Abstract
Sponge grounds are complex three-dimensional benthic habitats dominated by sponges. These sponge-dominated assemblages have been reported worldwide, from the intertidal zone to the deep sea. In shallow euphotic waters, dense sponge aggregations have been mainly found in tropical areas, and their presence is in some cases related to environmental degradation and coral decline. The Mediterranean Sea is globally recognised as a biodiversity hotspot, where light-exposed rocky reefs are typically dominated by photophilous algae. However, high local anthropogenic pressures, coupled with climate change, are leading to the reorganisation of benthic communities and the occurrence of regime shifts in several areas. Here we report the first description of unusual, shallow-water sponge grounds in Mediterranean light-exposed rocky reefs, in an area previously impacted by the destructive date-mussel fishery. These assemblages, found along the Apulian coast (central Mediterranean Sea), are now (2017) characterised by a mean coverage of sponges ranging between 3% and 33%, with maximum values up to 85%. Variation in the structure of assemblages and in the abundance of individual taxa between depths has been tested by multivariate and univariate techniques. The spatial characterisation has been complemented with the taxonomic analysis of the sponge assemblages, which resulted in the identification of 14 sponge taxa. These findings are compared with results of previous research in the same area and discussed with particular reference to the potential variables involved in sponge dominance and spatial distribution in the present system and elsewhere.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sponges diverged from other metazoans over 600 million years ago and are now common sessile organisms in tropical, temperate, and polar benthic ecosystems (Brien et al. 1973; Srivastava et al. 2010; Bell et al. 2015). Sponges are considered important members of benthic marine ecosystems, as a result of their extensive distribution, large biomass in many habitats, and the number of ecological roles that they play (Bell 2008; Heip et al. 2009).
Sponge aggregations may create complex three-dimensional habitats, which, when characterised by large sponge biomass and space occupancy, are described as ‘sponge grounds’ (Heyward et al. 2010; Hogg et al. 2010; Bo et al. 2012; Murillo et al. 2012; Beazley et al., 2013; Maldonado et al. 2017). Sponge grounds are found in a variety of habitats, from the intertidal to the deep-sea environment (Maldonado et al. 2017).
In shallow waters, dense sponge aggregations have been reported in polar, temperate, and tropical ecosystems, and mainly in shaded or dark habitats such as caves, mesophotic reefs, and understoreys (Dayton et al. 1974; Bell and Barnes 2000; Corriero et al. 2000; Aronson et al. 2002; Bell 2002; Heyward et al. 2010; Gerovasileiou and Voultsiadou 2012).
However, in shallow light-exposed reefs, sponge-dominated communities have been only described in tropical regions, including Indo-Pacific, Central Pacific, Caribbean, and Brazilian coasts (Maldonado et al. 2017). In these areas, sponges may have become dominant on shallow rocky substrates because of environmental degradation responsible for mass mortality of corals (Aronson et al. 2002; Kelmo et al. 2013; Farnham and Bell 2018). Furthermore, there is evidence that, as a result of extreme thermal events, some sponge species can proliferate extensively, becoming dominant in the marine environment (Aronson et al. 2002; Kelmo et al. 2013). In Brazilian and Belizean reefs, for example, the El Niño-Southern Oscillation event in 1998 had devastating effects on hermatypic corals, while sponge abundance increased (Aronson et al. 2002; Kelmo et al. 2013), probably due to the higher tolerance of sponges to rapid temperature variations compared with other sessile taxa (Bell et al. 2013). Similarly, in both the Indonesian Wakatobi Marine National Park and the Palmyra Atoll in the Central Pacific reef, degradation led to a drastic reduction of corals as the dominant organisms, and their replacement by sponges (Bell and Smith 2004; Knapp et al. 2013).
More recently, the wider accessibility of ROV technology has led to the discovery of sponge-dominated habitats in the deep seas of all the oceans (Krautter et al. 2001; Klitgaard and Tendal 2004; Bo et al. 2012; Göcke and Janussen 2013; Bertolino et al. 2015; Howell et al. 2016; Mcintyre et al. 2016). These habitats are now recognised as reservoirs of biodiversity by the United Nations Environment Programme (UNEP) (Hogg et al. 2010). The development of such sponge aggregations is typically shaped by geological, hydrological, and biological gradients, and they may be important to the nutrient circulation in abyssal environments (Hogg et al. 2010; Kutti et al. 2013; Cathalot et al. 2015; Goodwin et al. 2017; Maldonado et al. 2017).
The Mediterranean Sea is a temperate basin with a rich biota that includes cold-temperate and subtropical species (Bianchi et al. 2012). Despite the small size, it has a very high number of species and degree of endemism, and is globally recognised as a hotspot of biodiversity (Coll et al. 2010). Mediterranean light-exposed rocky reefs of the open coast are typically dominated by photophilous algae, while shaded or dark reefs can be dominated by sciaphilous algae or invertebrates (Pérès and Picard 1964; Ballesteros 2006; Danovaro 2013). However, due to severe demographic, urban, and industrial pressures, combined with global climate change, Mediterranean biodiversity has been changing at an unprecedented rate in recent years, with the occurrence of several shifts in its biological settings (Bianchi and Morri 2000; Cuttelod et al. 2009; Lejeusne et al., 2010; Claudet and Fraschetti 2010; Coll et al. 2010; Bianchi et al. 2014).
In the last decades of the past century, some areas of the Mediterranean Sea, including the Apulian coasts, have been greatly affected by the date-mussel Lithophaga lithophaga fishery (Fanelli et al. 1994). This heavily destructive practice involves the demolition of the rocky substrate using SCUBA, sledgehammers and underwater vehicles to collect the living molluscs (Guidetti et al. 2003). It was documented that, after the destruction of the rock and the consequent elimination of all the sessile organisms, the substrata have been quickly colonised by the sea urchins Arbacia lixula and Paracentrotus lividus. Sea urchins, through unselective grazing, prevented the recolonisation by perennial macroalgae (Russo and Cicogna 1991; Bonaviri et al. 2011; Guidetti 2011).
Here we report the first quantitative and taxonomic characterisation of an unusual shallow-water (3–7 m of depth) sponge ground from a light-exposed rocky reef of the Mediterranean Sea. Possible biotic and abiotic factors triggering sponge dominance in this area are discussed.
