Abstract
The bigfin reef squid Sepioteuthis lessoniana is a neritic squid widely distributed in the Indo-Pacific region and of interest to fisheries for its high commercial value. The age and growth of this squid has been studied along its distribution range, from Japan to Australia; however, the life-history parameters of this squid around Taiwan are unclear. In this study, growth and maturation of S. lessoniana in the waters off northern Taiwan between April 2009 and March 2010 was studied using statolith microstructure analysis. Statoliths of 142 females (88–355 mm mantle length, ML) and 129 males (85–401 mm ML) were read. The oldest female was mature at 216 days (355 mm ML), whereas the oldest male was mature at 209 days (345 mm ML). The squids hatch almost year-round, except in January and February, peaking in May and August–September. The ML-at-age data were best described by Schnute and linear functions for the females and males, respectively. The males attained a higher size-at-age than the females. Maturity ogives by age and ML class indicated that females mature later and at a larger size than males. Growth and maturation parameters of seasonal cohorts showed little difference, although squids hatching in warmer seasons tend to have a faster growth rate and higher size-at-age than those hatching in colder seasons for both sexes. The differences of life-history traits between seasonal cohorts could be inferred by progressive changes in the life-history traits of the squids hatching during transitional months, i.e. months between two peak seasons. These results provide essential life-history traits and improve our understanding of S. lessoniana populations in the waters off northern Taiwan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The bigfin reef squid Sepioteuthis lessoniana is a neritic squid widely distributed in Indo-West Pacific waters and regarded as a valuable fishery resource [1]. A species complex may exist in Australian waters based on genetic analysis [2], and Japanese waters based on various reproductive features and genetic analysis [3–5]. Nevertheless, recent study suggests a low genetic diversity for populations in the Japanese waters and significant separation for populations in Japanese waters from those in the East and South China Sea [6].
Sepioteuthis lessoniana occurs throughout the waters around Taiwan, usually caught as a by-catch of neritic trawl fisheries. The squid is also targeted by recreational fishers in the waters off northern Taiwan jigging from October through March. However, the fisheries biology and population structure of this squid around Taiwan remains poorly understood. The commercial value of S. lessoniana (ca. US$15 per kg; currency US$1 = NTD$30) is usually higher than other neritic squids (ca. US$4 per kg) [7]. Therefore, this squid is a marine resource with commercial potential in Taiwan. It is essential and critical to understand the life-history traits and population structure of S. lessoniana around Taiwan to develop approaches to conservation and management.
Squid statoliths are analogous to fish otoliths in structure and function [8], making them conveniently applicable to the techniques and methods which have been developed for otoliths [9, 10]. Enlargement over time and regular deposition make statoliths appropriate tissues for studying the age and growth of squids, and other life-history events which might be recorded in the statoliths [10]. Statoliths have been frequently used in age and growth studies of squids around the world [11–13].
The widespread distribution of S. lessoniana in neritic waters throughout the Indo-Pacific and those successfully maintained in aquaria make the species a useful model animal for exploring the influence of environmental conditions on growth and maturity [14–19]. Daily formation of growth increments within statoliths of S. lessoniana has been validated by chemical marking for populations in Australian waters [20]. Squids have been observed to grow rapidly and have short life spans with maximum age estimated to be ca. 200 days for populations in tropical waters (from Philippines [21] and from Thailand and Australia [17]). The spawning seasons of S. lessoniana ranged between April and October in Okinawa waters [22] and between July and November in Zanzibar waters [23]. Similar to other squids, growth of S. lessoniana was highly sensitive to the ambient environmental conditions such as water temperature [15, 17]. Therefore, growth and hence size-at-maturity may vary among populations experiencing different environmental conditions [18]. Study of life-history traits of a species throughout its distribution ranges is required to not only improve understanding of population parameters, but also to provide essential information for management [24].
