic acid. J Phytopathol 152:34–42
Calvo P, Nelson L, Kloepper JW (2014) Agricultural uses of plant biostimulants. Plant Soil 383:3–41
Chanthini K, Sreenath Kumar C, Jayasurya Kingsley S (2012) Antifungal activity of seaweed extracts against phytopathogen Alternaria solani. J Acad Indust Res 1:86–90
Chase AR (1989) Aliette 80WP and bacterial disease control-III. Phytotoxicity. Foliage Digest 12:4–5
Cherrab M, Bennani A, Charest PM, Serrhini MN (2002) Pathogenecity and vegetative compatibility of Verticillium dahlia Kleb. Isolates from olives in Morocco. J Phytopathol 150:703–709
Conrath U, Beckers GJM, Langenbach CJG, Jaskiewicz MR (2015) Priming for enhanced defense. Annu Rev Phytopathol 53:97–119
Cox S, Abu-Ghannam N, Gupta S (2010) An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seawees. Int Food Res J 17:205–220
Escobar M, Dandekar MA (2003) Agrobacterium tumefaciens as an agent of disease. Trends Plant Sci 8:380–386
Faize M, Burgos L, Faize L, Petri C, Barba-Espin G, Díaz-Vivancos P, Clemente-Moreno MJ, Alburquerque HJA (2012) Modulation of tobacco bacterial disease resistance using cytosolic ascorbate peroxidase and Cu,Zn-superoxide dismutase. Plant Pathol 5:858–866
Farrand SK (1990) Agrobacterium radiobacter K84: a model biocontrol system. In: Baker RR, Dunn PE (eds) New directions in biological control: alternatives for suppressing agricultural pests and diseases. Wiley-Liss, New York, pp. 679–691
Fradin EF, Thomma BPHJ (2006) Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahlia and V. albo-atrum. Mol Plant Pathol 7:71–86
Hernández-Herrera RM, Virgen-Calleros G, Ruiz-Lopez M, Zañudo-Hernández J, Delano-Frier JP, Sanchez-Hernández C (2014) Extracts from green and brown seaweeds protect tomato (Solanum lycopersicum) against necrotrophic fungus Alternaria solani. J Appl Phycol 26:1607–1614
Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H (2001) A large family of class III plant peroxidases. Plant Cell Physiol 42:462–468
Indira K, Balakrishnan S, Srinivasan M, Bragadeeswaran S, Balasubramanian T (2013) Evaluation of in vitro antimicrobial property of seaweed (Halimeda tuna) from Tuticorin coast, Tamil Nadu, southeast coast of India. Afr J Biotechnol 12:284–289
Jayaraj J, Wan A, Rahman M, Punja ZK (2008) Seaweed extract reduces foliar fungal diseases on carrot. Crop Prot 27:1360–1366
Jayaraj J, Norrie J, Punja ZK (2011) Commercial extract from the brown seaweed Ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. J Appl Phycol 23:353–361
Jiménez E, Dorta F, Medina C, Ramirez A, Ramirez I, Peña-Cortés H (2011) Antiphytopathogenic activities of macro-algae extracts. Mar Drugs 9:739–756
Klarzynski O, Plesse B, Joubert JM, Yvin JC, Kopp M, Kloareg B, Fritig B (2000) Linear beta-1,3 glucans are elicitors of defense responses in tobacco. Plant Physiol 124:1027–1037
Klosterman SJ, Atallah ZK, Vallad GE, Subbarao KV (2009) Diversity, pathogenicity, and management of Verticillium species. Annu Rev Phytopathol 47:39–62
Kumar CS, Sarada DVL, Rengasamy R (2008) Seaweed extracts control the leaf spot disease of the medicinal plant Gymnema sylvestre. Indian J Sci Technol 3:1–5
Kumari R, Kaur I, Bhatnagar AK (2011) Effect of aqueous extract of Sargassum johnstonii Setchell & Gardner on growth, yield and quality of Lycopersicon esculantum Mill. J Appl Phycol 23:623–633
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48:251–275
Li B, Lu F, Wei X, Zhao R (2008) Fucoidan: structure and bioactivity. Molecules 13:1671–1695
Masia A, Ventura M, Gemma H, Sansavini S (1998) Effect of some plant growth regulator treatments on apple fruit ripening. Plant Growth Regul 25:127–134
Mattner SW, Wite D, Riches DA, Porter IJ, Arioli T (2013) The effect of kelp extract on seedling establishment of broccoli on contrasting soil types in southern Victoria, Australia. Biol Agric Hortic 29:258–270
Mattner SW, Villalta ON, Wite D, Porter IJ, Arioli T (2014) In vitro suppression of Sclerotinia minor by a seaweed extract from Durvillaea potatorum and Ascophyllum nodosum. Aust Plant Dis Notes 9:137
Mayer A (2006) Polyphenol oxidases in plants and fungi: going places? Phytochemistry 67:2318–2331
Mercier L, Lafitte C, Borderies G, Briand X, Esquerre-Tugaye MT, Fournier J (2001) The algal polysaccharide carrageenans can act as an elicitor of plant defence. New Phytol 149:43–51
