Abstract
The beak is a hard structure of cephalopods with relatively constant shape, which records a lot of biological and ecological information. Based on samples collected by Chinese squid jigging vessels off the Peruvian Exclusive Economic Zone from July to October 2013, we explored the feasibility of age estimation and growth pattern with the upper beak of Dosidicus gigas. The internal rostral axis and longitudinal increments could be clearly identified on the upper beak rostrum sagittal sections of this species. Checks and abnormal structure were observed in some beaks. Ages estimated were from 123 to 298 days for females and from 106 to 274 days for males. Back-calculated hatching dates were from December 2012 to May 2013. All of the samples were from the austral summer/autumn spawning cohort. Hatching peak occurred between January and March. Growth in mantle length (ML) and body weight were best described by exponential functions and there were no significant differences between females and males. The maximum absolute daily growth rate and instantaneous growth rate of ML were 2.12 mm/day and 0.59/day, respectively. This study suggests that the upper beak could be used for estimating age, growth and population structure of D. gigas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The jumbo flying squid Dosidicus gigas is widely distributed throughout the Eastern Pacific Ocean, from California (37°N) south to the southern part of Chile (47°S). It is the largest and one of the most abundant species of the family Ommastrephidae [1, 2]. Dosidicus gigas spawn throughout the year, and grow fast with a life span of about 1 year [3]. The population structure of D. gigas is complicated, with large spatial variability and existence of multiple geographic stocks [4]. Based on the sizes of adult males and females, three intraspecific groups of D. gigas were distinguished [1]: a small-sized group (130–260 mm for males and 140–340 mm for females, respectively), a medium-sized group (240–420 mm for males and 280–600 mm for females, respectively) and a large-sized group (>400–500 mm for males and 550–650 to 1000–1200 mm for females, respectively). According to the back-calculated hatching dates, D. gigas could be separated into summer/autumn (December–May) and winter/spring (June–November) spawning cohorts [3]. The fishery biology of D. gigas in the Gulf of California, off the Peruvian and Chilean coast was studied extensively [1, 5–9], and spatial variations were found among geographic populations of D. gigas in the Eastern Pacific Ocean [4]. Dosidicus gigas is one of the most important and abundant species in Peruvian waters, and is caught by local and distant-water fishing vessels with high landings [1].
Using length-frequency analysis to estimate growth in previous studies [10, 11] was confirmed to be inappropriate for cephalopods, because cohorts of cephalopods tend to overlap in length groups [12, 13]. Statoliths are calcareous structures located in the equilibrium organs which serve to detect body acceleration and have been considered as ‘black boxes’ recording valuable information about cephalopod life history events [14]. The statolith is widely used in the age determination of cephalopods [5, 15–17]. Liu et al. [3] estimated the age of D. gigas off the Peruvian Exclusive Economic Zone (EEZ) using statoliths, and the estimated ages ranged from 144 to 633 days. Chen et al. [18] analyzed the statolith microstructure of D. gigas off the Chilean EEZ and back-calculated hatch dates, confirming that D. gigas had spring and autumn spawning cohorts. However, the statolith is tiny and hard to extract. It is also difficult to obtain the proper grinding plane in which growth increments are widest. The increments in statoliths are usually confusing and it is difficult to correctly count their numbers [19].
The beak is mainly composed of proteins and chitin fibers [20]. Like other hard structures, it also has a relatively constant shape and high stiffness [21–23]. Up to now, the beak was widely used in population identification, systematics and biomass estimation [4, 24, 25]. Besides, it is also used in age estimation due to the regular growth cycle of beak [26–29]. The daily deposition of Octopus vulgaris’s beak increments was validated in the rostrum sagittal section (RSS) by environmental marking [30]. Villegas Barcenas et al. [31] studied the beak RSSs increments of cultivated Octopus maya, and found that there was no significant difference between the number of beak increments and age. Therefore, it was confirmed that the beak RSS increments of Octopus maya were formed daily as a result of periodic deposition of increments. Hu et al. [32] developed the relationship between the number of beak and statolith increments, and found that the number of beak increments was lineally related to the number of statolith increments. The regression model was significant (p < 0.01) and had an r 2 value of 1 and slope close to 1. Therefore, they suggest that the beak can be used to estimate the age of D. gigas. Liu et al. [33] studied the bias of the increments in statolith and beak of four oceanic ommastrephid squid species, and found that increments counted on the upper beak RSS of D. gigas and O. bartramii were mostly similar to the ages determined by statolith, which confirms that the beak could yield reliable age estimation for both species.
