Abstract
Indonesian coral reefs are the epicenter of marine biodiversity, yet are under rapid anthropogenically induced decline. Therefore, ecological monitoring of high diversity taxa is paramount to facilitate effective management and conservation. This study presents an initial report from a comprehensive survey of shallow-water (0–15 m) gorgonian assemblage composition and structure across sites with varying habitat quality within the Wakatobi Marine National Park (WMNP), SE Sulawesi, Indonesia. Current estimates of over 90 morphospecies from 38 genera and 12 families within the calcaxonian, holaxonian and scleraxonian groups are reported. This extensive survey confirms high local gorgonian abundance, diversity and species richness in the absence of anthropogenic influence and increasing with depth. Notably, morphological variants of the zooxanthellate species Isis hippuris Linnaeus, 1758, and Briareum Blainville, 1830, drive site and habitat assemblage differences across environmental gradients. Azooxanthellate taxa, particularly within the Plexauridae, drive species richness and diversity with depth. Of the 14 predictor variables measured, benthic characteristics, water flow and natural light explained just 30% of gorgonian assemblage structure. Furthermore, zooxanthellate and azooxanthellate taxa partitioned distinct gorgonian communities into two trophic groups—autotrophs and heterotrophs, respectively—with contrasting diversity and abundance patterns within and between study sites. This study strongly supports the WMNP as an area of high regional gorgonian abundance and diversity. Varying ecological patterns across environmental clines can provide the foundation for future research and conservation management strategies in some of the most biodiverse marine ecosystems in the world.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Indonesian archipelago is a center of marine biodiversity, likely a consequence of geological and oceanographic processes influencing species diversification and persistence (Carpenter et al. 2011; Sanciangco et al. 2013) at local and regional scales. Eastern Indonesian reefs are particularly diverse, with low climatic variability and strong seasonal upwellings (Gieskes et al. 1988; Baars et al. 1990), yet ecological assessments are sparse (Tomascik et al. 2004). Increases in human population growth, continual marine resource exploitation through coral mining, and cyanide, dynamite and subsistence fisheries mean that such biodiverse ecosystems are being destroyed before their components are discovered (McManus 1997). Therefore, comparative assessment of coral reef communities relative to their environment, including the increasing assortment of anthropogenic influences, provides a valuable resource for conservation management.
Gorgonian corals (Cnidaria: Anthozoa: Octocorallia) are conspicuous, diverse and often dominant components of benthic marine environments, notably tropical shallow reefs, deep-sea habitats and mesophotic habitats (Cerrano et al. 2010; Rowley 2014b; Sánchez 2016). Numerous gorgonians are conservation ‘flagship’ species (e.g., Eunicella verrucosa Pallas, 1766; Tinsley 2005, Paramuricea clavata Risso, 1826; Linares et al. 2008a; Cerrano et al. 2010), being ecologically diverse, long-lived engineering taxa that maintain habitat heterogeneity and provide secondary space to other organisms (Buhl-Mortensen et al. 2010). Nevertheless, despite their ecological importance and diversity, there is still little information on gorgonians within the Indonesian archipelago (Tomascik et al. 2004).
Gorgonian corals are colonial suspension feeders primarily defined by a semirigid scleroproteinaceous (gorgonin) axis with varying amounts of calcification deposited primarily as magnesium calcite (Bayer 1961). The Octocorallia are characterized by polyps bearing eight pinnate tentacles, and eight mesenteries dividing the gastrovascular cavity (Bayer 1961). Originally classified under the order Gorgonacea, gorgonians currently comprise the subordinal groups, Holaxonia and Calcaxonia, and the group scleraxonians within the order Alcyonacea (Bayer 1981). Even though taxonomically obsolete, the term gorgonian continues to be used and indicates a specific structural group that is differentiated from the ‘true soft corals’ (of the subordinal group Alcyoniina characterized by lacking a skeletal axis). Taxonomic identification of Indo-Pacific gorgonians is, however, confounded by widespread homoplasy, considerable morphological variability, cryptic and sibling taxa (Knowlton 1993). Classified as ‘poorly known’ (van Ofwegen 2004), shallow-water gorgonian taxonomy within Central Indonesia remains in a state of flux requiring integrative systematic, molecular and ecological approaches.
Gorgonian ecology reflects, together or in part, reproductive strategies and changes along environmental gradients relative to individual species tolerances (Fabricius and Alderslade 2001). Environmental factors such as substrate type, light, temperature, sedimentation, salinity, current regime and flow rate (Garrabou et al. 2001) influence gorgonian demography. Biotic factors, including competition, predation, symbioses, reproduction, settlement and developmental properties, further refine local community structure (Sánchez 2004, 2016). Such factors have been shown to induce intra- and interspecific morphological variability (West 1997; Linares et al. 2008b; Prada et al. 2008), habitat selection and colony orientation (Sánchez et al. 2003). Nevertheless, gorgonians are typically associated with areas of low sedimentation and high water flow through strong currents and upwellings (Yoshioka and Yoshioka 1989); the largest planar arborescent colonies occur in healthy reef environments (Linares et al. 2008b). Complex habitats provide more vertical relief, colonizable area and microhabitat variability than soft benthic substrata (Etnoyer et al. 2010). Yet even with suitable substratum, most gorgonians are absent in the areas of high turbidity and sediment load, likely due to the physical impairment of settlement, feeding, reproduction and growth (Anthony and Fabricius 2000). In contrast, high-turbidity reefs in Singapore, for example, support healthy azooxanthellate gorgonian communities (Goh and Chou 1994). Reduced irradiance may therefore provide competitive release (Rogers 1990) for azooxanthellate taxa, turbid habitats being marginal for zooxanthellate gorgonians as they tend to follow similar depth ranges to scleractinian corals with no evidence of hard coral community replacement (Fabricius and Alderslade 2001). Moreover, there appears to be no evidence for negative associations with other benthic space competitors in other areas (e.g., Yoshioka and Yoshioka 1989).
Gorgonian distribution has been positively associated with substrate availability and type (Goh and Chou 1994), localized overlapping of species range sizes (as a function of temperature) and benthic–pelagic coupling (Matsumoto et al. 2007). Both azooxanthellate and zooxanthellate gorgonians show eco-phenotypic interactions that strongly correlate with depth (West et al. 1993) and size (Sebens 1982). Yet little is known of the ecology, reproductive strategies and relative range sizes for most gorgonian species in the Indo-Pacific.
Prominent drivers of gorgonian ecology, therefore, remain unclear; studies usually describe regional differences (Singapore: Goh and Chou 1994; Caribbean: Sánchez et al. 1997; Guam: Paulay et al. 2003; Hong Kong: Fabricius and McCorry 2006; Japan: Matsumoto et al. 2007; tropical America: Sánchez 2016). However, ecological factors that regulate species diversity, as well as consistency in species nomenclature, are of significant research and conservation importance, especially within the Indonesian archipelago, which is subject to continual overexploitation and habitat loss. Published expeditions within Central Indonesia, such as the ‘Siboga’ (Versluys 1902, 1906; Nutting 1910a, b, c, d, e, 1911; Stiasny 1937) and ‘Snellius’ (e.g., Stiasny 1940; Verseveldt 1966) sampled only deepwater and Alcyoniidae taxa, respectively, which are largely unrepresentative of shallow-water gorgonians on Indonesian reefs. Annual rapid assessment surveys are increasingly conducted by conservation agencies (e.g., World Wildlife Fund, The Nature Conservancy) throughout the Indonesian archipelago, with a view for sustainable conservation management. Such surveys are rudimentary with low taxonomic resolution for gorgonians. The disparity between gorgonian diversity and ecological assessment within Indonesia is therefore primarily due to taxonomic uncertainty (Bayer 1981), with concomitant difficulties in field identification (Fabricius and Alderslade 2001).
Little is known of gorgonian ecology within SE Sulawesi, Indonesia, despite their high regional abundance and diversity. The aims of this study therefore were to (1) characterize gorgonian assemblage composition and structure across a gradient of habitat quality within the Wakatobi Marine National Park (WMNP), (2) assess gorgonian diversity and abundance between reef habitats as a function of depth within each site, (3) describe differences in the distribution patterns of zooxanthellate (phototrophic) and azooxanthellate (heterotrophic) gorgonian taxa and (4) identify potential environmental driver(s) of gorgonian assemblage structure.
