Abstract
Habitat availability underpins the diversity and distribution of benthic marine communities. Sponges are significant structural components of seabeds; therefore, understanding sponge-community associations are important for the effective management of marine biodiversity. Invertebrate communities were quantified from 11 sponge species having distinct morphologies from Ningaloo Reef (tropical) and Rottnest Island (temperate), Western Australia. Communities from substrate adjacent to sponges were additionally sampled for comparisons to sponge-associated fauna. Gross and fine-scale morphological features of sponge host species were quantified to assess their effects on faunal abundance and diversity. A total of 3966 individuals from 125 taxa were extracted, showing low co-occurrences of taxa from both sponges and the surrounding substrate (Ningaloo 8.9%; Rottnest 11.2%). Four out of the 11 sponges supported higher fauna abundance compared to their surrounding substrate, including Haliclona sp. NTM148 (Ningaloo; 1.21 ± 0.54 N.cm−3, 60 × higher than substrate) and Monanchora clathrata (Rottnest; 2.87 ± 1.7 N.cm−3, 32 × higher than substrate). These communities were dominated by the barnacle Acastinae sp.4 (100%) and sedentary polychaete Spionidae sp. 1 (99%), respectively, highlighting strong host-specific associations. Sponge size (volume), % of internal space, minimum diameter of internal space, and gross morphological complexity were important at explaining variation in faunal assemblage, with larger sponges having more internal space of larger minimum canal diameter supporting higher community abundance. This study highlights the significance of large and long-lived sponges as sources of unique marine biodiversity that are yet to be discovered and the importance of sponge gardens for the conservation of cryptic marine biodiversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sponges are important components of tropical and temperate reefs, and can dominate the benthos in biomass and cover at some locations globally (Heyward et al. 2010; Maldonado et al. 2017). The significant abundance and diversity of sponges on corals reefs is particularly apparent in the Caribbean (Díaz and Rützler 2001). Sponges in the Indo-Pacific are predicted to increase over the next 2 decades following present day losses of corals to global warming (Bell et al. 2018; Hughes et al. 2018; Pawlik and McMurray 2019). In temperate waters, sponges have been reported to naturally dominate over other invertebrate taxa in species richness and cover (Roberts and Davis 1996). While biogenic structures provided by corals and kelp forests play important roles in providing shelter, habitat and food for a variety of associated organisms (Kerry and Bellwood 2012; Araujo et al. 2013), less is known on the role of sponges as habitats to other marine biodiversity in these ecosystems.
Sponges come in diverse shapes and sizes that include encrusting, massive, erect, and cup forms (Schönberg and Fromont 2014) and offer surfaces as substrate for the attachment and refuge for other marine taxa (Frith 1976; Neves and Omena 2003). For example, barnacles of the family Acastinae have been reported to occur in several tropical and temperate species of sponges in the Red Sea, Indian Ocean, and Pacific Ocean (Ilan et al. 1999; Yu et al. 2017), highlighting the wide geographic range of this sponge–barnacle association. Certain invertebrate taxa, such as the host-specific bivalve Vulsella vulsella, have been reported to form a mutualistic partnership with the sponge Spongia sp.; whereby, while the sponge provides structural habitat for the bivalve to live in, it in turn receives exhaled water from the bivalve that increased its own water circulation rates (Tsubaki and Kato 2014). This highlights the complex interactions between the sponge host and its associated assemblage that could yet be uncovered. In addition, the complex internal system of the poriferan aquiferous canals could offer further living spaces within the sponge for some invertebrate taxa. An excellent example of such utilisation of internal space is by multiple species of alpheid shrimps that form colonies with social complexities that parallel bees and termites (Duffy 1996).
Other physical characteristics of sponges that could influence the patterns of sponge-associated biodiversity include sponge volume (Koukouras et al. 1992, 1996; Ribeiro et al. 2003; Abdo 2007; Beepat et al. 2014). While larger sponges could host higher diversity and abundance of invertebrate communities, this relationship is not overarching when multiple sponge species with varying general body size are compared (Koukouras et al. 1985; Fiore and Jutte 2010) and highlights the importance of a host species-specific approach to understanding sponge-associated fauna (SAF) diversity. Environmental factors such as habitat type and water depth were also shown to influence patterns of SAF communities (Ribeiro et al. 2003; Beepat et al. 2014). Differences in the abundance and diversity of SAF may also be a consequence of biotic interactions such as predation (Abdo 2007) and allelochemical attractants (Villamizar and Laughlin 1991; Skilleter et al. 2005). Sponge-derived chemicals have been proposed to serve as attractants to some SAF, such as amphipods (Frith 1976). However, the full extent of the effects of sponge chemistry to SAF communities is poorly understood.
Most studies reported a common pattern which showed that SAF composition comprised of polychaetes, amphipods, decapods, molluscs, and ophiuroids (Koukouras et al. 1985, 1996; Villamizar and Laughlin 1991; Neves and Omena 2003; Ribeiro et al. 2003; Abdo 2007; Fiore and Jutte 2010). These studies also reported that these marine organisms use sponges as shelter, nurseries, or food source. However, whether these associations are ubiquitous across sponge species and geography (e.g., tropical and temperate reefs), and whether these associated communities are homogenous within sponge species is less understood. In addition, previous studies had focussed primarily on describing the faunal communities associated with the sponge host, but did not provide comparisons of the sponge-associated diversity to the alternative habitats that surrounds the sponge itself; information of which can be crucial to understanding the specificity of sponge utilisation by these communities.
In Western Australia, the Ningaloo Reef is a World Heritage area and is recognized as a biodiversity hotspot, with 331 sponge species reported to date (Heyward et al. 2010; Schönberg and Fromont 2011; Fromont et al. 2016). Likewise, temperate Australia harbours a high diversity of sponges and constitutes regions of high conservation values (Sorokin et al. 2007; Lemloh et al. 2009). In this study, we describe the composition of SAF from 11 species of sponges having distinct morphologies from Ningaloo Reef (tropical reef) and Rottnest Island (temperate reef), to determine whether SAF compositions varied between sponges with different morphological characters (volume, wet weight, dry weight, internal space, diameter space, and gross complexity) and between sponge species. Substrate was also collected as a habitat control to investigate the differences of SAF composition between the host sponge and its surrounding substrate.
Materials and methods
Sampling sites and collection method
Eleven common species of sponges across eight functional morphologies were collected from depths of 8‒10 m from tropical fringing coral reefs at Norwegian and Jane Bay in the Ningaloo Marine Park in summer 2017 (4‒11 January 2017; 22°39.753′ S, 113°38.376′ E; referred to herein as Ningaloo for brevity; nspecies = 5), and from temperate reefs off Parker Point, Rottnest Island, Western Australia in summer 2018 (18‒20 November 2018; 32°1.668′ S, 115°31.739′ E; referred to herein as Rottnest for brevity; nspecies = 6; Fig. 1). Collections were both made in summer to eliminate any potential confounding effects of seasonal variation on associated fauna assemblages. Five whole individuals of Rhabdastrella globostellata (massive simple, M-s), Pseudoceratina cf. verrucosa (cup barrel, C-b), Haliclona (Haliclona) sp. NTM148 (encrusting crust, EN-cr), Ceratopsion montebelloensis (erect laminar, E-lam), and Spheciospongia vagabunda (massive cryptic, M-crp) were collected using SCUBA from Ningaloo. Similarly, five whole individuals of Petrosia (Strongylophora) sp. WAM NG1 (massive simple, M-s), Cymbastela stipitata (cup wide, C-wd), Pericharax sp. (encrusting creeping, EN-cg), Tethya sp. KMB1 (massive ball, M-bl), and Mycale (Arenochalina) cf. mirabilis (erect laminar, E-lam), and four individuals of Monanchora clathrata (erect laminar, E-lam) were collected from Rottnest (Fig. 1). Operational taxonomic unit (OTU) names were assigned to sponge hosts where the group were considered to be unique, but further assessments are required to determine their species names; whereby Haliclona (Haliclona) sp. NTM148 representing an OTU originally deposited to the Museum and Art Gallery of the Northern Territory, and Petrosia (Strongylophora) sp. WAM NG1 and Tethya sp. KMB1 were OTUs originally deposited to the Western Australian Museum.