The waters off northern Taiwan, located at the southern edge of the East China Sea, are influenced by the Taiwan Strait waters as well as the seasonal intrusion of the Kuroshrio current [25] resulting in abundant primary productivity [26], and thus is one of the important fishing grounds of Taiwan [27]. Growth and maturation are critical life-history traits in population ecology and are essential information for approaches to conservation and management. Sepioteuthis lessoniana occur throughout the waters around Taiwan as a by-catch of multi-gear fisheries, while relevant information on population biology and ecology are scarce. The objective of this study was to examine growth and maturation of S. lessoniana in the waters off northern Taiwan, where this squid is targeted by recreational fishing, using statolith microstructure analysis. The results will provide basic information and improve our understanding of S. lessoniana populations in the waters off northern Taiwan.
Materials and methods
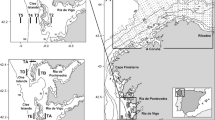
Samples of S. lessoniana were collected monthly from a day-trip vessel which jigged squid with wood-shrimp bait around Keelung Islet in the waters off northern Taiwan between April 2009 and March 2010 (Fig. 1). The squid samples were frozen immediately then thawed before examination in the laboratory. Specimens were sexed and measurements were obtained of the dorsal mantle length (ML), body weight (BW), gonad organs weight (GW, oviducts and oviducal glands, nidamental glands, and ovaries in females; Needham complex and testes in males). Measurements were recorded to the nearest 1 mm and 1 g for length and weight measurements, respectively.
Sexual maturity was determined with the naked eye following Boyle and Rodhouse [28] classification: stage I and II (immature); III (maturing); IV (mature); and V (spent). A pair of statoliths were extracted from each specimen, washed and stored in 96-well plates with liquid paraffin.
Each statolith (left side) was cleaned with xylene and ethanol and mounted on a glass slide with crystal bond. A transverse plane was obtained by grinding from the anterior side. The statolith was ground by hand with wet waterproof sandpaper (1,000, 2,400 and 4,000 grits, Buehler), and polished with 0.05 µm alumina powder and polish cloth. The prepared statoliths were examined under a compound microscope (Leica DM-2500) with a digital camera (Canon EOS-500D). Frames of statolith images were taken in series from the nucleus to margin of the lateral dome and saved as digital image files. Multiple frames of a statolith image were stitched together as a whole using Photoshop software, and the growth increments of the statolith were then traced and counted using Powerpoint software. The total number of increments (NI) within each statolith was marked and counted from the nucleus to the tip of the lateral dome. One observer marked and counted the samples most of the time. However, all the increments were marked on prepared statolith images and saved as image files which are traceable if any sample was questionable.
The formation of growth increments in statoliths of S. lessoniana has been validated to be daily [20]. Accordingly, the number of increments was considered to be equivalent to the age of the squid in days. Hatching dates were determined by subtracting estimated daily ages from the date of capture.
The relationship between ML and BW was fitted using a power function: BW = aMLb, where a and b are coefficients to be estimated. The effect of sex on the exponent b of the ML-BW relationship was examined by analysis of covariance (ANCOVA).
Four empirical equations (linear, exponential, power, and logistic) and two growth functions (von Bertalanffy and Gompertz) were fitted to the ML-at-age data. The equations are as follows.
Linear function:
exponential function:
power function:
logistic function:
von Bertalanffy function:
Gompertz function:
where L is mantle length (mm) at age t; t is age (day); a, b, c, are parameters to be estimated.
The Schnute function [29, 30] was also used.
Schnute function:
where t 1 and t 2 are the minimum and maximum ages of squid sampled, respectively. L 1 and L 2 are the estimated size at the minimum and maximum observed ages, respectively. Parameters g 1 and g 2 are growth rates to be estimated.
The parameters of the models were estimated by the least squares method (linear models) and non-linear least squares using the Gauss–Newton algorithm (non-linear models) incorporated in software R (Version 3.1.1; www.r-project.org).
The best growth function was identified using the Akaike information criterion (AIC) [31]:
where LL is the negative log-likelihood and P the number of parameters in each function.
Maturity ogives were estimated by fitting the logistic curve: Y = 1/(1 + Exp(−a(X − b))) to the proportion of mature squid (Y) by age or ML interval (X); where a is the slope of the curve and b is the age (t 50 %) and ML (ML50 %) at which 50 % of females or males were mature [32]. Parameters a and b were calculated by non-linear least squares using the Gauss–Newton algorithm incorporated in software R (Version 3.1.1).