Materials and methods
Study area
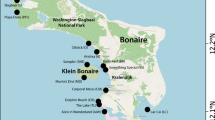
The WMNP (Tukang Besi Islands) is a remote island group of ca. 13,900 km2 in SE Sulawesi, Indonesia (Fig. 1a). Established in 1996, the WMNP is the second largest marine park in Indonesia containing ca. 600 km2 of the most biodiverse coral reefs in the world (Scaps and Denis 2007), with a low incidence of coral disease (0.57%; Haapkylä et al. 2007) and ENSO-induced bleaching events (Crabbe and Smith 2003; Haapkylä et al. 2007) likely due to local upwelling (in April–November; Gieskes et al. 1988; Baars et al. 1990; Tomascik et al. 2004). Approximately 100,000 people live within the Wakatobi Marine National Park, resulting in extensive subsistence marine resource dependence and destructive commercial fisheries in populated areas (Clifton 2013). Four sites were selected around the islands of Kaledupa (ca. 17,000 people) and Hoga (< 100 people; Fig. 1b) which have different levels of natural and anthropogenic disturbance (Electronic supplementary material, ESM, Fig. S1), and distance from shore. Sampela (impacted), an enclosed lagoon with an outer reef wall ca. 400 m from a Bajo (sea gypsy) village of ca. 1600 people, is subject to exploitation through coral mining, fishing and high sediment loading due to natural re-suspension, bioturbation through gleaning, and mangrove loss. Furthermore, community wastewater is released onto the reef (Haapkylä et al. 2007). Buoy 3, ca. 500 m offshore, is a moderately sheltered fringing reef with a sheer reef wall containing small cryptic overhang habitats. This site has an extended reef flat, which is subject to frequent gleaning of marine invertebrates by local inhabitants, in addition to recovering from coral mining and blast fishing since 2004. Pak Kasim’s, ca. 500 m offshore, is a topographically complex fringing reef, also subject to coral mining and blast fishing on the reef flat and crest until 2004. Ridge 1 (healthy), ca. 1 km offshore, is an exposed reef ridge with strong water currents (Fig. 1b) and upwelling with a small amount of blast fishing on the reef crest in 2004 (D. J. Smith pers. comm.). The reef slope can also be sheer with cryptic overhang habitats. All sites have a pronounced reef flat of < 3 m depth (Ridge 1 has a shallow reef plateau at ca. 3 m depth), reef crest (3–6 m depth) and slope (> 6 m depth) with varying levels of sedimentation draining from the reef flats during spring tides.
Sample collection
Gorgonian distribution and abundance
Surveys were conducted between June and September 2009 using SCUBA, snorkeling and scaled digital photography. Four 10 × 4 m belt transects were laid ca. 20 m apart, running parallel to the reef contour in each reef habitat (flat ≤ 3 m, crest ca. 6 m and slope ca. 12 m depth) within each site. The 12 transects (four at each depth/habitat: flat, crest and slope) were run at each study site, covering a total area surveyed of 1920 m2. Individual colonies encountered along each transect, including beneath canopy structures (Goatley and Bellwood 2011), were photographed using a Canon IXUS 900Ti, with WP-DC7 underwater housing and INON UWL-105 AD × 0.51 lens. Considering the propensity for asexual fragmentation in some octocorals (Fabricius and Alderslade 2001), an individual colony was defined as an independent colony unassociated with any other colony of the same phenotype (e.g., touching, as in encrusting forms of Briareum spp.). Each image was taken directly opposite and/or above each colony with a ruler for scale. Voucher specimens (branch clippings of ~2 to 8 cm in length) were preserved in 95% ethanol for taxonomic clarification and stored at the Bernice P. Bishop Museum, Honolulu, USA (Accession number: 2014.005). Sclerites were dissolved from the surrounding tissue in 5% sodium hypochlorite solution and visualized using optical microscopy. Gross taxonomic identification followed Bayer (1981), Fabricius and Alderslade (2001) and references therein (see ESM taxonomic notes). A thorough taxonomic account (morphological and molecular) of the gorgonian corals from this region is currently underway. Taxa were determined to be zooxanthellate through centrifugation as described in Rowley (2014a). Many colonies were identified based on colony and branching morphology, polyp characteristics and sclerite analysis, and assigned to ‘morphospecies’ within genera since most gorgonian species within the Indo-Pacific are undescribed and there are considerable crossover/intermediates with those that are. However, individuals were grouped in accordance with Bayer’s (1981) three-group system (suborders Holaxonia and Calcaxonia, and scleraxonians group) and the families and genera therein.
Environmental variables
Sites were characterized through the assessment of 14 environmental variables (Table 1). Benthic characteristics were determined using transects as described for gorgonian surveys and categorized according to English et al. (1997) using point-intercept transects with points every 0.5 m (Kingsford and Battershill 1998). Values are expressed as % cover (± SE). Rugosity was measured with a 7.30-m chain laid over three replicate transects per habitat and calculated using the ratio of contoured surface distance to linear distance (McCormick 1994). Rugosity was not measured for overhangs due to logistical constraints.
Suspended sedimentation rates were assessed using four standard 1-L sediment traps (English et al. 1997) deployed in each habitat at all sites for 10 d. Sediment and water were filtered (0.2 μm pore size), dried at 60 °C and weighed (g dry weight d−1). Sediment grain diameter for all samples was measured using Retsch Technology test sieves (aperture size range 2.0, 1, 0.5, 0.125, 0.25, 0.063, < 0.063 mm), logarithmically converted, expressed as phi (Φ) and classified under the Wentworth scale (Wentworth 1922). Water flow velocity (cm s−1) was measured using a General Oceanics flow meter with a low-velocity rotor and custom-made aluminum pipes for reef placement. Chlorophyll-a (µg L−1), salinity (PSU) and turbidity (NTU) were measured using RBR XR-420 CTD data loggers. Temperature (°C) and light (measured as lux and presented as Kd(PAR)) were measured using HOBO data loggers. The loggers were placed upright at each transect depth, recording every minute for up to 24-h cycles for at least the study period. Latitude and longitude were determined by a handheld GPS meter (Garmin eTrex). All variables except latitude and longitude were entered into the statistical models as raw values. Significant outliers were removed. Outliers included light measurements during days of persistent cloud cover and sediment traps containing fish and invertebrates that may have skewed the end results.
Data analyses
Data were analyzed using univariate (SPSS v18.0) and multivariate routines in the PRIMER-E v6.1.12 statistical package (Clarke and Gorley 2006), with PERMANOVA+ v1.02 extension (Anderson 2001). Gorgonian assemblage data were dispersion weighted, a transformation procedure that accounts for the variance structure of individual species (Clarke et al. 2006b). Differences in gorgonian assemblages were analyzed with a two-factor (site and habitat) crossed model with pairwise comparisons using 9999 permutations (PERMANOVA; Anderson 2001) based on a zero-adjusted Bray–Curtis similarity matrix (Clarke et al. 2006a). Results were visualized using a constrained canonical analysis of principal coordinates (CAP; Anderson and Willis 2003), which reveals real group differences from the maximum variation between groups. Prominent taxa contributing to dissimilarities among gorgonian assemblages were identified using similarity percentages (SIMPER; Clarke 1993). The influence of dominant species was further investigated using Pearson’s product-moment correlations for each species with each canonical axis (Anderson and Willis 2003) and displayed as a vector overlay on CAP ordinations. Morphospecies diversity indices were used to quantitatively assess gorgonian assemblage structure among the study sites and habitats; indices were species richness as the total number of morphospecies (S) present, and the Hill numbers N1, N2 and modified ratio N21’ (Peet 1974) to assess the influence (defined as the effect of certain morphospecies on the assemblage sampled) of rare and dominant species and taxonomic spread (equitability), respectively (Clarke and Gorley 2006). For example, the higher the mean value for each metric, the higher the influence of rare (N1) or dominant (N2) morphospecies sampled in an assemblage. Similarly, higher taxonomic spread (N21’) or evenness in a sample indicates a similar number of individuals per morphospecies and, therefore, low dominance of any one morphospecies. Conversely, a low level of evenness reveals that one or more morphospecies are represented by a higher number of individuals and are thus dominating the assemblage sampled. It is noteworthy that the diversity indices presented here each assess a specific objective, e.g., the effect that rare or dominant morphospecies have/has on the section of the assemblage sampled. Therefore, in this study four indices were selected to describe the assemblages sampled, which have varying species richness and diversity across sites and habitats. Species accumulation curves with eight estimates of morphospecies richness extrapolated to 48 samples (transects) were also assembled in PRIMER-E v6.1.12 (ESM Fig. S2). Replicates were permuted randomly 999 times. Zooxanthellate and azooxanthellate gorgonian distributions were tested for independence using the Wald–Wolfowitz (runs) test (SPSS v18.0; Wald and Wolfowitz 1943).
Predictor environmental variable(s) thought to influence the ecological structure of gorgonian assemblages were investigated using distance-based forward selection analysis of linear models (DISTLM forward; McArdle and Anderson 2001) based on a Euclidean distance matrix. Variables were normalized and conditionally tested using 9999 permutations of the residuals under a reduced model (Anderson 2001). This model was then replicated using only the primary environmental variables as identified by the initial ‘all variable’ DISTLM forward model. Results were visualized using the distance-based redundancy analysis ordination (dbRDA; McArdle and Anderson 2001).
