Abstract
The diversity of calcareous epiliths along a bathymetrical transect spanning the intertidal to bathyal (0–500 m) was investigated during a two-year carbonate cycling experiment deployed in the Faial Channel, Azores. The epilith inventory comprises 47 taxa of bryozoans, 9 serpulids, 3 bivalves, 3 cirripeds, 3 foraminiferans, 2 vermetid gastropods, and 1 scleractinian coral, along with 6 rhodophyte morphotypes. Colonised surface area, carbonate accretion rates, and biodiversity peak in the deeper euphotic zone (15 and 60 m), where mature biocoenoses were established after 2 years exposure, whereas colonisation was retarded at dysphotic and aphotic depths. Particularly in the photic zone, colonised surface area, accretion rates, as well as species richness and abundance were higher on down-facing compared to up-facing surfaces. Analyses of similarity (ANOSIM) and non-metrical multidimensional scaling (NMDS) of species abundance data revealed that water depth and substrate orientation were the dominant factors controlling the community structure as a result of direct (photosynthesis) and indirect (bioerosion pressure; nutrient supply) effects of the light regime, while exposure time and substrate type had little influence. The same hierarchy applies for the ichnodiversity of bioerosion traces, but with an inverse pattern in case of substrate orientation, reflecting the interaction of encrustation and bioerosion. Positive net carbonate production rates support the development of oyster bioherms and heterozoan-dominated carbonate/volcaniclastic sediments accumulating in the Faial Channel and adjacent slope. A comparison with biogenic sediments from other Macaronesian archipelagos and seamounts demonstrates the abundance and diversity of non-tropical heterozoan carbonates in oceanic islands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The warm-temperate ‘carbonate factory’ of the Azores Archipelago supports a heterozoan association defined by James (1997: p4) as “an association of benthic carbonate particles produced by organisms that are light-independent, plus or minus red calcareous algae”. These carbonate sediments are produced by a remarkably high biodiversity of carbonate-secreting organisms settling on available hard substrates (e.g. Martins 1990; Wisshak et al. 2010) – despite the spatial isolation from the continental shelf on both sides of the North Atlantic Ocean. These calcareous and sessile epilithobionts and episkeletobionts (sensu Taylor and Wilson 2002) are herein collectively referred to as ‘calcareous epiliths’. They comprise among others coralline algae, foraminiferans, serpulid worms, various groups of molluscs, bryozoans, crinoids, as well as scleractinian and stylasterid corals that colonise the volcanic bedrock or other epiliths. Taxonomic and ecological studies on the calcareous epiliths from Azorean waters are, however, largely restricted to shallow water in the reach of scuba divers (see Martins 1990 for a bibliography and Cardigos et al. 2006 for a compilation of non-indigenous species in the Azores). From deeper waters, a number of scientific cruises such as the classic Challenger, l’Hirondelle, and Princesse Alice expeditions (see Santos et al. 1995 for a review on the history of marine research in the Azores) have dredged benthic biota that found their way into the taxonomic literature on the respective organism groups, e.g. scleractinian corals in Zibrowius (1980), stylasterids in Zibrowius and Cairns (1992), bryozoans in Jullien and Calvet (1903), cirripeds in Young (1998) and others. Direct sea-floor observations at water depths deeper than 50 m via manned submersibles were previously limited to a number of missions with the French Archimède in the late 1960s (Laubier 1972; Pérès 1992). Most recently, the Azores came into focus of EU research consortia (e.g. HERMIONE and CoralFish) working on cold-water coral ecosystems, including ROV and submersible work on selected seamounts and island slopes (e.g. Santos et al. 2010; Braga-Henriques et al. 2012, 2013; Gomes-Pereira et al. 2012; Tempera et al. 2012).
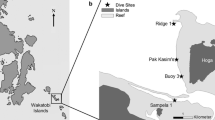
In order to study the biodiversity of hardground communities, and for budgeting carbonate production and degradation along a bathymetrical and hydrographical transect covering all photic zones from the intertidal down to aphotic bathyal depths (Fig. 1), a two-year carbonate cycling experiment was launched in 2006 with the support of the submersible ’LULA’. A general characterisation of the fauna and habitats encountered along the bathymetrical transect, the water mass properties, the light regime, and the carbonate system parameters, along with the evaluation of the carbonate budget based on the experimentally determined carbonate accretion and bioerosion rates, are given in Wisshak et al. (2010). The inventory of organisms involved in carbonate bioerosion, and the incipient trace fossils they produce, was the basis for a detailed ichnodiversity analysis (i.e. the diversity of ichnotaxa, analogous to biotaxa in biodiversity analyses) reported in Wisshak et al. (2011). Rounding up a paper trilogy we herein elaborate on a detailed biodiversity assessment of the calcareous epiliths found settling on the PVC and limestone substrates of the experimental platforms. The main factors included in this evaluation are substrate type and orientation, exposure period, and the depth distribution in relation to environmental gradients. Thereby, water depth and substrate orientation are ‘composite factors’ comprising influence of e.g. the light regime (controlling absence/presence of photoautotrophic organisms competing for space or exerting bioerosion pressure), hydrodynamic force, nutrient availability, or sedimentation rates. All of these factors are known to significantly influence larval settlement and survival of the adult organisms, thus controlling the establishment of epilithic hard bottom communities (e.g. Crisp 1974; Harris and Irons 1982; Glasby 2000; Field et al. 2007; Nicoletti et al. 2007; Davis 2009; Cowie 2010; see Wahl 2009 for a recent compilation on marine hard bottom communities and references therein). The results are integrated with the corresponding patterns observed for carbonate bioerosion as reported in Wisshak et al. (2010, 2011) for completing the picture of diversity of those hardground dwellers involved in carbonate production and degradation in the warm-temperate waters in the Azores. To put the present results in a wider perspective, a brief review of biosedimentary facies types recovered from Azorean and other Macaronesian volcanic islands and seamounts is added, providing indications of what kind of carbonate facies can be expected in this understudied non-tropical carbonate factory.
Material and methods
The experimental setup
Experimental settlement platforms equipped with up- and down-facing carbonate and PVC substrates, and a temperature data logger were mounted to cliff boulders in the intertidal zone and placed along a bathymetric transect at 0, 5, 15, 60, 150 and 500 m water depth in the southern Faial Channel by scuba diving and the submersible ‘LULA’ (Fig. 1). Two platforms were deployed at each depth station, the first one of which was recovered after one year and the second subset after 2 years of exposure. Settled calcifying organisms were recorded on five replicate settlement plates per depth, substrate type, substrate orientation, and exposure time. The PVC and limestone settlement plates were 10 × 10 × 1 cm in size, sealed on all but the upper side with epoxy resin, and mounted with a central nylon bolt in the three orientations up-facing, up-facing in a grazer exclusion cage, and down-facing.
At the 5-m station, a severe winter storm devastated one of the platforms and damaged the other, which was then recovered after only 3 months of exposure. It was thus only considered with respect to taxa records but was excluded from statistical analyses. The same applies for the grazer exclusion cages where non-calcified epiphytes on the cages and sediment baffling within the cages rendered the assessment of meaningful abundance data infeasible.
Carbonate accretion
PVC and limestone plates were treated with diluted hydrogen peroxide for up to 5 days, in order to remove most of the non-calcareous bioaccretion. Adding to the gravimetrically determined carbonate accretion rates expressed as grams per square metre and year (data from Wisshak et al. 2010; methods described therein), the carbonate accretion densities expressed as percent coverage on each settlement plate was determined with the image analysis software AnalySIS five (Olympus). This was achieved by automatically detecting the pronounced colour difference of the dark grey PVC versus the grey to white carbonate accretion on evenly lit digital photographs. Insufficient phase contrast prohibited the application of this method in case of the limestone substrates.
Epilith inventory
The spectrum of calcareous epiliths was recorded under a dissecting microscope and photographically documented applying multifocus imaging (motorised Leica M420 with Leica DFC 320 camera and IM50 software). The bryozoans were additionally examined with scanning electron microscopy (SEM) for confirming subtle, but taxonomically relevant, morphological details. For the calcareous algae, taxonomical assessment of the bleached skeletons via reflected light microscopy was not feasible and they were consequently classified by morphotypes. All other calcareous epiliths were, as far as possible, taxonomically identified to species level (bryozoans partly in open nomenclature owing to the lack of modern taxonomic revisions), and the number of individuals per settlement plate was counted. Hence, the smallest considered unit reflected in the abundance data set and addressed by the term ‘species richness’ (sensu lato) corresponds to the highest level of distinction achievable via stereo-microscopy and is herein for the sake of simplicity referred to as ‘taxon’. In some cases a taxon may thus comprise two or more species. The assessed specimen numbers, respectively colony numbers in case of bryozoans (see below), were averaged per settlement plate, orientation, and exposure time, before extrapolation to specimens per square metre and year. Bryozoan taxa were further classified according to their colony growth form. Erect colonies were subdivided into rigid delicate branching (de), rigid robust branching (ro), rigid fenestrate (fe), and flexible (fl) (for definitions see Nelson et al. 1988a). Encrusting colonies were subdivided into patches (pa), ribbons (ri), runners (ru), and spots (sp) (for definitions see Bishop 1989).
Statistical biodiversity analyses
Compilation of the data matrix and basic statistical analyses (sample unit means ± SD, species richness, Whittaker plots, octave class plots) was undertaken with Microsoft EXCEL. The determination of biodiversity and evenness indices as well as computation of rarefaction curves were carried out with the software package PAST 2.03 (Hammer et al. 2001; 2010) following the guidelines in Hammer and Harper (2006). Calculated indices include species richness (S), the total and the mean number of individuals (n), the Simpson index of dominance (D), the Shannon index (H’), and the Shannon index normalised for species richness known as Pielou’s equitability (J). All of these indices were computed for non-transformed abundance data excluding unspecified foraminiferans, as well as juvenile coralline algae, serpulids, and bryozoans. Rarefaction curves including the 95 % confidence interval (individual rarefaction) were computed using the total sum of individuals per depth station comprising both exposure periods.