The objectives of this study are to study the upper beak microstructure of D. gigas and analyze its feasibility to estimate the age and growth for a population in a specific area off the Peruvian EEZ. This study will provide a effective tools for determining the age and growth for oceanic squids.
Materials and methods
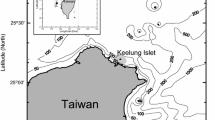
A total of 467 samples were taken from the catch of the Chinese commercial jigging vessel “Puyuan 802” off the Peruvian EEZ (79°57′–83°24′W and 10°54′–15°09′S) from July to October in 2013 (Fig. 1). About 30–40 squids were randomly sampled every fishing day. The sampled squids were immediately frozen on the vessel. In this study, according to the size of D. gigas, the upper beaks of 276 samples were used to estimate the age. The sampled squids were defrosted in the laboratory, and mantle length (ML) and body weight (BW) were measured to the nearest 1 mm and 1 g, respectively. Sex was identified and maturity stages were evaluated according to the maturity scale of Lipiński and Underhill [34], who defined I–II as immature, III as maturing, and IV–V as mature.
The beaks were extracted, then cleaned and preserved in 70 % alcohol for further processing. The increments of beak’s microstructure were located in the RSS (Fig. 2), which was obtained using the methodology modified by Perales-Raya et al. [35]. The rostrum area of upper beak was cut and then embedded in a small plastic mold of epoxy (UKORM) mixed with hardener. After hardening, the piece was ground with 600 and 1200 grit waterproof sandpaper. Both sides of the sagittal section were polished with 0.05 μm aluminum oxide powder (BAIKOWSKI) after reaching the sagittal pane (Fig. 2). Increments were counted along the internal rostral axis (Fig. 3a), which continues from the anterior to the posterior part of the hood [36]. To avoid tip erosion effects, the first increments were counted in the dorsal area of the rostral sections (Fig. 3a, d). The beak RSS increments were deposited daily, which has been confirmed for D. gigas [32, 33]. The number of increments for each beak was counted independently by two readers. The average was adopted if counts of rings from the two readers were within 10 % of the mean [13]. The hatching date was back-calculated from the number of increments and the date of capture.
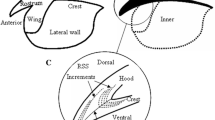
Location of increments in upper beak for Dosidicus gigas [35]
The upper beak microstructure of Dosidicus gigas. a The rostrum sagittal sections of upper beak; b checks of upper beak microstructure; c the bands which intersect with growth increments; d the growth increments in the dorsal region of the hood; e abnormal structure [32]
Based on the back-calculated hatching dates, spawning cohorts were defined. Linear, power, exponential and logarithmic curves were fitted to age–ML and age–BW data [13, 18, 37]. The Akaike information criterion (AIC) was calculated for each model, and the model with the smallest AIC value was selected to describe the growth [18]. The analysis of covariance (ANCOVA) was used to evaluate the differences in growth curves (age–ML and age–BW) between females and males. Instantaneous growth rate (G) and absolute daily growth rate (DGR, mm/day or g/day) were calculated for each 30-day interval by sex. The G and DGR were calculated using the following equations [38, 39]:
where S 1 and S 2 were estimated ML or BW at the beginning (t 1) and end (t 2) of time interval, respectively.
Results
Rostrum sagittal sections (RSS) of upper beak
The increments of upper beak RSS continued from the rostral tip to the joining point of the hood and the crest, and were composed of light and dark bands (Fig. 3a). The increments of hood and lateral wall regions were distributed symmetrically on the both sides of internal rostral axis. The term “longitudinal increments” is used for these thin and closed portions of the bands, which are deposited parallel to the beak edges (Fig. 3a).