Materials and methods
The study area
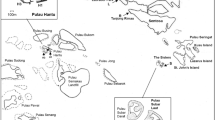
This study was carried out in August 2017, by SCUBA diving, along the south-western coast of the Salento Peninsula (Fig. 1). Sampling was performed in the framework of the project Biodiversity MARE Tricase (Micaroni et al. 2018a, 2018b). The estimated area characterised by the presence of extensive sponge assemblages corresponds to ~ 26 km2. Sampling was conducted at the two sites where the shallow-water sponge grounds were present according to previous observations: site 1 (~ 1 km long), located at Santa Caterina, Nardò, Lecce, and site 2 (~ 6 km long), located at Porto Cesareo, Lecce. The two sites are ~ 13 km apart (Fig. 1), and site 2 is within the marine protected area of Porto Cesareo.
The coastline at the study area shows an alternation of sandy beaches and rocky shores, while the sampled shallow subtidal rocky habitat is characterised by well-illuminated and moderately exposed calcareous plateaus. Detailed information on the hydrological regime of the study area can be found in Parenzan (1983) and Rivaro et al. (2004). The benthic assemblages are typical of Mediterranean barrens, with a few erect macroalgae, including articulated corallines Amphiroa sp., sheet-like brown algae of the genus Dictyota, and the ‘peacock’s tail’ brown alga Padina pavonica (Linnaeus) Thivy, 1960; main invertebrates include the sea urchins Paracentrotus lividus (Lamarck, 1816) and Arbacia lixula (Linnaeus, 1758), the endolithic bivalve Lithophaga lithophaga (Linnaeus, 1758), encrusting bryozoans, and sponges (Guidetti et al. 2003). Among the most common sponge species reported in the area there are Crambe crambe (Schmidt, 1862), Phorbas sp., Chondrilla nucula Schmidt, 1862, Sarcotragus spp., Ircinia spp., and Cliona spp. (Guidetti et al. 2003).
Sampling design and collection of data
Data were collected at three depths (3 m, 5 m, and 7 m) along two belt transects (50 m long) in each of the two study sites. Within each transect, 10 photo-quadrats (0.25 m2, a few meters apart) were taken at random using a Nikon AW130 camera. The Image-J software package was used to estimate the percentage cover of all sessile species present. Samples of sponges were collected for taxonomic identification and fixed in 80% ethanol. Skeleton and spicule preparations were made using standard methods (Rützler 1978). The taxonomic identification was based on Systema Porifera (Hooper and Van Soest 2002), Fauna d’Italia (Pansini et al. 2011), and the World Porifera Database (WPD) (Van Soest et al. 2019).
Due to the indistinguishable external morphology of some specimens, it was necessary to combine some species into operational taxonomic units (OTUs), such as ‘black massive sponges’ (BMS), which included Spongia officinalis, Sarcotragus spinosulus, and Ircinia variabilis; the ‘encrusting red sponges’ morphological group (ERS) counted Crambe crambe, Phorbas fictitius (Bowerbank, 1866), Clathria (Microciona) sp., Hymeniacidon perlevis (Montagu, 1814), and Hemimycale columella (Bowerbank, 1874). OTUs as morphospecies are known to be generally effective for the identification of patterns of distribution of benthic invertebrates (Brind’Amour et al. 2014), marine sponges in particular (De Voogd and Cleary 2008; Schlacher et al. 2010; Downey et al. 2018), at the same time avoiding the need for destructive sampling in concerned habitats.
Statistical analysis
Permutational multivariate analysis of variance (PERMANOVA, Anderson 2001) based on Bray-Curtis untransformed dissimilarities was used to examine differences in assemblage structure among depths and transects, separately for each site. The analysis was based on a two-way model including the crossed factors ‘Depth’ (fixed, three levels: 3 m, 5 m, 7 m) and ‘Transect’ (random with two levels), with the ten photo-quadrats sampled in each transect providing the replicates. When relevant, paired t tests were done for post-hoc comparisons of significant multivariate differences between depths.
Multivariate patterns of ‘average’ assemblages in each combination of transect and depth, separately for each site, were visualised by non-metric multidimensional scaling (nMDS) based on Bray-Curtis untransformed dissimilarities. The SIMPER procedure (Clarke 1993) was used to quantify the absolute (δi) and the percent (δi %) contribution of each taxon to the total dissimilarity between depths, using a cut-off of 90% of cumulative dissimilarity for excluding low contributions.
Data on the percentage cover of each sessile taxon identified as relevant by SIMPER were analysed with analysis of variance (ANOVA) based on the same model as that used for PERMANOVA. When relevant, the ‘Depth x Transect’ term was eliminated from the linear model to test for the effect of ‘Depth’, according to Winer et al. (1991) and Underwood (1997). Before each ANOVA, the assumption of homogeneity of variances was checked with Cochran’s C test. Log transformation of the data was performed when necessary. When heterogeneity of variances could not be removed by transformation, untransformed data were analysed and non-significant results (at p > 0.05) were considered robust as the probability of Type II error is not affected by heterogeneity of variances (Underwood 1997). The Student-Newman-Keuls test was used for post-hoc comparisons between depths, when relevant.
Results
Assemblage description
The benthic assemblages at both sites were characterised by a large abundance of sponges and ephemeral algae (see Appendix 1). Site 1 had the highest abundance of sponges over the barren substrate, with a mean cover ranging from 11% to 33% and maximum values in some quadrats up to 85%. In site 2, the abundance of sponges was slightly lower, with mean cover of 3–30%, although patches with a high sponge density (up to 78%) were found. The taxonomic survey resulted in the identification of 14 sponge taxa, 12 identified at the species level and 2 at the genus level (Table 1). The most abundant sponge species was C. nucula, which accounted for 26–96% and 0–76% of the total sponge cover in site 1 and site 2, respectively. The second most abundant sponge taxon was the BMS, which reached cover values of 0.04–3.9% and 0–5.9% in site 1 and site 2, respectively. At site 1, none of the other sponge species exceeded 1% of cover, while at site 2 a considerable cover contribution was provided by Aplysina aerophoba (mean cover: 0.02–3.9%), Cliona viridis (mean cover: 0.09–2.0%), and Ircinia variabilis (mean cover: 0–1.3%).
Among algae, the most abundant were the filamentous turf-forming green algae (hereafter indicated just as turf), with a mean cover of 5–76%, followed by Padina pavonica (0.2–44%) and Amphiroa sp. (0.02–10%). A considerable proportion of the substrate, however, was characterised by bare rock (2–58%). Sea urchins did not generally exceed 10 individuals/m2. The only exception was transect 2 of site 1 at 3 m depth, where the abundance of urchins reached 19 individuals/m2 (see Appendix 3). It is worth noting that sponges and algae appeared patchily and inversely distributed: areas with high coverage of sponges alternated with areas characterised by relatively more abundant algae (Fig. 2).