Normal distribution of the abundance data set was rejected applying the Shapiro-Wilk test with the software STATISTICA 9, calling for a non-parametrical community structure analysis. To test for significant differences in the community composition between depths, orientations, substrate types, and exposure times, analysis of similarity (ANOSIM) with 999 permutations was applied with the software PRIMER 6.1.3. These were computed via a Bray-Curtis similarity matrix based on log-transformed abundance data in order to reduce the influence of taxa with very high abundances (Clarke and Warwick 2001). Additionally, non-metric multidimensional scaling plots (NMDS) were computed to visualise similarity clusters and for indirect gradient analysis (Minchin 1987).
Sediment sampling
To broaden the scope of this settlement experiment, we included a sediment sample taken with LULA at the 150 m station in the Faial Channel, complemented by a selection of sediment samples taken with a Van Veen grab during RV Victor Hensen cruise VH97 on various Macaronesian seamounts and islands. The samples were sieved under running water for separating the different grain size classes. The facies designation of these samples is based on the coarse-grained (>2 mm) sediment fraction.
Results and discussion
Carbonate accretion
When visually comparing the experimental substrates after bleaching, distinct differences in carbonate accretion patterns are obvious in respect of water depth. Differences concerning substrate orientation are less obvious and mainly reflected by species composition, whereas a temporal pattern is not evident at first sight (Fig. 2). The carbonate accretion was quantified in two complementing ways: by the gravimetrical determination of carbonate accretion rates expressed in grams carbonate built up per square metre and year, reported in Wisshak et al. (2010), and as accretion densities expressed as percent coverage reported herein (results of both methods are compiled in Table 1 and Fig. 3 for comparison). Carbonate accretion densities vary from virtually zero to a maximum mean of about 83 % on the down-facing PVC substrates at the 60 m station after one and two years of exposure, respectively. Mean densities are generally highest at the 15- and 60-m stations, and higher on the down-facing compared to the up-facing substrates. In the intertidal and 150-m stations, less than 10 %, and at the bathyal 500-m station less than 1 % of the surface area were covered by calcareous epiliths (Fig. 3a, c). This pattern closely mirrors the carbonate accretion rates reported in Wisshak et al. 2010 (Fig. 3b-c). The coefficient of variation (CV) of the accretion density is lowest on the most densely colonised substrates at the 15- and 60-m station, with values between 5 and 25 %. The sparsely colonised intertidal and 500-m stations, in contrast, yield heterogeneous values in the range of 14 to 167 %, reflecting a considerable patchiness in colonisation. The mean accretion density naturally increased with exposure time except for the up-facing substrates at the 15-m station where less surface area was covered on the platforms deployed for 2 years (Fig. 3a). This pattern parallels the obtained accretion rates only partially with contradicting trends at the intertidal and at the 500-m stations (Fig. 3b). This is because the accretion rates do not solely depend on the available surface area, but also on the density and height of the encrusting skeletons. The increase in surface area cover within the second year of exposure is comparatively low (Fig. 3a), and an increase in mean accretion rate, such as at the 60-m station, was mainly realised in the vertical axis.
Mean carbonate accretion rates and accretion densities (see Table 1 for SDs) along the bathymetrical transect from the intertidal down to bathyal 500 m depth; a mean accretion densities (% of surface cover) for the two exposure periods and substrate orientations; b mean accretion rates (data from Wisshak et al. 2010) for the two exposure periods and substrate orientations; c mean total carbonate accretion rates (data from Wisshak et al. 2010) and accretion densities
Epilith inventory and abundance
In the following account, the spectrum of taxa in the various groups of organisms is outlined with respect to their occurrence and abundance along the bathymetrical transect, their substrate orientation or type, and the exposure time. A complete list of the recorded taxa and a semi-quantitative summary of the fully quantitative abundance matrix is given in Table 2. Species richness (excluding juveniles, the records of the damaged 5-m platform, and records from the caged substrates) and biodiversity indices for the various organism groups are given in Table 3, and are graphically displayed in Fig. 4. Special emphasis is put on bryozoans as the most diverse organism group, for which additional numerical data are given in Table 4.
Coralline algae
For the quantitative analysis the coralline algae were distinguished by their morphotypes since characters required for identification to generic or even species level are not discernible with confidence via stereo-microscopy only. At least two of the six recorded morphotypes contain more than a single species. Tentative identifications can be made for ‘morphotype 1’ (Lithophyllum sp. + L ithophyllum pustulatum), ‘morphotype 2’ (Lithophyllum sp.), ‘morphotype 3’ (Hydrolithon sp.), and ‘morphotype 6’ (Corallina elongata + Haliptilon sp.). With the exception of ‘morphotype 4’, which was absent at 0 m, all morphotypes were present from 0 to 60 m. At the latter station, only the illuminated up-facing substrates were colonised by the phototrophic algae. This light-dependent orientation preference is also seen at the 15-m station, with the highest abundance of most morphotypes recorded on the up-facing substrates and only very rare occurrences on the down-facing substrates (‘morphotype 4’ and ‘morphotype 6’ only). The occurrence or mean abundance was similar between the 1 and 2 yrs platforms as well as between the limestone and the PVC substrates (Tables 2–3).
Foraminiferans
Sessile foraminiferans were common at all subtidal stations and were found particularly abundant on the up-facing substrates at the 150- and 500-m stations. However, most were too small to allow confident identification or counting at species level via stereo-microscopy, and were thus lumped for quantification purposes. Only three large and conspicuous species were discerned as Discoramulina bollii, Gypsina vesicularis, and Miniacina miniacea. The bryozoan-like D. bollii was found commonly at the 15 to 150-m stations where it showed a clear preference for down-facing substrates. In contrast, G. vesicularis was rare to very rare with a preference for up-facing substrates. M. miniacea was found most abundant on the down-facing substrates at 15 m water depth and decreased in number towards the bathyal 500-m station. Distinct differences with respect to substrate type or exposure time were not apparent (Tables 2–3).
Corals
Only few individuals (7 in total) of early ontogenetic and thus indistinguishable calyxes of the solitary scleractinians Desmophyllum sp. and Caryophyllia sp. were recorded in a depth range of 15 to 500 m. With one exception they were all found settling on the 2-year platforms and showed a preference for limestone substrates but did not exhibit a preference with respect to substrate orientation.
Serpulids
With a total of 9 species, serpulid worms represent the second-most diverse group recorded on the settlement platforms with a bathymetrical range of 15 to 500 m. The overall species richness of serpulids is highest at 150 m with a co-occurrence of 6 species. By far the most common species at the 15 to 150-m stations (both orientations and exposure times) is Spirobranchus polytrema. At 150 m it co-occurs with Hyalopomatus ?marenzelleri, which is the dominant serpulid at the bathyal 500-m station. Further relevant species are the rare Vermiliopsis striaticeps at the 15- and 60-m stations, the rare Hydroides azoricus at the 60- and 150-m stations, Filograna gracilis which is restricted to 150 m depth, the very rare living fossil Neomicrorbis azoricus found exclusively at the deepest station, and the conspicuous Vitreotubus digeronimoi which was also found restricted to the 500-m station. Neither a temporal pattern nor a substrate type preference in the occurrence and abundance of serpulids was apparent (Tables 2–3).
Molluscs
A total of 5 species were found cemented onto the experimental substrates. None were recorded in the intertidal 0-m station, and species richness declined from 3 species at 15 m to only one species at the bathyal 500-m station. The bivalve Chama gryphoides was very rare at the 60- and 150-m stations and was only found on the 2 year experimental platforms. Pycnodontine oysters are represented by two species – Neopycnodonte cochlear was very common at 60 and 150 m, whereas only a single juvenile specimen of its deep-sea relative Neopycnodonte zibrowii was found at the 500-m station, few metres away from the type locality of this species (Wisshak et al. 2009a). Vermetid gastropods were found at the 15- and 60-m stations on both substrate orientations and exposure times. Vermetus rugulosus co-occurs with Vermetus triquetrus at the 15-m station, whereas only the latter species was also recorded at the 60-m station. While the mollusc diversity increased between one and two years of exposure, there were only small differences with respect to substrate type and orientation.
Cirripeds
Three species of cirripeds were recorded on the settlement plates. The intertidal station was dominated by the balanid Chthamalus stellatus which increased in number with exposure time. At the 15- and 60-m stations Balanus trigonus and Verruca spengleri co-occurred. The latter species was particularly common at the 15-m station where it showed a distinct preference for the down-facing substrates, whereas at 60 m neither one of the species exhibited a preferred orientation, nor a dependency on substrate type or exposure time.
Bryozoans
With a total of 47 taxa (8 Cyclostomata, 39 Cheilostomata) recorded over the two-year time span of the experiment, bryozoans form the most speciose group (Fig. 5a). The number of taxa was highest at 150 m depth (30 taxa), with slightly fewer taxa occurring at 60 m (26 taxa) and 15 m (22 taxa). At 500 m merely eight taxa were recorded (Table 4). The platforms at 5 m were colonised by only two species but were too badly damaged for reliable abundance data. No bryozoans were recorded at the intertidal 0-m station.