In analyzing the upper beak RSS, the pigmentation of the hood region was darker than that for the lateral wall region (Fig. 3a). Checks were found universally in the upper beak microstructure, and a check was generally composed of several bands (increments). The light bands of checks were lighter than the general light bands and the bands of checks were darker than the general dark bands (Fig. 3b). There were not only growth increments, but also bands that intersect with growth increments in the upper beak RSS (Fig. 3c). The bands were narrow between two adjacent increments and were thin in the dorsal border of hood. The rostrum was generally eroded during the feeding process. Therefore, to avoid the tip erosion effects, we counted the first increments in the dorsal region of the rostral sections (Fig. 3d). A special structure was found in the upper beak RSS of a male D. gigas with its increments of dorsal region being obstructed and becoming distorted (Fig. 3e).
Size-structure
For female squids, ML ranged from 209 to 405 mm, the average ML reached 275.0 ± 34.5 mm, and the 230–290 mm size range contained 69.5 % of the females (Fig. 4a). For males, ML ranged from 205 to 396 mm, the average ML of males reaching 258.3 ± 30.4 mm was less than the average ML of females, and the dominant ML ranged from 230 to 290 mm, consisting of 77.3 % of the males (Fig. 4b). For females, the percentage of maturing and mature squid was 4.4 %, and the ML ranged from 211 to 356 mm. For males, the percentage of maturing and mature squid was 31.2 %, and ML ranged from 205 to 321 mm.
Age-structure
The ages of female specimens ranged from 123 to 298 days and the average age was 195.8 ± 29.0 days. Females of 150 to 240 days old consisted of 89.4 % of the total number of female squid (Fig. 5a). The age of males varied from 106 to 274 days and the average age reached 183.4 ± 30.0 days. The age range of 150–240 days included 87.5 % of the males (Fig. 5b). The maturing and mature squids ranged from 180 to 235 days for females and 135 to 225 days for males (Fig. 5).
Hatching time and spawning cohort
The hatching dates ranged from 2 December 2012 to 19 May 2013 (Fig. 6). Therefore, all of the samples were from the summer/autumn spawning cohort. A distinct hatching peak was observed between January and March for squids collected during July and October in 2013, and squids hatched between January and March consisted of 83.7 % of the total samples (Fig. 6).
Growth models
The age–ML data were best described by exponential curves that had the lowest AIC values, and there were no significant differences between females and males (ANCOVA, F = 0.701, p = 0.918) (Table 1; Fig. 7). The age–BW data were also best fitted by exponential curves and there were no significant differences between females and males (ANCOVA, F = 0.576, p = 0.984) (Table 1; Fig. 8).
Growth rates
There were no significant differences in DGR and G between females and males (t test, p > 0.05). Therefore, growth rates were analyzed for sex-combined a data. The maximum DGR and G of ML and BW occurred in 271–300 days (Table 2). In this study, the average DGR and G of ML were 1.05 mm/day and 0.34/day, respectively. The maximum DGR and G in ML reached 2.12 mm/day and 0.59/day, respectively (Table 2).
Discussion
The light and dark bands were alternant in the upper beak RSS. Regular activity patterns and endogenous rhythms are induced by light–dark cycles in some cephalopods, and this endogenous rhythm was probably reflected on the beaks [36, 40]. The increments were distributed symmetrically on both sides of the internal rostral axis (Figs. 2, 3a). Compared to the pigmentation of hood region, the pigmentation of lateral wall area was more lightly deposited (Fig. 3a). Therefore, the increments in the lateral wall region were easily observed. However, the increments were generally overlapping and difficult to count accurately. Meanwhile, the increments of border in the lateral wall region were usually incomplete. Thus, age determination should be done by counting the increments of the hood region.