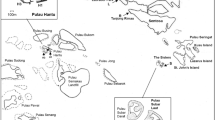
Map of the west coast of Australia showing the locations of the Ningaloo Reef and Rottnest Island sampling sites (red dots) and key townships (blue dots). The five tropical sponge species sampled from Ningaloo Reef comprised Pseudoceratina cf. verrucosa, Spheciospongia cf. vagabunda, Rhabdastrella globostellata, Ceratopsion montebelloensis and Haliclona (Haliclona) sp. NTM184. The six temperate sponge species sampled from Rottnest Island comprised Petrosia (Strongylophora) sp. WAM NG1, Pericharax sp., Cymbastela stipitata, Mycale (Arenochalina) cf. mirabilis, Tethya sp. KMB1, and Monanchora clathrata. Texts in parentheses after species names represent the functional morphology of the sponges: C-b—Cup barrel, C-wd—Cup wide, M-crp—Massive cryptic, M-s—Massive simple, M-bl—Massive ball, E-lam—Erect laminar, EN-cr—Encrusting crust, and EN-cg—Encrusting creeping (Schönberg and Fromont 2014). Inset shows an overview map of Australia with the region indicated with a dashed red bounding box
For the collections, individual sponges were first photographed in situ and a numbered Calico bag was then placed over the entire sponge. Sponges were removed from the substrate using a flat -bladed scraper and the bag immediately sealed underwater to trap any escaping biota. To assess if there were differences in the abundance and diversity of taxa between sponges and the surrounding available habitats, the most dominant substrate was additionally sampled immediately adjacent (< 1 m) to the individual sponges in separate Calico bags. The substrate types that were collected included sand, rubble, consolidated reef and macroalgae. Macroalgae occurring adjacent to the sponge were sampled using the same method used for sponge collection. For sandy and rubbly substrate, the bag was positioned onto the matrix and the top 2‒5 cm layer from an area of ~ 500‒900 cm2 transferred into the bag using a trowel. For consolidated substrate (i.e., reef), the bag was positioned over the matrix and a hammer and chisel used to chip off the surface of the reef from an area similar to that sampled for the unconsolidated substrate. The volumes of the different substrate types were determined by volumetric displacement of freshwater at 25 °C in a 1,000 ml glass graduated cylinder, whereby a displacement of 1 mL of water was assumed to be 1 cm3 of substrate volume. The wet weight of the substrate samples ranged from 26 to 680 g (mean and SE = 295.8 ± 35.8 g, n = 25) for Ningaloo and 2.8 to 207.8 g (mean and SE = 53.7 ± 7.6 g, n = 29) for Rottnest (Supplementary Table 1). The volume of substrate ranged from 15 to 350 cm3 (147.32 ± 18.51 cm3, n = 25) for Ningaloo and 3 to 110 cm3 (44.9 ± 4.0 cm3, n = 29) for Rottnest. Bags containing the sponge and substrate were preserved in 70% ethanol on board the research vessel pending the assessment of sponge-associated fauna (SAF).
Extraction and taxonomic identification of fauna from sponges and substrate
To extract fauna, preserved sponges were first removed from the Calico bag and shaken vigorously in seawater in a plastic bag to dislodge any organisms that were attached onto the sponge surface. The resulting seawater was then sieved through a 1 mm mesh to collect any fauna and these were subsequently transferred into a container with fresh 70% ethanol. Additionally, any fauna that were > 1 mm and in the Calico bag were transferred to the 70% ethanol using a fine pair of forceps. Barnacles that were embedded into the pinacoderm of the sponge were carefully excised using a scalpel blade. To extract fauna that were living internally of the sponges, sponge samples were cut to 1 cm-thick slices using a serrated knife and any fauna > 1 mm residing within the internal aquiferous canals extracted using a fine pair of forceps. Where canals could not be visually inspected due to their orientation through the 1 cm slice (e.g., at an angle through the slice) a gentle stream of seawater was applied to expel any hidden fauna. Fauna that were collected externally and internally of the sponge were consolidated within the same container of 70% ethanol, and were collectively defined as sponge-associated fauna, as external fauna here may represent diversity that were living in the sponge prior to the collection, but have exited the sponge between collection and sample preservation. SAF were stored in 70% ethanol pending taxonomic identification. For consolidated substrate types, including consolidated reef matrix and macroalgae, these were similarly vigorously agitated in seawater to dislodge biota that were attached to their surfaces and sieved through a 1 mm mesh. These substrates were then visually inspected, and any remaining biota picked using a fine pair of forceps. Unconsolidated substrate, such as sand, was thoroughly picked for any visible (> 1 mm) biota. Invertebrate fauna was identified to family, and where possible to the genus level. Within the family- and genus-level groupings, taxa having distinct morphological characters were given unique identifiers and treated as operational taxonomic units (OTUs) to facilitate further biodiversity assessment. Although OTUs cannot provide information on specific biological traits of specific species, OTU data are useful to enhance our understanding of biodiversity, distributions, and ecology of sponge-associated fauna. Species (OTU) richness and total counts of SAF were recorded for each sponge replicate.
Quantification of sponge morphological variables
To assess if sponge morphological characters had any effects on the abundance and diversity of associated biodiversity, wet and dry weight, volume, gross complexity, and the dimensions and proportion of inhabitable internal spaces (i.e., aquiferous canals) were quantified for each sponge sample. Sponge wet weight was quantified using a Shimadzu ELB3000 balance, and dry weight was quantified after drying the sponge biomass in an oven at 60 °C for 48 h. Sponge volume was determined by summing the volume of individual sponge slices calculated by volumetric displacement of freshwater at 25 °C in a 1,000 ml glass graduated cylinder, whereby a displacement of 1 mL of water was assumed to be 1 cm3 of sponge volume. The 1 cm slices of sponge tissue were photographed and images analysed using ImageJ to acquire measurements of internal spaces and gross complexity (Schindelin et al. 2012). The maximum diameter and area of aquiferous canals that were > 0.5 mm in diameter in each slice were quantified. To derive the percentage of the sponge body that comprised aquiferous canals, the total percentage of area of empty spaces were averaged across the total number of slices for each of the sponge samples. The mean, median, minimum, maximum and standard deviation of the diameter of internal spaces (mm) for each sponge sample were calculated using the total number of occurrences across all the slices.
The gross complexity index (GCI) was developed to represent sponge morphological complexity as a numerical value, in contrast to qualitative descriptions (i.e., functional morphology categories). The index was derived by dividing the surface area of a sponge by its own volume, whereby a larger index value would correspond to greater gross complexity (e.g., more convoluted or having more rugose features), thus potentially being more conducive for hosting associated fauna. Commonly used techniques for the quantification of surface areas for other benthic taxa (e.g., scleractinian corals; Veal et al. 2010) such as wax dipping, were unsuitable for sponges due to their soft and porous bodies, and this study did not have access to more advance techniques such as photogrammetry and X-ray–CT. Therefore, for sponge hosts having erect and massive body types with thickness of > 1 cm, including P. cf. verrucosa, S. cf. vagabunda, R. globostellata, P. (Strongylophora) sp. WAM NG1, Pericharax sp., M. (Arenochalina) cf. mirabilis, Tethya sp. KMB1, and M. clathrata, the surface area of individual sponges was estimated using the aforementioned 1 cm slices, and employing the formula: SA = ∑ (Ca*), (Cb*1)…(Cz*1), whereby SA is the surface area in cm2, C is the circumference of slice a to z, and 1 is the thickness of individual slices in cm. For sponges having thin (< 1 cm) lamellate body types (including C. montebelloensis and C. stipitata), surface areas were calculated as the sum of area of all exposed lamellar surfaces. Likewise, for H. (Haliclona) sp. NTM184, which occurred as thin crusts on the reef matrix, surface area was calculated from the exposed surface of the sponge not attached to the substrate. While our approach may not generate the accuracy of surface area estimates that are comparable to techniques developed for rigid bodied taxa, these estimates are nevertheless useful for quantitative assessments of the effects of gross morphologies on sponge-associated fauna communities.