Biodiversity assessment; a the cumulative species richness of the various organism groups at the 5 depth stations; b selected biodiversity indices, namely the Simpson index D, the Shannon index H’, Pielou’s equitability J, and the logarithm of the species richness S (=H’ max); c the mean and total number of counted individuals
Colony growth forms include a variety of encrusting (e.g. Escharina vulgaris) as well as erect flexible (e.g. Bugula spp.), erect rigid robust branching (e.g. Omalosecosa sp.), and erect rigid fenestrate (Reteporella sp.) types (Tables 2 and 4). Within the dominant encrusting growth form (35 taxa), several of the cyclostome species produce small "spot" colonies (e.g. Conocavea richardi). However, most cheilostome species form colonies of the multiserial "patch"-type comprising several dozens of zooids, while two species (E. vulgaris, Stephanollona contracta) produce extensive patches up to some 4 cm in diameter, and consist of hundreds of zooids after one year of exposure time. The uniserial encrusting type termed "runner" is represented by six species (e.g. Hippothoa aff. divaricata), while Celleporina cf. laciniosa forms narrow oligoserial strips of the “ribbon” type.
For reasons of uniform data handling, bryozoan colonies were counted as individuals. This method has, however, several drawbacks concerning the assessment of the area covered, the significance of taxa to carbonate production, and estimating the faunal dominance of certain taxa. A single colony of the patch growth type may cover significantly more space and produce more carbonate than numerous spot colonies. Similarly, an erect, tree-like colony usually covers less substrate space than an encrusting colony but may produce significantly more carbonate. Other colony types (e.g. encrusting runners) have a great colony perimeter but the zooids are widely spaced (i.e. the zooid density per given area is low), and are usually very lightly calcified. As a result, the numerical abundance data presented in Table 2 may differ from the interpretation of the results that also integrate information from general optical impressions of the settlement plates. For instance, whereas the highest number of taxa was recorded at 150 m, the abundance of bryozoan colonies was greatest at 60 m, and the largest area covered by bryozoans was at 15 m, which is not evident from the quantitative analysis alone.
At 15 m, the runner Aetea cf. sica, the spot-like ‘Tubulipora’ sp., the erect-flexible Scrupocellaria sp. 2 and Crisia sp., as well as the patch-producing S. contracta and E. vulgaris, were numerically the most abundant bryozoans, with the latter two species forming the largest colonies. At 60 m ‘Tubulipora’ sp. was among the most common organisms, while the erect rigid Tubuliporidae indet. 3, and the erect flexible Crisia sp. also contributed significantly to the bryozoan abundance at this depth. Colonies of the uniserial H. aff. divaricata occasionally covered the entire plates, although the thinly calcified zooids are small and zooid density is very low. Stephanollona contracta and E. vulgaris were present as well but with distinctly smaller colonies than at 15 m while covering a larger area than most of the above-mentioned species. Although relatively few in numbers and covering a small area, the erect rigid colonies of Celleporina laciniosa and Omalosecosa sp., as well as the erect fenestrate Reteporella sp., form a conspicuous part of the bryozoan fauna at this depth. The platforms at 150 m depth showed a drastic decrease in colony size and area covered, particularly of the encrusting colonies, resulting in a significantly reduced substrate cover, whereas bryozoan species richness was greatest at this depth for both exposure times (Table 4). Species with spot colonies like ‘Tubulipora’ sp. and ?Disporella sp., the erect Crisia sp., and the runner H. aff. divaricata were most abundant. At 500 m, very few bryozoans were present, mostly comprising cyclostome spot colonies.
With increasing depth (15–500 m) there is a decrease in overall area covered by bryozoans (Fig. 2); a general decrease in colony size within single species (e.g. E. vulgaris); and a change in predominance of taxa with encrusting patch colonies to those with erect, spot and runner colonies (Table 4). Moreover, the ratio between cheilostomes and cyclostome taxa decreases from 4.5 at 15 m to 0.6 at 500 m (Table 4), which is mainly due to a slight increase in the number of cyclostome taxa from 15 to 150 m, and to a drastic decrease in cheilostome taxa (3 taxa) at 500 m. Most taxa (23 taxa) were present only at a single depth station, whereas 11 taxa occur at two, and 9 taxa occur at three stations. All four taxa that occur at all four stations from 15 to 500 m (‘Tubulipora’ sp., Callopora sp., Crisia sp., Stomatopora sp.), and also some of those that are present at three stations (among others: Anascan indet., Crisia sp. 2, ?Disporella sp.) presumably comprise more than one species as characteristic features were absent and/or their morphologies were difficult or impossible to distinguish with a light microscope. The only verified species occurring at three depth stations are E. vulgaris and H. aff. divaricata (both from 15 to 150 m), as well as Reteporella sp. (60 to 500 m).
Orientation preferences are species specific and evident in several taxa, with a number of taxa occurring mainly on the up-facing plates, whereas significantly more bryozoan colonies were generally found on the down-facing substrates from 15 to 150 m. Among the more commonly occurring species, only Beania cf. mirabilis and Micropora sp. were exclusively found on the up-facing substrates, whereas Buffonellaria cf. acorensis was exclusively encrusting the down-facing plates. Escharina vulgaris and H. aff. divaricata were more abundant on the down-facing than on the up-facing substrates throughout their range from 15 to 150 m. Several taxa (e.g. Aetea cf. sica, ‘Tubulipora’ sp., S. contracta) showed a preference for the down-facing substrates at shallow depths (15 m) while occurring either on both orientations or preferentially on the up-facing substrates at greater depths (60–150 m). At all stations from 15 to 150 m the ancestrulae (the initial zooid of a colony formed by metamorphosis of the larva) or juvenile colonies of both cyclostome and cheilostome bryozoans were distinctly more abundant on the down-facing substrates, while they were evenly distributed at 500 m. A number of species did not show any obvious preference throughout their depth range (Chorizopora aff. brongniartii, Nimba praetexta, Puellina (Cribrilaria) sp., Reteporella sp., Stomatopora sp.).
The majority of taxa were present in both years while six were recorded on the 1 yr platforms only (Bugula dentata, Catenicella sp., Haplopoma sp., Microporella sp. 2, Reptadeonella sp., Schizobrachiella sanguinea), and three were present only on the 2 yrs platforms (Celleporina cf. laciniosa, Tubuliporidae indet. 2). All of these taxa were rarely or very rarely observed on the settlement plates. Differences in preferential settlement on limestone vs. PVC plates were not observed in bryozoans.
Epilithic biocoenoses and carbonate production
Broadening the perspective from individual groups of organisms to the community level, we can characterise the biocoenoses of calcareous epiliths developed at the various depth stations with emphasis on carbonate production (large and/or dense skeletons versus small and/or porous ones) rather than absolute abundance, and with a focus on the factors bathymetry and substrate orientation.
At the wave-exposed, intertidal 0-m station the biocoenosis was poor in taxa with only one recorded cirriped and six morphotypes of coralline red algae. Carbonate was chiefly produced by the balanid Chthamalus stellatus in concert with large and thick thalli of different taxa of the coralline alga Lithophyllum. All calcareous epiliths were heavily infested with microbial euendoliths, and the encrusting algae appear strongly affected and fragmented by hydrodynamic force as well as by the action of grazers such as limpets, chitons, and echinids (Wisshak et al. 2011). Grazed substrate surface was rapidly recolonized by coralline algae as recorded by the high abundance of juvenile colonies.
In contrast, at shallow euphotic 15 m depth a rich biocoenosis was developed after only one year of exposure, with a total of 40 recorded taxa. The illuminated up-facing substrates were colonised by all morphotypes of coralline algae that produce only thin thalli, and thus contributed little to carbonate production. Only Corallina elongata and Haliptilon sp. formed erect and segmented thalli in relevant quantity. Carbonate was mainly produced by the ubiquitous serpulid Spirobranchus polytrema, the tubes of which reached more than 2 cm in length during the first year of exposure. The biocoenosis was complemented by a large number of small foraminiferans, rare vermetids, very rare balanids, and an anecdotal occurrence of solitary scleractinians. Bryozoans were rare albeit fairly diverse (19 taxa). A noteworthy contribution to overall carbonate production was only provided by patch colonies of Copidozoum sp. All calcareous epiliths were affected by bioeroding microbial communities but indications for grazing pressure were far less prevalent compared to the intertidal zone (Wisshak et al. 2011). On the down-facing substrates, the autophototrophic coralline algae were subordinate to large foraminiferans such as Miniacina miniacea, which, in contrast to the likewise common Discoramulina bollii, contributed considerably to the carbonate production at this depth station. Main carbonate producers were, however, the serpulid Spirobranchus polytrema and the cirriped Verruca spengleri. Other significant carbonate producers were the oyster Neopycnodonte cochlear and the vermetid Vermetus rugulosus. Among the 25 taxa of bryozoans, E. vulgaris and particularly S. contracta were relatively abundant and highly competitive relative to other bryozoans and groups of organisms, producing large colonies and contributing a significant proportion to the overall carbonate accretion. Although producing small spot-like colonies, due to their abundance ‘Tubulipora’ sp. was also an important carbonate producer, particularly on the 2-year plates. Other common but distinctly less calcified bryozoan taxa on the down-facing plates were the relatively large colonies of the erect flexible Crisia sp. and Scrupocellaria spp.