Results
Study site characteristics
Environmental variables characterizing each study site reveal a gradient of habitat quality from the impacted site Sampela to the healthy reefs of Ridge 1 (Tables 1 and 2). Live benthic variable abundance, particularly soft coral cover, increased toward Ridge 1, while abiotic variables and dead coral decreased toward Ridge 1(Table 1). Hard coral cover also increased toward Ridge 1, but peaked at Buoy 3 due to a dominance of encrusting Montipora colonies on the reef slope (Haapkylä et al. 2007). Rugosity also increased from low (0.82 ± 0.04) at Sampela to complex (0.61 ± 0.03) at Ridge 1 (Table 1). Daily sedimentation rate, turbidity, light attenuation and water flow also followed the same trend, with sediment grain size markedly smaller at Sampela than at the other sites. Sediment was of limestone origin except at Sampela, which had smaller, darker and slightly oily sediment particles (S Rowley pers. obs.). The annual range of both salinity and temperature at each site was small, with the lowest temperature at Ridge 1 (Table 1). Chlorophyll-a varied across sites, the greatest variance being at the far ends of the gradient.
Gorgonian distribution and abundance
A total of 3483 gorgonian colonies were documented in this study; 126, 441, 1171 and 1745 recorded at Sampela, Buoy 3, Pak Kasim’s and Ridge 1, respectively (Fig. 2; Table 2). To date, over 90 gorgonian morphospecies from 38 genera and 12 families within the suborders/group Calcaxonia, Holaxonia and Scleraxonia have been identified (Table 2). Species richness and diversity followed a typical pattern of increase from the impacted site Sampela to the healthy site Ridge 1 (Fig. 2; Table 2). This pattern of increased species richness and diversity was similarly replicated with depth except at Sampela, where most colonies and species were found on the reef crest and flat (Fig. 2). The contrast between the relatively constant evenness values (N21’) with increases in both rare (N1) and dominant (N2) morphospecies as depth increases and disturbance decreases (Fig. 2) suggests that zooxanthellate and azooxanthellate gorgonians have different distributions in the WMNP. Diversity indices highlight the differences between the two trophic groups (Fig. 3; azooxanthellate diversity increases with depth as evenness decreases due to differential distribution between taxa). Buoy 3 is an exception; here, individuals were distributed relatively evenly among morphospecies. In contrast, zooxanthellate morphospecies diversity decreased with depth, with evenness relatively constant. This pattern was very weak at Sampela and Buoy 3 due to the small number of zooxanthellate taxa present at depth at these sites (Fig. 5a, g; Table 2).
Colony density (colonies 20 m−2) followed a very similar pattern to species richness across all sites and habitats (ESM Fig. S2a–c). The highest density of gorgonian colonies was on the reef slope at Ridge 1 (171 ± 1 20 m−2; ESM Fig. S2a) largely composed of azooxanthellate taxa (101 ± 0.8 20 m−2; ESM Fig. S2c). Pak Kasim’s had the greatest density on the reef crest (101 ± 1.7 20 m−2) dominated by zooxanthellate taxa (76 ± 1.4 20 m−2; ESM Fig. S2b). Overall colony density at the degraded site Sampela was greatest on the reef crest and completely attributed to zooxanthellate colonies (13.25 ± 1.7 20 m−2; ESM Fig. S2a, b), which was also the case on the reef flat at Buoy 3 (29.75 ± 2.4 20 m−2; ESM Fig. S2a, b).
Gorgonian community structure
There were clear differences in gorgonian community structure among sites and habitats within the WMNP (Figs. 2, 3, 4, 5 and 6). Seven families from the Calcaxonia (Ellisellidae, Isididae), Holaxonia (Acanthogorgiidae, Plexauridae) and Scleraxonia (Briareidae, Subergorgiidae and Melithaeidae) characterized reef habitats from low diversity and abundance at the impacted site Sampela to high diversity and abundance at Ridge 1 (Fig. 3). Furthermore, morphospecies accumulation indices confirm that richness was predominantly represented by azooxanthellate taxa, particularly a high number of rare taxa observed in just one or two samples (ESM Fig. S3a, b; Table S1).
Mean zooxanthellate (a, c, e, g) and azooxanthellate (b, d, f, h) gorgonian species richness (a, b), Hill’s diversity indices N1 for the influence of rare species (c, d), N2 for the influence of dominant species (e, f) and modified ratio for evenness N21’ (g, h) across sites and habitats. Errors are standard errors Sa, Sampela; B3, Buoy 3; PK, Pak Kasim’s; R1, Ridge 1
Constrained CAP ordinations based on dispersion-weighted pretreated data and zero-adjusted Bray–Curtis distance matrices, of (a, c) zooxanthellate and (b, d) azooxanthellate gorgonian assemblages between sites (a, b) and habitats (c, d). Vector overlays show species contributing the most difference among the a priori groups tested
The Isididae at Sampela were dominant across the flats and crest (11.5 ± 1 and 11.5 ± 1.4 20 m−2, respectively), with occasional Briareidae on the slope (2 ± 1 20 m−2). At Buoy 3, Isididae were dominant on the reef flat (30 ± 2 20 m−2), Acanthogorgiidae characterized overhangs on the reef crest (20 ± 1.5 20 m−2), and Plexauridae on the reef slope (18 ± 1 20 m−2). Pak Kasim’s was dominated by high numbers of the Isididae on the reef flat and crest (68 ± 3 20 and 58 ± 2 20 m−2, respectively), with Plexauridae and Briareidae on the reef slope (56 ± 2 20, 27 ± 1 20 m−2, respectively). Isididae and Briareidae had the greatest relative abundance on the ridge top at Ridge 1 (48 ± 2 20, 30 ± 3 20 m−2, respectively), Plexauridae, Isididae, Briareidae and Melithaeidae on the reef crest (49 ± 2, 36 ± 3, 28 ± 1 and 27 ± 1 20 m−2, respectively), and Plexauridae and Briareidae on the reef slope (63 ± 0.1 20, 67 ± 1 20 m−2). In sum, phototrophic/zooxanthellate taxa were low in species diversity (Fig. 5; ESM Fig. S3c), but had the greatest relative abundance on the reef flats and crest for Isididae and reef slope for Briareidae (Fig. 3). Heterotrophic/azooxanthellate taxa, especially within the family Plexauridae, contributed greatest to the increased biodiversity with depth (Fig. 3).
Colony size is not reported in this study (it was recorded; these data will be reported in a future study), but numerous azooxanthellate species were small (< 10 cm high) and located within sheltered crevices, overhangs, or at the base or under other coral colonies (e.g., the soft coral Sarcophyton Lesson, 1834, and tabulate scleractinian Acropora Oken, 1815). Observations at depths greater than those reported here suggest a continual increase in gorgonian diversity, abundance and size, plus a remarkable frequency of new recruits (< 5 cm tall, generalized estimate across taxa from a combination of repeated annual surveys [Rowley unpublished data] and published gorgonian studies e.g., Bramanti et al. 2005; Linares et al. 2008b). Additional gorgonian species present within the WMNP not encountered during the surveys are documented in Table 2. Additional observations to note include colony asexual fragmentation by Junceella fragilis (also noted by Fabricius and Alderslade 2001) and the morphotypes of Isis hippuris and Briareum spp. Ad hoc measurements and tagging of fragments of Isis morphotypes at each study site revealed upward growth irrespective of site or depth (SJ Rowley unpublished data). Finally, small colonies of Acanthogorgia and Bebryce were frequently encountered at the base of large (> 50 cm in height) Annella reticulata Ellis and Solander 1736, A. mollis Nutting 1910, and Melithaea spp. colonies. This contrasted with occasional observations of the same species on the open reef, where it would invariably be heavily infested with fouling organisms (ESM Fig. S4).
Gorgonian abundance varied significantly among all sites and habitats with no interaction effects (PERMANOVA: pseudo-F = 7.938, P < 0.0001; pseudo-F = 6.714, P < 0.0001). Pairwise comparisons revealed significant differences were between all sites and habitats, most notably Sampela and Ridge 1, and the reef flat and slope, respectively (Fig. 4). CAP analyses were consistent with these results, where strong allocation success (number of correct allocations to each factor level) clearly defined distinct assemblage variability between sites and habitats (Fig. 4; Table 2). SIMPER further revealed that particular morphotypes within the zooxanthellate taxa Isis hippuris Linnaeus, 1758, and Briareum Blainville, 1834, accounted most for the differences in gorgonian assemblages among sites and habitats (Table 3). Specifically, I. hippuris colonies with long thick branches (hereafter I. hippuris[LT]) were prevalent on the reef flat at Sampela (Fig. 4a, b), whereas low-lying branching Briareum species (hereafter Briareum sp.B) were more abundant toward the reef slope, particularly at Ridge 1 (Fig. 6a, c). In contrast, I. hippuris colonies that were planar or multiplanar with short branches (hereafter I. hippuris[N]) were more abundant on the reef crest (Fig. 4b). Encrusting Briareum colonies (denoted Briareum sp.E) were more abundant on the reef flat, particularly at Ridge 1 (Fig. 4a, b). In addition, the azooxanthellate Acanthogorgia sp.2 contributed considerably to the difference between the reef crest and flat (Fig. 4b). This was due to its exclusive and abundant presence on the ceilings of caves and overhangs, characteristic of Buoy 3.