The bands intersecting with growth increments were found in the upper beak RSS (Fig. 3c). These bands were thin and dense, which probably resulted from the deposition and growth of the upper beak in another direction. The rostrum tip was usually lost during the feeding process, which made it impossible to count the increments located at the rostrum tip. To avoid the tip erosion effects, the first increments were counted in the dorsal region of the rostral sections (Fig. 3a, d). A similar result was also observed in the beak RSS of Octopus vulgaris [35]. Checks were prevalent in the upper beak RSS (Fig. 3b), and were considered to record life history events of the octopus [30, 41]. Therefore, checks might also reflect stressful events of squids. It is possible that environmental stresses, such as temperature shocks, storms or unsuccessful attacks by predators, provide sufficient stress to induce check formation within the beak RSS [14]. In this study, there was an extremely special structure that was located at the upper beak RSS (Fig. 3e). The increments of the dorsal area of the hood were seemingly obstructed, became distorted and then restored to a normal condition, which was probably induced by some ambient environmental stress. Therefore, the beak also has the potential for studying the life history of squid by analyzing its trace element, just like the statolith in previous studies [42, 43].
For females, there were only a few maturing and mature squids throughout the ML (Fig. 4a). For male samples, however, the maturing and mature squids consisted of 48.0 % of samples of ML from 200 to 260 mm and consisted of 17.1 % of samples with the ML ranging from 260 to 410 mm, respectively (Fig. 4b). Therefore, the squids sampled in this study were probably composed of small-sized and medium-sized groups based on the classification standard of Nigmatullin et al. [1]. In previous studies, the small-sized and medium-sized groups were also observed off the Peruvian coast [1, 44]. In some studies, temperature and food availability were considered as the main factors leading to the existence of different groups as a result of phenotypic size variability, but with no definitive conclusion [45–47]. However, Arkhipkin et al. [48] studied the effect of ambient temperature on adult size and life span of D. gigas, and demonstrated that the significant negative effect of sea surface temperatures on the life span led to a shorter life cycle of squid with a smaller size or a longer life cycle of squid with a larger size. Besides, it was considered that the small-sized group was found predominantly in the near-equatorial area, those of the large-sized group live in the northern and southern peripheries of the range, and those of the medium-sized group occur within the whole species range (except in the most high-latitudinal parts) [1]. Squids probably mature quickly at a smaller size in high temperature areas [6, 48]. On the contrary, squids mature late and at a larger size in low temperature areas.
This study suggests a hatching peak between January and February. A similar result was also found in the Peruvian waters [3]. Besides, squids hatched in summer/autumn and recruited to the fishery in winter/spring were found based on the hatching dates and the dates of capture in this study. However, in this study, the squids were sampled only in 4 months. The low abundance in 2013 led to a relatively short fishing season compared to previous years [3]. Dosidicus gigas spawns throughout the year [3]. However, the hatching peak would vary in different geographic areas. Off the western coast of the Baja California Peninsula, Mejia-Rebollo et al. [49] suggested that the hatching peak of D. gigas was from January to March. Off the Chilean EEZ, Chen et al. [18] stated that the prevailing hatching time was from August to November. Environment factors such as temperature and salinity were probably different in the same time frame among different geographic areas. Therefore, the hatching peaks of D. gigas differ among geographic areas.
In this study, all of the samples were identified to be of a summer/autumn spawning cohort. The age–ML data and age–BW data were both best fitted with exponential models (Figs. 7, 8). Meanwhile, Liu et al. [3] found that the age–ML data and age–BW data were best described by exponential functions for summer/autumn spawning cohort of D. gigas, based on statolith microstructure off the Peruvian EEZ. The growth curve in this study is close to that in Liu et al. [3], from 120 to 300 days (Fig. 9). Therefore, this also indicates that the beak microstructure can be used to study the age and growth of D. gigas. But the sampled squids in this study did not cover all the life history stages. Thus, the application of the models developed in this study should be restricted to the size ranges included by this study. Therefore, the squids of all ontogenetic stages should be collected using fishing gear such as purse nets to research the growth of the whole lifespan.