Multivariate structure of assemblages
At both sites, the structure of sponge assemblages (combining both the identity and the relative abundance of constituent taxa) varied depending on the combination of depths and transects (Table 2). At site 1 (Santa Caterina), assemblages differed between 3 and 7 m depth in transect 2 and between 5 and 7 m depth in both transects. At site 2 (Porto Cesareo), they differed between 3 and 5 m depth in transect 1, between 3 and 7 m depth in both transects, and between 5 and 7 m depth in transect 2. No significant differences were found in all other combinations of depths and transects (Table 2). These multivariate patterns were graphically evident in terms of both a spatial separation and a different dispersion of centroids corresponding to the different combination of levels of the examined factors in the nMDS plots (Fig. 3a, b).
Abundance of individual taxa
Overall, five sessile taxa were identified as contributing most to differences between depths at each site. These included the sponges C. nucula and BMS, the brown alga P. pavonica, algal turf, and the calcareous red alga Amphiroa sp. (see Appendix 2).
At Santa Caterina (site 1), in particular, the abundance of both C. nucula and P. pavonica did not vary among depths, although the first species was heterogeneously distributed between transects independently of depth (Table 3 and Fig. 4a, b). Both P. pavonica and turf were affected by the combination of depths and transects (Table 3), although with contrasting patterns. Along transect 1, P. pavonica was comparably abundant at all depths, while along transect 2, it progressively decreased with the reduction of depth (Fig. 4c). The cover of turf, instead, was larger at 7 m than at 5 m depth along transect 1, and larger at 3 m compared with 7 m depth along transect 2, with the third examined depth being inconsistently ranked between the other two in both transects (Fig. 4d). Finally, a main effect of ‘Depth’ was detected for Amphiroa sp., which showed consistently larger abundance at 5 m compared with 3 m and 7 m depth. This result should be interpreted with caution due to a significant violation of the assumption of homogeneity of variances (Table 3). The graphs, however, confirmed that the pattern was not critically biased by heteroscedasticity (Fig. 4e).
Mean (+ SE, n = 10) abundance of individual taxa, illustrating differences between depths and transects at site 1. Different letters above bars represent means differing significantly at p < 0.05 (SNK tests). Only comparisons within each transect are appropriate in c and d. Note that different graphs are on different scales
At Porto Cesareo (site 2), almost all relevant sessile taxa (Chondrilla nucula, BMS, turf, and Amphiroa sp.) varied interactively with depths and transects, with the only exception being P. pavonica that did not show any significant differences between depths or transects (Table 4 and Fig. 5a–e). Specifically, C. nucula was more abundant at 3 m than at 5 m and 7 m depth along transect 1, while it did not vary with depth along transect 2 (Fig. 5a). The BMS were similarly abundant across depths in transect 1, while the cover of this taxon was larger at 5 m compared with the two other sampled depths along transect 2 (Fig. 5b). Comparable abundance among depths was shown by turf and Amphiroa sp. along transect 1 and transect 2, respectively. Along transect 2, the cover of turf was larger at 5 m compared with 7 m depth (with no alternative hypothesis for 3 m depth: Fig. 5d), while Amphiroa sp. was more abundant at 3 m compared with both the other depths (Fig. 5e).
Mean (+ SE, n = 10) abundance of individual taxa, illustrating differences between depths and transects at site 2. Different letters above bars represent means differing significantly at p < 0.05 (SNK tests). Only comparisons within each transect are appropriate in a, b, d, and e. Note that different graphs are on different scales
Discussion
This study represents the first taxonomic and quantitative characterisation of a shallow-water light-exposed sponge ground in the Mediterranean Sea. The faunal lists available for the western coast of the Salento Peninsula indicate that the structure of the present sponge ground matches to a large degree the typical species composition of Mediterranean rocky subtidal and barren habitats (Corriero et al. 1984; Parenzan 1984; Corriero et al. 2004; Costa et al. 2018; Longo et al. 2018).
The distinctiveness of the present assemblages is represented by the spatial dominance of sponges that were observed to occupy up to 85% of the substratum, with a mean percentage cover up to 33% along 50 m transects. This abundance is very unusual for the Mediterranean Sea, where shallow and relatively sheltered rocky shores are commonly dominated by macroalgae (Pérès and Picard 1964; McQuaid and Branch 1985; Kraufvelin et al. 2010). At both study sites, sponge aggregations were grouped between 3 and 7 m depth and were mainly constituted by C. nucula and “black massive sponges”.
The dominant sponge species within the examined assemblages, however, was C. nucula. This photophilous species hosts photosynthetic cyanobacteria and contains toxic chemical compounds that make it a strong competitor for space, without reported predators in the Mediterranean Sea (Vicente 1990; Arillo et al. 1993; Milanese et al. 2003). In general, C. nucula was patchily distributed on the seafloor. This sponge, however, had a similar distribution across depths and transects at site 1, while it was relatively more abundant at 3 m depth at site 2, but only along transect 2. These results agree with previously reported evidence that composition of sponge assemblages is shaped by factors acting at small scales (Bell 2007). Wave exposure is another factor that can affect the composition of benthic communities (Sebens 1991). In Santa Caterina (site 1), in particular, transect 2 was located northward of transect 1. Here, transect 2 showed a larger abundance of both the seasonal alga P. pavonica and the BMS compared to transect 1. This pattern could be driven by the effect of the prevalent coastal winds that, in the Salento Peninsula, blow from north-east (Mangia et al. 2004), potentially making the two transects subject to different hydrodynamic and sedimentation regimes. Such environmental factors are known drivers of variation of benthic assemblages in general (Rosenberg 1995; Guichard and Bourget 1998; Airoldi 2003), and sponge assemblages in particular (Sebens 1991; Bell and Barnes 2000).
In tropical areas, the occurrence of shallow-water sponge grounds has been mainly associated with environmental degradation and coral decline due to extreme thermal events and other anthropogenic impacts (Bell and Smith 2004; Kelmo et al. 2013; Maldonado et al. 2017).In the Caribbean Sea, for instance, a species of Chondrilla rapidly increased in abundance and became dominant in the reef after thermal anomalies due to the 1998 El Niño event that had caused severe bleaching and mass mortality of corals (Aronson et al. 2002). Chondrilla remained the dominant component on the substratum for 10 years, until 2009 when an earthquake killed almost all the organisms in the area (Norström et al. 2009; Aronson et al. 2012).