Zooxanthellate versus azooxanthellate gorgonians
The dominance of the zooxanthellate gorgonians I. hippuris (1094 colonies) and Briareum spp. (792 colonies) obscured distribution patterns of azooxanthellate taxa (Fig. 4). A total of 1896 zooxanthellate and 1587 azooxanthellate gorgonian colonies were surveyed. Calcaxonians, holaxonians and scleraxonians were represented by both zooxanthellate and azooxanthellate taxa with six genera belonging to four families and 31 genera belonging to 10 families, respectively. Taxonomic richness and diversity of azooxanthellate species largely replicated that of Fig. 2 (and ESM Fig. S2a), increasing toward Ridge 1 and with depth (Fig. 5). Zooxanthellate taxonomic richness and diversity also increased with site, but showed an inverse relationship with depth, being greatest at the reef crest and flat (Fig. 5).
The distributions of zooxanthellate and azooxanthellate taxa were non-random [Wald–Wolfowitz (runs) test, P < 0.001]. The relative abundance of both zooxanthellate and azooxanthellate taxa differed significantly across sites (PERMANOVA, pseudo-F = 9.476, P < 0.0001 and pseudo-F = 3.997, P < 0.0001, respectively) and habitats (PERMANOVA, pseudo-F = 7.716, P < 0.0001 and pseudo-F = 4.687, P < 0.0001, respectively). Yet an interaction effect (pseudo-F = 1.925; P = 0.012) between sites and habitats for azooxanthellate taxa revealed that significance levels were principally driven by zooxanthellate gorgonians. Results were further supported by CAP analyses; allocation success was weaker for azooxanthellate taxa at Pak Kasim’s, Buoy 3 and the reef crest (Table 3). CAP and SIMPER analyses confirmed previous results of I. hippuris[LT] on the reef flats at Sampela, and I. hippuris[N] toward the reef crest (Fig. 6a, c; Table 3). Briareum spp. followed a typical pattern of encrusting on the reef flats at Ridge 1, with low-lying branching colonies characterizing the reef crest and slope, a pattern particularly replicated at Pak Kasim’s (Fig. 6a, c). It is notable that both Briareum spp. and I. hippuris colonies had different coloration at depth and areas of high turbidity; Briareum colonies were typically magenta on the reef flat and crest and brown or gray on the reef slope and at Sampela. Similarly, I. hippuris colonies were mustard yellow on the reef flat and crest, but beige on the reef slope, particularly at Sampela.
Azooxanthellate species within five families principally defined the reef slope (Fig. 6d) except at Sampela. However, only two azooxanthellate colonies (Melithaea ochracea Linnaeus, 1758, and an Astrogorgia sp.) were encountered during the survey at Sampela (Table 2). Species richness and diversity were similar to the first model (Fig. 2) for the crest and slope except at Sampela (Fig. 5b, d, f, h). The pattern of Acanthogorgia sp.2 on the reef crest at Buoy 3 (Fig. 6b, d) was replicated by Melithaea sp.2 at Ridge 1, which also inhabited the ceilings of caves, overhangs and crevices. Both species are undescribed. Melithaea sp.1 showed distinct assemblages on the ridge top at Ridge 1 (Fig. 6d). However, most azooxanthellate taxa inhabited the reef slope with similar assemblage composition and distribution patterns across Buoy 3 and Pak Kasim’s as evident by the reduced allocation success (an indicator of reduced site and habitat distinction; Table 3) and site x habitat interaction.
Environmental variables
Biotic variables (sponges, algae, ascidians, mollusks), sediment grain size, light and rugosity explained 34.04% of the variability in gorgonian assemblage structure (pseudo-F = 3.864, P < 0.001; Fig. 7a, c). Benthic covariates of gorgonian assemblages and low influential variables (temperature, salinity) were omitted from a repeated analysis revealing light, sediment grain size, rugosity and water flow explained 41.65% of gorgonian assemblage variability (pseudo-F = 3.645, P < 0.001; Fig. 7b, d). The same model was applied separately to zooxanthellate and azooxanthellate species. Biotic variables, water flow and light explained 33.7% of the variability in zooxanthellate communities (pseudo-F = 4.732, P = 0.001), whereas rugosity, biotic variables and sediment grain size explained 28.24% of the variability in azooxanthellate communities (pseudo-F = 2.221, P < 0.001). Results from the abated model suggested that water flow, light and chlorophyll-a (40.15%; pseudo-F = 4.232, P = 0.001), rugosity, sediment grain size and light (45.50%; pseudo-F = 2.222, P = 0.001) had a significant influence on zooxanthellate and azooxanthellate distributions, respectively.
Distance-based redundancy ordinations of the best predictor variables for differences in gorgonian assemblages among sites (a full variables; b abated variables) and habitats (c full variables; d abated variables) within the Wakatobi Marine National Park. Vector overlays depict both direction and strength of the most influential variables on the dbRDA axes
Discussion
Current estimates of over 90 gorgonian species and distinct morphotypes from 38 genera and 12 families were documented across shallow (0–15 m) coral reefs within the WMNP, Indonesia. This study strongly supports the WMNP as an area of high regional gorgonian abundance and diversity comparable with previously described shallow-water gorgonians across the Indo-Pacific comprising ~50 genera within 14 families (Grasshoff 1999; Fabricius and Alderslade 2001; Samimi-Namin et al. 2011; Reijnen et al. 2014). Distinct community types across sites and habitats along an environmental gradient are characterized by contrasting distributions between zooxanthellate and azooxanthellate gorgonians. This pattern results in part from variations in habitat complexity, water flow and natural light.
Gorgonian assemblage structure
Gorgonian distribution within the WMNP followed a gradient of low diversity and abundance at the impacted site Sampela to high diversity and abundance at Ridge 1. Species richness and diversity increased with depth, a pattern consistent with previous research on azooxanthellate benthic invertebrates within the area (e.g., Porifera: Bell and Smith 2004), yet the inverse was true for zooxanthellate gorgonians, also seen for Scleractinia (Haapkylä et al. 2007). Similarly, gorgonian diversity is greater at depth in other areas (Singapore: Goh and Chou 1994; Caribbean: Sánchez et al. 1997; Marianas: Paulay et al. 2003; Hong Kong: Fabricius and McCorry 2006; Japan: Matsumoto et al. 2007; Palau: Fabricius et al. 2007; Philippines: Rowley 2014b) with concomitant zooxanthellate octocoral abundance in the shallows (Great Barrier Reef: Fabricius and Klumpp 1995; Thailand: Chanmethakul et al. 2010).
Gorgonian populations within the WMNP reached a mean density of up to 9 colonies m−2 on the reef slope at Ridge 1. They were dominated primarily by plexaurids and branching Briareum. Similarly, densities of I. hippuris colonies on the reef flat and crest of Sampela, even though greater than other benthic taxa at this site, were still comparatively lower (1.7 m−2) than at the other study sites (up to 9 m−2 at Pak Kasim’s). Densities were nevertheless greater than the anthropogenically impacted gorgonian populations reported at other geographic locations (Bramanti et al. 2014; Etnoyer et al. 2016). Similar densities to those seen at Ridge 1, particularly for taxa in the Plexauridae, have also been found at shallow depths (< 10 m) in the Caribbean (Etnoyer et al. 2010; Lenz et al. 2015), as well as for the endemic and azooxanthellate precious coral Corallium rubrum Linnaeus, 1758, in the Mediterranean (Bramanti et al. 2014). However, the Plexaurids of the WMNP were azooxanthellate and increased in colony density and species diversity with depth, unlike those of the Caribbean and tropical Atlantic, which are zooxanthellate. Nonetheless, increased octocoral ecological assessments particularly at greater depths may lead to the discovery of new species (e.g., Etnoyer et al. 2010) in the WMNP. This pattern is also seen in other Indo-Pacific locations (e.g., Philippines: Rowley 2014b), providing opportunities for phylogeographic comparisons.
Differences in gorgonian assemblage structure between sites and habitats were driven by morphotypes of the zooxanthellate isidid I. hippuris and morphotypes of the genus Briareum. The dominance of I. hippuris on shallow reef flats may be due, in part, to differential disturbance levels among study sites. As posited by the intermediate disturbance hypothesis, continual disturbance maintains species diversity, stability and biodiversity within a reef community (Connell 1978; Aronson and Precht 1995; Bohn et al. 2014). For example, strong upwelling and water currents at Ridge 1 are frequent yet not necessarily devastating disturbances. Frequent colonization of patches of disturbed reef would permit higher species diversity while preventing competitive dominance, a pattern seen at Ridge 1. Extreme disturbances at the degraded site Sampela include resource exploitation, high sedimentation rates and benthic grazing by the echinoderm Diadema spp. (Hodgson 2008), which is reflected in the impoverished gorgonian communities.
Habitat structural complexity, measured as colonizable area, substratum type and light intensity, can determine settlement choices and profoundly influence benthic community structure on coral reefs (Sánchez et al. 1997; Linares et al. 2008b). Yet the combination of predictor biotic variables, sediment grain size, rugosity and light explained only 23% of gorgonian assemblage structure across clear environmental clines. Evidently, two inherently related patterns are occurring. First, benthic variables such as sponges, algae, hard and soft coral, and all members of coral reef benthic communities co-vary with gorgonian distribution. Remodeling without these covariates revealed that sediment grain size, light, rugosity and chlorophyll-a still only explained 25% of gorgonian assemblage structure. Second, this suggests that zooxanthellate and azooxanthellate gorgonian distributions contrast with each other, essentially reflecting two different trophic groups, heterotrophs and phototrophs. This is more likely due to differential resource use relative to natural light as a function of bathymetry, than to interspecific competitive forces among shallow-water gorgonians.
