Abstract
Hydrochemical characteristics of groundwater in the vulnerable tribal region of Gosthani river basin extended in Eastern Ghats hard rock terrain, Andhra Pradesh state, Southern India were determined. The occurrence of groundwater in hard rock terrain of this region is predominantly controlled by joints, fractures, porosity, and thickness of the weathered zone. The geoenvironmental conditions within aquifer are influencing the occurrence and concentration of chemical parameters of groundwater. In order to evaluate the quality of groundwater in the area, 24 groundwater samples were collected and analyzed for various physicochemical parameters such as pH, EC, TDS, TA, TH, Ca2+, Na+, K+, Fe, SO42−, NO3−, Cl−, and F−. Mg2+, HCO3− plus CO32−, Gibbs ratios, CAI, %Na, SAR, RSC, PI, KR, and CR were computed. The results revealed that the groundwater in the area is soft to hard water type. The abundance of the major cations are Ca2+>Na+>Mg2+ and major anions are HCO3−>Cl−>NO3−. The dominant hydrochemical facies of groundwater is Ca-Na-HCO3 and Ca-HCO3-Cl type. In the study area, the vulnerable tribal population lives in remote and inaccessible areas and is mainly dependent on groundwater for drinking and other purposes. The result of low concentrations of various chemical ions in the water suggested that less enrichment of mineral content caused various health hazards in the indigenous tribal population. Soil erosion, deforestation, and traditional shifting cultivation activities are encouraging for improper leaching of chemical constituents into the groundwater. Further, the high levels of iron found in 87.5% of samples are possibly by the source rock interaction with water. Overall, the assessment of water quality using WQIA and other methods indicates that some samples in the study region are chemically unsuitable for drinking and irrigation purposes. The present work provides baseline information in solving problems due to quality deterioration of the groundwater for implementing sustainable management practices in improving the living conditions of the tribes of this region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is a vital resource for human survival and it supports various forms of life and economic activities of the globe. The accessibility of surface and groundwater resources is very limited, and preserving both the resources in terms of quality as well as quantity is critical for human and also for environmental health. The groundwater is generally believed to be free from the contamination and thus considered safe for drinking. UNICEF reported that 663 million people across the world used unimproved drinking water sources, including unprotected wells, springs, and surface water (WHO-UNICEF 2015). Environmental risk factors such as physical, chemical, and biological hazards are directly affecting the human health. These factors depend on unsafe drinking water, poor sanitation, and insufficient hygiene leading to transmission of diseases like diarrhea, cholera, dysentery, hepatitis, and typhoid. Underprivileged people especially children are more vulnerable to bear the health burden associated with poor quality of water and sanitation. In India, it is estimated that around 37.7 millions are affected by waterborne diseases each year causing more than 1.5 million deaths primarily among children under 5 years of age. About 75% of rural households of tribal populations do not have proper sanitation facility and most of them depend on contaminated water obtained from unsafe sources, thereby exposing themselves to water-induced diseases (UNICEF 2013). Thus, proper assessment and reporting of underground and surface water quality is an important issue in developing countries like India and particularly in tribal regions where the communities are under developed (Thakur et al. 1991).