The deep euphotic 60-m platforms yielded a diverse biocoenosis (46 taxa). On the up-facing substrates the deepest coralline red algae were present (all morphotypes) but thalli were thin and thus insignificant for the carbonate produced at this depth station. The large foraminiferan Miniacina miniacea is occasionally abundant. Carbonate production was again dominated by the large and massive tubes of Spirobranchus polytrema, whereas the two co-occurring serpulids were only rare to very rare. The second principal carbonate producer was the oyster Neopycnodonte cochlear, which formed valves of up to 3 cm in diameter during the 2-year experimental period. The most diverse group were again the bryozoans (26 taxa), although colony size in the encrusting taxa, and therefore their contribution to carbonate production, significantly decreased at this depth. Again, ‘Tubulipora’ sp. was present with numerous small colonies, while E. vulgaris and S. contracta were the species with the largest patch colonies. Instead, large erect and heavily calcified colonies, such as those of C. laciniosa, Omalosecosa sp., and Reteporella sp., first occurred at this depth. Although present with relatively few colonies, their contribution to carbonate accretion was significantly larger than that of encrusting species. On the down-facing substrates Spirobranchus polytrema is again a key carbonate producer but is outcompeted by the oyster Neopycnodonte cochlear, which is responsible for the fact that at this station the highest rate of carbonate accretion was recorded. Other large calcareous epiliths such as the deepest occurrences of cirripeds and vermetids, as well as large foraminiferans, and serpulids other than S. polytrema were of subordinate importance. With 17 bryozoan taxa occurring on the down-facing plates, the diversity was distinctly lower than on the up-facing substrates, which is surprising because the diversity on the lower surface was significantly higher at 15 m and more or less equal on both surfaces at 150 and 500 m. The erect robust C. laciniosa and the fenestrate Reteporella sp. yielded noteworthy contributions to carbonate production, as did the small but abundant colonies of Tubuliporidae indet. 3, and particularly ‘Tubulipora’ sp.
At the dysphotic to aphotic 150-m station, the biocoenosis was diverse (41 taxa) but carbonate production was strongly reduced. On the up-facing substrates, light levels were too low to support coralline red algal growth. Spirobranchus polytrema was still a relevant carbonate producer in concert with Neopycnodonte cochlear. Also Miniacina miniacea was present and serpulids were diverse. The bryozoans (24 taxa) gained in relative importance in the significantly reduced overall carbonate production. The fenestrate Reteporella sp. and the Tubuliporidae were major carbonate producers. On the down-facing substrates, a very similar general composition of the biocoenosis was encountered but abundance and carbonate accretion were higher. Chief carbonate producer was N. cochlear whereas S. polytrema was replaced by Hyalopomatus ?marenzelleri as the most productive serpulid. With 24 taxa the bryozoans were still extremely diverse at this depth although most taxa were present with only a few colonies. Again, colonies were generally very small apart from the runner H. aff. divaricata, which covered large areas of the settlement plates but did not significantly contribute to carbonate production.
At the bathyal limit of the bathymetrical transect, the epilithic biocoenosis was reduced in diversity (17 taxa) and carbonate production was extremely low. Only the serpulid Hyalopomatus ?marenzelleri was abundant and produced skeletons of relevant size. All other faunal elements were very rare and even the bryozoans were strongly reduced in diversity (5 taxa) and abundance, with the cyclostome ‘Tubulipora’ sp. as the only noteworthy carbonate producer. On the down-facing substrates, abundances were slightly higher, also in case of the dominant H. ?marenzelleri.
Statistical biodiversity analyses
A total of 74 taxa were recorded on the settlement plates. This number must be regarded as conservative because several of our taxa or morphotypes are likely to comprise two or more species. Bryozoans are clearly the most speciose group among the encrusting fauna (47 taxa ≈ 64 %), with serpulids (9 taxa ≈ 12 %), coralline algae (6 morphotypes ≈ 8 %), molluscs (5 taxa ≈ 7 %), as well as foraminiferans and cirripeds (3 taxa ≈ 4 % each) being subordinate. Of the 74 taxa, 70 occurred on the 1-year platforms, and 66 on the 2-years platforms.
Biodiversity indices and abundance models
Abundance data from the different substrate orientations, substrate types, depth stations, and exposure times are summarised in Table 3. The total and mean number of specimens show a drastic increase from the 0- to 15-m station, with approximately the same number at the 60-m station, and a distinct drop towards the 150-m station, and again to the 500-m station (Fig. 5c). The trend in species richness parallels this pattern between the 0- and 60-m stations, as there are an equally high number of taxa also at the 150-m station (due to the peak in bryozoan diversity), dropping off only towards the 500-m station (Fig. 5a). Faunal evenness/equitability was found highest, and dominance lowest, at the 150-m station (Table 3, Fig. 5b).
Whittaker plots for the various depth stations (Fig. 6a-b) are very similar for one and two years of exposure. At all depth stations, the log-transformed abundance per rank plots along a straight line (e.g. 150 m) or are slightly S-shaped (15 and 60 m). Hence, the communities combine characters of the geometric model (Motomura 1932) and the log-normal model (Preston 1962). In accordance to the Whittaker plots, histograms for the number of taxa within octaves with power-of-two abundance classes (Fig. 6c) are closer to normal distribution for the 15- and 60-m station. At 0 and 500 m, the number of taxa and specimens is too low to draw reliable conclusions. This is also reflected in the rarefaction analysis for the five depth stations: At the 15 to 150-m stations, where many thousand specimens were recorded, the rarefaction curve levels out and the confidence interval is narrow, indicating that the picture we gather from these samples is close to complete. In contrast, bearing limitations of rarefaction analysis as a predictive tool in mind (Tipper 1979), the steep slope of the distal rarefaction curve at 500 m suggests that considerably more taxa could have been expected with larger sample size. The rarefaction curve for the 0-m station, however, flattens out despite the relatively small sample size, indicating that the low number of recorded taxa is probably more realistic. Standardising for sample size to the smallest n = 658 (500-m station) yields standardised species counts S(n) of 5.9 ± 0.3 at 0 m, 27.3 ± 1.8 at 15 m, 23.1 ± 2.0 at 60 m, 28.5 ± 1.8 at 150 m, and the counted 16 taxa at the 500-m station. Hence the overall bathymetrical trend is retained but the contrast between the 15- to 150-m stations, and again to the 500-m station is less pronounced than apparent from the epilith inventory only.
Significance of substrate type, spatial and temporal variability
Analysis of similarity (ANOSIM) was applied in order to test for significance in trends with respect to four considered factors: water depth (composite factor comprising gradients in various environmental parameters), substrate type, substrate orientation, and exposure time (Table 5). When considering data from all depth stations, ANOSIM yields significant differences for all four factors, whereby depth is the most significant (highest R values and p = 0.001), followed by orientation and exposure time, and the lowest significance (very low R values, albeit p = 0.002) for substrate type. When comparing sub-datasets, the differences are most significant (high R values and p = 0.001) for the pairwise comparisons among the various depth stations with the lowest level of dissimilarity indicated between the 15- and 60-m stations, and the 60- and 150-m stations. A correspondingly high degree of dissimilarity was computed for substrate orientation (high R values and p = 0.001), which is most pronounced at the 15- and 60-m stations and weakest at the 500-m station. The degree of dissimilarity is less pronounced for exposure time (intermediate to low R values and p values up to 0.025). Substrate type shows the lowest dissimilarity with only three of the depth stations being significantly different (low R values and p values up to 0.045), and, in turn, a high degree of similarity (very low R values and p values > 0.2) for the 15- and 60-m stations.
A very similar pattern is evident when visualising the abundance data set via non-metrical multidimensional scaling (NMDS) based on Bray-Curtis distance measure (Fig. 7) as a tool for similarity assessments and indirect gradient analysis (Minchin 1987). The plot of the entire data set (Fig. 7a) shows an array of discrete clusters in the actual bathymetrical sequence of the different depth stations. The clusters of the 15-, 60-, and 150-m stations are denser and located closer to each other (high degree of similarity both within and among depth stations) compared to the 0- and 500-m stations. For evaluating patterns per depth, further NMDS plots were computed for the different depth stations (Fig. 7b-f). Data from the intertidal 0-m station show differences between the two substrate types and little influence of exposure time. The 15 m data form a discrete cluster in down- and up-facing substrates indicating a distinct orientation preference; trends within these two clusters are not resolvable on this scale. At 60 and 150 m, again clusters are developed for the two substrate orientations but less pronounced compared to 15 m. Within each orientation the two exposure periods form sub-clusters indicating some degree of temporal pattern. Substrate type, in contrast, does not appear to have an effect. Finally, the plot for the 500-m station shows scattered clusters with slight overlap for the two substrate orientations, indicating only a moderate difference, while a temporal effect or a dependency on the substrate type are not evident.
Non-metrical multidimensional scaling plots (NMDS) for the species abundance data set, based on the Bray-Curtis similarity measure; a complete data set showing discrete clusters for the different depth stations as a reflection of the bathymetrical gradient; b-f separate NMDS for the various depth stations illustrating distinct differences in taxon composition for the two substrate orientations, particularly at the 15- to 150-m stations. Differences with respect to exposure time (circles versus triangles) are only distinct for the 60- and 150-m stations, and substrate type (light grey versus dark grey symbols) appears to have little effect
In summary, ANOSIM and NMDS plots draw a coherent picture that indicates a relative importance of the four observed factors (in sequence of decreasing relevance) water depth, substrate orientation, exposure time, and substrate type.
Integrating calcareous epilith biodiversity and bioerosion ichnodiversity
The diversity of calcareous epiliths and the ichnodiversity of bioerosion traces are complementing aspects of benthic biodiversity which are rarely considered in concert. We thus conclude the paper trilogy with a comparison of the present calcareous epilith diversity analysis with the corresponding bioerosion ichnodiversity analysis recorded in the limestone substrate as reported earlier by Wisshak et al. (2011), and we set the biodiversity patterns in context of the environmental gradients as well as carbonate budget quantifications presented in Wisshak et al. (2010).