Principal component analysis (PCA) was performed to extract the variation in six life-history traits (BW, ML, number of increments, statolith radius, average growth rate and GSI) between monthly groups (micro-cohorts) of the squid. The score of the first two PCs of each group was applied to analyze the similarity of growth parameters between groups by cluster analysis with Euclidean distance and unweighted pair-group average method.
Results
Of the 341 S. lessoniana specimens collected, 271 statoliths (79.5 %) were appropriately prepared for reading (Table 1), which consisted of 142 females and 129 males. ML ranged from 88 to 355 mm and from 85 to 401 mm for females and males, respectively. No spent individual was found of either sex. The smallest individuals were collected in August for both sexes and the largest ones were collected in March (male) and April (female, Fig. 2). The estimated coefficients presented an allometric relationship between ML and BW of S. lessoniana. The slopes of the ML-BW relationship differed significantly between the two sexes (ANCOVA, F 1, 267 = 4.94, P = 0.027); females are heavier than males at the same ML (females: BW = 0.0003ML2.70, R 2 = 0.995; males: BW = 0.0003ML2.65, R 2 = 0.994).
Age and hatching dates
Of the 271 prepared squid, estimated ages ranged from 77 to 216 days. The youngest female, immature at 77 days and 100 mm ML was collected in August; and the youngest male, immature at 86 days and 85 mm ML was collected in August. The oldest female, mature at 216 days and 355 mm ML was collected in April; and the oldest male, mature at 209 days and 345 mm ML was collected in April. Squids collected from April 2009 to March 2010 had hatched almost year-round, except in January–February (Fig. 3). The hatching month peaked in August and September, while a minor peak in May was noted.
Growth pattern
All parameters of growth functions fitted to ML-at-age data of the squid are tabulated in Table 2. The parameters for both sexes failed to converge when fitted to a von Bertanlanffy growth function, although the parameters can be estimated. The age-at-ML data was best described by Schnute function (followed by logistic function) and linear equation (followed by logistic function) based on the AIC values (Table 2). The scatter-plot of ML-at-age data for females showed an asymptotic pattern (Fig. 4a), while that for males showed a high variation after 130 increments (Fig. 4b).
Age- and ML-at-maturity
Age- and ML-at-maturity for both sexes were estimated by fitting logistic curves to the proportion of mature squid by age (in days) or size class. Maturity ogives by age group explained 97.7 and 93.4 % of the variance in maturity for females and males, respectively (Fig. 5a). Females matured at 153 days, while males matured ca. 10 days earlier (at 143 days) than females. Maturity ogives by size class explained 91.4 and 96.5 % of the variance in maturity for females and males, respectively (Fig. 5b). ML-at-maturity was estimated to be 244 and 232 mm for females and males, respectively.
Growth and maturation for seasonal cohorts
Two seasonal cohorts were distinguished by natural break and information from local fishermen in distribution of hatching month for S. lessoniana (Fig. 3): the spring cohort (SC) hatched from March to July, and the autumn cohort (AC) hatched from August to December. ML-at-age data of AC females were best described by a power equation and those data of others (AC males and both sexes for SC) were best described by linear equations (Fig. 6).
Maturity ogives of females by age group explained 95.9 and 96.8 % of the variance in maturity for females of AC and SC, respectively (Fig. 7a). Age-at-maturity was estimated to be 150 and 154 days for AC and SC, respectively. Maturity ogives of males by age group explained 76.5 and 98.2 % of the variance in maturity for AC and SC, respectively (Fig. 7b). Age-at-maturity was estimated to be 138 and 141 days for AC and SC, respectively. Maturity ogives of females by size group explained 94.0 and 53.0 % of the variance in maturity for females of AC and SC, respectively (Fig. 7c). ML-at-maturity was estimated to be 248 and 237 mm for AC and SC, respectively. Maturity ogives of males by size group explained 86.7 and 90.6 % of the variance in maturity for females of AC and SC, respectively (Fig. 7d). ML-at-maturity was estimated to be 231 and 240 mm for AC and SC, respectively.