Geochemical processes generally occur within the groundwater and reactions from these processes with underground materials are influencing the quality of water. It provides probable clue in the genesis of different chemical constituents of water. The chemistry of groundwater in a particular area varies based on the composition of the precipitation, mineralogy, chemistry of the country rocks, the geological processes within aquifer, and the anthropogenic activities. The interaction between all these factors leads to formation of various water types. Geochemical characterization of groundwater in different environments was reported and described in many areas including India and other parts of the world (Jagannadha Sarma and Swamy 1981; Apambire et al. 1997; Satyanarayana and Perikali 2003; Glynn and Plummer 2005; Subramani et al. 2010; Singaraja et al. 2014; Ruiz et al. 2015; Alharbi 2018). High concentrations of various parameters in groundwater with special reference to fluoride (Valenzuela et al. 2006; Routroy et al. 2013), arsenic (Mandal et al. 1996; Fendorf et al. 2010; Kanoua and Merkel 2017), and iron (Promma et al. 2007; Goyal et al. 2010; Ivanova et al. 2014) were reported in various regions. Deficiency or excess of fluoride in the natural waters is closely associated with human health. Fluoride in potable water at the level of 1 mg/l is essential for human life as it enhances the bone development and prevents dental caries. Excess concentrations of fluoride lead to bone decay, dental fluorosis, and white patches, staining, mottling, and pitting of teeth (Bo et al. 2003). Further, the presence of certain trace elements like iron in groundwater forms an essential nutritional component for the growth and survival of certain species of biota. The concentration of these elements may be controlled by biological processes (Oborn and Hem 1962). Excess iron (> 1.0 mg/l) in groundwater was reported in more than 0.11 million habitations in India (CGWB 2010). Nitrate, which is important constituent of groundwater, originates from natural and anthropogenic sources. The anthropogenic source of contamination is mainly from fertilizers, farm manure, domestic wastewater, and sewage, etc. (Jalali 2005). Various chemical constituents of groundwater exceed the permissible limits of World Health Organization (WHO 2011) or Bureau of Indian Standards (BIS 2012) and are expected to have health hazards. The evaluation of water quality with the help of various analytical parameters consisting of huge data that are presented in large tables may create ambiguity in analysis. To overcome this problem, several researchers had applied different methodologies for expressing water quality in a practical, logical, and comparative way (Singh et al. 2004; Masoud 2014; Belkhiri and Narany 2015). Water quality index (WQI) is one such method expressing the quality of water in terms of grades by considering all parameters into an account (Brown et al. 1970; Bhargava 1985; Smith 1990; Swamee and Tyagi 2000; Khan et al. 2003; Dwivedi and Pathak 2007; Mahapatra et al. 2012; Gulgundi and Shetty 2018).
Undoubtedly, the quality of groundwater plays a significant role in the assessment of health condition of the people. Though several studies have reported on the surface and groundwater quality of the Eastern Ghats terrain of India, but very few studies are available on the groundwater quality in tribal regions of the Eastern Ghats of Visakhapatnam district, Andhra Pradesh (A.P), Sothern India (Sundaray et al. 2006; Rao et al. 2012). The present paper describes the chemical characteristics of groundwater in a vulnerable tribal region of Eastern Ghats hard rock terrain of A.P. An attempt had also been made to rate the quality of groundwater through WQIA method and assess its suitability for drinking and irrigation purposes.
Study area
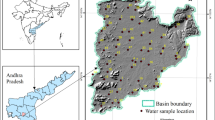
The study area, located in the Eastern Ghats of Visakhapatnam and Vizianagaram districts of A.P., Southern India, about 70 km northwest of Visakhapatnam city, covering an area of 321 km2 (18° 08′ 30″–18° 20′ 54″ N latitudes and 82° 56′ 10″–83° 13′ 33″ E longitudes), is a representative basin in the hard rock terrain (Fig. 1). The river Gosthani, originates at an altitude of 1402 m amsl, and its tributaries flow towards Bay of Bengal. The water of the river is used for irrigation and also the drinking purpose for the people of Visakhapatnam city. The area is underlain by hard crystalline rocks, mainly Khondalites and Charnockites of Archaean age. Calc-granulites and crystalline limestones are exposed and have well-developed karstic features such as stalactites and stalagmites. The bauxitic laterite cappings overlies the Khondalites are exhibiting in brown to red color. More than 70% of the basin area is covered by dissected hills that indicate the low potential for groundwater prospecting. The occurrence and movement of groundwater in a hard rock terrain are restricted to fractures and joints in unweathered portion and porous zones of weathered formations. The thickness of the weathered material is about 15–30 m in the topographic lows. Extraction of groundwater is through dug wells and bore wells as observed in the study area. The water levels during pre-monsoon vary from 2.1 to 8.2 mbgl and in post-monsoon ranges from 1.7 to 6.2 mbgl. The discharge of open wells and bore wells varies from 20 to 100 m3/day and from 240 to 1200 m3/day, respectively. In general, the scope for groundwater development is very limited due to hard rock terrain. Soils consisting of sandy loam, loamy sand, and clay loam with brown to red color are covered by dense forest. Tropical deciduous forest with scrubs is covering 57% of the total area. Overall agricultural activity depends mainly on rainfall and the availability of water from rivulets and springs. Predominant tribal groups, namely, Bhagata, Gadaba, Poorja, Valmiki, Khond, Kammara, Kotia, Mali, Konda Dora, Manne Dora, and Reddi Dora, are identified in the study area. The traditional economic activities practiced by the tribes in this region are hunting, cultivation, cattle-herding, folk-artists, simple artisan, and agricultural and non-agricultural labourers. Paddy is the predominant crop and is being cultivated by using terrace method in the valley parts. Minor produce collected from the forest such as adda leaf, tamarind, hill broom, honey, and other medicinal plants are sold in the weekly markets (Nageswara Rao and Swarna Latha 2017).