Statistical analyses
All multivariate statistical analyses were performed in PRIMER v7 (Clarke and Gorley 2015) and PERMANOVA + (Anderson et al. 2008) using the Bray–Curtis resemblance matrix of the fourth-root transformed biodiversity data and 9999 permutations (Clarke and Gorley 2015) unless specified. To assess the differences in associated fauna diversity among sponge host species, and between sponges and their adjacent substrates, associated fauna count data were first standardised to sample (sponge or substrate) volumes prior to analyses (N.cm−3). As singleton taxa, defined as taxon whereby only one individual occurred in one or several sponge or substrate samples, were dominant in the dataset (82 out of 125, 65.6%), analyses were performed separately on (1) the entire dataset, (2) the dataset with singleton taxa excluded, and (3) the dataset with singleton taxa only, where appropriate. A principle coordinate ordination (PCO) analysis using the entire dataset was first performed to assess broad scale patterns in associated faunal diversity across sponge species and locations, with accompanying correlation vector analysis to identify OTUs that were most associated to the cluster groupings. A two-way nested PERMANOVA was performed on the singleton excluded data, with ‘Habitat types’ nested within ‘Location’, to assess differences in associated fauna between locations and habitat types. Pairwise tests for ‘Habitat types’ within ‘Location’ were subsequently performed to assess specific differences among sponge host species and their adjacent substrates. A shade plot and group average cluster analysis of the singleton only data using the Jaccard similarity matrix were performed to assess if there were any location or habitat specific patterns in singleton taxa distribution.
To assess if sponge morphological characters were important in explaining the occurrence and abundance of associated faunal taxa, distance-based linear models (DISTLMs) were performed on raw counts of the singleton excluded data from sponges as response variables and sponge morphological data as explanatory variables (environmental). DISTLMs were broadly performed on data across all sponges across the two locations, and also separately within locations. Prior to DISTLMs, sponge morphological variables were assessed for collinearity using a draftsman plot and correlation analyses, and collinear variables omitted from the analyses (Pearson’s R > 0.9; Supplementary Table 2). The final six non-collinear test variables comprised volume (cm3), gross complexity index (GCI), mean, minimum and standard deviation diameter of internal spaces (mm), and the percentage of internal space. Morphological variables were normalised prior to performing DISTLM, and the forward, backward, and best selection procedures and AIC criterion used to identify the best model. Distance-based redundancy analyses (dbRDA) were subsequently performed to produce ordinations of the data onto a two-dimensional space and constrained by the significant explanatory variables. Pearson’s correlation analyses were performed, on univariate fauna abundance and species richness of the singleton excluded data, to assess their relationships to significant sponge morphological variables identified by DISTLM, across all sponges from both locations (broad phyla level), across sponges within locations (community level), and within sponge host species (species level).
Results
Broad patterns of sponge-associated fauna diversity
A total of 3966 individuals from 125 taxa were extracted from sponges and their surrounding habitats across Ningaloo and Rottnest. Broadly, the diversity included 37 operational taxonomic units (OTUs) of Crustacea, 27 Polychaeta, 20 Echinodermata, 12 Ascidiacea, 11 Mollusca, 6 Porifera, 5 Bryozoa, 3 Pisces, 2 Foraminifera, 1 Platyhelminthes, and 1 Sipuncula taxa (Supplementary Table 3). Sponge-associated fauna comprised 3673 individuals (92.6% of all individuals extracted from both sponge and substrate) from 67 taxa. Collectively across the two locations, the number of unique taxa that were sampled only from sponges or substrate was relatively even (52 and 59 taxa, respectively), with 14 taxa that co-occurred on both sponges and substrate. When singleton taxa were excluded from the dataset, the number of taxa that co-occurred on both sponge and substrate remained relatively consistent (12 taxa, 28% of diversity); however, sponge and substrate-specific taxa were reduced to 18 (42%) and 13 taxa (30%), respectively (Supplementary Table 3). Similar patterns of biodiversity were observed when locations were considered separately, with the co-occurrence of associated fauna (excluding singleton taxa) on both sponges and substrate at Ningaloo representing 11.1% of total diversity (ntaxa: sponge = 12, substrate = 12, both = 3), and at Rottnest representing 32% of total diversity (ntaxa: sponge = 9, substrate = 8, both = 8), highlighting that majority of the diversity were either sponge or substrate specific at the time of sampling.
A PCO plot of the entire associated fauna biodiversity data (standardised to sample volume, i.e., sponge or substrate) showed tight clustering of associated biodiversity for Ningaloo sponges and substrate, while the Rottnest samples were more widely distributed along the PCO2 axis with some overlap of biodiversity with Ningaloo samples (Fig. 2a). The species richness of associated fauna from individual samples ranged from 0 S.cm−3 (that include 6 Ningaloo sponges, 5 Ningaloo substrates, 6 Rottnest sponge and 2 Rottnest substrate samples) to 0.67 S.cm−3 in a sample of macroalgal substrate from Rottnest. Notably, substrate comprising macroalgae from Rottnest yielded the highest species richness by volume in the dataset and recorded 0.1 ± 0.2 S.cm−3 (nsample = 27). The sponge that recorded the highest species richness by volume was an individual of C. stipitata from Rottnest (0.3 S.cm−3), followed by an individual of Pericharax sp. (0.1 S.cm−3) and an individual of Tethya sp. KMB1 (0.09 S.cm−3), both also from Rottnest. The abundance of associated fauna by sample volume ranged from 0 N.cm−3 to a maximum 7.7 N.cm−3 (Fig. 2b), with the sample having the highest density of fauna recorded in an individual of the sponge M. clathrata from Ningaloo. Contrary to species richness, the abundance of associated fauna was found to be higher in sponge samples, with an individual of Haliclona sp. NTM148 from Ningaloo and another individual of M. clathrata recording 3.2 and 2.4 N.cm−3, respectively. The substrate having the highest abundance of associated fauna by volume was a sample of macroalgae from Rottnest (1.5 N.cm−3). Correlation vector analysis found the association of Leucothoidae sp. 1, Polynoidae sp., Isopoda sp. 1, and Alpheidae sp. 1 to the Rottnest diversity, and Alpheidae sp. 3 to the Ningaloo diversity (Fig. 2a; Pearson’s R > 0.2).
Principal component ordinations (PCO) of the fourth-root transformed Bray–Curtis similarity matrix of the entire associated biodiversity counts standardised to the volume of sample sponge host or substrate between locations and habitat types (sponges and substrate). Each bubble represents an individual sponge or substrate sample. a Species richness of associated fauna per volume (S.cm−3) as bubbles and b total abundance of associated per volume (N.cm−3) as bubbles. Correlation vector overlay shows taxa having Pearson’s value of R > 0.2
Sponge-specific associated fauna diversity
The overall species richness and abundance of sponge faunal assemblage was compared to the faunal assemblage from the sponge’s surrounding substrate to assess if sponges harboured higher density and/or diversity than nearby alternative habitats. The mean species richness of associated fauna was lower in all the sponge hosts from both locations when compared to their surrounding substrate, with Sp:SB S.cm−3 ratios ranging from 0.1 to 1 (Table 1). On the other hand, there were pronounced sponge species-specific patterns in the abundances of associated fauna biodiversity between sponges and their surrounding substrate at both locations, which showed both elevations and depressions in the number of associated individuals on sponges compared to substrate (Sp:Sb N.cm−3; Table 1). Three out of five sponge species at Ningaloo supported higher abundances of fauna compared to their surrounding substrate with Haliclona sp. NTM148 recording > 60 × higher mean abundance than the surrounding substrate and represented the highest difference between sponge-substrate associated faunal abundance for any of the sponge species across locations. This was followed by P. cf. verrucosa (23 ×) and R. globostellata (13 ×), highlighting these species to be important habitats for some of the associated taxa. The other two Ningaloo sponge species, S. cf. vagabunda and C. montebelloensis, did not support faunal assemblage greater than their surrounding substrate, recording Sp:Sb N.cm−3 ratios of 1 and 0.2, respectively. At Rottnest, only M. clathrata hosted more fauna (~ 32 ×) than its surrounding substrate, while the rest of the five Rottnest sponge species hosted lower abundance of fauna compared to their surrounding substrate.