In order to extract the variation between monthly groups (micro-cohorts) of the squid, six life-history traits (BW, ML, number of increments, statolith radius, average growth rate and GSI) were selected to perform principal component analysis (PCA). The first two factors (PC1 and PC2) explained 85.9 and 87.6 % of the total variance in life-history traits for females and males, respectively (Table 3). The first factor (PC1) was positively correlated with six variables (BW, ML, NI, SR, GSI and average growth rate, GR) for females and males, while the second factor (PC2) was positively correlated with GR and GSI for females and males, respectively. The monthly groups from the same season for both sexes of the squids showed similar distribution patterns in the bi-plot composed of PC1 and PC2, AC in the positive direction along PC1, whereas SC was in the negative direction along PC1 (Fig. 8). Monthly groups of the squids showed two extreme conditions of growth parameters: smaller size and slower growth rate for groups hatched in March–May (negative PC1), whereas larger size and faster growth rate for groups hatched in September–October (positive PC1). Nevertheless, squids hatched in June–August and November showed a transitional pattern in growth parameters between these two extreme conditions. Dendrograms derived from cluster analysis based on PCA scores illustrated the similarity in growth parameters between monthly groups of the squids (Fig. 9). In general, squids hatched in the two peak seasons (March–May vs September–October) were of high similarity in growth parameters, while those hatched in transitional months were of high variation in growth parameters. Clusters of monthly groups of the squids could correspond to seasonal cohorts in females, whereas a high similarity of growth parameters was found for squids hatched in transitional months in males.
Scores (in average ± standard error by month) and loadings in two principal components from principal component analysis for spring cohort (open ellipses) and autumn cohort (shaded ellipses) of Sepioteuthis lessoniana in the waters off northern Taiwan based on six biological measurements. (BW body weight, ML mantle length, NI number of increments, SR statolith radius, GSI gonadosomatic index, GR average growth rate)
Discussion
Sepioteuthis lessoniana off northern Taiwan hatched almost year-round. The ML-at-age data were best described by Schnute and linear functions for females and males, respectively. The females mature later and at a larger size than the males, although the males attained a larger size-at-age than the females. Growth and maturation parameters of two seasonal cohorts (SC and AC) were further analyzed: AC tended to grow faster while maturing at a larger size than SC. Nevertheless, the life-history traits of the seasonal cohorts were complicated and could be inferred by progressive changes in the life-history traits of the squids hatching in the transitional months, i.e. months between two peak seasons. These results provide essential information for S. lessoniana populations off northern Taiwan.
Age and hatching dates
Age and growth of S. lessoniana has been studied for populations from tropical to sub-tropical waters [17, 20, 21]. Geographical differences in estimated maximum life span of S. lessoniana have been shown in previous studies, with estimated maximum age at 124–136 days for squids in the Philippines and Thailand waters, while maximum age was 153–224 days for squids in tropical and subtropical Australian waters [17, 21]. Thus, a short life span (less than 200 days) was suggested for S. lessoniana [12]. In the present study, estimated ages for mature squid ranged from 113 to 216 days, which covered the maximum ages of squids from tropical and subtropical waters. The waters off northern Taiwan are at a latitude of 25°10′N, approximately the same latitude as Hervey Bay in Australia (subtropical waters [17]), although in the opposite hemisphere. The monthly mean sea surface temperature (SST) off northern Taiwan ranged from 19.1 to 28.6 °C between 2008 and 2010 which showed a wider range than the monthly mean temperature in Hervey Bay (from 23 to 27.2 °C in 1998 [17]). The great variation of monthly SST off northern Taiwan may result in a wider range of maximum ages of S. lessoniana.
Sepioteuthis lessoniana in the waters off northern Taiwan hatched almost year-round, excluding January and February. A similar hatching pattern was observed for S. lessoniana populations in Palk Bay in the Indian Ocean [33], whereas specific spawning seasons were suggested for populations in Zanzibar (from July to November [23] ) and in Japanese waters (from April to October [22] ). Year-round hatching of intra-annual cohorts or “micro-cohorts” results in continuous recruitment for short-lived species such as squids [34, 35]. The micro-cohort structuring of squids has been evident in many species, particularly those under commercial exploitation, e.g. Dosidicus gigas in the Gulf of California [36], Illex argentinus in the Southwest Atlantic [37], I. coindetii in the Strait of Sicily [38], and Loligo vulgaris in Galician [39] and Portuguese [40] waters. The composite intra-annual structures and plasticity of life-history parameters of squids may advance adaption and sustainability of the population that are sensitive to changes in environmental conditions and are under fishery exploitation [18, 24, 41, 42].