The total number of calcareous epilith taxa amounts to 74 and compares to a total of 56 ichnospecies and -forms. The intersection set, i.e. the number of bioerosion traces that are produced by calcareous epiliths, amounts to 7 and comprises the attachment scars of bryozoans (Finichnus dromeus, F. peristoma, and F. isp.), foraminiferans (Kardopomorphos isp. and the ‘Gypsina attachment form’), vermetid gastropods (Renichnus arcuatus), and verrucid cirripeds (Centrichnus concentricus). Only 8 % of the spectrum of calcareous epiliths, the 6 morphotypes of calcareous red algae, are photoautotrophs that rely on the sun as primary energy source, as opposed to the chemotrophic calcareous epiliths that fuel their metabolism with organic matter. Among the bioeroders, the respective percentage is higher (32 %) with 11 traces attributed to euendolithic cyanobacteria, and 7 microborings to chlorophyte algae). On the scale of the individual depth stations, the dominance of heterozoans is naturally limited to the up-facing substrates in the shallow euphotic zone where the PAR is large enough to support a larger percentage of photozoans. The much reduced species richness at the intertidal station for instance is dominated by calcareous red algae (83 %) among the epiliths, and by cyanobacteria and chlorophytes (77 %) among the endoliths. Already at the shallow euphotic 15 m station the rhodophytes are subordinate (15 %) and photoautotrophic euendoliths are reduced to 33 % of the ichnospecies richness, underlining the dominance of heterozoans among the carbonate-producing as well as carbonate-degrading biota in the Azores.
The statistical analyses (NMDS plots and ANOSIM) of the calcareous epiliths and the bioerosion traces recorded in the limestone substrate (Wisshak et al. 2011) indicate that the same relative relevance of factors apply for both components of hardground biont biodiversity. These are a strong influence of water depth and substrate orientation but little effect of exposure time and substrate type (accretion only). This is in coherence also with the patterns expressed in the quantified bioerosion and carbonate accretion rates, as reported in Wisshak et al. (2010), and the carbonate densities reported herein.
As for the composite factors water depth and substrate orientation, the most relevant underlying factor for the distribution of both, calcareous epiliths and bioeroding biota, most probably is the light regime. In shallower water and on up-facing substrates, increased availability of light promotes growth of photoautotrophic bioeroding microphytes and quickly growing fleshy as well as calcareous epilithic algae that lead to competition with slowly growing, calcifying heterotrophic organisms (Wisshak et al. 2011). This is in good accordance to the observed significant differences in bioeroder abundance and bioerosion rates on the one hand, and calcifier abundance and accretion rates on the other hand, as observed on up- versus down-facing substrates in shallow water, and a more homogenous settlement in deeper water (Fig. 8b-c). By promoting the growth of photoautotrophic bioeroders and calcifiers, the light availability not only influences the abundance and rates, but also the ichnodiversity and biodiversity. This is reflected in the general decrease in ichnodiversity from shallow euphotic towards aphotic depth stations (Fig. 8b), clearly confirming the results of previous bioerosion experiments along extended bathymetric transects from various biogeographical settings (e.g. Golubic et al. 1975; Zeff and Perkins 1979; Budd and Perkins 1980; Vogel et al. 2000; Wisshak et al. 2005). It is as well reflected in the higher ichnodiversity on up-facing substrates with a higher diversity (and abundance) of traces produced by photoautotrophic microphytes and traces left by grazers feeding upon them, compared to the shaded down-facing substrates (Fig. 8b) as reported in (Wisshak et al. 2011). For the calcareous epiliths in contrast, the light regime has little direct influence on their biodiversity trends regarding water depth and substrate orientation, owing to the low fraction of photoautotrophs (calcareous algae) among them (see above). Nevertheless, there are relevant indirect effects of light energy since a preference for the shaded down-facing substrates may well be controlled by avoidance of competition with photoautotrophic macroalgae. Another indirect effect is explained by the hypothesis put forward in Wisshak et al. (2010) of a causal relationship between bioerosion pressure (reflected in bioeroder abundance and bioerosion rates) on the abundance of calcareous epiliths and linked carbonate accretion rates. Interestingly this effect is mirrored also in the relation of bioerosion ichnodiversity and diversity of calcareous epiliths: While as a general trend, the diversity of calcareous epiliths and the bioerosion ichnodiversity do positively correlate to some extent (Fig. 8a) and slopes of linear regressions are very similar for the up-facing substrates (r 2 = 0.57; p = 0.01; F = 8.06) as well as on the down-facing substrates (r 2 = 0.57; p = 0.03; F = 10.76), the trend lines are clearly offset in favour of ichnodiversity in case of the up-facing substrates. The direct comparison of carbonate accretion and bioerosion rates for the various depth stations draws a comparable picture with an inverse relationship for the two substrate orientations (Fig. 8c).
Integration of calcareous epilith biodiversity and bioerosion ichnodiversity; a moderately positive correlation of species richness and ichnospecies richness for up-facing and down-facing substrates; b species richness and ichnospecies richness in relation to bathymetry, with an inverse pattern with regards to the comparison of up- and down-facing substrates; c comparison of carbonate accretion and bioerosion rates at the various depth stations, with the same inverse pattern as for the species richness
Apart from the direct and indirect effects of the light regime, two other underlying factors have a relevant influence on the distribution of the filter feeders that dominate the calcareous epiliths: water temperature and the supply of nutrition in form of phytoplankton and other particulate organic matter (POM) such as phytodetritus. In the Azores setting, temperatures and their seasonal fluctuation strongly decrease towards deeper waters (see Fig. 3 in Wisshak et al. 2010), potentially slowing down growth of calcifiers and the establishment of mature epilithic communities, as reflected in the strong decrease in epilith abundance (Fig. 5) as well as carbonate accretion rates and densities (Fig. 3) from the 60 m station towards deeper waters. This trend may be enhanced by a decrease in phytoplankton and POM availability with depth and a strong seasonal fluctuation in flux (e.g. Duineveld et al. 2004), which in the Azores is recorded by the seasonal establishment of a pronounced deep chlorophyll maximum (DCM) at around 100 m water depth (roughly coinciding with the depth of the seasonal thermocline) and a decrease in primary production below this depth (Piazena et al. 2002). Inorganic nutrients, in contrast, are of subordinate importance for filter feeders and show a depletion at the 60 m station with either a decrease (ammonium and nitrite) or increase (nitrate and phosphate) towards the 500 m stations (see figure and Table 2 in Wisshak et al. 2010). Despite the strong bathymetrical trend in carbonate accretion rates and densities as well as the orientation preference in calcareous epiliths, their biodiversity, in contrast, is less affected (Figs. 5 and 8), particularly when considering the standardised species counts (see above).
Comparison to SSETI findings
A remarkable suite of similarities in calcareous epilith biodiversity patterns and gradients arises when comparing the results from the Azores experiment with the findings of the most elaborate settlement experiment in recent years, the ‘Shelf and Slope Experimental Taphonomy Initiative’ (SSETI; see Parsons-Hubbard et al. 2011 for a summary and further references), that was carried out over the course of two decades in the Bahamas and Gulf of Mexico along a similar total bathymetrical range (15 to 570 m water depth). Despite a differing experimental approach (loose, tethered, and bagged mollusc shells) and a focus mainly on taphonomy, several patterns are shared between the two experiments and regions, particularly with respect to the calcareous epiliths and a limited set of bioerosion traces as analysed by Brett et al. (2011): In concordance to our results from the shallower stations, the main suite of encrusters established after only one year of exposure with only minor additions of taxa in the following years. The increase in coverage was thus found nonlinear over a timescale of months to years but species richness showed more variable trends as overgrowth of existing and exposed skeletons, as well as partial burial in sediment (not an issue in the Azores experiment) naturally became more important over time. Serpulids played an important role in coverage and carbonate production, with relatively uniform percentages of contribution at the different depth stations. Bryozoans were the most speciose phylum in both experiments, whereas they contributed relatively little to carbonate production/spatial coverage over time compared to serpulids and foraminiferas (in the Bahamas) or bivalves (in the Azores), and their average colony size decreased with increasing depth. Also in good accordance to the present findings, density of encrusters was found highest at the shallow subtidal sites, dominated by photoautotrophic organisms, while mean coverage values declined drastically with depth, whereas the peak in species richness was found in intermediate 73 m water depth. Consequently, their NMDS plots also identified water depth and the related photic zonation as main controlling factor, both for the % coverage and the species richness. Brett et al. (2011) concur in our interpretation that the light regime and the linked nutrient supply, i.e. a decrease in primary production with depth, are the controlling underlying factors. In addition, they propose the possibility of a thermocline effect, i.e. a limit in distribution of larvae by creating a density barrier. This hypothesis could in part also explain the marked differences in carbonate accretion rates and densities recorded at the 60 versus 150 m stations in the Azores experiment.
Carbonate production and sedimentation on oceanic islands and seamounts
Comparing the assemblages on the experimental platforms with naturally occurring calcareous epiliths, calcareous bioherms, and resulting sediments from the Faial Channel allows us to demonstrate that the artificial assemblages reflect the adjacent natural associations. Identifying the main carbonate producers and facies types prevailing in the Azores and other oceanic islands or seamounts has received little attention (e.g. Scoffin et al. 1980; Henrich et al. 1995; Basso 1998; Johnson et al. 2012) but is of considerable importance for our understanding of modern carbonate systems in non-tropical settings as well as for the interpretation of fossil deposits on such islands (Westphal et al. 2010). Such non-tropical fossil carbonates of Neogene age are present in all Macaronesian archipelagos: in the Azores (Ávila et al. 2009a; Ávila et al. subm.), Madeira (Johnson et al. 2011), the Canaries (Meco et al. 2007; Johnson et al. 2012), and Cape Verde (Johnson et al. 2012). Although they may provide environments that are stable in ecological terms, volcanic oceanic islands are considered ephemeral structures from a geological point of view (Ramalho et al. 2013). Besides changes in eustatic sea-level, uplift and subsidence of the volcanic cone during different stages of its history, as well as the presence of narrow shelves and steep slopes render the sedimentary accommodation space highly variably in time and space (Donovan 2002; Donovan et al. 2013; Quartau et al. 2010, 2012; Meireles et al. 2013).