Host species-specific faunal associations were assessed for Ningaloo and Rottnest sponges. For Ningaloo sponges, the mean abundances (N) of fauna ranged from 0.01 ± 0.01 N.cm−3 in S. cf. vagabunda to 1.21 ± 0.54 N.cm−3 in Haliclona sp. NTM148 (Table 1). In addition to Haliclona sp. NTM148, both R. globostellata and P. cf. verrucosa also supported relatively high abundance of fauna (0.38 ± 0.12 N.cm−3 and 0.23 ± 0.05 N.cm−3 respectively). Some of the Ningaloo sponges, were dominated by a single associated faunal group. For example, S. cf. vagabunda and Haliclona sp. NTM148 communities were represented solely by polychaete worms and crustaceans, respectively, while R. globostellata and P. cf. verrucosa communities were dominated (> 95%) by polychaete worms and crustaceans, respectively (Fig. 3 see Supplementary Table 3 for the full list of associated biodiversity). Specifically, the alpheid shrimp Alpheidae sp. 3 dominated the P. cf. verrucosa assemblage and occurred at mean abundance of 0.22 ± 0.05 N.cm−3 (95% of total abundance), while the sedentary worm Spionidae sp. 1 dominated the R. globostellata assemblage (0.37 ± 0.11 N.cm−3, 96% of total abundance; Supplementary Table 3). The barnacle Acastinae sp. 4 was the only taxa found associated to Haliclona sp. NTM148 (100% of total abundance), and similarly, the polychaete Phyllodocida sp. 3 for S. cf. vagabunda (100% of total abundance). Alpheidae sp. 3, Spionidae sp. 1, Acastinae sp. 4, and Phyllodocida sp.3 were never recorded from Ningaloo substrate samples, highlighting that these taxa were sponge specific.
Mean percentages of abundance (N) and species (OTU) richness (S) of the 11 major taxonomic groups of fauna, standardised to the sample volume (sponge or adjacent substrate samples), including the Polychaeta, Crustacea, Pisces, Ascidiacea, Mollusca, Platyhelminthes, Sipuncula, Foraminifera, Bryozoa, Porifera, and Echinodermata at Ningaloo Reef. Sponge host species a R. globostellata, b S. cf. vagabunda, c P. cf. verrucosa, d Haliclona sp. NTM148, and e C. montebelloensis. Please refer to Supplementary Table 3 for the mean counts per volume sample (N.cm−3) of the full biodiversity list
The range of mean faunal abundances among Rottnest sponge species was greater than that found for Ningaloo sponges, from 0.03 ± 0.006 in Petrosia sp. WAM NG1 to 2.87 ± 1.7 N.cm−3 in M. clathrata (Table 1). Unlike Ningaloo sponges where sponge communities may be represented by a single taxon (e.g., Haliclona sp. NTM148 and S. cf. vagabunda), sponge communities of Rottnest comprised of at least two taxa (Fig. 4). Polychaete worms, specifically Spionidae sp. 1, were found strongly associated with M. clathrata (2.84 ± 1.7 N.cm−3, 99% of total abundance, Fig. 4). Similarly, Petrosia sp. WAM NG1 were dominated by crustaceans (87.5%), with the barnacle Acastinae sp. 6 dominating other species in the Crustacea (0.02 ± 0.005 N.cm−3, 70% total abundance). Spionidae sp. 1 and Acantinae sp. 6 were not recorded from any Rottnest substrate samples. For the other four Rottnest sponges, the dominance by a particular taxa group was variable between sponge species, with the Polychaeta (24%), Crustacea (27%), and Bryozoa (21%) forming the majority of the Tethya sp. KMB1 assemblage, Crustacea (77%) for the M. cf. mirabilis assemblage, Echinodermata (65%) for the Pericharax sp. assemblage, and Foraminifera (60%) and Crustacea (40%) for the C. stipitata assemblage.
Mean percentages of abundance (N) and species (OTU) richness (S) of the 11 major taxonomic groups of fauna, standardised to the volume of samples (sponge or adjacent substrate), including the Polychaeta, Crustacea, Pisces, Ascidiacea, Mollusca, Platyhelminthes, Sipuncula, Foraminifera, Bryozoa, Porifera, and Echinodermata at Rottnest Island. Sponge host species a Tethya sp. KMB1, b M. cf. mirabilis, c Pericharax sp., d M. clathrata, e C. stipitata, and f Petrosia sp.WAM NG1. Please refer to Supplementary Table 3 for the mean counts per volume sample (N.cm−3) of the full biodiversity list
A global test of the associated faunal communities density data (N.cm−3), using a dataset that excluded singleton taxa, found that both ‘Location’ and specific ‘Habitat types’ (sponge species and adjacent substrate) affected associated faunal communities significantly (two-way nested PERMANOVA: Location, Pseudo-F(1,86) = 3.23, P = 0.001; Habitat type (Location), Pseudo-F(20,86) = 3.32, P = 0.0001). The differences found in associated faunal communities between Rottnest and Ningaloo were expected due to the large geographical separation between the two sampling locations (> 1000 km separation) spanning tropical and temperate ecosystems. Pairwise tests for ‘Habitat types’ within ‘Location’ subsequently found that the faunal communities associated with some sponge host species were significantly different to other co-occurring sponge species and their surrounding substrate (Table 2). For example, at Rottnest, the faunal assemblages associated with M. clathrata and Petrosia sp. WAM NG1 were significantly different (P < 0.05) to that found in any other sponge species and substrate (Table 2A). Similarly, faunal assemblages that were associated with R. globostellata, C. cf. verrucosa, and Haliclona sp. NTM148 at Ningaloo were significantly different (P < 0.05) to that found in any other sponge species and substrate. Pairwise analyses additionally indicated where associated fauna communities were not statistically different between specific sponge host species and their adjacent substrate. This was apparent for Tethya sp. KMB1, Pericharax sp. and C. stipitata at Rottnest, and S. cf. vagabunda and C. montebelloensis at Ningaloo (Table 2), and indicate that any differences detected between the faunal communities associated with these sponge species and other sponges, or substrate, are likely driven by local substrate effects rather than by the sponge hosts themselves. Assessment of the dataset comprising only of singleton taxa found that the majority (88%) of the diversity was found only in one sample (Fig. 5). Shade plot and cluster analyses of the singleton diversity found no clear distribution patterns of taxa to sponge host species or substrate type, at either location (Fig. 5).
Shade plot of the occurrence of singleton taxa in the dataset, showing samples (sponge and substrate) ordered based on the cluster analysis dendogram of the Jaccard similarity matrix, with the location, sample type, and sponge host species indicated at the top of the figure. Only samples that contained singleton taxa are presented. The associated faunal operational taxonomic unit (OTU) and their respective phyla are indicated on the left of the figure. Fauna are ordered by their occurrences across samples, from 1 (occurred in a single sample) to 5 (occurred in 5 samples), demarcated by the horizontal dashed blue lines
Effects of sponge morphology on associated faunal diversity
Mean sponge volume ranged from 18 cm3 in the smallest species (C. stipitata) to 532 cm3 in the largest species (Petrosia sp. WAM NG1, Table 3), with volume strongly correlated to wet and dry weights (Pearson’s r > 0.9; Supplementary Table 2). The gross complexity index (surface area/volume; GCI) identified C. montebelloensis as having the highest complexity (412.92 ± 44.45) and supported its convoluted and multi-lobed erect laminar morphology (Table 3; see Fig. 1 for an image of the sponge). The massive-simple sponge Petrosia WAM NG1 had the lowest GCI of 8.35 ± 0.85. Out of all the sponge species, P. cf. verrucosa had the highest percentage (25.06 ± 2.33%) of its body dedicated to the aquiferous system, followed by M. cf. mirabilis cf. (16.91 ± 2.66%, Table 3). The mean diameter of internal space was largest in M. cf. mirabilis cf. (9.67 ± 1.04 mm) and ranged between 1.03 and 1.34 mm for P. cf. verrucosa cf., M. clathrata and Petrosia WAM NG1 (Table 3). The rest of the sponge species had dense mesohyll and had no habitable internal spaces that were larger than 0.1 mm, and included S. cf. vagabunda, Haliclona sp. NTM148, C. montebelloensis, Tethya sp. KMB1, Pericharax sp., and C. stipitata (Table 3).