The domination of C. nucula in the present Apulian study system is consistent with such observations, although in a different environmental and biological context. To the best of our knowledge, no historical quantitative data are available for the area examined in our study. According to the earliest naturalistic studies made in the area, however, the shallow rocky reefs were reported to be characterized by photophilous macroalgae (Solazzi 1967 and 1968; Parenzan 1973 and 1983). More recently, the whole Salento Peninsula was affected by the heavily destructive date-mussel fishery that originated persistent barren habitats (Russo and Cicogna 1991; Fanelli et al. 1994; Guidetti et al. 2003; Bonaviri et al. 2011; Guidetti 2011). Our area of study was found to be one of the most heavily damaged (Fanelli et al. 1994). Under such circumstances, it is plausible that sponges took advantage of the increased availability of free space. Indeed, the sea urchins Arbacia lixula and Paracentrotus lividus are common at the study sites and are known to feed intensely on algae and other invertebrates such as bryozoans, hydrozoans, barnacles, and polychaetes (Privitera et al. 2008; Wangensteen et al. 2011). Sponges are known to compete for the substrate with other sessile organisms (Dayton et al. 1974; Jackson and Buss 1975). However, especially on moderately exposed rocky shores of temperate seas, macroalgae are among the strongest space occupiers (Palumbi 1985; Davis et al. 1997; Cebrian and Uriz 2006; Baldacconi and Corriero 2009; Cárdenas et al. 2016). Therefore, it is likely that their anthropogenic removal could trigger the dominance by sponges.
Furthermore, a comparison with data from 2002, shortly after the ban of the L. lithophaga fishery, seems to indicate a reduction of sea urchins and of the percentage cover of bare substrate. At La Strea, Porto Cesareo (close to transect 1 at site 2), between 5 and 7 m depth, an average of ~ 20 sea urchins per m2 was reported in 2002 (Guidetti et al. 2003). Fifteen years later, we found an average of just two urchins per m2. This reduction may be associated with the reduction in the percentage of bare substrate, which changed from 90 to 95% in 2002 (Guidetti et al. 2003) to 11–14% in 2017. Such drastic ecological change may explain the large occurrence of ephemeral and seasonal algae, typical of impacted and urbanized environments, in the assemblage described here (Hay 1981; Piazzi et al. 1999; Benedetti-Cecchi et al. 2001; Bulleri et al. 2002).
In addition to biological interactions, ocean warming associated with global change could have provided a further contribution to the establishment of the examined sponge ground. Thermal anomalies have already led to mass mortality events and species shifts in the Mediterranean Sea and worldwide (Coma et al. 2009; Hoegh-Guldberg and Bruno 2010; Rivetti et al. 2014). Recent studies indicate that certain sponge species are less sensitive to ocean warming and acidification compared with many other benthic organisms (Bell et al. 2013). Therefore, they could benefit from environmental conditions related to global change compared with other organisms (Bell et al. 2018b). This type of evidence is available also for the eastern Mediterranean Sea (Bianchi et al. 2014). Furthermore, Bertolino et al. (2017a, b) suggest that, on a millennial time-scale, seawater warming coincides with changes in sponge assemblage composition and an overall increase of sponge richness.
Therefore, there are reasons to believe that the assemblages reported in this study would represent not just an isolated and temporary phenomenon, but also an important early-warning indicator of a future scenario in temperate shallow subtidal environments similarly to impacted coral reef systems (Bell et al. 2018a). However, manipulative experiments are necessary to unambiguously test for the previously discussed models and hypotheses. These must be specifically designed to unravel the causal role of biotic and abiotic factors and their possible interactions that allow the formation and the plausible persistence of these shallow-water sponge grounds. In conclusion, information from studies such as the present one, possibly expanded to larger spatial and temporal scales (Dayton et al. 2016), is essential to guarantee that effective measures include a representative sample of target organisms, of their natural variability, and of the responsible factors even if not yet fully known (Benedetti-Cecchi et al. 2003).
References
Airoldi L (2003) The effects of sedimentation on rocky coast assemblages. Oceanogr Mar Biol 41:169–171. https://doi.org/10.2980/1195-6860(2006)13[119:eonsot]2.0.co;2
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26(1):32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Arillo A, Bavestrello G, Burlando B, Sara M (1993) Metabolic integration between symbiotic cyanobacteria and sponges: a possible mechanism. Mar Biol 117:159–162. https://doi.org/10.1007/bf00346438
Aronson RB, Precht W, Toscano M, Koltes KH (2002) The 1998 bleaching event and its aftermath on a coral reef in Belize. Mar Biol 141:435–447. https://doi.org/10.1007/s00227-002-0842-5
Aronson RB, Precht WF, Macintyre IG, Toth LT (2012) Catastrophe and the life span of coral reefs. Ecology 93:303–313. https://doi.org/10.1890/11-1037.1
Baldacconi R, Corriero G (2009) Effects of the spread of the alga Caulerpa racemosa var. cylindracea on the sponge assemblage from coralligenous concretions of the Apulian coast (Ionian Sea, Italy). Mar Ecol 30:337–345. https://doi.org/10.1111/j.1439-0485.2009.00282.x
Ballesteros E (2006) Mediterranean coralligenous assemblages: a synthesis of present knowledge. Oceanogr Mar Biol 44:123–195. https://doi.org/10.1201/9781420006391.ch4