Zooxanthellate versus azooxanthellate gorgonians
The dominance of zooxanthellate taxa driving separation between reef areas and location obscured azooxanthellate distribution patterns. Trophic group separation (zooxanthellate = phototrophy; azooxanthellate = heterotrophy) revealed a clear environmental gradient interaction with depth. Thus, the groups displayed contrasting patterns, with azooxanthellate species richness and diversity increasing with depth, consistent with other areas (Goldberg 1973; Goh and Chou 1994; Sánchez et al. 1997; Paulay et al. 2003; Fabricius and McCorry 2006; Matsumoto et al. 2007; this study), and the opposite pattern for zooxanthellate taxa.
Zooxanthellate gorgonian assemblages
Zooxanthellate species are primarily responsible for differences between site and habitat. Distinct I. hippuris morphologies showed patterns of variability both within and among sites, most notably bushy colonies with long thick branches (I. hippuris[LT]) on the reef flat at Sampela and planar short tightly packed branched colonies (I. hippuris[N]) at Ridge 1. Colony form can depend on feeding strategy, and the same genotype can show different resource allocation patterns in different environments (Weiner 2004; Rowley 2014a). Alternatively, morphological variants in sympatry are common in Cnidaria (Knowlton 1993; Prada et al. 2008) and may be indicative of phenotypic plasticity or incipient ecological divergence in response to natural light and water flow. Variation through increased branching surface area enhancing photosynthetic efficiency in shallow-water branching taxa (Hennige et al. 2008; Rowley 2014a), coupled with a dual mode of reproduction (external brooding and asexual fragmentation; Rowley 2014a), may likely explain the biological success of I. hippuris across environmental clines within the WMNP.
The zooxanthellate gorgonian I. hippuris is a gonochoristic (Simpson 1906) external brooder (Rowley 2014a), yet also displays considerable fragmentation. Asexual propagation through fragmentation is not uncommon in gorgonians. Because of being exposed to various levels of disturbance, clones within a species can vary in their sensitivity to various types of disturbances either across sites or habitats within sites (Coffroth and Lasker 1998). However, vegetative fragments of I. hippuris were present on the reef flats across all study sites, suggesting that morphotypes were not disturbance sensitive. Asexual propagation through fragmentation facilitates rapid post-disturbance recovery (Dauget 1992), which can result in high local population abundance as evident by I. hippuris on the reef flats in the WMNP.
The asexual fragmentation observed in Isis may enable colonization of a disturbed area, particularly in the absence of other taxa or suitable substratum for settlement. However, densities of I. hippuris[LT] colonies at Sampela were low compared to the other sites. Therefore, even though I. hippuris[LT] was the dominant taxon on the reef flats at Sampela it was still not immune to the frequency of high disturbances. In contrast to Sampela, Buoy 3 and Pak Kasim’s, which are both subject to past destructive fishing practices and bleaching on the former reef flats (ending in 2004; D. J. Smith pers. comm.), have higher gorgonian abundance, diversity and colony density. However, considerable loose rubble and anthropogenic gleaning on the shallow (~1–3 m) reef flats of Buoy 3 likely impede settlement success even at low levels of turbulence (Goh and Chou 1994), thus resulting in minimal recovery and gorgonian presence. Yet high I. hippuris abundance on the deeper (~3–5 m) reef flats at Pak Kasim’s may indicate a combination of the lack of gleaning at this depth, an absence of displacement of vegetative propagules by disturbance, and the fact that this species is an r-selected strategist. Therefore, the response of I. hippuris colonies at Pak Kasim’s to reef recovery is apparently unencumbered by inhibitors to settlement and growth. Hence, it thrives in elevated current flow, low turbidity and minimal loose substratum. This pattern may also be true for Briareum sp.B on the reef slopes of Pak Kasim’s and Ridge 1. A combination of reduced disturbance (no gleaning, reduced hydrodynamics, low sedimentation), an increase in competitor release from other benthic zooxanthellate taxa (e.g., scleractinians) with increased depth, and an ability to bud asexually may, in part, explain the abundance of Briareum sp.B at these sites.
Colony density can be a function of various aspects of disturbance: type, frequency, intensity and type of substratum for species capable of asexual/vegetative propagation (Coffroth and Lasker 1998). Clonal propagation of tolerant clones may eventually lead to phenotypic and physiological adaptation. Colonies of I. hippuris[LT] at Sampela had a distinct morphology of long-branched bushy colonies which may well have become adapted to a turbid reef environment, whereas short branched planar colonies (I. hippuris[N]) are found on healthy reefs (Rowley 2014a; Rowley et al. 2015). Similarly, in the Caribbean, closely related species within the zooxanthellate genus Antillogorgia Bayer, 1951, also partitioned between two contrasting environments, with colony densities at a lagoonal site still lower than that I. hippuris at Sampela (Sánchez et al. 1997). Densities were nevertheless greater for the anthropogenically impacted gorgonian populations reported in other geographic locations (Bramanti et al. 2014; Etnoyer et al. 2016). Therefore, gorgonian assemblages within the WMNP show high regional abundance and diversity compared to other geographic locations, as well as the monospecific patches of I. hippuris morphotypes at Sampela, a pattern also as seen in other gorgonian taxa (e.g., Bramanti et al. 2014).
The zooxanthellate genus Briareum also influenced separation between the factors site and habitat. Whereas I. hippuris and scleractinian corals were most abundant on the shallow reef flats and crest, low-lying branched Briareum sp.B were more abundant on the reef slope, particularly at Pak Kasim’s and Ridge 1. Furthermore, numerous asexual fragments and juvenile colonies were encountered. This pattern mirrored its Atlantic congener, Briareum asbestinum Pallas, 1766, which reproduces through asexual fragmentation and external brooding producing low-dispersal philopatric larvae (Brazeau and Harvell 1994).
The dual reproductive strategy (external brooding and asexual budding) observed in Briareum spp. may likely explain, in part, the relative success of this species at depths where few zooxanthellate taxa are encountered and azooxanthellate diversity is high. Briareum morphotypes also displayed habitat specificity with branching taxa at depth and encrusting types on the high flow reef flat/ridge top. Encrusting morphologies reduce drag in such high flow environments (Bell and Smith 2004). However, habitats characterized by low wave action, high turbidity and sedimentation rates have also been shown to favor encrusting Briareum spp. (Fabricius and Alderslade 2001; Fabricius and De’ath 2004), likely due to morphological and behavioral preadaptations such as phenotypic and photoacclimatory plasticity, colony and polyp size, reproductive strategy and recruitment survival (Anthony 2000). Yet such patterns are in direct contrast with those in this study. Furthermore, Briareum spp. abundance was considerably lower compared to I. hippuris at Sampela; three of the seventeen colonies encountered were encrusting. Thus, branching and lobe-like, upward-projecting Briareum morphologies may well be selected for in low light and water flow, high turbidity and sedimented environments, reducing sediment smothering with increased surface area-to-volume ratio for photosynthetic efficiency akin to I. hippuris.
Azooxanthellate gorgonian assemblages
Azooxanthellate gorgonian assemblage structure showed a relatively consistent pattern across sites and habitats except at Sampela. However, an amplitudinal/additive interaction (i.e., not due to ‘crossing-over’) revealed that proportionality of abundance between sites and habitats changed markedly for some taxa. Nevertheless, azooxanthellate gorgonians showed assemblage patterns consistent with an environmental decline from the healthy, high-energy Ridge 1 to the depauperate reef communities at Sampela. Community structure of azooxanthellate taxa varied little within the deeper depths with only Plexauridae and Melithaeidae present across all sites. Species within the most diverse family, Plexauridae, drove diversity with depth, a pattern generally observed in other azooxanthellate families (Goh and Chou 1994; Fabricius and McCorry 2006; Matsumoto et al. 2007; this study). Increased diversity and a high frequency of recruits with depth suggest a deeper refugium and competitor release from zooxanthellate corals. This pattern is similarly replicated by sponge taxa (Bell and Smith 2004) inferring no or positive interactions between these two benthic groups (McLean and Yoshioka 2007), both of which typically have powerful secondary metabolites. Moreover, increased azooxanthellate diversity with depth may represent a consistent biological source pool. Such taxa are invaluable given past sea level variance in addition to current and future natural and anthropogenic disturbance, particularly with regards to the insidious effects of destructive fishing practices and global climate change.