Growth pattern of S. lessoniana
Most squids are generally short-lived (usually 1 year) and have a semelparous life history, i.e. adults die after spawning [28]. The lifetime energy budget of squids seems to have evolved through physiological progenesis, i.e. the allocation of their energy resources among growth components is an essential characteristic of the early life of iteroparous forms [43]. Theoretically, the whole ontogenesis of any species could be subdivided into several periods of growth (‘stanzas’, [44]), which usually correspond with major ontogenetic events like hatching, sexual maturation, or shifts of habitats [44]. Growth within each stanza of an individual may follow a sigmoid curve, or so-called Sachs cycle [44]. The lower part (early phase) of the growth trajectory may be described by exponential functions, whereas the upper part (final phase) can be described by one of the asymptotic functions [30, 44]. Squids have been identified as having either two (embryonic and post-embryonic) or three growth stanzas (embryonic, paralarval and juvenile-adult) during their ontogenesis [30].
In the present study, the size-at-age data of the entire squid samples (with mixed cohorts) were best described by Schnute and linear functions for females and males, respectively. However, the size-at-age data of seasonal cohorts of the squids were best described by linear or power equations. A power function was suggested for populations of S. lessoniana occurring in tropical and subtropical waters of Thailand and Australia [17]. The asymptotic growth trajectory for the entire squid samples might be a composite pattern from mixing several micro-cohorts which experienced a fast and short life history [17, 45].
Maturation of S. lessoniana
The age-at-maturity of S. lessoniana off northern Taiwan was at ca. 153 and 143 days for females and males, respectively, whereas ML-at-maturity was 244 and 232 mm for females and males, respectively (Fig. 5). A similar ML-at-age was found in Japanese waters (200–250 mm for females [22]), while a smaller size-at-maturity was found in Zanzibar waters (ca. 162 mm for females [23]). It is evident that populations of S. lessoniana in tropical waters are smaller-sized and mature earlier compared to populations in subtropical waters [17]. Squids dwelling in cooler waters could grow more slowly, mature later and have a longer life span than their counterparts in warmer waters, such as Zanzibar.
A higher variability in age-at-maturity than in size-at-maturity of S. lessoniana off northern Taiwan was found for males, while that was not the case for females (Fig. 5). Several studies have found that maturation of squid is more strongly influenced by size rather than age [20, 46]. Maturation of S. lessoniana has been shown to be a size-related process, where the weight of reproductive organs is highly correlated to body weight, rather than with age [18]. A high variation in age-at-maturation of S. lessoniana off northern Taiwan suggested that the population might be composed of multiple inter-annual cohorts experiencing different environmental conditions and with distinct growth rates [18, 40], and hence varying age-at-maturity.
Seasonal variation in growth and maturation
Growth of squids might be influenced by water temperature [47, 48], as well as food availability [16]. Ambient water temperature has been shown to have a positive correlation with growth rates, in particular during early life stages, in a range of squid species, e.g. Loligo pealei [49]; Lolliguncula brevis [46]; Loligo gahi [50]; Sepioteuhtis lessoniana [15]. The differences in growth patterns of squid may reflect differences in life-history traits for populations dwelling in different environmental conditions [18, 51]. Two growth strategies for populations of S. lessoniana from two extremes of environmental conditions have been proposed [17]: the ‘hot’ strategy with a small body size and a short life span for squid in equatorial waters (Thailand), and the ‘cool’ strategy with a larger body size and a longer life span for squid in subtropical waters (Hervey Bay). The life-history traits of S. lessoniana in Townsville, which is located around 19°13′S in the tropical waters between Thailand and Hervey Bay, showed an alternation between two growth strategies depending on season of hatching [17]. This may explain the differences in growth pattern of squid in this study to a degree.