Based on the results in this study, significant differences of growth rates were not found between females and males. The maximum DGR and G of ML and BW were reached in 271–300 days (Table 2). The maximum DGR in ML and BW reached 2.12 mm/day and 22.47 g/day, respectively. However, the results might be biased because fewer samples ranged between 271 and 300 days old. In previous studies, off Costa Rica Dome EEZ, the maximum DGR (1.46 mm/day) and G (0.52/day) in ML occurred in 181–210 days old for females, and the maximum DGR (2.07 mm/day) and G (0.85/day) were reached in 151–180 days old for males [3]. In Baja California waters, the maximum DGR (2.09 mm/day) in ML occurred at 220 days old in females, and the maximum DGR (2.1 mm/day) reached at 200 days in males [49]. Therefore, the growth rates of squid might be different among spawning cohorts and geographic populations of D. gigas due to the variation of oceanographic environments in different areas.
In summary, the age, growth and population structure of D. gigas were successfully estimated using the microstructure of the upper beak for RSS in this study. We recommend that the beak be used in the study of age, growth and population structure of Ommastrephidae squid. A sampling program covering large areas and a long time period needs to be conducted in the future for evaluating potential spatio-temporal variability in age and growth of D. gigas.
References
Nigmatullin CM, Nesis K, Arkhipkin A (2001) A review of the biology of the jumbo squid Dosidicus gigas (Cephalopoda: Ommastrephidae). Fish Res 54:9–19
Taipe A, Yamashiro C, Mariategui L, Rojas P, Roque C (2001) Distribution and concentrations of jumbo flying squid (Dosidicus gigas) off the Peruvian coast between 1991 and 1999. Fish Res 54:21–32
Liu B, Chen X, Chen Y, Tian S, Li J, Fang Z, Yang M (2013) Age, maturation, and population structure of the Humboldt squid Dosidicus gigas off the Peruvian Exclusive Economic Zones. Chin J Oceanol Limnol 31:81–91
Liu B, Fang Z, Chen X, Chen Y (2015) Spatial variations in beak structure to identify potentially geographic populations of Dosidicus gigas in the Eastern Pacific Ocean. Fish Res 164:185–192
Masuda S (1998) Growth and population structure of Dosidicus gigas in the southeastern Pacific Ocean. In: Okutani T (ed) Large pelagic squids, Japan Marine Fishery Resources Research Center, Japan, pp 107–118
Argüelles J, Rodhouse P, Villegas P, Castillo G (2001) Age, growth and population structure of the jumbo flying squid Dosidicus gigas in Peruvian waters. Fish Res 54:51–61
Morales-Bojórquez E, Cisneros-Mata MA, Nevárez-Martı́ MO (2001) Review of stock assessment and fishery biology of Dosidicus gigas in the Gulf of California, Mexico. Fish Res 54:83–94
Markaida U (2006) Population structure and reproductive biology of jumbo squid Dosidicus gigas from the Gulf of California after the 1997–1998 El Niño event. Fish Res 79:28–37
Liu B, Chen X, Lu H, Chen Y, Qian W (2010) Fishery biology of the jumbo flying squid Dosidicus gigas off the Exclusive Economic Zone of Chilean waters. Sci Mar 74:687–695
Nesis K (1970) Biology of the Peru–Chilean giant squid, Dosidicus gigas. Okeanologiya 10:140–152 (in Russian, English abstract)
Caddy J (1983) On the fishery and biology of the giant squid Dosidicus gigas in the Gulf of California, Mexico. FAO Fish Tech Paper 231:306–340
Jackson GD, Choat JH (1992) Growth in tropical cephalopods: an analysis based on statolith microstructure. Can J Fish Aquat Sci 49:218–228
Yatsu A, Midorikawa S, Shimada T, Uozumi Y (1997) Age and growth of the neon flying squid, Ommastrephes bartramii, in the North Pacific Ocean. Fish Res 29:257–270
Arkhipkin AI (2005) Statoliths as ‘black boxes’ (life recorders) in squid. Mar Freshw Res 56:573–583