Marginal tests from DISTLM of the multivariate dataset (counts), excluding singleton associated taxa from all sponge species from Ningaloo and Rottnest and using the six non-collinear sponge morphological variables, found that most of the variables were significant at explaining faunal assemblages (see Supplementary Tables 4‒6 for the detailed summary statistics). Further sequential tests using the forward, backward, and best selection procedures all identified sponge total volume (cm3), gross complexity index (GCI), minimum diameter of internal space (mm), and the percentage of available internal space to be most relevant in explaining the patterns of sponge-associated fauna (Supplementary Table 4‒6). When SAF diversity were assessed separately within locations, DISTLMs identified sponge volume, gross complexity index, and % internal space as significant in explaining sponge-associated biodiversity for Ningaloo sponges, and only gross complexity index (GCI) was identified as significant for explaining patterns of associated diversity for Rottnest sponges (Supplementary Table 4‒6).
A dbRDA ordination of the data from the two locations constrained by sponge total volume (cm3), minimum diameter of internal space (mm), the percentage of available internal space, and gross complexity index explained 82.8% of the fitted variation and 19.6% of the total variation in the multivariate sponge-associated fauna data (Fig. 6a). There was an obvious separation of the Ningaloo P. cf. verrucosa samples along the axis of the % internal space vector, with a corresponding correlation vector overlay of the SAF occurrences identifying the snapping shrimp Alpheidae sp. 3 to have the strongest positive relationship to this morphological variable (Fig. 6a); highlighting that the occurrence and abundance of Alpheidae sp. 3 is linked to the availability of space in the sponge host. The amphipod OTU Leucothoidae sp. 1, which was found associated with most sponges and substrate at Rottnest (Supplementary Table 3), were found in higher abundances in sponges having larger volumes and having internal spaces with larger diameters such as M. cf. mirabilis (Fig. 6a).
Distance-based redundancy analysis (dbRDA) ordinations of the sponge-associated fauna data constrained by sponge morphological variables identified as significant in explaining variability in the data by DISTLM analysis, for a the dataset excluding singleton taxa across 11 sponge species from Ningaloo Reef and Rottnest Island, constrained by the percentage of internal space, sponge volume (cm3), the minimum diameter of internal space, and gross complexity index, and b data for Ningaloo sponges only, constrained by the percentage of internal space, sponge volume (cm3), and gross complexity index. Correlation vector overlays (Pearson’s R > 0.3) show the relationships of the sponge morphological variables and associated fauna compositions to the data space. dbRDA ordination for Rottnest Island alone is not presented as only a single sponge morphological variable (gross complexity index, GCI) was significant
A dbRDA ordination of only Ningaloo sponges constrained to % internal space, sponge volume, and gross complexity index explained 88.3% of the fitted variation and 33% of the total variation in the data. Similar to dbRDA analysis using the full dataset, there was an obvious separation of the P. cf. verrucosa samples driven by higher % internal space and larger volume and strong association of Alpheidae sp. 3 to this OTU (Fig. 6b). For the other four Ningaloo sponge species, separation of the data occurred along the sponge volume and gross complexity index axes, with the sedentary worm Spionidae sp.1 most strongly correlated to larger sized R. globostellata having low gross complexity index (Fig. 6b). The barnacle Acastinae sp. 1, snapping shrimp Alpheidae sp. 2 and decapod Hippidae sp.2 were correlated to a lesser extent to R. globostellata (Fig. 6b). No dbRDA was performed for Rottnest sponges as only one explanatory variable (GCI) was significant, with a negative relationship observed between GCI values and SAF richness. The R2 value from DISTLM analysis of the Rottnest sponges data only was lower (0.11) compared to that for all sponges (0.236) and Ningaloo sponges only (0.373), highlighting the weaker relationship between the sponge-associated diversity and sponge morphological variables assessed for Rottnest sponges.
Analyses using univariate dataset of the faunal assemblages, excluding singletons, found that fauna species richness was positively correlated to host volume broadly across the two locations (df = 52, r = 0.71, p < 0.0001), and also within each of the locations with the strength of the relationship larger for Ningaloo sponges (Rottnest: df = 27, r = 0.71, p < 0.0001; Ningaloo: df = 23, r = 0.77, p < 0.0001; Table4 and Supplementary Fig. 1). A positive correlation of fauna abundance volume was found but only for Ningaloo sponges (df = 23, r = 0.65, p < 0.001; Table 4). Significant correlations between fauna abundance and/ or richness and host volume were detected at the species level, but were restricted to Petrosia sp. WAM NG1 and M. clathrata at Rottnest, and P. cf. verrucosa and C. montebelloensis at Ningaloo, highlighting the importance of this relationship at the community level, and to some extent at the species level (Table 4).
A positive but weak relationship between fauna abundance to percent internal space was only found at the community level at Ningaloo (df = 24, r = 0.435, p < 0.05; Supplementary Fig. 1). No significant correlations of abundance and species richness to minimum space diameter were found at either the community or species level (Table 4). These results highlight some positive effects of internal space characteristics of sponge host on fauna abundance, at the community level at Ningaloo; however, these effects were not as pronounced as that found for volume on biodiversity. Of note, majority of the sponge host (6 out of 11 species) had no available internal space (see Table 3). A weak negative correlation of fauna species richness to host gross complexity of Rottnest sponge community was found (df = 28, r = − 0.58, p < 0.01), with a strong negative relationship found for gross complexity and abundance of Petrosia WAM NG1 at the species level (df = 4, r = − 0.91, p < 0.05; Table 4 and Supplementary Fig. 1), further highlighting the importance of body size rather than morphological complexity for associated fauna abundance and species richness. Nevertheless, a positive relationship of the abundance of associated fauna to gross complexity of Pericharax sp. was detected (df = 4, r = 0.90, p > 0.05), indicating host species-specific effects of gross complexity on associated fauna communities (Table 4, Supplementary Fig. 1).
Discussion
The invertebrate assemblages that were found living in association with sponges (sponge-associated fauna, SAF) from tropical Ningaloo Reef and temperate Rottnest Island, and across 11 sponge species with different morphologies were diverse, with approximately 3700 individuals collected from 67 taxa. Out of this diversity, 52 taxa were only found on the host and not from the immediate surrounding substrate. When singleton taxa (66% of the associated diversity) were omitted, sponge-specific taxa constituted majority of the diversity with 42% of taxa occurring only on sponges compared to that were 28% shared across sponges and substrate. While the abundance of SAF (standardised to sample volume) could reach up to 60 × of that found associated to the surrounding substrate, these assemblages were, in most cases, dominated by a single taxon. For example, the alpheid shrimp Alpheidae sp. 3 that was associated with the cup-barrel sponge Pseudoceratina cf. verrucosa, represented 95% of total SAF abundance for the host species. Similar patterns were found for the tropical Rhabdastrella globostellata and temperate Monanchora clathrata (with the polychaete Spionida sp.1 contributing to 96% and 99% of total abundance, respectively), Haliclona NTM148 (barnacle Acastinae sp.4 contributing to 100% of total abundance) and temperate Petrosia WAM NG 1 (barnacle Acastinae sp.6 contributing to 77% of total abundance). In all these cases, these associated fauna were never found in substrate samples, demonstrating that these associations were not only host specific but high numbers could be supported by the sponge host, thus highlighting the importance of sponges as habitat for some marine biodiversity in shallow tropical and temperate reefs. Broadly across Ningaloo Reef and Rottnest Island, sponge-associated fauna were generally dominated by Crustacea and Polychaeta, and support the close associations of these groups to sponges from other global regions previously studied (Amsler et al. 2009; Padua et al., 2016; Kersken et al. 2014; García-Hernández et al. 2019).