Temporal variability in growth parameters of squids has been shown, with a faster growth rate and higher ML-at-age for squids hatching in the warmer season than for those hatching in the colder season [50, 52]. Nevertheless, environmental conditions may affect growth patterns of squids through the life cycle. The warm cohort of Loligo vulgaris, hatching in warmer waters while subadults and adults live in colder waters, has higher ML-at-age, a longer life span and matures at a smaller size and earlier age than the cold cohort, hatching in colder waters while subadults and adults live in warmer waters [40, 51]. In this study, the squids hatching in warmer waters (from August to October) have higher growth rates and larger sizes than those hatching in colder waters (from March to May, Fig. 8). This suggests that populations of S. lessoniana off northern Taiwan may show similar growth strategies to the seasonal cohorts of L. vulgaris in Portuguese waters. However, little difference in growth and maturation parameters between seasonal cohorts of S. lessoniana was found (Figs. 6, 7). The differences in life-history traits between seasonal cohorts could be inferred by progressive changes in the life-history traits of the squids hatching in the transitional months (June–July for females and June–August for males, Fig. 9), when the oceanographic conditions shift from northeasterly to southwesterly monsoon [53, 54]. Nevertheless, it should be noted that a smaller difference between seasonal cohorts might have been induced by the low sample size; thus, further study may be required to examine explicitly the temporal variability in life-history parameters of S. lessoniana off northern Taiwan.
References
Jereb P, Roper CFE (eds) (2010) Cephalopods of the world: an annotated and illustrated catalogue of cephalopod species known to date, vol 2. Myopsid and oegopsid squids. FAO Species Catalogue for Fishery Purposes. No. 4. FAO, Rome
Triantafillos L, Adams M (2005) Genetic evidence that the northern calamary, Sepioteuthis lessoniana, is a species complex in Australian waters. ICES J Mar Sci 62:1665–1670
Segawa S, Hirayana S, Okutani T (1993) Is Sepioteuthis lessoniana in Okinawa a single species? In: Okutani T, O’Dor RK, Kubodera T (eds) Recent advances in cephalopod fisheries biology. Tokai University Press, Tokyo, pp 513–521
Izuka T, Segawa S, Okutano T, Numachi K (1994) Evidence on the existence of three species in the oval squid Sepioteuthis lessoniana complex in Ishigaka Island, Okinawa, Southwestern Japan, by isozyme analysis. Jpn J Malacol Venus 53:217–228
Izuka T, Segawa S, Okutani T (1996) Biochemical study of the population heterogeneity and distribution of the oval squid Sepioteuthis lessoniana complex in southwestern Japan. Am Malacol Bull 12:129–135
Aoki M, Imai H, Naruse T, Ikeda Y (2008) Low genetic diversity of oval squid, Sepioteuthis cf. lessoniana (Cephalopoda: Loliginidae), in Japanese waters inferred from a mitochondrial DNA non-coding region. Pac Sci 62:403–411
Anonymous (2012) Fisheries Statistical Yearbook, Taiwan, Kinmen and Matsu Area 2011. Fisheries Agency, Council of Agriculture, Executive Yuan, Kaohsiung (in Chinese with English abstract)
Jackson GD, Alford RA, Choat JH (2000) Can length frequency analysis be used to determine squid growth?—an assessment of ELEFAN. ICES J Mar Sci 57:948–954
Jackson GD (1994) Application and future potential of statolith increment analysis in squid and sepioids. Can J Fish Aquat Sci 51:2612–2625
Arkhipkin AI (2005) Statoliths as ‘black boxes’ (life recorders) in squid. Mar Freshw Res 56:573–583
Arkhipkin AI (2004) Diversity in growth and longevity in short-lived animals: squid of the suborder Oegopsina. Mar Freshw Res 55:341–355
Jackson GD (2004) Advances in defining the life histories of myopsid squid. Mar Freshw Res 55:357–365