Uozumi Y, Ohara H (1993) Age and growth of Nototodarus sloanii (Cephalopoda: Oegopsida) based on daily increment counts in statoliths. Bull Jpn Soc Sci Fish 59:1469–1477
Jackson GD (1994) Application and future potential of statolith increment analysis in squids and sepioids. Can J Fish Aquat Sci 51:2612–2625
González AF, Castro BG, Guerra A (1996) Age and growth of the short-finned squid Illex coindetii in Galician waters (NW Spain) based on statolith analysis. ICES J Mar Sci: J Cons 53:802–810
Chen X, Lu H, Liu B, Chen Y (2011) Age, growth and population structure of jumbo flying squid, Dosidicus gigas, based on statolith microstructure off the Exclusive Economic Zone of Chilean waters. J Mar Biol Assoc UK 91:229–235
Arkhipkin AI, Shcherbich ZN (2012) Thirty years’ progress in age determination of squid using statoliths. J Mar Biol Assoc UK 92:1389–1398
Miserez A, Li Y, Waite JH, Zok F (2007) Jumbo squid beaks: inspiration for design of robust organic composites. Acta Biomater 3:139–149
Clarke MR (1962) The identification of cephalopod ‘beaks’ and the relationship between beak size and total body weight. British Museum, UK
GroÈger J, Piatkowski U, Heinemann H (2000) Beak length analysis of the Southern Ocean squid Psychroteuthis glacialis (Cephalopoda: Psychroteuthidae) and its use for size and biomass estimation. Polar Biol 23:70–74
Ikica Z, Vuković V, Đurović M, Joksimović A, ŠIFNER SK (2014) Analysis of beak morphometry of the horned octopus Eledone cirrhosa, Lamarck 1798 (Cephalopoda: Octopoda), in the south-eastern Adriatic Sea. Acta Adriat 55:43–56
Jackson GD (1995) The use of beaks as tools for biomass estimation in the deepwater squid Moroteuthis ingens (Cephalopoda: Onychoteuthidae) in New Zealand waters. Polar Biol 15:9–14
Ogden RS, Allcock A, Wats P, Thorpe J (1998) The role of beak shape in octopodid taxonomy. S Afr J Mar Sci 20:29–36
Hernández-López JL, Castro-Hernández JJ, Hernández-García V (2001) Age determined from the daily deposition of concentric rings on common octopus (Octopus vulgaris) beaks. Fish Bull 99:679–684
Canali E, Ponte G, Belcari P, Rocha F, Fiorito G (2011) Evaluating age in Octopus vulgaris: estimation, validation and seasonal differences. Mar Ecol Prog Ser 441:141–149
Castanhari G, Tomás ARG (2012) Beak increment counts as a tool for growth studies of the common octopus Octopus vulgaris in southern Brazil. Bol Inst Pesca, São Paulo 38:323–331
Rodríguez-Domínguez A, Rosas C, Méndez-Loeza I, Markaida U (2013) Validation of growth increments in stylets, beaks and lenses as ageing tools in Octopus maya. J Exp Mar Biol Ecol 449:194–199
Perales-Raya C, Almansa E, Bartolomé A, Felipe BC, Iglesias J, Sánchez FJ, Carrasco JF, Rodríguez C (2014) Age validation in Octopus vulgaris beaks across the full ontogenetic range: beaks as recorders of life events in octopuses. J Shellfish Res 33:481–493
Villegas Barcenas G, Perales-Raya C, Bartolome A, Almansa E, Rosas C (2014) Age validation in Octopus maya (Voss and Solis, 1966) by counting increments in the beak rostrum sagittal sections of known age individuals. Fish Res 152:93–97
Hu GY, Chen XJ, Liu BL, Fang Z (2015) Microstructure of statolith and beak for Dosidicus gigas and its determination of growth increments. J Fish China 39(3):361–370 (In Chinese)
Liu BL, Chen XJ, Chen Y, Hu GY (2015) Determination of squid age using upper beak rostrum sections: technique improvement and comparison with the statolith. Mar Biol 162:1685–1693
Lipiński M, Underhill L (1995) Sexual maturation in squid: quantum or continuum? S Afr J Mar Sci 15:207–223
Perales-Raya C, Bartolomé A, García-Santamaría MT, Pascual-Alayón P, Almansa E (2010) Age estimation obtained from analysis of octopus (Octopus vulgaris Cuvier, 1797) beaks: improvements and comparisons. Fish Res 106:171–176