Beazley LI, Kenchington EL, Murillo FJ, Sacau MDM (2013) Deep-sea sponge grounds enhance diversity and abundance of epibenthic megafauna in the Northwest Atlantic. ICES J Mar Sci 70:1471–1490. https://doi.org/10.1093/icesjms/fst124
Bell JJ (2007) The ecology of sponges in Lough Hyne Marine Nature Reserve (south-west Ireland): past, present and future perspectives. J Mar Biol Assoc UK 87:1655–1668. https://doi.org/10.1017/S0025315407058171
Bell JJ (2008) The functional roles of marine sponges. Estuar Coast Shelf Sci 79:341–353. https://doi.org/10.1016/j.ecss.2008.05.002
Bell JJ, Barnes DK (2000) The distribution and prevalence of sponges in relation to environmental gradients within a temperate sea lough: vertical cliff surfaces. Divers Distrib 6:283–303. https://doi.org/10.1046/j.1472-4642.2000.00091.x
Bell JJ, Bennett HM, Rovellini A, Webster NS (2018a) Sponges to be winners under near-future climate scenarios. BioScience 68:955–968. https://doi.org/10.1093/biosci/biy142
Bell JJ, Davy SK, Jones T, Taylor MW, Webster NS (2013) Could some coral reefs become sponge reefs as our climate changes? Glob Chang Biol 19:2613–2624. https://doi.org/10.1111/gcb.12212
Bell JJ, McGrath E, Biggerstaff A, Bates T, Cárdenas CA, Bennett H (2015) Global conservation status of sponges. Conserv Biol 29:42–53. https://doi.org/10.1111/cobi.12447
Bell JJ, Rovellini A, Davy SK, Taylor MW, Fulton EA, Dunn MR, Bennett HM, Kandler MN, Luter HM, Webster NS (2018b) Climate change alterations to ecosystem dominance: how might sponge-dominated reefs function? Ecology 99:1920–1931. https://doi.org/10.1002/ecy.2446
Bell JJ, Smith D (2004) Ecology of sponge assemblages (Porifera) in the Wakatobi region, south-east Sulawesi, Indonesia: richness and abundance. J Mar Biol Assoc UK 84:581–591. https://doi.org/10.1017/S0025315404009580h
Bell JJ (2002) The sponge community in a semi-submerged temperate sea cave: density, diversity and richness. Mar Ecol 23:297–311. https://doi.org/10.1046/j.1439-0485.2002.02784.x
Benedetti-Cecchi L, Bertocci I, Micheli F, Maggi E, Fosella T, Vaselli S (2003) Implications of spatial heterogeneity for management of marine protected areas (MPAs): examples from assemblages of rocky coasts in the Northwest Mediterranean. Mar Environ Res 55:429–458. https://doi.org/10.1016/S0141-1136(02)00310-0
Benedetti-Cecchi L, Pannacciulli F, Bulleri F, Moschella PS, Airoldi L, Relini G, Cinelli F (2001) Predicting the consequences of anthropogenic disturbance: large-scale effects of loss of canopy algae on rocky shores. Mar Ecol Prog Ser 214:137–150. https://doi.org/10.3354/meps214137
Bertolino M, Bo M, Canese S, Bavestrello G, Pansini M (2015) Deep sponge communities of the Gulf of St Eufemia (Calabria, southern Tyrrhenian Sea), with description of two new species. J Mar Biol Assoc UK 95:1371–1387. https://doi.org/10.1017/S0025315413001380
Bertolino M, Cattaneo-Vietti R, Costa G, Pansini M, Fraschetti S, Bavestrello G (2017a) Have climate changes driven the diversity of a Mediterranean coralligenous sponge assemblage on a millennial timescale? Palaeogeogr Palaeoclimatol Palaeoecol 487:355–363. https://doi.org/10.1016/j.palaeo.2017.09.020
Bertolino M, Costa G, Carella M, Cattaneo-Vietti R, Cerrano C, Pansini M, Quarta G, Calcagnile L, Bavestrello G (2017b) The dynamics of a Mediterranean coralligenous sponge assemblage at decennial and millennial temporal scales. PLoS One 12:0177945. https://doi.org/10.1371/journal.pone.0177945
Bianchi CN, Corsini-Foka M, Morri C, Zenetos A (2014) Thirty years after-dramatic change in the coastal marine habitats of Kos Island (Greece), 1981–2013. Mediterr Mar Sci 15:482–497. https://doi.org/10.12681/mms.678
Bianchi CN, Morri C (2000) Marine biodiversity of the Mediterranean Sea: situation, problems and prospects for future research. Mar Pollut Bull 40:367–376. https://doi.org/10.1016/S0025-326X(00)00027-8
Bianchi CN, Morri C, Chiantore M, Montefalcone M, Parravicini V, Rovere A (2012) Mediterranean Sea biodiversity between the legacy from the past and a future of change. Life in the Mediterranean Sea: a look at habitat changes:1–55
Bo M, Bertolino M, Bavestrello G, Canese S, Giusti M, Angiolillo M, Pansini M, Taviani M (2012) Role of deep sponge grounds in the Mediterranean Sea: a case study in southern Italy. Hydrobiologia 687:163–177. https://doi.org/10.1007/s10750-011-0964-1
Bonaviri C, Fernández TV, Fanelli G, Badalamenti F, Gianguzza P (2011) Leading role of the sea urchin Arbacia lixula in maintaining the barren state in southwestern Mediterranean. Mar Biol 158:2505. https://doi.org/10.1007/s00227-011-1751-2
Brien P, Lévi C, Sara M, Tuzet O, Vacelet J, Grassé PP (1973) Spongiaires. Traité de Zoologie 3:1–716
Brind'Amour A, Laffargue P, Morin J, Vaz S, Foveau A, Le Bris H (2014) Morphospecies and taxonomic sufficiency of benthic megafauna in scientific bottom trawl surveys. Cont Shelf Res 72:1–9. https://doi.org/10.1016/j.csr.2013.10.015
Bulleri F, Bertocci I, Micheli F (2002) Interplay of encrusting coralline algae and sea urchins in maintaining alternative habitats. Mar Ecol Prog Ser 243:101–109. https://doi.org/10.3354/meps243101
Cárdenas CA, Davy SK, Bell JJ (2016) Influence of canopy-forming algae on temperate sponge assemblages. J Mar Biol Assoc UK 96:351–362. https://doi.org/10.1017/S0025315414002057
Cathalot C, Van Oevelen D, Cox TJ, Kutti T, Lavaleye M, Duineveld G, Meysman FJ (2015) Cold-water coral reefs and adjacent sponge grounds: hotspots of benthic respiration and organic carbon cycling in the deep sea. Front Mar Sci 2:37. https://doi.org/10.3389/fmars.2015.00037
Cebrian E, Uriz MJ (2006) Grazing on fleshy seaweeds by sea urchins facilitates sponge Cliona viridis growth. Mar Ecol Prog Ser 323:83–89. https://doi.org/10.3354/meps323083
Clarke KR (1993) Nonparametric multivariate analyses of changes in community structure. Aust J Ecol1 8:117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Claudet J, Fraschetti S (2010) Human-driven impacts on marine habitats: a regional meta-analysis in the Mediterranean Sea. Biol Conserv 143:2195–2206. https://doi.org/10.1016/j.biocon.2010.06.004