Arkhipkin AI, Shcherbich ZN (2012) Thirty years’ progress in age determination of squid using statoliths. J Mar Biol Assoc UK 92:1389–1398
Ikeda Y, Wada Y, Arai N, Sakamoto W (1999) Note on size variation of body and statoliths in the oval squid Sepioteuthis lessoniana hatchlings. J Mar Biol Assoc UK 79:757–759
Forsythe JW, Walsh LS, Turk PE, Lee PG (2001) Impact of temperature on juvenile growth and age at first egg-laying of the Pacific reef squid Sepioteuthis lessoniana reared in captivity. Mar Biol 138:103–112
Jackson GD, Moltschaniwskyj NA (2001) The influence of ration level on growth and statolith increment width of the tropical squid Sepioteuthis lessoniana (Cephalopoda: Loliginidae): an experimental approach. Mar Biol 138:819–825
Jackson GD, Moltschaniwskyj NA (2002) Spatial and temporal variation in growth rates and maturity in the Indo-Pacific squid Sepioteuthis lessoniana (Cephalopoda: Loliginidae). Mar Biol 140:747–754
Pecl G (2001) Flexible reproductive strategies in tropical and temperate Sepioteuthis squids. Mar Biol 138:93–101
Ikeda Y, Kobayashi M (2010) Statolith growth of juvenile oval squid Sepioteuthis lessoniana (Cephalopoda: Loliginidae) with special reference to ambient thermal condition. Mar Biol Res 6:485–495
Jackson GD (1990) Age and growth of the tropical nearshore loliginid squid Sepioteuthis lessoniana determined from statolith growth-ring analysis. Fish Bull (US) 88:113–118
Balgos MC, Pauly D (1998) Age and growth of the squid Sepioteuthis lessoniana in N.W. Luzon, Philippines. S Afr J Mar Sci 20:449–452
Segawa S (1987) Life history of the oval squid, Sepioteuthis lessoniana in Kominato and adjacent waters central Honshu, Japan. J Tokyo Univ Fish 74:67–105
Mhitu H, Mgaya Y, Ngoile M (2001) Growth and reproduction of the bigfin squid, Sepioteuthis lessoniana, in the coastal waters of Zanzibar. In: Richmond MD, Francis J (eds) Marine science development in Tanzania and eastern Africa: proceedings of the 20th anniversary conference on advances in marine science in Tanzania. IMS/WIOMSA, Zanzibar, pp 289–300
Pecl GT, Moltschaniwskyj NA, Tracey SR, Jordan AR (2004) Inter-annual plasticity of squid life-history and population structure: ecological and management implications. Oecologia 139:515–524
Tang T-Y, Tai J-H, Yang Y-J (2000) The flow pattern north of Taiwan and the migration of the Kuroshio. Cont Shelf Res 20:349–371
Gong G-C, Liu K-K, Pai S-C (1995) Prediction of nitrate concentration from two end member mixing in the southern East China Sea. Cont Shelf Res 15:827–842
Liao C-H, Lee M-A, Lan Y-C, Lee K-T (2006) The temporal and spatial change in position of squid fishing ground in relation to oceanic features in the northeastern waters of Taiwan. J Fish Soc Taiwan 33:99–113
Boyle PR, Rodhouse PG (2005) Cephalopods: ecology and fisheries. Blackwell Publishing, Oxford
Schnute J (1981) A versatile growth model with statistically stable parameters. Can J Fish Aquat Sci 28:1128–1140
Arkhipkin AI, Roa-Ureta R (2005) Identification of ontogenetic growth models for squid. Mar Freshw Res 56:371–386
Haddon M (2001) Modelling and quantitative methods in fisheries. Chapman and Hall, London
King M (2007) Fisheries biology, assessment and management, 2nd edn. Blackwell Publishing, Oxford
Rao KV (1954) Biology and fishery of the Palk-Bay squid, Sepioteuthis arctipinnis Gould. Indian J Fish 1:37–67
Boyle PR, Boletzky Sv (1996) Cephalopod populations: definition and dynamics. Phil Trans R Soc Lond B 351:985–1002
Caddy JF (1991) Daily rings on squid statoliths: an opportunity to test standard population models? In: Jereb P, Ragonese S, Boletzky Sv (eds) Squid age determination using statoliths. N.T.R. - I.T.P.P. Special Publication, Mazzara del Vallo, pp 53–124