Acanthogorgia sp.2 was the only azooxanthellate species driving differences between and within factor levels in the full statistical model. This is because of its exclusive abundance on the ceilings of caves and overhangs on the reef crest at Buoy 3. This specialized distribution may be due to within-overhang microhabitats, pre-settlement larval preferences such as negative phototaxis (Sánchez et al. 1997), geotaxis or differential mortality following settlement in other areas. Interestingly, species of Acanthogorgia and Bebryce were frequently encountered at the base of large, chemically well-defended gorgonians such as Annella reticulata, A. mollis (Puglisi et al. 2002), Melithaea spp. and the soft coral Sarcophyton (Fleury et al. 2006). Such taxa may affect recruitment (Yoshioka and Yoshioka 1989) through waterborne exudates facilitating spatial refugia from predation, competition (Hay 1986) or fouling. Nonetheless, individual colonies of Acanthogorgia and Bebryce, which were encountered on the open reef, not close to other taxa, were heavily infested with fouling organisms. Preferential settlement is, at present, unknown for such taxa and would certainly warrant further study. Furthermore, azooxanthellate Caribbean gorgonian larvae prefer to settle on consolidated topographically complex reefs and have longer pelagic larval duration (PLD; Sánchez et al. 1997) than zooxanthellate taxa. Yet both fitness enhancement through substratum selection and PLD are unknown for Indonesian gorgonians. In this study, diversity and abundance increased markedly with habitat complexity toward Ridge 1 and with depth. This bioenvironmental cline suggests selection and post-settlement success for sites with high topographic complexity and consolidated substratum. In contrast, low relief, unconsolidated fine-grained substratum coupled with low water flow, high sediment rate, continuous anthropogenic disturbance and high grazing activity from Diadema spp. at Sampela (Hodgson 2008) likely act in concert with reduced larval availability, settlement and survival to result in low biodiversity at the Sampela end of the gradient.
Predictor variables highlight water flow, light and chlorophyll-a for zooxanthellate species, and rugosity, sediment grain size and light for azooxanthellate species. High water motion and localized upwelling further enhanced by strong water currents at Ridge 1 fertilize the reef with deep nutrients for primary productivity and enhanced food availability (Sebens 1984), maximizing species biodiversity and abundance. Therefore, increased azooxanthellate species richness and diversity on the ridge top at Ridge 1, coupled with slightly reduced zooxanthellate species abundance compared to Pak Kasim’s, are indicative of a natural reef environment on Ridge 1 with overall reduced species dominance. Taken together, sedimentation, rugosity, light and water flow have been shown to be major factors controlling local gorgonian populations (Sánchez et al. 1997; Linares et al. 2008b). This pattern seems true, in part, across environmental gradients within the WMNP. However, differences between zooxanthellate and azooxanthellate gorgonians and coral reef benthic variables may account for the large amount of variation in gorgonian assemblage structure unexplained by the predictor variable model.
Conservation implications
Ongoing quantitative ecological and concomitant taxonomic analyses (combining morphological and molecular techniques, e.g., McFadden et al. 2014) are necessary for the conservation of tropical marine biodiversity. However, the social and economic situation in the WMNP appears complex, with an increasing shift from subsistence to income fisheries through economic development (Pilgrim et al. 2007; Clifton 2013). This discard of folklore and marked decrease in coral reef fish abundance within the region (Exton 2010) inevitably leads to alternative adaptations and ecological shifts. Here, morphotypes of taxa such as I. hippuris or Briareum can assist in reef health assessments, particularly as such patterns are of increasing conservation management importance, with I. hippuris now under a 5-yr moratorium from exploitation (Ministry of Marine and Fisheries Ministerial Decree No. 46/KEPMEN-KP/2014; Nagib Edrus and Suman 2013). Furthermore, the ubiquitous and diverse forms of gorgonians over wide geographic ranges suggest that separate species exist at each location with almost ‘one for each reef’ (Grasshoff 2001; Rowley et al. 2015). Therefore, biodiversity assessments cannot rely on the bulk sampling methods of the past, which provide a narrow biodiversity estimate unable to capture rare taxa (e.g., CoBabe and Allmon 1994; Buzas et al. 2002). Henceforth, ongoing ecological monitoring and thorough taxonomic analyses within and among locations are of vital importance.
Government agencies and local communities acknowledge the disturbing reality of human encroachment on reefs within the WMNP. Enforcement is often favored over community education (Clifton 2003), yet neither is sufficiently implemented due to budgetary and organizational constraints and lack of political willingness (von Heland et al. 2014). Well-meaning remedial fisheries management strategies such as no-take zone (Unsworth et al. 2007) and payoff strategies have been implemented, but withdrawn instilling false hope and a lack of trust in cross-cultural cooperation. Interestingly, Barnes-Mauthe et al. (2013) demonstrated that regular temporary octopus fishery closures and local community involvement in both fisheries monitoring and education led to significant increases in catch and local income, fostering trust and cooperation with local communities in Madagascar. However, increased illegal fishing and overfishing on ‘open days’ thwarted conservation efforts over time (Benbow et al. 2014; Oliver et al. 2015). One can only hope that ongoing education and financial support may be of some benefit to local WMNP communities, yet in the face of human necessity and dogmatic perception it is hard to predict and sadly out of the scope of this research.
In summary, gorgonian distribution patterns within the WMNP followed a gradient from low diversity and abundance at the impacted site at Sampela to high diversity and abundance at Ridge 1. Moreover, this environmental gradient response interacted with habitat, primarily as a function of depth (thus light) structuring zooxanthellate and azooxanthellate taxa on shallow and slope reef habitats, respectively. In this initial study in the WMNP, light availability and benthic competitors appear to define the distribution and abundance of most gorgonian taxa. Most notable are morphological variants of the zooxanthellate species I. hippuris and morphotypes of the genus Briareum, such biological success likely being a consequence of dual reproductive strategies (sexual and asexual reproduction) and morphological responses to different environments. Tests of physiological resilience of respective morphotypes would be informative for management plans and coral reef biodiversity assessments. By determining species delineation and/or potential ‘eco-morphotype’ environmental specificity, monitoring of gorgonian taxa, in particular I. hippuris, could therefore greatly assist environmental impact assessments and identify areas of habitat degradation.
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson MJ, Willis TJ (2003) Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84:511–524
Anthony KRN (2000) Enhanced particle-feeding capacity of corals on turbid reefs (Great Barrier Reef, Australia). Coral Reefs 19:59–67
Anthony KRN, Fabricius KE (2000) Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J Exp Mar Bio Ecol 252:221–253
Aronson RB, Precht WF (1995) Landscape patterns of reef coral diversity: a test of the intermediate disturbance hypothesis. J Exp Mar Bio Ecol 192:1–14
Baars MA, Sumoto AB, Oosterhuis SS, Arinardi OH (1990) Zooplankton abundance in the eastern Banda Sea and northern Arafura Sea during and after the upwellings season, August 1984 and February 1985. Netherlands Journal of Sea Research 25:527–543
Barnes-Mauthe M, Oleson KLL, Zafindrasilivonona B (2013) The total economic value of small-scale fisheries with a characterization of post-landing trends: an application in Madagascar with global relevance. Fish Res 147:175–185
Bayer FM (1961) The shallow-water Octocorallia of the West Indian region. Martinus Nijhoff, The Hague
Bayer FM (1981) Key to the genera of Octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa), with diagnoses of new taxa. Proceedings of the Biological Society of Washington 94:901–947
Bell JJ, Smith DJ (2004) Ecology of sponge assemblages (Porifera) in the Wakatobi region, south-east Sulawesi, Indonesia: richness and abundance. J Mar Biol Assoc UK 84:581–589