Coll M, Piroddi C, Steenbeek J, Kaschner K, Lasram FBR, Aguzzi J, Ballesteros E, Bianchi CN, Corbera J, Dailianis T, Danovaro R, Estrada M, Froglia C, Galil BS, Gasol JM, Gertwagen R, Gil J, Guilhaumon F, Kesner-Reyes K, Kitsos MS, Koukouras A, Lampadariou N, Laxamana E, López-Fé de la Cuadra CM, Lotze HK, Martin D, Mouillot D, Oro D, Raicevich S, Rius-Barile J, Saiz-Salinas JI, San Vicente C, Somot S, Templado J, Turon X, Vafidis D, Villanueva R, Voultsiadou E (2010) The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS One 5:11842. https://doi.org/10.1371/journal.pone.0011842
Coma R, Ribes M, Serrano E, Jiménez E, Salat J, Pascual J (2009) Global warming-enhanced stratification and mass mortality events in the Mediterranean. Proc Natl Acad Sci 106:6176–6181. https://doi.org/10.1073/pnas.0805801106
Corriero G, Gherardi M, Giangrande A, Longo C, Mercurio M, Musco L, Marzano CN (2004) Inventory and distribution of hard bottom fauna from the marine protected area of Porto Cesareo (Ionian Sea): Porifera and Polychaeta. Ital J Zool 71:237–245. https://doi.org/10.1080/11250000409356578
Corriero G, Pansini M, Sarà M (1984) Sui poriferi della insenatura della Strea a Porto Cesareo (Lecce) Thalassia Salentina 14:3–10
Corriero G, Scalera Liaci L, Ruggiero D, Pansini M (2000) The sponge community of a semi-submerged Mediterranean cave. Mar Ecol 21:85–96. https://doi.org/10.1046/j.1439-0485.2000.00655.x
Costa G, Bertolino M, Pinna S, Bonaviri C, Padiglia A, Zinni M, Pronzato R, Manconi R (2018) Mediterranean sponges from shallow subtidal rocky reefs: Cystoseira canopy vs barren grounds. Estuar Coast Shelf S 207:293–302. https://doi.org/10.1016/j.ecss.2018.04.002
Cuttelod A, García N, Malak DA, Temple HJ, Katariya V (2009) The Mediterranean: a biodiversity hotspot under threat. Wildlife in a Changing World - an analysis of the 2008 IUCN Red List of Threatened Species 89
Danovaro R (2013) Biologia marina. Biodiversità e funzionamento degli ecosistemi marini. Città Studi Edizioni, Turin, Italy
Davis AR, Roberts DE, Cummins SP (1997) Rapid invasion of a sponge-dominated deep-reef by Caulerpa scalpelliformis (Chlorophyta) in Botany Bay, New South Wales. Aust J Ecol 22:146–150. https://doi.org/10.1111/j.1442-9993.1997.tb00653.x
Dayton P, Jarrell S, Kim S, Thrush S, Hammerstrom K, Slattery M, Parnell E (2016) Surprising episodic recruitment and growth of Antarctic sponges: implications for ecological resilience. J Exp Mar Biol Ecol 482:38–55. https://doi.org/10.1016/j.jembe.2016.05.001
Dayton PK, Robilliard GA, Paine RT, Dayton LB (1974) Biological accommodation in the benthic community at McMurdo Sound, Antarctica. Ecol Monogr 44:105–128. https://doi.org/10.2307/1942321
De Voogd NJ, Cleary DF (2008) An analysis of sponge diversity and distribution at three taxonomic levels in the Thousand Islands/Jakarta Bay reef complex, West-Java, Indonesia. Mar Ecol 29:205–215. https://doi.org/10.1111/j.1439-0485.2008.00238.x
Downey RV, Fuchs M, Janussen D (2018) Unusually diverse, abundant and endemic deep–sea sponge fauna revealed in the Sea of Okhotsk (NW Pacific Ocean). Deep-Sea Res Pt II 154:47–58. https://doi.org/10.1016/j.dsr2.2018.02.005
Fanelli G, Piraino S, Belmonte G, Geraci S, Boero F (1994) Human predation along Apulian rocky coasts (SE Italy): desertification caused by Lithophaga lithophaga (Mollusca) fisheries. Mar Ecol Prog Ser 1–8. https://doi.org/10.1016/0006-3207(96)83262-9
Farnham ES, Bell JJ (2018) Spatial variation in a shallow-water sponge-dominated reef in Timor-Leste (East Timor). Pac Sci 72:233–244. https://doi.org/10.2984/72.2.5
Gerovasileiou V, Voultsiadou E (2012) Marine caves of the Mediterranean Sea: a sponge biodiversity reservoir within a biodiversity hotspot. PLoS One 7:e39873. https://doi.org/10.1371/journal.pone.0039873
Göcke C, Janussen D (2013) Sponge assemblages of the deep Weddell Sea: ecological and zoogeographic results of ANDEEP I–III and SYSTCO I expeditions. Polar Biol 36:1059–1068. https://doi.org/10.1007/s00300-013-1329-1
Goodwin CE, Berman J, Downey RV, Hendry KR (2017) Carnivorous sponges (Porifera: Demospongiae: Poecilosclerida: Cladorhizidae) from the Drake Passage (Southern Ocean) with a description of eight new species and a review of the family Cladorhizidae in the Southern Ocean. Invertebr Syst 31:37–64. https://doi.org/10.1071/IS16020
Guichard F, Bourget E (1998) Topographic heterogeneity, hydrodynamics, and benthic community structure: a scale-dependent cascade. Mar Ecol Prog Ser 171:59–70. https://doi.org/10.3354/meps171059
Guidetti P (2011) The destructive date-mussel fishery and the persistence of barrens in Mediterranean rocky reefs. Mar Pollut Bull 62:691–695. https://doi.org/10.1016/j.marpolbul.2011.01.029
Guidetti P, Fraschetti S, Terlizzi A, Boero F (2003) Distribution patterns of sea urchins and barrens in shallow Mediterranean rocky reefs impacted by the illegal fishery of the rock-boring mollusc Lithophaga lithophaga. Mar Biol 143:1135–1142. https://doi.org/10.1007/s00227-003-1163-z
Hay ME (1981) The functional morphology of turf-forming seaweeds: persistence in stressful marine habitats. Ecology 62:739–750. https://doi.org/10.2307/1937742
Heip C, Hummel H, van Avesaath P, Appeltans W, Arvanitidis C, Aspden R, Austen M, Boero F, Bouma TJ, Boxshall G, Buchholz F, Crowe T, Delaney A, Deprez T, Emblow C, Feral JP, Gasol JM, Gooday A, Harder J, Ianora A, Kraberg A, Mackenzie B, Ojaveer H, Paterson D, Rumohr H, Schiedek D, Sokolowski A, Somerfield P, Sousa Pinto I, Vincx M, Weslawski J, Nash R (2009) Marine biodiversity and ecosystem functioning. Printbase, Dublin, Ireland
Heyward AA, Fromont JJ, Schoenberg CC, Colquhoun JJ, Radford BB, Gomez OO (2010) The sponge gardens of Ningaloo reef, Western Australia. Open Mar Biol J 4:3–11. https://doi.org/10.2174/1874450801004010003