The experiment in the southern Faial Channel showed that diverse assemblages of calcareous epiliths support a net carbonate production across the entire bathymetrical transect, apart from the intertidal station, where strong hydrodynamic force in concert with high rates of bioerosion lead to a net erosive regime (Wisshak et al. 2010). The surplus in carbonate is most pronounced in the deep euphotic zone (60-m-station) where carbonate accretion rates peak with a mean of 645 g m−2 yr−1, about twenty-fold the respective mean bioerosion rate at this depth (Wisshak et al. 2010). As discussed in the previous section, the biogenic carbonate material produced by the calcareous epiliths in our settlement experiment is dominated by heterozoan taxa and is thus in good accordance with the ‘heterozoan association’ defined by James (1997). The disarticulated or detached carbonate skeletons provide the raw material for deposition of carbonate-rich sediments on the surrounding seafloor and potential downslope export to deeper waters. In the central Faial Channel between 50 to 150 m depth, below the fair-weather wave base, the gently sloping surface topography provides suitable accommodation space for calcareous sediment mainly composed of sand- and gravel-sized biogenic skeletal components, and shaped by tidal currents to form extensive mega-ripple beds that blanket the volcanic bedrock (Fig. 9a). The carbonate skeletal components are mixed with varying amounts of volcaniclastic material that often dominates the finer fractions of the sediment. The skeletal material largely derives from benthic islands of hard ground colonised or built up by the oyster Neopycnodonte cochlear and the clam Chama gryphoides, in concert with a diverse suite of bryozoans, serpulids, cirripeds, vermetids, foraminiferans, and occasionally solitary corals. These oyster bioherms elevate about 1 m above the surrounding sediment and often reach more than 10 m in lateral extent (Fig. 9a-b; see also Wisshak et al. 2010). On the steep slope of the southern Faial Channel at >300 m depth, carbonate production on settlement plates and in the natural surroundings is greatly reduced. However, volcanic bedrock cliffs and overhangs harbour a peculiar association of the large, slowly growing and extremely long-lived oyster Neopycnodonte zibrowii (Fig. 9c), and the sessile cyrtocrinid Cyathidium foresti (see Wisshak et al. 2009a,b), as well as cold-water corals such as the scleractinian Desmophyllum dianthus (Marali et al. 2013) or the calcareous hydrocoral Errina dabneyi (Wisshak et al. 2009c). Their disarticulated or detached skeletons accumulate at the base of the hard ground features and mix with the surrounding volcaniclastic-dominated sediment fans.
Carbonate production within oyster bioherms in the Faial Channel (a-c), and sediment composition on various Macaronesian oceanic islands and seamounts (d-l; 1-cm-grid in background), documented and sampled with submersible LULA in the course of the experiment and with a Van Veen grab during Victor Hensen cruise VH97 on various Macaronesian islands and seamounts (given with the station number, coordinates, and water depth); a Carbonate sands surrounding Neopycnodonte cochlear / Chama gryphoides bioherms and the experimental platforms at 60 m depth; b Corresponding oyster bioherms in 150 m depth colonised by a diverse set of hydrozoans, sponges, serpulids and bryozoans; c Hundreds of deep-sea oysters Neopycnodonte zibrowii thriving at a steep escarpment in 470 m depth; d Sediment sample (>2 mm fraction) recovered in close vicinity to the 150 m station; e-l Samples (>2 mm fraction) from Lanzarote, Amanay Bank, Gettysburg Peak, Gorringe Bank, and Dacia Seamount
A sediment sample taken near the 150 m station (Fig. 9d) reveals that, overall, the calcareous epiliths from the settlement plates are also represented in the taphocoenosis, forming a ‘bryomol’ facies association sensu Nelson et al. (1988b). The gravel-sized (>2 mm) bioclasts, which are added to by volcanic clasts, predominantly consist of erect and, to a much lesser extent, encrusting bryozoans and bivalves, with minor contributions by serpulids and foraminiferans (M. minacea). Apart from the abundant erect Adeonellopsis sp. and Diporula hastigera, all other taxa have also been found on the settlement plates at the 60 and/or 150 m stations. Despite the fact that bryozoans dominate the gravel-sized sediment, the major long-term carbonate producers from 15 to 500 m are bivalves, both on the settlement plates and in the natural setting.
Biogenic sediments from other subtropical Macaronesian islands and seamounts (Fig. 6) reveal similar skeletal assemblages dominated by heterozoan organisms with varying contributions by coralline red algae. A sample from the photic zone at Lanzarote (Fig. 9e) provides an example of typical shallow-water maerl sediment dominated by calcareous red algal fragments, with major contribution by molluscs (Cerithiidae, Trochidae, and Phasianellidae) and accessory echinoids (‘rhodalgal’ association sensu Carannante et al. 1988). In a more diverse assemblage from deeper water (105 m), sampled at Amanay Bank the maerl is complemented by the foraminiferan M. miniacea, as well as molluscs, bryozoans and serpulids (Fig. 9f). M. miniacea plays an important, albeit usually neglected, role in overall carbonate production in the Macaronesian and adjacent regions of the NE Atlantic (Milliman 1976). This becomes particularly evident on Gorringe Bank. In a sample from Gettysburg Peak at its southwestern limit, it occurs together with molluscs, bryozoans, serpulids, balanids and echinoids in an exclusively heterozoan skeletal assemblage at 63 m depth (Fig. 9g). From a different location and depth (86 m) on Gorringe Bank stems an example where M. miniacea is the dominant carbonate producer (Fig. 9h), with molluscs, erect bryozoans, and serpulids as additional components. Sediment with almost equal proportions of erect and massive bryozoans, M. miniacea, bivalves, serpulids, and balanids (Fig. 9i), was recovered from another station on Gorringe Bank at 135 m depth. On Dacia Seamount, a peculiar assemblage was sampled at 108 m depth with bivalves and tubes of the infaunal serpulid Ditrupa, and only minor contribution by coralline red algae, M. miniacea, molluscs, bryozoans, and sessile serpulids (Fig. 9j). Another Gorringe Bank sample (302 m) shows a typical bryomol association (Fig. 9k), almost exclusively with fragments of erect bryozoans and bivalves, and few pteropods. The abundance of (planktonic) pteropods commonly increases with water depth such as in the deepest of the present samples, recovered from 440 m at Gettysburg Peak (Fig. 9l).
These examples show that non-tropical biogenic carbonate sediments are not restricted to continental coast- and shelf environments but also commonly occur on oceanic islands and seamounts, and that a wide range of heterozoan organisms contribute to their formation. But how do diverse associations of sessile benthic organisms establish on remote oceanic islands in the first place? Colonisation is rendered difficult by the sheer distance to the nearest source regions of potential propagules: the Azores are separated by >800 km of open ocean and abyssal depths from the nearest island (Madeira), by ~1,400 km to nearest continental shelf (NW Africa, Portugal), and by some 4,000 km from the opposite North American coastline. Yet, Late Miocene assemblages in Santa Maria Island (Ávila et al. subm.), as well as the modern benthic associations encountered on the settlement plates, are comparable in composition and diversity to shelf communities to a certain extent. For instance, congenerics of most bryozoan taxa present on the settlement plates occur in shallow water on the continental shelf, and some of the species (e.g. E. vulgaris, S. sanguinea, Schizoporella dunkeri, S. contracta) are indistinguishable from European shelf populations. The Miocene carbonates at Santa Maria are a coquina predominantly composed of large oysters and pectinids (forming a ‘bimol’ skeletal assemblage sensu Hayton et al. 1995), accompanied by echinoids, balanids, bryozoans, serpulids, brachiopods, gastropods, and rare vertebrate remains (whale bones, fish and shark teeth) (Ávila et al. subm). Thus, both fossil and the Recent epilithic faunas are composed of, and the carbonate production is dominated by, taxa with long-lived, planktotrophic larvae (bivalves, balanids, serpulids), whereas, paradoxically, the most speciose group Bryozoa (in the fossil record and on the settlement plates) have short-lived, lecitotrophic larvae (Johannesson 1988). Colonisation of the Azores and other remote islands may thus be achieved to a considerable extent by mature colonies rafting on natural floating objects (wood, pumice, algal fronds and holdfasts) during the Neogene, and also on artificial substrates and ship hulls in historical times (e.g. Barnes 2002; Thiel and Gutow 2005; Cardigos et al. 2006). Considering that some of the taxa with planktotrophic larvae may have also arrived on the islands on rafts in a juvenile stage or as adults highlights the importance of this means of dispersal of shallow-water species (e.g. Ávila 2006; Ávila et al. 2009b).
Conclusions
-
1.
The temperate carbonate factory in the Azores harbours a high diversity of epilithic organisms engaged in carbonate build-up, comprising 47 taxa of bryozoans, 9 serpulids, 3 bivalves, 3 cirripeds, 3 foraminiferans, 2 vermetid gastropods, and 1 scleractinian coral, along with 6 morphotypes of calcareous algae that were present on experimental substrates exposed for two years along a transect from intertidal to bathyal depths.
-
2.
Colonised surface area, carbonate accretion rates, as well as biodiversity were most pronounced in case of mature biocoenoses established on experimental platforms in the deeper parts of the euphotic zone at 15 and 60 m water depth, whereas at the dysphotic 150 m station, and even more so at the aphotic 500 m station, colonisation was much slower, forming opportunistic biocoenoses only after 2 years of exposure.
-
3.