Raya C, Hernández-González C (1998) Growth lines within the beak microstructure of the octopus Octopus vulgaris Cuvier, 1797. S Afr J Mar Sci 20:135–142
Schwarz R, Perez JAA (2010) Growth model identification of short-finned squid Illex argentinus (Cephalopoda: Ommastrephidae) off southern Brazil using statoliths. Fish Res 106:177–184
Forsythe J, Van Heukelem W (1987) Growth. In Boyle PR (ed) Cephalopod life cycles, Vol. II: comparative reviews, Academic Press, London, pp 135–156
Arkhipkin A, Mikheev A (1992) Age and growth of the squid Sthenoteuthis pteropus (Oegopsida: Ommastrephidae) from the Central-East Atlantic. J Exp Mar Biol Ecol 163:261–276
Cobb CS, Pope SK, Williamson R (1995) Circadian rhythms to light–dark cycles in the lesser octopus, Eledone clrrhosa. Mar Freshw Behav Physiol 26:47–57
Perales-Raya C, Jurado-Ruzafa A, Bartolomé A, Duque V, Carrasco MN, Fraile-Nuez E (2014) Age of spent Octopus vulgaris and stress mark analysis using beaks of wild individuals. Hydrobiologia 725:105–114
Arkhipkin AI, Campana SE, FitzGerald J, Thorrold SR (2004) Spatial and temporal variation in elemental signatures of statoliths from the Patagonian longfin squid (Loligo gahi). Can J Fish Aquat Sci 61:1212–1224
Zumholz K, Hansteen TH, Piatkowski U, Croot PL (2007) Influence of temperature and salinity on the trace element incorporation into statoliths of the common cuttlefish (Sepia officinalis). Mar Biol 151:1321–1330
Arkhipkin A, Murzov S (1986) Age and growth patterns of Dosidicus gigas (Ommastrephidae). Present state of fishery for squids and prospects of its development. VNIRO Press, Moscow, pp 107–123
Hoving HJT, Gilly WF, Markaida U, Benoit-Bird KJ, Brown ZW, Daniel P, Field JC, Parassenti L, Liu B, Campos B (2013) Extreme plasticity in life-history strategy allows a migratory predator (jumbo squid) to cope with a changing climate. Global Change Biol 19:2089–2103
Keyl F, Argüelles J, Mariátegui L, Tafur R, Wolff M, Yamashiro C (2008) A hypothesis on range expansion and spatio-temporal shifts in size-at-maturity of jumbo squid (Dosidicus gigas) in the eastern Pacific Ocean. CalCOFI Rep 49:119–128
Tafur R, Keyl F, Argueelles J (2009) Reproductive biology of jumbo squid Dosidicus gigas in relation to environmental variability of the northern Humboldt Current System. Mar Ecol Prog Ser 400:127
Arkhipkin A, Argüelles J, Shcherbich Z, Yamashiro C (2014) Ambient temperature influences adult size and life span in jumbo squid (Dosidicus gigas). Can J Fish Aquat Sci 72:400–409
Mejia-Rebollo A, Quiñónez-Velázquez C, Salinas-Zavala CA, Markaida U (2008) Age, growth and maturity of jumbo squid (Dosidicus gigas d’Orbigny, 1835) off the western coast of the Baja California Peninsula. CalCOFI Rep 49:256–262
Acknowledgments
The support of the scientific survey made by Puyuan 802 is gratefully acknowledged. This work was funded by the National Science Foundation of China (NSFC41306127 and NSFC41276156), National Science Foundation of Shanghai (13ZR1419700), the innovation Program of Shanghai Municipal Education Commission (13YZ091), and the Shanghai Leading Academic Discipline Project (Fisheries Discipline). The involvement of Y. Chen was supported by SHOU International Center for Marine Studies and Shanghai visiting 1000 Talent Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Co-first authors: G. Hu and Z. Fang.
Rights and permissions
About this article
Cite this article
Hu, G., Fang, Z., Liu, B. et al. Age, growth and population structure of jumbo flying squid Dosidicus gigas off the Peruvian Exclusive Economic Zone based on beak microstructure. Fish Sci 82, 597–604 (2016). https://doi.org/10.1007/s12562-016-0991-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-016-0991-y