Markaida U, Quiñónez-Velázquez C, Sosa-Nishizaki O (2004) Age, growth and maturation of jumbo squid Dosidicus gigas (Cephalopoda: Ommastrephidae) from the Gulf of California, Mexico. Fish Res 66:31–47
Arkhipkin AI (1993) Age, growth, stock structure and migratory rate of prespawning short-finned squid, Illex argentinus, based on statolith ageing investigation. Fish Res 16:313–338
Arkhipkin AI, Jereb P, Ragonese S (2000) Growth and maturation in two successive seasonal groups of the short-finned squid, Illex coindetii from the Strait of Sicily (central Mediterranean). ICES J Mar Sci 57:31–41
Rocha F, Guerra A (1999) Age and growth of two sympatric squid Loligo vulgaris and Loligo forbesi, in Galician waters (north-west Spain). J Mar Biol Assoc UK 79:697–707
Moreno A, Pereira J, Cunha MM (2005) Environmental influences on age and size at maturity of Loligo vulgaris. Aquat Living Resour 18:377–384
Boyle PR, Pierce GJ, Hastie LC (1995) Flexible reproductive strategies in the squid Loligo forbesi. Mar Biol 121:501–508
Pecl GT, Jackson GD (2008) The potential impacts of climate change on inshore squid: biology, ecology and fisheries. Rev Fish Biol Fish 18:373–385
Rodhouse PG (1998) Physiological progenesis in cephalopod molluscs. Biol Bull 195:17–20
Ricker WE (1979) Growth rates and models. In: Hoar WS, Randall DJ, Brett JR (eds) Fish physiology, vol VIII., Biogenic and growthAcademic Press, Orlando, pp 677–743
Jackson GD, O’Dor RK (2001) Time, space and the ecophysiology of squid growth, life in the fast lane. Vie Milieu 51:205–215
Jackson GD, Forsythe JW, Hixon RF, Hanlon RT (1997) Age, growth and maturation of Lolliguncula brevis (Cephalopoda: Loliginidae) in the Northwestern Gulf of Mexico with a comparison of length-frequency vs. statolith age analysis. Can J Fish Aquat Sci 54:2920–2929
Forsythe JW (1993) A working hypothesis of how seasonal temperature change may impact the field growth of young cephalopods. In: Okutani T, O’Dor RK, Kubodera T (eds) Recent advances in fisheries biology. Tokai University Press, Tokyo, pp 133–143
Forsythe JW (2004) Accounting for the effect of temperature on squid growth in nature: from hypothesis to practice. Mar Freshw Res 55:331–339
Brodziak JKT, Macy WKIII (1996) Growth of long-finned squid, Loligo pealei, in the northwest Atlantic. Fish Bull (US) 94:212–236
Hatfield EMC (2000) Do some like it hot? Temperature as a possible determinant of variability in the growth of the Patagonian squid, Loligo gahi (Cephalopoda: Loliginidae). Fish Res 47:27–40
Moreno A, Azevedo M, Pereira J, Pierce GJ (2007) Growth strategies in the squid Loligo vulgaris from Portuguese waters. Mar Biol Res 3:49–59
Natsukari Y, Nakanose T, Oda K (1988) Age and growth of the loliginid squid Photololigo edulis (Hoyle 1885). J Exp Mar Biol Ecol 116:177–190
Jan S, Wang J, Chern C-S, Chao S-Y (2002) Seasonal variation of the circulation in the Taiwan Strait. J Mar Syst 35:249–268
Liang W-D, Tang TY, Yang YJ, Ko MT, Chuang W-S (2003) Upper-ocean currents around Taiwan. Deep-Sea Res 50:1085–1105
Acknowledgments
We thank the fishermen of Ho-ping Island for assistance with the collection of squid specimens. We also thank Ms. B.-W. Lee at the National Taiwan Ocean University for assistance in sample preparation and data processing. This study was partially funded by the National Science Council, Executive Yuan of Republic of China (Taiwan), through the grants: No. NSC100-2313-B-019-007 and NSC101-2313-B-019-001. The constructive comments from two anonymous reviewers are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, CS., Chen, JY. & Lin, CW. Variation in life-history traits for micro-cohorts of Sepioteuthis lessoniana in the waters off northern Taiwan. Fish Sci 81, 53–64 (2015). https://doi.org/10.1007/s12562-014-0831-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-014-0831-x