Rarefaction analysis with standardised taxon counts indicate that the actual highest species richness might be present at the 150 m station where also the lowest degree of dominance is indicated by a suite of biodiversity indices.
-
4.
A general orientation preference for down-facing substrates, particularly in the photic zone, is expressed in higher rates of carbonate accretion, higher accretion densities, and higher species richness as well as abundance compared to up-facing surfaces.
-
5.
Statistical community structure analyses (ANOSIM and NMDS) qualify water depth (and linked environmental gradients) and substrate orientation as the main factors governing the observed biodiversity patterns, while the influence of exposure time and substrate type were subordinate.
-
6.
Integrating calcareous epilith diversity and bioerosion ichnodiversity shows the same hierarchy in controlling factors for both components of benthic biodiversity. Species and ichnospecies richness correlate with a dominance of bioeroders on the up-facing substrates and of calcareous epiliths on the down-facing substrates. This inverse pattern is also expressed in the respective abundances, carbonate accretion and bioerosion rates, reflecting the interaction of carbonate build-up and degradation in the warm-temperate Azores waters.
-
7.
Most important underlying factor, acting directly and indirectly, is the light regime. In shallower water and on up-facing substrates, increased PAR promotes growth of photoautotrophic bioeroding microphytes and quickly growing fleshy and calcareous epiliths (algae) that enhance competition with slowly growing, heterotrophic calcareous epiliths, and lead to an increased bioerosion pressure by microendoliths and grazers feeding upon them. Additional factors with strong bathymetrical gradients are temperature and the availability of particulate organic matter.
-
8.
The composition of the epilithic assemblages on the settlement plates is well reflected on natural hard grounds such as abundant oyster bioherms as well as in the surrounding carbonate sediments of ‘bryomol’ facies type. The biogenic sediments are mixed with volcaniclastic components and deposited in the accommodation space provided by the Faial Channel and along the adjacent slope. Net carbonate production rates along the entire subtidal bathymetrical transect peak in the deep euphotic zone, below the fair-weather wave base and under reduced rates of bioerosion.
-
9.
Examples of biogenic sediments from other subtropical Macaronesian archipelagos and seamounts reveal similar heterozoan assemblages, and show that non-tropical biogenic carbonate sediments are commonly occurring on oceanic islands and seamounts with a wide range of heterotrophic organisms, which are occasionally complemented by calcareous red algae, contributing to their formation.
-
10.
While Neogene to Recent key carbonate producers in the Azores are generally dominated by taxa with long-lived, planktotrophic larvae (bivalves, balanids, serpulids), the most speciose group Bryozoa has short-lived, lecitotrophic larvae. Together with the high number of conspecifics or closely related species on the European shelf, this highlights the importance of rafting on natural and artificial floating objects as mode of dispersal to this remote oceanic archipelago.
References
Ávila SP (2006) Oceanic islands, rafting, geographical range and bathymetry: a neglected relationship? Occup Publ Ir Biogeogr Soc 9:22–39
Ávila SP, Ramalho RS, Habermann J, Quartau R, Kroh A, Meireles R, Berning B, Kirby M, Zanon V, Goss A, Rebelo AC, Melo C, Madeira P, Cordeiro R, Bagaço L, da Silva CM, Cachão M, Madeira J (subm.) A late Miocene storm-induced shell bed (coquina) from the central North Atlantic: taphonomy, palaeoecology and palaeoenvironmental reconstruction. Palaeogeogr Palaeoclimatol Palaeoecol
Ávila SP, Madeira P, Zazo C, Kroh A, Kirby M, da Silva CM, Cachão M, de Frias Martins AM (2009a) Palaeoecology of the Pleistocene (MIS 5.5) outcrops of Santa Maria Island (Azores) in a complex oceanic tectonic setting. Palaeogeogr Palaeoclimatol Palaeoecol 274:18–31
Ávila SP, da Silva CM, Schiebel R, Cecca F, Backeljau T, de Frias Martins AM (2009b) How did they get here? The biogeography of the marine molluscs of the Azores. Bull Soc Geol Fr 180:295–307
Barnes DKA (2002) Invasions by marine life on plastic debris. Nature 416:808–809
Basso D (1998) Deep rhodolith distribution in the Pontian Islands, Italy: a model for the paleoecology of a temperate sea. Palaeogeogr Palaeoclimatol Palaeoecol 137:173–187
Bishop JDD (1989) Colony form and the exploitation of spatial refuges by encrusting Bryozoa. Biol Rev 64:197–218
Braga-Henriques A, Carreiro-Silva M, Tempera F, Porteiro FM, Jakobsen K, Jakobsen J, Albuquerque M, Santos RS (2012) Carrying behaviour in the deep-sea crab Paromola cuvieri (Northeast Atlantic). Mar Biodivers 42:37–46
Braga-Henriques A, Porteiro FM, Ribeiro PA, de Matos V, Sampaio Í, Ocaña O, Santos RS (2013) Diversity, distribution and spatial structure of the cold-water coral fauna of the Azores (NE Atlantic). Biogeosciences 10:529–590
Brett CE, Parsons-Hubbard KM, Walker SE, Ferguson C, Powell EN, Staff G, Ashton-Alcox KA, Raymond A (2011) Gradients and patterns of sclerobionts on experimentally deployed bivalve shells: synopsis of bathymetric and temporal trends on a decadal time scale. Palaeogeogr Palaeoclimatol Palaeoecol 312:278–304
Budd DA, Perkins RD (1980) Bathymetric zonation and paleoecological significance of microborings in Puerto Rican shelf and slope sediments. J Sed Petrol 50:881–904
Carannante G, Esteban M, Milliman JD, Simone L (1988) Carbonate lithofacies as paleolatitude indicators: problems and limitations. Sed Geol 60:333–346
Cardigos F, Tempera F, Ávila SP, Goncalves JM, Colaco A, Santos RS (2006) Non-indigenous marine species of the Azores. Helgol Mar Res 60:160–169
Clarke KR, Warwick RM (2001) Changes in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth
Cowie PR (2010) Biofouling patterns with depth. In: Dürr S, Thomason JC (eds) Biofouling. Wiley-Blackwell, Chichester, pp 87–99
Crisp DJ (1974) Factors influencing the settlement of marine invertebrate larvae. In: Grant PT, Mackie AM (eds) Chemoreception in marine organisms. Academic Press, pp 177–265
Davis AR (2009) The role of mineral, living and artificial substrata in the development of subtidal assemblages. In: Wahl M (ed) Marine hard bottom communities: patterns, dynamics, diversity, and change. Ecological Studies 206. Springer, Berlin Heidelberg, pp 19–38
Donovan SK (2002) Island shelves, downslope transport and shell assemblages. Lethaia 35:277
Donovan SK, van Waveren IM, Portell RW (2013) Island slopes and jumbled shell beds. J Geol Soc Lond 170:527–534
Duineveld GCA, Lavaleye MSS, Berghuis EM (2004) Particle flux and food supply to a seamount cold-water coral community (Galicia Bank, NW Spain). Mar Ecol Prog Ser 277:13–23
Field SN, Glassom D, Bythell J (2007) Effects of artificial settlement plate materials and methods of deployment on the sessile epibenthic community development in a tropical environment. Coral Reefs 26:279–289
Glasby TM (2000) Surface composition and orientation interact to affect subtidal epibiota. J Exp Mar Biol Ecol 248:177–190
Golubic S, Perkins RD, Lukas KJ (1975) Boring microorganisms and microborings in carbonate substrates. In: Frey RW (ed) The study of trace fossils. Springer, Berlin Heidelberg, pp 229–259
Gomes-Pereira JN, Tempera F, Ribeiro PA, Porteiro FM (2012) Notes on fauna associated with an opportunistic artificial reef near cold-water corals. Arquipelago 29:11–18
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4, 9 pp., http://palaeo-electronica.org/2001_1/past/issue1_01.htm
Hammer Ø, Harper DAT, Ryan PD (2010) PAST – PAleontological STatistics, ver. 2.03. http://folk.uio.no/ohammer/past/
Hammer Ø, Harper DAT (2006) Paleontological data analysis. Blackwell, Oxford
Harris LG, Irons KP (1982) Substrate angle and predation as determinants in fouling community succession. In: Cairns JJ (ed) Artificial substrates. Ann Arbor Science, Michigan, pp 115–174
Hayton S, Nelson CS, Hood SD (1995) A skeletal assemblage classification system for non-tropical carbonate deposits based on New Zealand Cenozoic limestones. Sed Geol 100:123–141
Henrich R, Freiwald A, Betzler C, Bader B, Schäfer P, Samtleben C, Brachert TC, Wehrmann A, Zankl H, Kühlmann DHH (1995) Controls on modern carbonate sedimentation on warm-temperate to arctic coasts, shelves and seamounts in the Northern Hemisphere: implications for fossil counterparts. Facies 32:71–108
James NP (1997) The cool-water carbonate depositional realm. In: James NP, Clarke JAD (eds) Cool-water carbonates. SEPM Special Publication 56, 1–20, Tulsa
Johannesson K (1988) The paradox of Rockall: why is a brooding gastropod (Littorina saxatilis) more widespread than one having a planktonic larval dispersal stage (L. littorea)? Mar Biol 99:507–513