The suitability of sponges as habitat for other marine diversity could be related to several physical characteristics of the sponge. This study demonstrated that the availability of internal space offered by the sponge aquiferous canals and the minimum diameter of those canals can influence the abundance and species richness of SAF. Specifically, the high abundance of the mobile snapping Alpheid shrimps in sponges having high proportion of internal space of minimum internal canal diameter of > 1.3 mm, such as in the tropical P. cf. verrucosa, highlights the importance of the sponge aquiferous system in providing refugia for these colony forming decapod crustaceans (Duffy 1996; Koukouras et al. 1996). Importantly, the presence of gravid female shrimps in our samples was consistent to studies that reported sponges as important nurseries for SAF communities (Ribeiro et al. 2003; Abdo 2007; Padua et al., 2016). Of note, high numbers of copepod crustaceans have also been reported to internally inhabit sponges that lack a defined aquiferous canal system but having some level of internal cavity, such as in the shallow water Caribbean calcareous sponge Clathrina lutea having a simple asconoid body plan (García-Hernández et al. 2019). Likewise, snapping shrimps and polychaete worms have been reported to utilise the internal cavities of sponges at depths of hundreds of metres in the Mediterranean (Ilan et al. 1994), further highlighting the importance of sponge internal spaces for marine invertebrate diversity across depth and global regions. Due to their immense pumping capacity, sponges are important in the marine detrital cycle, particularly in shallow ecosystems, as they filter and uptake dissolved organic matter that is then made available as food source to other marine invertebrates as cellular detritus (through cellular turnover, de Goeij et al. 2013; Rix et al. 2018; Lesser et al. 2019); thus supplying an important source of food for its inhabitants (Wulff 2006).
While internal space provided by the sponge aquiferous system was ideal for hosting mobile taxa such as shrimps, some associated taxa were less reliant on this anatomical feature. This was apparent in the tropical haplosclerid sponge Haliclona (Haliclona) NTM148 which did not display any large internal habitable space, but harboured high numbers of the symbiotic barnacle Acastinae sp. 4 (up to 114 barnacles in a sponge host). While the precise settlement behaviours of sponge-associated barnacles to their hosts are presently unknown, it is likely that colonisation occurs through direct attachment to the sponge pinacoderm and burrowing into the mesohyll where all adult barnacles were found. Interestingly, high numbers of a related taxon, Acastinae sp. 6, were found in the temperate haplosclerid sponge Petrosia (Strongylophora) WAM NG1 (up to 40 barnacles in a specimen of sponge), thus supporting the strong symbiotic relationship between acastinid barnacles and haplosclerid sponges across broad geographical scales (> 1000 km; Van Syoc et al. 2015). In addition, large numbers of sedentary spionid polychaetes inhabited the surfaces of the tropical Rhabdastrella globostellata and temperate Monanchora clathrata (up to 1000 individuals on the latter species) and, interestingly, were never found on the surfaces of the other co-occurring sponge species investigated. While, the precise mechanism and specificity of symbiotic associations between these polychaete worms to those sponge species are unclear from the present study, the effect of sponge-specific allelochemical interactions that could promote or inhibit their host colonisation patterns could not be excluded (Jackson and Buss 1975; Koukouras et al. 1992; Chanas and Pawlik 1995; Skilleter et al. 2005).
Another important morphological feature of sponges that promoted faunal associations and diversity is host volume. Here, we found that larger sponges supported higher abundance and diversity of SAF, both at the level of the broader sponge assemblage (i.e., when multiple sponge host species considered) and within individual sponge species. Of note, SAF abundance and diversity to host size associations were more apparent for Ningaloo than Rottnest sponges. Specifically, at Ningaloo, larger individuals of P. verrucosa (~ 900 cm3) can host up to 5 × more individuals of alpheid shrimps, and are 4 × more diverse, than smaller conspecifics (~ 250 cm3), while larger Petrosia (Strongylophora) WAM NG 1 at Rottnest (~ 1200 cm3) could harbour up to 4 × more acastinid barnacles than smaller conspecifics (~ 350 cm3). Similarly, in a study on the associated assemblages of the sponge Spheciospongia vesparia by Westinga and Hoetjes (1981), a logarithmic increase of SAF taxa, and linear relationships of taxa abundance and biomass, with sponge volume was reported. Logically, a large-sized sponge could offer more space, both externally and internally, for SAF fauna to colonise. Likewise, a larger sponge that is assumed to be older could also be colonised by a broader suite of fauna over time, thus explaining the higher diversity of SAF that these sponges host. In some sponge species, such as Paraleucilla magna, a larger host volume did support higher diversity of SAF due to the range of microhabitats that were available from having more folds in its growth form (Padua et al. 2016). A larger body size, however, did not correspond to a higher abundance of SAF in P. magna, which was attributed to potential negative interactions of certain species occupying larger sponges that may have out-competed other co-habiting fauna. While these competitive interactions were not formally tested by Padua et al. (2016), the relatively small individual sizes of P. magna (range of 0.3‒37 cm3, compared to the mean size range of sponge species in this study of 18‒532 cm3) may indicate that the total carrying capacity for SAF for this species may have been reached across the range of size class sampled, thus inter-species interactions (e.g., competition and predation) becoming more important at structuring SAF assemblages than host physical characters alone. Nevertheless, at the sponge-community level, the findings from this present study demonstrate the importance of large and potentially long-lived sponges as habitat for marine invertebrate diversity, supporting similar reports for sponges globally (Frith 1976; Koukouras et al. 1992; Duarte and Nalesso 1996; Ribeiro et al. 2003; Cerrano et al. 2006; Ávila and Ortega-Bastida 2015), and highlights their high conservation value at both locations.
While larger sponges are better habitats for marine invertebrate taxa, the relationships between SAF abundance and diversity to sponge species gross morphological complexity were not as clear. Both positive and negative correlations of SAF abundance to sponge host morphological complexity were detected at the species level for Pericharax sp. and Petrosia sp. WAM NG1, respectively, and SAF diversity was negatively correlated to gross complexity at the community level at Rottnest. This inconsistency was further exemplified in the highly convoluted and multi-lobated erect laminar sponge C. montebelloensis from Ningaloo that recorded up to 11 × lower SAF abundance, compared to the barrel sponge P. cf. verrucosa and massive R. globostellata that were far less morphologically complex. This supports findings in a study by Koukouras et al. (1992), who used a similar surface area to biomass ratio as a proxy to complexity, which found the index to be an unreliable predictor of SAF abundance and diversity when inter-species sponge communities were compared. In contrast, several works that have utilised qualitative inter- and intra-species host complexity have reported positive correlations of SAF abundance and diversity to higher morphological complexity (Frith 1976; Koukouras et al. 1985; Klitgaard 1995; Neves and Omena 2003). These contrasting results suggest that gross morphological complexity alone may be insufficient as a predictor of SAF abundance and diversity at the host assemblage level; as the Porifera itself is a relatively diverse phylum (i.e. > 9000 described species) that possess diverse gross morphologies and skeletal construction, and complex metabolic pathways that could either promote or inhibit the colonisation by specific SAF (Bakus et al. 1986; Kubanek et al. 2002).