Benbow S, Humber F, Oliver TA, Oleson KL, Raberinary D, Nadon M, Ratsimbazafy H, Harris A (2014) Lessons learnt from experimental temporary octopus fishing closures in south-west Madagascar: benefits of concurrent closures. African Journal of Marine Science 36:31–37
Bohn K, Pavlick R, Reu B, Kleidon A (2014) The strengths of r- and K-selection shape diversity–disturbance relationships. PLoS One 9:e95659
Bramanti L, Magagnini G, De Maio L, Santangelo G (2005) Recruitment, early survival and growth of the Mediterranean red coral Corallium rubrum (L 1758), a 4-year study. J Exp Mar Bio Ecol 314:69–78
Bramanti L, Vielmini I, Rossi S, Tsounis G, Iannelli M, Cattaneo-Vietti R, Priori C, Santangelo G (2014) Demographic parameters of two populations of red coral (Corallium rubrum L. 1758) in the north-western Mediterranean. Mar Biol 161:1015–1026
Brazeau DA, Harvell CD (1994) Genetic structure of local populations and divergence between growth forms in a clonal invertebrate, the Caribbean octocoral Briareum asbestinum. Mar Biol 119:53–60
Buhl-Mortensen L, Vanreusel A, Gooday AJ, Levin LA, Priede IG, Buhl-Mortensen P, Gheerardyn H, King NJ, Raes M (2010) Biological structures as a source of habitat heterogeneity and biodiversity on the deep ocean margins. Mar Ecol 31:21–50
Buzas MA, Collins LS, Culver SJ (2002) Latitudinal difference in biodiversity caused by higher tropical rate of increase. Proc Natl Acad Sci U S A 99:7841–7843
Carpenter KE, Barber PH, Crandall ED, Ablan-Lagman MCA, Ambariyanto Mahardika GN, Manjaji-Matsumoto BM, Juinio-Meñez MA, Santos MD, Starger CJ, Toha AHA (2011) Comparative phylogeography of the Coral Triangle and implications for marine management. J Mar Biol 2011:396982
Cerrano C, Danovaro R, Gambi C, Pusceddu A, Riva A, Schiaparelli S (2010) Gold coral (Savalia savaglia) and gorgonian forests enhance benthic biodiversity and ecosystem functioning in the mesophotic zone. Biodivers Conserv 19:153–167
Chanmethakul T, Chansang H, Watanasit S (2010) Soft coral (Cnidaria: Alcyonacea) distribution patterns in Thai waters. Zool Stud 49:72–84
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Clarke KR, Gorley RN (2006) PRIMER v6: User manual/tutorial, 2nd edn. PRIMER-E Ltd, Plymouth, UK
Clarke KR, Somerfield PJ, Chapman MG (2006a) On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray-Curtis coefficient for denuded assemblages. J Exp Mar Bio Ecol 330:55–80
Clarke KR, Chapman MG, Somerfield PJ, Needham HR (2006b) Dispersion-based weighting of species counts in assemblage analyses. Mar Ecol Prog Ser 320:11–27
Clifton J (2003) Prospects for co-management in Indonesia’s marine protected areas. Mar Policy 27:389–395
Clifton J (2013) Refocusing conservation through a cultural lens: improving governance in the Wakatobi National Park, Indonesia. Mar Policy 41:80–86
CoBabe EA, Allmon WD (1994) Effects of sampling on paleoecologic and taphonomic analyses in high-diversity fossil accumulations: an example from the Eocene Gosport Sand, Alabama. Lethaia 27:167–178
Coffroth MA, Lasker HR (1998) Population structure of a clonal gorgonian coral: the interplay between clonal reproduction and disturbance. Evolution 52:379–393
Connell JH (1978) Diversity in tropical forest and coral reefs. Science 199:1302–1310
Crabbe JC, Smith DJ (2003) Computer modeling and estimation of recruitment patterns of non-branching coral colonies at three sites in the Wakatobi Marine Park, S.E. Sulawesi, Indonesia; implications for coral reef conservation. Comput Biol Chem 27:17–27
Dauget J-M (1992) Effets d’un changement d’orientation de la colonie sure la morphologie de Isis hippuris Linne, 1758 (Gorgonacea): note preliminaire. Bulletin de la Société Zoologique de France 117:375–382
English SA, Wilkinson C, Baker VJ (1997) Survey manual for tropical marine resources. Australian Institute of Marine Science, Townsville, Australia, p 390
Etnoyer PJ, Wirshing HH, Sánchez JA (2010) Rapid assessment of octocoral diversity and habitat on Saba Bank, Netherlands Antilles. PLoS One 5:e10668
Etnoyer PJ, Wickes LN, Silva M, Dubick JD, Balthis L, Salgado E, MacDonald IR (2016) Decline in condition of gorgonian octocorals on mesophotic reefs in the northern Gulf of Mexico: before and after the Deepwater Horizon oil spill. Coral Reefs 35:77–90
Exton DA (2010) Nearshore fisheries of the Wakatobi. In: Clifton J, Unsworth RKF, Smith DJ (eds) Marine research and conservation in the Coral Triangle: the Wakatobi National Park. Nova Science Publishers, Hauppauge, NY, pp 193–207
Fabricius KE, Klumpp DW (1995) Widespread mixotrophy in reef-inhabiting soft corals: the influence of depth, and colony expansion and contraction on photosynthesis. Mar Ecol Prog Ser 125:195–204
Fabricius KE, Alderslade P (2001) Soft corals and sea fans: a comprehensive guide to the tropical shallow-water general of the central-west Pacific, the Indian Ocean and the Red Sea. Australian Institute of Marine Science, Townsville, p 264
Fabricius KE, De’ath G (2004) Identifying ecological change and its causes: a case study on coral reefs. Ecol Appl 14:1448–1465
Fabricius KE, Alderslade P, Williams GC, Colin PL, Golbuu Y (2007) Octocorallia in Palau, Micronesia: effects of biogeography and coastal influences on local and regional biodiversity. In: Kayanne H, Omori M, Fabricius K, Verheij E, Colin P, Golbuu Y, Yurihira H (eds) Coral reefs of Palau. Palau International Coral Reef Centre, Palau, pp 79–92
Fabricius KE, McCorry D (2006) Changes in octocoral communities and benthic cover along a water quality gradient in the reefs of Hong Kong. Mar Pollut Bull 52:22–33
Fleury B, Coll J, Sammarco P (2006) Complementary (secondary) metabolites in a soft coral: sex-specific variability, inter-clonal variability, and competition. Mar Ecol 27:204–218
Garrabou J, Perez T, Sartoretto S, Harmelin JG (2001) Mass mortality event in red coral Corallium rubrum populations in the Provence region (France, NW Mediterranean). Mar Ecol Prog Ser 217:263–272
Gieskes WWC, Kraay GW, Nontji A, Setiapermana D, Sutomo D (1988) Monsoonal alterations of a mixed and a layer structure in the phytoplankton of the euphotic zone of the Banda Sea (Indonesia); a mathematical analysis of algal pigment fingerprints. Netherlands Journal of Sea Research 22:123–137
Goatley CHR, Bellwood DR (2011) The roles of dimensionality, canopies and complexity in ecosystem monitoring. PLoS One 6:e27307
Goh NKC, Chou LM (1994) Distribution and biodiversity of Singapore gorgonians (sub-class Octocorallia)—a preliminary survey. Hydrobiologia 285:101–109
Goldberg WM (1973) The ecology of the coral–octocoral communities off the southeast Florida coast: geomorphology, species composition, and zonation. Bull Mar Sci 23:465–488
Grasshoff M (1999) The shallow water gorgonians of New Caledonia and adjacent islands (Coelenterata: Octocorallia). Senckenb Biol 78:1–245
Grasshoff M (2001) Taxonomy, systematics, and octocorals: to Frederick M. Bayer, October 31st 2001. Bulletin of the Biological Society of Washington 10:3–14
Haapkylä J, Seymour AS, Trebilco J, Smith DJ (2007) Coral disease prevalence and coral health in the Wakatobi Marine National Park, south-east Sulawesi, Indonesia. J Mar Biol Ass UK 87:403–414
Hay ME (1986) Associational plant defenses and the maintenance of species diversity: turning competitors into accomplices. Am Nat 128:617–641
Hennige SJ, Smith DJ, Perkins R, Consalvey M, Paterson DD, Suggett DJ (2008) Photoacclimation, growth and distribution of massive coral species in clear and turbid waters. Mar Ecol Prog Ser 369:77–88
Hodgson A (2008) Spatial variation in reef echinoid abundance and diversity with habitat, fish community structure and anthropogenic pressure. Bachelors dissertation, Newcastle University, UK
Kingsford M, Battershill C (1998) Studying temperate marine environments: a handbook for ecologists. Canterbury University Press, New Zealand
Knowlton N (1993) Sibling species in the sea. Annu Rev Ecol Syst 24:189–216
Lenz EA, Bramanti L, Lasker HR, Edmunds PJ (2015) Long-term variation of octocoral populations in St. John, US Virgin Islands. Coral Reefs 34:1099–1109
Linares C, Coma R, Zabala M (2008a) Restoration of threatened red gorgonian populations: an experimental and modelling approach. Biol Conserv 141:427–437
Linares C, Coma R, Garrabou J, Díaz D, Zabala M (2008b) Size distribution, density and disturbance in two Mediterranean gorgonians: Paramuricea clavata and Eunicella singularis. J Appl Ecol 45:688–699
Matsumoto AK, Iwase F, Imahara Y, Namikawa H (2007) Bathymetric distribution and biodiversity of cold-water octocorals (Coelenterata: Octocorallia) in Sagami Bay and adjacent waters of Japan. Bull Mar Sci 81:231–252
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 82:290–297
McCormick MI (1994) Comparison of field methods for measuring surface topography and their associations with a tropical reef fish assemblage. Mar Ecol Prog Ser 112:87–96