Materials and methods
A total of 24 groundwater samples were collected in the month of April 2014 covering whole part of the study area (Fig. 1). In order to avoid contamination, proper care was taken in collecting water samples and clean 1-L acid-washed polyethylene bottles were used with strict adherence to the sampling protocol to draw the groundwater from wells. Samples were preserved in the laboratory for reporting the water quality analysis. The bottles were tightly capped to protect samples from atmospheric carbon dioxide, adequately labeled and preserved in the refrigerator by adding appropriate reagents until they were taken to the laboratory for measurement. Before taking readings, pumping was carried out until the meter readings were stable for each parameter. The collected water samples were analyzed in the laboratory for various hydrochemical parameters using standard methods (APHA 1998). pH and electrical conductivity (EC) were determined using digital pH and EC meters, respectively. TDS was measured using evaporation method. Total alkalinity (TA) as CaCO3 was determined by titrating with HCl. Total hardness (TH) as CaCO3 and calcium (Ca2+) were measured titrimetrically using standard EDTA. Sodium (Na+) and potassium (K+) were measured by flame photometry. Sulfate (SO42−) and nitrate (NO3−) were determined by spectrophotometer. Chloride (Cl−) was measured by standard AgNO3 titration. Fluoride (F−) and iron (Fe) were analyzed by selective ion meter and 1-10 phenanthroline solution, respectively. Care was taken that the pH, EC, TA, TH, and Ca2+ were analyzed within 24 h of sampling. The quantity controls of the measurements consisted of duplicate samples. Bicarbonate (HCO3−) plus carbonates (CO32−) and magnesium (Mg2+) were estimated using the formulas TA × 1.31 (Hem 1985) and [TH-(2.5 × CaH)]/4.1 (Todd and Mays 2005), respectively. Geochemical facies type, Gibbs ratios, chloro-alkaline indices (CAI), salinity hazard, percent sodium (%Na), sodium adsorption ratio (SAR), residual sodium carbonate (RSC), permeability index (PI), Kelley ratio (KR), and corrosivity ratio (CR) were computed for the purpose of groundwater usage for agriculture purpose. All the parameters were compared with the guidelines suggested by the Bureau of Indian Standards (BIS 2012). Analysis among various physicochemical parameters was carried out with SPSS statistical software package. Aquachem and ArcGIS softwares were used for making various diagrams and maps. Using the approach of Freeze and Cherry (1979), the analysis accuracy was checked for the water samples by using the charge balance. The charge balance values for all the samples vary by about 1–10%, which indicates that the analysis results are good. The error in the water sample analysis is due to the reagents employed, limitations of the methods, the instruments used and the presence of impurities in distilled water, etc.
WQI computation
Water quality index is a reliable means for understanding the overall quality of various parameters present in the water. Weighted arithmetic water quality index (WQIA) method was used to compute the overall water quality index of the groundwaters in the study area. Various physicochemical parameters occur in different ranges which are expressed in different units and have behavior in terms of concentration-impact relationship. Therefore, prior to index formulation, all parameters have been transformed into a single scale. Weights have been assigned according to the importance of chemical constituent which affects the quality. WQI is a unit less single dimensional numbers between 0 and 100. As per WQI, the maximum permissible value is 100. Values 100 and above indicate pollution and are unfit for human consumption. Therefore a numerical index is used as a management tool in water quality assessment. The main advantage of this method is to incorporate multiple water quality parameters data into a mathematical equation that rates the quality of water with a numerical number. A total of eleven important parameters chosen for calculation of quality index were pH, TDS, TA, TH, Ca2+, Mg2+, Fe, Cl−, NO3−, SO42−, and F−. Water quality standards (BIS 2012) were used for selected parameters to compute the water quality index by the following expression.
where Wi is the relative weight, qi is the water quality rating, wi is the unit weight, va is the actual value obtained from laboratory analysis, vs is the standard value of ith parameter, and vi is the ideal value (pH = 7 and 0 for all parameters). The computed WQI for various sampling stations were grouped into five categories, namely, excellent, good, poor, very poor, and unfit for human consumption.
Results and discussion
The concentration of various ions present in groundwater samples is given in Table 1. Cation and anion dominance of the groundwaters in the study area are Ca2+>Na+>Mg2+>K+ and HCO3−>Cl−>NO3−>SO42−.