Compared to other habitat forming taxa such as scleractinian corals, where the diversity of associated fauna could be reliably predicted by the complexity in branching patterns of coral species and colonies (Stella et al. 2010), the ecology of habitat utilisation in sponge-dominated habitats is evidently more complex, thus requiring further detailed assessments into finer aspects of morphology such as internal habitable space, and investigations into secondary metabolite production and organism interactions. While the effects of seasonal and diurnal movements of mobile SAF on assemblage compositions were not assessed in this present study, we highlight the importance of sponges as habitats for marine invertebrates at both tropical and temperate locations in Western Australia. Of note, novel species are routinely being discovered in sponges (Musco and Giangrande 2005; Lattig and Martin 2011; Myers and George 2017) and more focus needs to be placed on sponges in biodiversity assessments considering the growing evidence of their role in harbouring cryptic diversity. More importantly, this study demonstrates the significance of large and long-lived sponges as sources of unique associated marine biodiversity that are potentially yet to be discovered and highlights the importance of protecting these unique sponge gardens and beds for the conservation of marine biodiversity.
Availability of data and material
The full dataset is provided as Supplementary Material to the manuscript.
References
Abdo DA (2007) Endofauna differences between two temperate marine sponges (Demospongiae; Haplosclerida; Chalinidae) from southwest Australia. Mar Biol 152:845–854. https://doi.org/10.1007/s00227-007-0736-7
Amsler MO, Mcclintock JB, Amsler CD, Angus RA, Baker BJ (2009) An evaluation of sponge-associated amphipods from the Antarctic Peninsula. Antarct Sci 21(6):579. https://doi.org/10.1017/S0954102009990356
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth
Araujo RM, Bartsch I, Bekkby T, Erzini K, Sousa-Pinto I (2013) What is the impact of kelp forest density and/or area on fisheries? Environ Evid 2:15. https://doi.org/10.1186/2047-2382-2-15
Ávila E, Ortega-Bastida AL (2015) Influence of habitat and host morphology on macrofaunal assemblages associated with the sponge Halichondria melanadocia in an estuarine system of the southern Gulf of Mexico. Mar Ecol 36(4):1345–1353. https://doi.org/10.1111/maec.12233
Bakus GJ, Targett NM, Schulte B (1986) Chemical ecology of marine organisms: an overview. J Chem Ecol 12:951–987. https://doi.org/10.1007/BF01638991
Beepat SS, Appadoo C, Marie DEP, Paula JPM, Çinar ME, Sivakumar K (2014) Macrofauna associated with the sponge Neopetrosia exigua (Kirkpatrick, 1900) from Mauritius. West Indian Ocean J Mar Sci 13:133–142
Bell JJ, Bennett HM, Rovellini A, Webster NS (2018) Sponges to be winners under near-future climate scenarios. Bioscience 68:955–968. https://doi.org/10.1093/biosci/biy142
Cerrano C, Calcinai B, Pinca S, Bavestrello G (2006) Reef sponges as hosts of biodiversity: cases from North Sulawesi. In Xth Coral Reef Symposium, Okinawa, pp 208–213
Chanas B, Pawlik JR (1995) Defenses of Caribbean sponges against predatory reef fish. II. Spicules, tissue toughness, and nutritional quality. Mar Ecol Prog Ser 127:195–211. https://doi.org/10.3354/meps127195
Clarke K, Gorley R (2015) Getting started with PRIMER v7. PRIMER-E: Plymouth, Plymouth Marine Laboratory
de Goeij JM, van Oevelen D, Vermeij MJ, Osinga R, Middelburg JJ, de Goeij AF, Admiraal W (2013) Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science 342:108–110. https://doi.org/10.1126/science.1241981
Díaz MC, Rützler K (2001) Sponges: an essential component of Caribbean coral reefs. Bull Mar Sci 69:535–546
Duarte L, Nalesso R (1996) The sponge Zygomycale parishii (Bowerbank) and its endobiotic fauna. Estuar Coast Shelf Sci 42:139–151. https://doi.org/10.1006/ecss.1996.0011
Duffy JE (1996) Eusociality in a coral-reef shrimp. Nature 381:512. https://doi.org/10.1038/381512a0
Fiore CL, Jutte PC (2010) Characterization of macrofaunal assemblages associated with sponges and tunicates collected off the southeastern United States. Invertebr Biol 129:105–120. https://doi.org/10.1111/j.1744-7410.2010.00184.x
Frith DW (1976) Animals associated with sponges at North Hayling, Hampshire. Zool J Linn Soc 58:353–362. https://doi.org/10.1111/j.1096-3642.1976.tb01005.x
Fromont J, Abdul Wahab M, Gomez O, Ekins M, Grol M, Hooper J (2016) Patterns of sponge biodiversity in the pilbara. Northwest Aust Divers. https://doi.org/10.3390/d8040021
García-Hernández JE, Hammerman NM, Cruz-Motta JJ, Schizas NV (2019) Associated organisms inhabiting the calcareous sponge Clathrina lutea in La Parguera. Puerto Rico Caribb J Sci 49(2–3):239–254. https://doi.org/10.18475/cjos.v49i2.a12
Heyward A, Fromont J, Schoenberg C, Colquhoun J, Radford B, Gomez O (2010) The sponge gardens of Ningaloo reef, Western Australia. Open Mar Biol J 4:3–11. https://doi.org/10.2174/1874450801004010003
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80–83. https://doi.org/10.1126/science.aan8048
Ilan M, Ben-Eliahu MN, Galil B (1994) Three deep water sponges from the eastern Mediterranean and their associated fauna. Ophelia 39(1):45–54. https://doi.org/10.1080/00785326.1994.10429901
Ilan M, Loya Y, Kolbasov G, Brickner I (1999) Sponge-inhabiting barnacles on Red Sea coral reefs. Mar Biol 133:709–716. https://doi.org/10.1007/s002270050512
Jackson J, Buss L (1975) Alleopathy and spatial competition among coral reef invertebrates. PNAS 72:5160–5163. https://doi.org/10.1073/pnas.72.12.5160
Kerry J, Bellwood D (2012) The effect of coral morphology on shelter selection by coral reef fishes. Coral Reefs 31:415–424. https://doi.org/10.1007/s00338-011-0859-7
Kersken D, Göcke C, Brandt A, Lejzerowicz F, Schwabe E, Seefeldt MA, Veit-Köhler JD (2014) The infauna of three widely distributed sponge species (Hexactinellida and Demospongiae) from the deep Ekström Shelf in the Weddell Sea, Antarctica. Deep Sea Res Part II 108:101–112. https://doi.org/10.1016/j.dsr2.2014.06.005
Klitgaard AB (1995) The fauna associated with outer shelf and upper slope sponges (Porifera, Demospongiae) at the Faroe Islands, northeastern Atlantic. Sarsia 80:1–22. https://doi.org/10.1080/00364827.1995.10413574
Koukouras A, Voultsiadou-Koukoura E, Chintiroglou H, Dounos C (1985) Benthic bionomy of the North Aegean Sea lll. A comparison of the macobenthic animal assemblages associated with seven sponge species. Cah Biol Mar 26:301–319
Koukouras A, Russo A, Voultsiadou-Koukoura E, Dounas C, Chintiroglou C (1992) Relationship of sponge macrofauna with the morphology of their hosts in the North Aegean Sea. Int Rev Ges Hydrobiol Hydrogr 77:609–619. https://doi.org/10.1002/iroh.19920770406
Koukouras A, Russo A, Voultsiadou-Koukoura E, Arvanitidis C, Stefanidou D (1996) Macrofauna associated with sponge species of different morphology. Mar Ecol 17:569–582. https://doi.org/10.1111/j.1439-0485.1996.tb00418.x
Kubanek J, Whalen KE, Engel S, Kelly SR, Henkel TP, Fenical W, Pawlik JR (2002) Multiple defensive roles for triterpene glycosides from two Caribbean sponges. Oecologia 131:125–136. https://doi.org/10.1007/s00442-001-0853-9
Lattig P, Martin D (2011) Sponge-associated Haplosyllis (Polychaeta: Syllidae: Syllinae) from the Caribbean Sea, with the description of four new species. Sci Mar 75(4):733–758. https://doi.org/10.3989/scimar.2011.75n4733