Hoegh-Guldberg O, Bruno JF (2010) The impact of climate change on the world’s marine ecosystems. Science 328:1523–1528. https://doi.org/10.1126/science.1189930
Hogg MM, Tendal OS, Conway KW, Pomponi SA, van Soest RWM, Gutt J, Krautter M, Roberts JM (2010) Deep-seas Sponge grounds: reservoirs of biodiversity. UNEP Document Repository. http://hdl.handle.net/20.500.11822/8579
Hooper JN, van Soest RW (2002) Systema Porifera. A guide to the classification of sponges. Springer, Boston, USA
Howell KL, Piechaud N, Downie AL, Kenny A (2016) The distribution of deep-sea sponge aggregations in the North Atlantic and implications for their effective spatial management. Deep-Sea Res Part 1 Oceanogr res pap 115:309–320. https://doi.org/10.1016/j.dsr.2016.07.005
Jackson JBC, Buss LEO (1975) Alleopathy and spatial competition among coral reef invertebrates. Proc Natl Acad Sci 72:5160–5163. https://doi.org/10.1073/pnas.72.12.5160
Kelmo F, Bell JJ, Attrill MJ (2013) Tolerance of sponge assemblages to temperature anomalies: resilience and proliferation of sponges following the 1997–8 El-Nino southern oscillation. PLoS ONE 8:76441. https://doi.org/10.1371/journal.pone.0076441
Klitgaard AB, Tendal OS (2004) Distribution and species composition of mass occurrences of large-sized sponges in the northeast Atlantic. Prog Oceanogr 61:57–98. https://doi.org/10.1016/j.pocean.2004.06.002
Knapp IS, Williams GJ, Carballo JL, Cruz-Barraza JA, Gardner JP, Bell JJ (2013) Restriction of sponges to an atoll lagoon as a result of reduced environmental quality. Mar Pollut Bull 66:209–220. https://doi.org/10.1016/j.marpolbul.2012.08.017
Kraufvelin P, Lindholm A, Pedersen MF, Kirkerud LA, Bonsdorff E (2010) Biomass, diversity and production of rocky shore macroalgae at two nutrient enrichment and wave action levels. Mar Biol 157:29–47. https://doi.org/10.1007/s00227-009-1293-z
Krautter M, Conway KW, Barrie JV, Neuweiler M (2001) Discovery of a “living dinosaur”: globally unique modern hexactinellid sponge reefs off British Columbia, Canada. Facies 44:265–282. https://doi.org/10.1007/BF02668178
Kutti T, Bannister RJ, Fosså JH (2013) Community structure and ecological function of deep-water sponge grounds in the Traenadypet MPA—northern Norwegian continental shelf. Cont Shelf Res 69:21–30. https://doi.org/10.1016/j.csr.2013.09.011
Lejeusne C, Chevaldonné P, Pergent-Martini C, Boudouresque CF, Pérez T (2010) Climate change effects on a miniature ocean: the highly diverse, highly impacted Mediterranean Sea. Trends Ecol Evol 25:250–260. https://doi.org/10.1016/j.tree.2009.10.009
Longo C, Cardone F, Pierri C, Mercurio M, Mucciolo S, Marzano CN, Corriero G (2018) Sponges associated with coralligenous formations along the Apulian coasts. Mar Biodivers 48:2151–2163. https://doi.org/10.1007/s12526-017-0744-x
Maldonado M, Aguilar R, Bannister RJ, Bell JJ, Conway KW, Dayton PK, Díaz C, Gutt J, Kelly M, Kenchington ELR, Leys SP, Pomponi SA, Rapp HT, Rützler K, Tendal OS, Vacelet J, Young CM (2017) Sponge grounds as key marine habitats: a synthetic review of types, structure, functional roles, and conservation concerns. Marine Animal Forests, Springer, Cham:1–39. https://doi.org/10.1007/978-3-319-21012-4_24
Mangia C, Martano P, Miglietta MM, Morabito A, Tanzarella A (2004) Modelling local winds over the Salento peninsula. Meteorol Appl 11:231–244. https://doi.org/10.1017/S135048270400132X
McIntyre FD, Drewery J, Eerkes-Medrano D, Neat FC (2016) Distribution and diversity of deep-sea sponge grounds on the Rosemary Bank Seamount, NE Atlantic. Mar Biol 163:143. https://doi.org/10.1007/s00227-016-2913-z
McQuaid CD, Branch GM (1985) Trophic structure of rocky intertidal communities: response to wave action and implications for energy flow. Mar Ecol Prog Ser 22:153–161. https://doi.org/10.3354/meps022153
Micaroni V, Strano F, Di Franco D, Crocetta F, Grech D, Piraino S, Boero F (2018a) Project Biodiversity MARE Tricase: a biodiversity inventory of the coastal area of Tricase (Ionian Sea, Italy)- Mollusca: Heterobranchia. Eur Zool J 85(1):180–193. https://doi.org/10.1080/24750263.2018.1462413
Micaroni V, Strano F, Di Franco D, Langeneck J, Gravili C, Bertolino M, Costa G, Rindi F, Froglia C, Crocetta F, Giangrande A, Nicoletti L, Medagli P, Zuccarello V, Arzeni S, Bo M, Betti F, Mastrototaro F, Lattanzi L, Piraino S, Boero F (2018b) Project BiodiversityMARE Tricase: biodiversity research, monitoring and promotion at MARE Outpost (Apulia, Italy). Rend Lincei-Sci Fis Nat 29(3):599–604. https://doi.org/10.1007/s12210-018-0726-3
Milanese M, Chelossi E, Manconi R, Sara A, Sidri M, Pronzato R (2003) The marine sponge Chondrilla nucula Schmidt, 1862 as an elective candidate for bioremediation in integrated aquaculture. Biomol Eng 20:363–368. https://doi.org/10.1016/S1389-0344(03)00052-2
Murillo FJ, Muñoz PD, Cristobo J, Ríos P, González C, Kenchington E, Serrano A (2012) Deep-sea sponge grounds of the Flemish Cap, Flemish Pass and the Grand Banks of Newfoundland (Northwest Atlantic Ocean): distribution and species composition. Mar Biol Res 8:842–854. https://doi.org/10.1080/17451000.2012.682583
Norström AV, Nyström M, Lokrantz J, Folke C (2009) Alternative states on coral reefs: beyond coral-macroalgal phase shifts. Mar Ecol Prog Ser 376:295–306. https://doi.org/10.3354/meps07815
Palumbi SR (1985) Spatial variation in an alga-sponge commensalism and the evolution of ecological interactions. Amer Nat 126:267–274. https://doi.org/10.1086/284414
Pansini M, Manconi R, Pronzato R (2011) Porifera I. Calcarea, Demospongiae (partim), Hexactinellida, Homoscleromorpha. Calderini-Il Sole 24 Ore, Bologna, Italy
Parenzan P (1973) Biocenosi bentoniche della costa neretina da Porto Cesareo a Gallipoli (Golfo di Taranto). 5° Congresso Nazionale della Società Italiana di Biologia Marina, Nardò