Johnson ME, da Silva CM, Santos A, Baarli BG, Cachão M, Mayoral E, Rebelo A, Ledesma-Vázquez J (2011) Rhodolith transport and immobilization on a volcanically active rocky shore: Middle Miocene at Cabeço das Laranjas on Ilhéu de Cima (Madeira Archipelago, Portugal). Palaeogeogr Palaeoclimatol Palaeoecol 300:113–127
Johnson ME, Baarli BC, Cachão M, da Silva CM, Ledesma Vázquez J, Mayoral EJ, Ramalho RS, Santos A (2012) Rhodoliths, uniformitarianism, and Darwin: Pleistocene and Recent carbonate deposits in the Cape Verde and Canary archipelagos. Palaeogeogr Palaeoclimatol Palaeoecol 329(330):83–100
Jullien J, Calvet L (1903) Bryozoaires provenant des campagnes de l'Hirondelle (1886–1888). Rés Camp Sci Prince de Monaco 23:1–188
Laubier L (1972) Mission 1969 di bathyscaphe "Archimede" aux Acores observations faites au cours des plongees 1 et 2. Centre National por l'Exploration des Oceans, Serie: Resultats des campagnes à la mer 65–71
Marali S, Wisshak M, López Correa M, Freiwald A (2013) Skeletal microstructure and stable isotope signature of three bathyal solitary cold-water corals from the Azores. Palaeogeogr Palaeoclimatol Palaeoecol 373:25–38
Martins HR (1990) A selected bibliography of the marine fauna and flora of the Azores. Arquipélago 8:35–44
Meco J, Scaillet S, Guillou H, Lomoschitz A, Carracedo JC, Ballester J, Betancort J-C, Cilleros A (2007) Evidence for long-term uplift on the Canary Islands from emergent Mio-Pliocene littoral deposits. Global Planet Chang 57:222–234
Meireles RP, Quartau R, Ramalho RS, Rebelo AC, Madeira J, Zanon V, Ávila SP (2013) Depositional processes on oceanic island shelves – evidence from storm-generated Neogene deposits from the mid-North Atlantic. Sedimentology 60:1769–1785
Milliman JD (1976) Miniacina miniacea: modern foraminiferal sands on the outer Moroccan shelf. Sedimentology 23:415–419
Minchin PR (1987) An evaluation of the relative robustness of techniques for ecological ordination. Vegetation 69:89–107
Motomura I (1932) A statistical treatment of associations. Zool Mag 44:379–383
Nelson CS, Hyden FM, Keane SL, Leask WL, Gordon DP (1988a) Application of bryozoan zoarial growth-form studies in facies analysis of non-tropical carbonate deposits in New Zealand. Sed Geol 60:301–322
Nelson CS, Keane SL, Head PS (1988b) Non-tropical carbonate deposits on the modern New Zealand shelf. Sed Geol 60:71–94
Nicoletti L, Marzialetti S, Paganelli D, Ardizzone GD (2007) Long-term changes in a benthic assemblage associated with artificial reefs. Hydrobiologia 580:233–240
Parsons-Hubbard KM, Brett CE, Walker SE (2011) Taphonomic field experiments and the role of the Shelf and Slope Taphonomical Initiative. Palaeogeogr Palaeoclimatol Palaeoecol 312:195–208
Pérès JM (1992) Le bathyscaphe Francais Archimède aux Acores: Étude bionomique et ecologique du benthos profond. Acoreana Suppl 1992:237–264
Piazena H, Perez-Rodrigues E, Häder D-P, Lopez-Figueroa F (2002) Penetration of solar radiation into the water column of the central subtropical Atlantic Ocean – optical properties and possible biological consequences. Deep-Sea Res II 49:3513–3528
Preston FW (1962) The canonical distribution of commonness and rarity. Ecology 43(185–215):410–432
Quartau R, Trenhaile AS, Mitchell NC, Tempera F (2010) Development of volcanic insular shelves: insights from observations and modelling of Faial Island in the Azores Archipelago. Mar Geol 275:66–83
Quartau R, Tempera F, Mitchell NC, Pinheiro LM, Duarte H, Brito PO, Bates R, Monteiro JH (2012) Morphology of the Faial Island shelf (Azores): the interplay between volcanic, erosional, depositional, tectonic and mass-wasting processes. Geochem Geophys Geosyst 13, Q04012. doi:10.1029/2011GC003987
Ramalho R, Quartau R, Trenhaile AS, Mitchell NC, Woodroffe CD, Ávila SP (2013) Coastal evolution in oceanic islands: a complex interplay between volcanism, erosion, sedimentation and biogenic production. Earth Sci Rev 127:140–170
Santos RS, Hawkins S, Monteiro LR, Alves M, Isidro EJ (1995) Marine research, resources, and conservation in the Azores. Aquat Conserv 5:311–354
Santos RS, Tempera F, Colaço A, Cardigos F, Morato T (2010) Spotlight: Dom João de Castro Seamount. Oceanography 23:200–201
Scoffin TP, Alexandersson ET, Bowes GE, Clokie JJ, Farrow GE, Milliman JD (1980) Recent, temperate, sub-photic, carbonate sedimentation: Rockall Bank, northeast Atlantic. J Sed Petrol 50:331–356
Taylor PD, Wilson MA (2002) A new terminology for marine organisms inhabiting hard substrates. Palaios 17:522–525
Tempera F, Giacomello E, Mitchell NC, Campos AS, Braga-Henriques A, Bashmachnikov I, Martins A, Mendonça A, Morato T, Colaço A, Porteiro FM, Catarino D, Gonçalves J, Pinho MR, Isidro IJ, Santos RS, Menezes G (2012) Mapping the Condor seamount seafloor environment and associated biological assemblages (Azores, NE Atlantic). In: Harris PT, Baker EK (eds) Seafloor geomorphology as benthic habitat: Geohab atlas of seafloor geomorphic features and benthic habitats. Elsevier, London, pp 807–818
Thiel M, Gutow L (2005) The ecology of rafting in the marine environment. II. The rafting organisms and community. Oceanogr Mar Biol 43:279–418
Tipper JC (1979) Rarefaction and rarefiction - the use and abuse of a method in paleoecology. Paleobiology 5:423–434
Vogel K, Gektidis M, Golubic S, Kiene WE, Radtke G (2000) Experimental studies on microbial bioerosion at Lee Stocking Island, Bahamas and One Tree Island, Great Barrier Reef, Australia: implications for paleoecological reconstructions. Lethaia 33:190–204
Wahl M (2009) Marine hard bottom communities: patterns, dynamics, diversity, and change. Ecological Studies 206. Springer, Berlin Heidelberg
Westphal H, Halfar J, Freiwald A (2010) Heterozoan carbonates in subtropical to tropical settings in the present and past. Int J Earth Sci 99:153–169
Wisshak M, Gektidis M, Freiwald A, Lundälv T (2005) Bioerosion along a bathymetric gradient in a cold-temperate setting (Kosterfjord, SW Sweden): an experimental study. Facies 51:93–117
Wisshak M, López Correa M, Gofas S, Salas C, Taviani M, Jakobsen J, Freiwald A (2009a) Shell architecture, element composition, and stable isotope signature of the giant deep-sea oyster Neopycnodonte zibrowii sp. n. from the NE Atlantic. Deep-Sea Res I 56:374–404
Wisshak M, Neumann C, Jakobsen J, Freiwald A (2009b) The ‘living-fossil community’ of the cyrtocrinid Cyathidium foresti and the deep-sea oyster Neopycnodonte zibrowii (Azores Archipelago). Palaeogeogr Palaeoclimatol Palaeoecol 271:77–83
Wisshak M, López Correa M, Zibrowius H, Jakobsen J, Freiwald A (2009c) Skeletal reorganisation affects geochemical signals, exemplified in the stylasterid hydrocoral Errina dabneyi (Azores Archipelago). Mar Ecol Prog Ser 397:197–208
Wisshak M, Form A, Jakobsen J, Freiwald A (2010) Temperate carbonate cycling and water mass properties from intertidal to bathyal depths (Azores). Biogeosciences 7:2379–2396
Wisshak M, Tribollet A, Golubic S, Jakobsen J, Freiwald A (2011) Temperate bioerosion: Ichno- and biodiversity from intertidal to bathyal depths (Azores). Geobiology 9:492–520
Young PS (1998) Cirripedia (Crustacea) from the "Campagne Biacores" in the Azores region, including a generic revision of Verrucidae. Zoosystema 20:31–92
Zeff ML, Perkins RD (1979) Microbial alteration of Bahamian deep-sea carbonates. Sedimentology 26:175–201
Zibrowius H (1980) Les Scléractinaires de la Méditerranée et de l'Atlantique nord-orental. Mem Inst Oceanogr Monaco 11:1–227
Zibrowius H, Cairns SD (1992) Revision of the Northeast Atlantic and Mediterranean Stylasteridae (Cnidaria: Hydrozoa). Mem Mus Nat Hist Natl A 153:1–136
Acknowledgments
Carrying out the experiment was only possible with the commitment of the Rebikoff Niggeler Foundation and their submersible ‘LULA’. Taxonomic identifications greatly profited from the expertise of H. Zibrowius (serpulids, scleractinian corals), J.-G. Harmelin (cyclostome bryozoans), J. Aguirre and M. Verlaque (coralline algae), and S. Schiaparelli (vermetid gastropods). I. Pyko helped with the assessment of carbonate accretion densities, and N. Belou supported the statistical analyses (ANOSIM). Sediment samples were taken during RV Victor Hensen cruise VH97, funded by the Deutsche Forschungsgemeinschaft (He 1671/1). The manuscript was improved based on the thorough reviews and valuable comments provided by Sérgio Ávila and anonymous referees. The EU FP7 Synthesys program allowed BB to study bryozoan type material at the Muséum National d'Histoire Naturelle, Paris (FR-TAF-1902). The settlement experiment was financed by the Deutsche Forschungsgemeinschaft (DFG Fr 1134/12).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wisshak, M., Berning, B., Jakobsen, J. et al. Temperate carbonate production: biodiversity of calcareous epiliths from intertidal to bathyal depths (Azores). Mar Biodiv 45, 87–112 (2015). https://doi.org/10.1007/s12526-014-0231-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-014-0231-6