Moerschbacher B, Heck B, Kogel KH, Obst O, Reisener H (1986) An elicitor of the hypersensitive lignification response in wheat leaves isolated from the rust fungus Puccinia graminis f. sp. tritici. II. Induction of enzymes correlated with the biosynthesis of lignin. Z Naturforsch 41c:839–844
Naseem M, Kunz M, Dandekar T (2014) Probing the unknowns in cytokinin-mediated immune defense in Arabidopsis with systems biology approaches. Bioinform Biol Insights 8:35–44
Orzali L, Forni C, Riccion L (2014) Effect of chitosan seed treatment as elicitor of resistance to Fusarium graminearum in wheat. Seed Sci Technol 42:132–149
Paudel S, Rajotte EG, Felton GW (2014) Benefits and costs of tomato seed treatment with plant defense elicitor for insect resistance. Arthropod Plant Interact 8:539–545
Paulert R, Ebbinghaus D, Urlass C, Moerschbacher B (2010) Priming of the oxidative burst in rice and wheat cell cultures by ulvan, a polysaccharide from green macroalgae, and enhanced resistance against powdery mildew in wheat and barley plants. Plant Pathol 59:634–642
Prabha V, Prakash DJ, Sudha PN (2013) Analysis of bioactive compounds and antimicrobial activity of marine algae Kappaphycus alvarezii using three solvent extracts. IJPSR 4:306–310
Raghavendra VB, Lokesh S, Prakash HS (2007) Dravya, a product of seaweed extract (Sargassum wightii), induces resistance in cotton against Xanthomonas campestris pv. malvacearum. Phytoparasitica 35:442–449
Reitz SR, Trumble SR (1996) Effects of cytokinins containing seaweed extract on Phaseolus lunatus L.: influence on nutrient availability and apex removal. Bot Mar 39:33–38
Rekanovic E, Milijasevic S, Todorovic B, Potocnik I (2007) Possibilities of biological and chemical control of Verticillium wilt in pepper. Phytoparasitica 35:436–441
Ryder MH, Jones DA (1991) Biological control of crown gall using Agrobacterium strains K84 and K1026. Aust J Plant Physiol 18:571–579
Sigee DC (1993) Bacterial plant pathology: cell and molecular aspects. Cambridge University Press, Cambridge
Stadnik MJ, de Freitas MB (2014) Algal polysaccharides as source of plant resistance inducers. Trop Plant Pathol 39:111–118
Suleria HAR, Osborne S, Masci P, Gobe G (2015) Marine-based nutraceuticals: an innovative trend in the food and supplement industries. Mar Drugs 13:6336–6351
Talboys PW (1984) Chemical control of Verticillium wilts. Phytopathol Mediterr 23:163–175
Tian L, Wang KR, Lu JY (1998) Effect of carbendazim and tricyclazole on microsclerotia and melanin formation of Verticillium dahliae. Acta Phytopathologica Sinica 28:263–268
Varahalarao V, Chandrasekhar NK (2009) In vitro antimicrobial potentiality of some marine algae against selected phytopathogens. Biomed Pharmacol J 2:277–280
Wite D, Mattner SW, Porter IJ, Arioli T (2015) The suppressive effect of a commercial extract from Durvillaea potatorum and Ascophyllum nodosum on infection of broccoli by Plasmodiophora brassicae. J Appl Phycol 27:2157–2161
Worrall D, Holroyd GH, Moore JP, Glowacz M, Croft P, Taylor JE, Paul ND, Roberts MR (2012) Treating seeds with activators of plant defence generates longlasting priming of resistance to pests and pathogens. New Phytol 193:770–778
Zhang X, Ervin EH, Schmidt RE (2003) Physiological effects of liquid applications of seaweed extract and humic acid on creeping bentgrass. J Amer Soc Hort Sci 128:492–496
Zine H, Rifai LA, Faize M, Smaili A, Makroum K, Belfaiza M, Kabil EM, Koussa T (2016) Duality of acibenzolar-S-methyl in the inhibition of pathogen growth and induction of resistance during the interaction tomato/vertcillium dahliae. Eur J Plant Pathol 145:61–69
Zucconi F, Pera A, Forte M, De Bertoli M (1981) Evaluating toxicity in immature compost. Biocycle 22:54–57
Acknowledgments
This research was supported by the Moroccan CNRST (Centre National de Recherche Scientifique et Technique) within the frame work of the RS/2011/22 project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 11587 kb)
Rights and permissions
About this article
Cite this article
Esserti, S., Smaili, A., Rifai, L.A. et al. Protective effect of three brown seaweed extracts against fungal and bacterial diseases of tomato. J Appl Phycol 29, 1081–1093 (2017). https://doi.org/10.1007/s10811-016-0996-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0996-z