Lemloh ML, Fromont J, Brummer F, Usher KM (2009) Diversity and abundance of photosynthetic sponges in temperate Western Australia. BMC Ecol 9:4. https://doi.org/10.1186/1472-6785-9-4
Lesser MP, Mueller B, Pankey MS, Macartney KJ, Slattery M, de Goeij JM (2019) Depth-dependent detritus production in the sponge Halisarca caerulea. Limnol Oceanogr 65(6):1200–1216. https://doi.org/10.1002/lno.11384
Maldonado M, Aguilar R, Bannister RJ, Bell JJ, Conway KW, Dayton PK, Díaz C, Gutt J, Kelly M, Kenchington EL (2017) Sponge grounds as key marine habitats: a synthetic review of types, structure, functional roles, and conservation concerns. In: Rossi S, Bramanti L, Gori A, Orejas C (eds) Marine animal forests: the ecology of benthic biodiversity hotspots, pp 145–183. https://doi.org/10.1007/978-3-319-21012-4_24
Musco L, Giangrande A (2005) A new sponge-associated species, Syllis mayerin. sp.(Polychaeta: Syllidae), with a discussion on the status of S. armillaris (Müller, 1776). Sci Mar 69(4):467–474. https://doi.org/10.3989/scimar.2005.69n4467
Myers AA, George AM (2017) Amphipoda living in sponges on the Great Barrier Reef, Australia (Crustacea, Amphipoda). Zootaxa 4365(5):571–584. https://doi.org/10.11646/zootaxa.4365.5.4
Neves G, Omena E (2003) Influence of sponge morphology on the composition of the polychaete associated fauna from Rocas Atoll, northeast Brazil. Coral Reefs 22:123–129. https://doi.org/10.1007/s00338-003-0295-4
Padua A, Lanna E, Klautau M (2016) Macrofauna inhabiting the sponge Paraleucilla magna (Porifera: Calcarea) in Rio de Janeiro, Brazil. J Mar Biolog Assoc UK 96(3):605. https://doi.org/10.1017/S0025315412000434
Pawlik JR, McMurray SE (2019) The emerging ecological and biogeochemical importance of sponges on coral reefs. Annu Rev Mar Sci 12:315–337. https://doi.org/10.1146/annurev-marine-010419-010807
Ribeiro SM, Omena EP, Muricy G (2003) Macrofauna associated to Mycale microsigmatosa (Porifera, Demospongiae) in Rio de Janeiro State, SE Brazil. Estuar Coast Shelf Sci 57:951–959. https://doi.org/10.1016/S0272-7714(02)00425-0
Rix L, de Goeij JM, van Oevelen D, Struck U, Al-Horani FA, Wild C, Naumann MS (2018) Reef sponges facilitate the transfer of coral-derived organic matter to their associated fauna via the sponge loop. Mar Ecol Prog Ser 589:85–96. https://doi.org/10.3354/meps12443
Roberts D, Davis A (1996) Patterns in sponge (Porifera) assemblages on temperate coastal reefs off Sydney, Australia. Mar Freshw Res 47:897–906. https://doi.org/10.1071/MF9960897
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676. https://doi.org/10.1038/nmeth.2072
Schönberg CHL, Fromont J (2011) Sponge gardens of Ningaloo Reef (Carnarvon Shelf, Western Australia) are biodiversity hotspots. Hydrobiologia 687:143–161. https://doi.org/10.1007/978-94-007-4688-6_13
Schönberg C, Fromont J (2014) Sponge functional growth forms as a means for classifying sponges without taxonomy. The Ningaloo Atlas Available at https://eatlas.org.au/node/33141 (Accessed 5 April 2019)
Skilleter GA, Russell BD, Degnan BM, Garson MJ (2005) Living in a potentially toxic environment: comparisons of endofauna in two congeneric sponges from the Great Barrier Reef. Mar Ecol Prog Ser 304:67–75. https://doi.org/10.3354/meps304067
Sorokin S, Fromont J, Currie D (2007) Demosponge biodiversity in the benthic protection zone of the Great Australian Bight. T Roy Soc South Aust 131:192–204. https://doi.org/10.1080/03721426.2007.10887083
Stella JS, Jones GP, Pratchett MS (2010) Variation in the structure of epifaunal invertebrate assemblages among coral hosts. Coral Reefs 29:957–973. https://doi.org/10.1007/s00338-010-0648-8
Tsubaki R, Kato M (2014) A novel filtering mutualism between a sponge host and its endosymbiotic bivalves. PLoS ONE 9:e108885. https://doi.org/10.1371/journal.pone.0108885
Van Syoc RJ, Van Soest RW, Xavier JR, Hooper JN (2015) A phylogenetic overview of sponge-inhabiting barnacles and their host specificity (Crustacea, Cirripedia). Proc Calif Acad Sci 4:331–357
Veal CJ, Holmes G, Nunez M, Hoegh-Guldberg O, Osborn J (2010) A comparative study of methods for surface area and three-dimensional shape measurement of coral skeletons. Limnol Oceanogr-Meth 8(5):241–253. https://doi.org/10.4319/lom.2010.8.241
Villamizar E, Laughlin R (1991) Fauna associated with the sponges Aplysina archeri and Aplysina lacunosa in a coral reef of the Archipielago de Los Roques, National Park, Venezuela. In: Reitner J, Keupp H (eds) Fossil and recent sponges. Springer, Berlin, Heidelberg, pp 522–542. https://doi.org/10.1007/978-3-642-75656-6_44
Westinga EPHC, Hoetjes PC (1981) The intrasponge fauna of Spheciospongia vesparia (Porifera, Demospongiae) at Curaçao and Bonaire. Mar Biol 62(2–3):39–150. https://doi.org/10.1007/BF00388176
Wulff JL (2006) Ecological interactions of marine sponges. Can J Zool 84:146–166. https://doi.org/10.1139/z06-019
Yu M-C, Kolbasov GA, Hosie AM, Lee T-M, Chan BK (2017) Descriptions of four new sponge-inhabiting barnacles (Thoracica: Archaeobalanidae: Acastinae). Zootaxa 4277:151–198. https://doi.org/10.11646/zootaxa.4277.2.1
Acknowledgements
We would like to thank the Master and crew of the Australian Institute of Marine Science RV Solander for facilitating fieldwork and sample collections at Ningaloo Reef. Thank you to Nick Thake and John Statton for field assistance at Ningaloo Reef and Rottnest Island, respectively. We would also to thank Belinda Alvarez for formally identifying the sponges used in this study. Collections were performed under permits provided by the Western Australian Department of Parks and Wildlife and Western Australian Fisheries, DPaW SF010984, WA Fisheries Exemption 2885 and WA Fisheries Exemption 3021. YYC was supported by a full scholarship for her MSc from the Ministry of Education of Taiwan. We would like to thank the Associate Editor and 4 Reviewers who all provided helpful comments to improve the manuscript.
Funding
YYC was supported by a full scholarship from the Ministry of Education of Taiwan. The research was partly funded by the Australian Institute of Marine Science.
Author information
Authors and Affiliations
Contributions
Y-YC conducted field and lab work, and statistical analyses, and drafted the manuscript, JP and GK contributed to the draft of the manuscript, MAAW conceived the project, conducted field and lab work, statistical analyses, and drafted the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest. All data from this research are available on request.
Ethical approval
This research was conducted in accordance with the University of Western Australia’s policies, procedures, and guidelines. No specific permissions were required. Collections were performed under permits provided by the Western Australian Department of Parks and Wildlife and Western Australian Fisheries, DPaW SF010984, WA Fisheries Exemption 2885 and WA Fisheries Exemption 3021.
Additional information
Responsible Editor: M. G. Chapman.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by J M A. Vega, G. Paulay and undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chin, YY., Prince, J., Kendrick, G. et al. Sponges in shallow tropical and temperate reefs are important habitats for marine invertebrate biodiversity. Mar Biol 167, 164 (2020). https://doi.org/10.1007/s00227-020-03771-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-020-03771-1