McFadden CS, Brown AS, Brayton C, Hunt CB, van Ofwegen LP (2014) Application of DNA barcoding in biodiversity studies of shallow-water octocorals: molecular proxies agree with morphological estimates of species richness in Palau. Coral Reefs 33:275–286
McLean EL, Yoshioka PM (2007) Associations and interactions between gorgonians and sponges. In: Custodio MR, Lobo-Hadju G, Hadju E, Muricy E (eds) Porifera research: biodiversity, innovation and sustainability. Museu Nacional, Rio de Janeiro, pp 443–448
McManus JW (1997) Tropical marine fisheries and the future of coral reefs: a brief review with emphasis on Southeast Asia. Coral Reefs 16:S121–S127
Nagib Edrus I, Suman A (2013) Policy synthesis on protection and conservation for octocorallian fauna (Isis hippuris Linnaeus 1758). Jurnal Kebijakan Perikanan Indonesia 5:107–112
Nutting CC (1910a) The Gorgonacea of the Siboga Expedition III. The Muriceidae. Siboga-Expeditie Monographie XIII, Brill, Leiden, The Netherlands, p 108
Nutting CC (1910b) The Gorgonacea of the Siboga Expedition IV. The Plexauridae. Siboga-Expeditie Monographie Monographie XIII, Brill, Leiden, The Netherlands, p 20
Nutting CC (1910c) The Gorgonacea of the Siboga Expedition V. The Isidae. Siboga- Expeditie Monographie XIII,, Brill, Leiden, The Netherlands, p 24
Nutting CC (1910d) The Gorgonacea of the Siboga Expedition VI. The Gorgonellidae. Siboga-Expeditie Monographie XIII, Brill, Leiden, The Netherlands, p 39
Nutting CC (1910e) The Gorgonacea of the Siboga Expedition VII. The Gorgoniidae. Siboga-Expeditie Monographie Monographie XIII, Brill, Leiden, The Netherlands, p 10
Nutting CC (1911) The Gorgonacea of the Siboga Expedition VIII. The Scleraxonia. Siboga-Expeditie Monographie Monographie XIII, Brill, Leiden, The Netherlands, p 62
Oliver TA, Oleson KL, Ratsimbazafy H, Raberinary D, Benbow S, Harris A (2015) Positive catch and economic benefits of periodic octopus fishery closures: do effective, narrowly targeted actions ‘catalyze’ broader management? PLoS One 10:e0129075
Paulay G, Puglisi MP, Starmer JA (2003) The non-scleractinian Anthozoa (Cnidaria) of the Mariana Islands. Micronesica 35–36:138–155
Peet RK (1974) The measurement of species diversity. Annu Rev Ecol Syst 5:285–307
Pilgrim SE, Cullen LC, Smith DJ, Pretty J (2007) Hidden harvest or hidden revenue? Local resource use in a remote region of Southeast Sulawesi, Indonesia. Indian Journal of Traditional Knowledge 6:150–159
Prada C, Schizas NV, Yoshioka PM (2008) Phenotypic plasticity or speciation? A case of a clonal marine organism. BMC Evol Biol 8:47
Puglisi MP, Paul VJ, Biggs J, Slattery M (2002) Co-occurrence of chemical and structural defenses in the gorgonian corals of Guam. Mar Ecol Prog Ser 239:105–114
Reijnen BT, McFadden CS, Hermanlimianto YT, van Ofwegen LP (2014) A molecular and morphological exploration of the generic boundaries in the family Melithaeidae (Coelenterata: Octocorallia) and its taxonomic consequences. Mol Phylogenet Evol 70:383–401
Rogers CS (1990) Responses of coral reefs and reef organisms to sedimentation. Mar Ecol Prog Ser 62:185–202
Rowley SJ (2014a) Gorgonian responses to environmental change on coral reefs in SE Sulawesi, Indonesia. PhD thesis, Victoria University of Wellington, New Zealand, p 213
Rowley SJ (2014b) Refugia in the ‘twilight zone’: discoveries from the Philippines. The Marine Biologist 2:16–17
Rowley SJ, Pochon X, Watling L (2015) Environmental influences on the Indo-Pacific octocoral Isis hippuris Linnaeus, 1758 (Alcyonacea: Isididae): genetic fixation or phenotypic plasticity? PeerJ 3:e1128
Samimi-Namin K, van Ofwegen LP, Wilson SC, Claereboudt MR (2011) The first in situ and shallow-water observation of the genus Pseudothelogorgia (Octocorallia: Keroeididae). Zool Stud 50:265
Sánchez JA (2004) Evolution and dynamics of branching colonial form in marine modular cnidarians: gorgonian octocorals. Hydrobiologia 530–531:283–290
Sánchez JA (2016) Diversity and evolution of octocoral animal forests at both sides of tropical America. In: Rossi S, Bramanti L, Gori A, Orejas C (eds) Marine animal forests. The ecology of benthic biodiversity hotspots, Springer International, pp 1–33
Sánchez JA, Diaz JM, Zea S (1997) Gorgonian communities in two contrasting environments on oceanic atolls of the southwest Caribbean. Bull Mar Sci 61:453–465
Sánchez JA, Zeng W, Coluci VR, Simpson C, Lasker HR (2003) How similar are branching networks in nature? A view from the ocean: Caribbean gorgonian corals. J Theor Biol 222:135–138
Sanciangco JC, Carpenter KE, Etnoyer PJ, Moretzsohn F (2013) Habitat availability and heterogeneity and the Indo-Pacific warm pool as predictors of marine species richness in the tropical Indo-Pacific. PLoS One 8:e56245
Scaps P, Denis V (2007) Association between the scallop, Pedum spondyloideum, (Bivalva: Pteriomorphia: Pectinidae) and scleractinian corals from the Wakatobi Marine National Park (southeastern Sulawesi, Indonesia). The Raffles Bulletin of Zoology 55:371–380
Sebens KP (1982) The limits to indeterminate growth: an optimal size model applied to passive suspension feeders. Ecology 63:209–222
Sebens KP (1984) Water flow and coral colony size: inter habitat comparisons of the octocoral Alcyonium siderium. Proc Natl Acad Sci U S A 81:5473–5477
Simpson JJ (1906) The structure of Isis hippuris, Linnaeus. Zool J Linn Soc 29:421–434
Stiasny G (1937) Die Gorgonacea der Siboga-Expedition. Suppl. II, Revision der Scleraxonia, etc. Siboga-Expeditie Monographie, Brill, Leiden, The Netherlands, p 133
Stiasny G (1940) Die Gorgonarien Sammlung der Snellius-Expedition. Temminckia 5:191–256
Tinsley P (2005) Worbarrow reefs seafan project 2003–2005. Dorset Wildlife Trust, http://www.seasearch.org.uk/downloads/Worbarrow%20Reefs%20Study.pdf
Tomascik T, Mah AJ, Nontji A, Kasim Moosa M (2004) The ecology of the Indonesian seas part one. Oxford University Press, p 642
Unsworth RK, Powell A, Hukom F, Smith DJ (2007) The ecology of Indo-Pacific grouper (Serranidae) species and the effects of a small scale no take area on grouper assemblage, abundance and size frequency distribution. Mar Biol 74:53–62
van Ofwegen LP (2004) The present status of taxonomic knowledge of octocorals in Indonesia. In: Tomascik T, Mah AJ, Nontji A, Kasim Moosa M (eds) The ecology of the Indonesian seas part one. Oxford University Press, Oxford, pp 345–346
Verseveldt J (1966) Biological results of the Snellius Expedition XXII. Octocorallia from the Malay Archipelago (Part II). Zoologische Verhandelingen Leiden 80:1–109
Versluys J (1902) Die Gorgoniden der Siboga-Expedition. I. Die Chrysogorgiiden, volume 13. Monographie Siboga-Expeditie, Brill, Leiden, The Netherlands, p 120
Versluys J (1906) Die Gorgoniden der Siboga Expedition II. Die Primnoidae. Siboga- Expeditie Monographie XIII, 178. pls. 1_10
von Heland F, Clifton J, Olsson P (2014) Improving stewardship of marine resources: linking strategy to opportunity. Sustainability 6:4470–4496
Wald A, Wolfowitz J (1943) An exact test for randomness in the non-parametric case based on serial correlation. Annals of Mathematical Statistics 14:378–388
Weiner J (2004) Allocation, plasticity and allometry in plants. Perspectives in Plant Ecology, Evolution and Systematics 6:207–215
Wentworth CK (1922) A scale of grade and class terms for clastic sediments. J Geol 30:377–392
West JM (1997) Plasticity in the sclerites of a gorgonian coral: tests of water motion, light level, and damage cues. Biol Bull 192:279–289
West JM, Harvell CD, Walls AM (1993) Morphological plasticity in a gorgonian coral (Briareum asbestinum) over a depth cline. Mar Ecol Prog Ser 94:61–69
Yoshioka PM, Yoshioka BB (1989) Effects of water motion, topographic relief and sediment transport on the distribution of shallow-water gorgonian community. Mar Ecol Prog Ser 54:257–264
Acknowledgements
This study was made possible by the generous support of the Wakatobi Government, the Indonesian staff at ALAM of the Wakatobi Marine National Park, and the Wallacea Foundation, particularly Pak Iwan, Arif and Azrul. The State Ministry of Research and Technology (RISTEK) granted research permits to Prof. D. J. Smith, under whose auspices this work was conducted. This manuscript benefited from spirited discussion and guidance from Profs. S. M. Stanley, S. K. Davy, J. Gardner, C. Birkeland, C. Todd, L. Watling, Drs. J. J. Bell, A. Rowden, R. L. Pyle and G. Williams, as well as insightful comments from two anonymous reviewers. S. J. R was supported by a Victoria University Wellington Doctoral Research Scholarship and the Coral Reef Research Unit, with sponsorship from P Duxfield at Cameras Underwater for camera equipment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Topic Editor Dr. Alastair Harborne
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rowley, S.J. Environmental gradients structure gorgonian assemblages on coral reefs in SE Sulawesi, Indonesia. Coral Reefs 37, 609–630 (2018). https://doi.org/10.1007/s00338-018-1685-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-018-1685-y