Parenzan P (1983) Puglia marittima: aspetti geologici e biologia marina (20 anni di ricerche naturalistiche nei mari pugliesi). Congedo Editore, Galatina, Italy
Parenzan P (1984) L'insenatura della Strea di Porto Cesareo. Thalassia Salentina 14:28–38
Pérès JM, J Picard (1964) Nouveau manuel de bionomie benthique de la mer Méditerranée. Recueil des Travaux de la Station marine d’Endoume 31:1–137
Piazzi L, Pardi G, Cinelli F (1999) Algal vertical zonation and seasonal dynamics along a subtidal cliff on Gorgona Island (Tuscan Archipelago, Italy). Plant Biosyst 133:3–13. https://doi.org/10.1080/11263509909381528
Privitera D, Chiantore M, Mangialajo L, Glavic N, Kozul W, Cattaneo-Vietti R (2008) Inter-and intra-specific competition between Paracentrotus lividus and Arbacia lixula in resource-limited barren areas. J Sea Res 60:184–192. https://doi.org/10.1016/j.seares.2008.07.001
Rivaro P, Ianni C, Massolo S, Ruggieri N, Frache R (2004) Spatial and seasonal variability of dissolved oxygen and nutrients in the Southern Adriatic coastal waters. Chem Ecol 20:279–307. https://doi.org/10.1080/02757540410001670191
Rivetti I, Fraschetti S, Lionello P, Zambianchi E, Boero F (2014) Global warming and mass mortalities of benthic invertebrates in the Mediterranean Sea. PLoS ONE 9:115655. https://doi.org/10.1371/journal.pone.0115655
Rosenberg R (1995) Benthic marine fauna structured by hydrodynamic processes and food availability. J Sea Res 34:303–317. https://doi.org/10.1016/0077-7579(95)90040-3
Russo GF, Cicogna F (1991) In: Boudouresque CF, Avon M, Gravez V (eds) The date mussel (Lithophaga lithophaga), a 'case' in the gulf of Naples. Les espèces marines à protéger, en Méditerranée, pp 141–150
Rützler K (1978) Sponges in coral reefs. In Stoddart D.R. and Johannes R.E. (eds) Coral Reefs: Research Methods. Monographs on Oceanografic methodologies. Paris, UNESCO 5
Schlacher TA, Williams A, Althaus F, Schlacher-Hoenlinger MA (2010) High-resolution seabed imagery as a tool for biodiversity conservation planning on continental margins. Mar Ecol 31:200–221. https://doi.org/10.1111/j.1439-0485.2009.00286.x
Sebens KP (1991) Habitat structure and community dynamics in marine benthic systems. In: Habitat structure. Springer, Berlin, pp 211–234. https://doi.org/10.1007/978-94-011-3076-9_11
Solazzi A (1967) Primi dati sulle alghe macroscopiche bentoniche della Costa Neretina. Giorn Bot Ital 101:425–426. https://doi.org/10.1080/11263506409430539
Solazzi A (1968) Flora e vegetazione macroscopica bentonica della costa neretina (Lecce). Atti e relazioni Acc Pugliese delle Scienze ns Cl Sc Fis Med Nat 26:905–935
Srivastava M, Simakov O, Chapman J, Fahey B, Gauthier MEA, Mitros T, Richards GS, Conaco C, Dacre M, Hellsten U, Larroux C, Putnam NH, Stanke M, Adamska M, Darling A, Degnan SM, Oakley TH, Plachetzki DC, Zhai Y, Adamski M, Calcino A, Cummins SF, Goodstein DM, Harris C, Jackson DJ, Leys SP, Shu S, Woodcroft BJ, Vervoort M, Kosik KS, Manning G, Degnan BM, Rokhsar DS (2010) The Amphimedon queenslandica genome and the evolution of animal complexity. Nature 466:720–726. https://doi.org/10.1038/nature09201
Underwood AJ (1997) Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge, UK
Van Soest RWM, Boury-Esnault N, Hooper JNA, Rützler K, de Voogd NJ, Alvarez B, Hajdu E, Pisera AB, Manconi R, Schönberg C, Klautau M, Picton B, Kelly M, Vacelet J, Dohrmann M, Díaz MC, Cárdenas P, Carballo JL, Ríos P, Downey R (2019) World Porifera database. http://www.marinespecies.org/porifera
Vicente VP (1990) Overgrowth activity by the encrusting sponge Chondrilla nucula on a coral reef in Puerto Rico. In: Rutzler K (ed) New perspectives in sponge biology. Smithsonian Institution Press, Washington D.C., pp 436–442
Wangensteen OS, Turon X, García-Cisneros A, Recasens M, Romero J, Palacín C (2011) A wolf in sheep’s clothing: carnivory in dominant sea urchins in the Mediterranean. Mar Ecol Prog Ser 441:117–128. https://doi.org/10.3354/meps09359
Winer BJ, Brown DR, Michelis KM (1991) Statistical principles in experimental design. McGraw-Hill, New York, USA
Acknowledgements
This study was performed in the framework of the project Biodiversity MARE Tricase (http://www.biodiversitymaretricase.org/). We thank the Consorzio di Gestione Area Marina Protetta di Porto Cesareo and its staff for the authorisation to work in the marine protected area and for their support in the sampling activities. We would also like to express our gratitude to Prof G. Bavestrello and Prof J. Bell for comments that greatly improved the manuscript.
Funding
This study was partially supported by the PADI Foundation Grant n. 28815.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and institutional guidelines for animal testing, animal care, and use of animals were followed by the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements. The study is compliant with CBD and Nagoya protocols.
Data availability
All data generated or analysed during this study are included in this published article.
Author Contribution
FS, VM and MB conceived and designed research. FS and VM conducted field samplings and wrote the manuscript. GC collected qualitative and quantitative data from photo-quadrats and trough taxonomic identification of sponge species. IB contributed to the experimental design and to the statistical analysis. All authors contributed, read and approved the manuscript.
Additional information
Communicated by D. Janussen
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Francesca Strano and Valerio Micaroni are first co-authors.
Rights and permissions
About this article
Cite this article
Strano, F., Micaroni, V., Costa, G. et al. Shallow-water sponge grounds along the Apulian coast (central Mediterranean Sea). Mar. Biodivers. 50, 7 (2020). https://doi.org/10.1007/s12526-019-01026-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-019-01026-x