Classification of groundwater through hydrochemical diagrams
Hydrochemical diagrams assist in understanding the evolutionary trends of chemical constituents of water and effectively portray hydrochemical data. A form of trilinear diagrams for graphical representation of major ions to describe water chemistry is developed by various scientists (Hill 1940; Langelier and Ludwig 1942; Piper 1944; Durov 1948; Lloyd 1965; Romani 1981; Chadha 1999). One such important trilinear diagram is the Piper diagram which is widely used to study the similarities and differences in the chemical composition of waters and also to classify them into certain water types. Accordingly, the groundwaters were categorized into different hydrochemical facies using the Piper’s trilinear diagram (Piper 1944).
Hydrochemical facies
Based on the plots of major cations Ca2+, Mg2+, Na+, and K+ and major anions Cl−, HCO3−, and SO42− content in the groundwater of Piper’s trilinear diagram (1944), seven hydrochemical facies have been identified (Fig. 2a). The trilinear diagram of Piper helps to understand the chemical relationships of groundwaters in more definite terms. The diagram indicates that majority of groundwater samples fall in the fields of 1, 3, and 5 suggesting that alkaline earth exceeds alkalies, weak acids exceeds strong acids, and carbonate hardness (secondary alkalinity) exceeds 50%, respectively (Table 2). However, some samples having high sulfate concentration falls in field 4 indicating strong acids exceeds weak acids, and mixed water having no one cation-anion pair exceeds 50% which represents field 9. Fifty percent of samples show that the groundwater is Ca-Na-HCO3-Cl type followed by Ca-HCO3-Cl and Ca-Na-Cl-HCO3.
Mechanisms controlling the groundwater chemistry
Gibbs classification
To understand the groundwater chemistry and relationship of the chemical constituents of water to their respective aquifers such as atmospheric precipitation, rock–water interaction, and rate of evaporation, Gibbs (1970) had proposed a diagram in which ratio of dominent cations (ratio I) and anions (ratio II) are plotted against the value of TDS. These two ratios are calculated using the following equations:
Ratio I = (Na++K+)/(Na++K++Ca++) and ratio II = Cl−/(Cl−+HCO3−) where the concentration of ions are in meq/l.
The calculated results are presented in Table 3. The ratio of major cations (ratio I) and anions (ratio II) of the water samples was plotted against TDS values for the present study (Fig. 2b). The ratio I and II for cations and anions vary from 0.18 to 0.53 and 0.2 to 0.62, respectively. It is found that most of the water samples fall under the rock dominance category. The rock-dominance suggests that the rock–water interaction is the major source of dissolved ions over the control of hydrochemical process. The rock–water interaction generally includes the chemical weathering of rocks, dissolution-precipitation of secondary carbonates, and ion exchange between water and clay minerals.
Chloro-alkaline indices
Chloro-alkaline indices (CAI) are widely used to evaluate the ion exchange reactions between groundwater and its host rocks. Schoeller (1967) proposed the indices which can be computed by using the following equations:
CAI 1 = [Cl−−(Na++K+)]/Cl− and CAI 2 = [Cl−− (Na++K+)]/(SO42−+HCO3−+CO32−+NO3−) where the concentration of ions are in meq/l.
The CAI values may be positive or negative, depending upon the exchange of alkalies from the host rock with alkaline earths in water and vice versa. The calculated values of CAI 1 and CAI 2 vary from − 1.32 to 0.60 and − 0.33 to 0.9 (Table 3). The diagram reveals that most of the samples show positive values and few samples illustrate negative values in the study area (Fig. 3). This observation mainly indicates that normal or base exchange reaction, in which alkaline earths have been exchanged for alkalies, is the dominant process in the groundwater. It explains that the water is considered as a base exchange softened type.
Groundwater use for domestic purposes
The range of concentrations of chemical parameters in groundwater samples and their effect on human health are presented in Table 4. Hydrogen ion concentration (pH) of the water ranges between 6.1 and 8.4 in the study area. pH indicates the strength of the water to react with the acidic or alkaline material present in the water (Hem 1985). The combination of CO2 with water forms carbonic acid, which affects the pH of the water. Acidic groundwater with low pH has an increased capacity for dissolving minerals. pH of natural unpolluted groundwater is generally between 6.0 and 8.5 indicating that the groundwater of the study area is good (Weiner 2000). Alkalinity varies from 36 to 158 mg/l in the study area. Alkalinity greater than 25 mg/l as CaCO3 is beneficial to water quality. However, high alkalinity (> 250 mg/l) gives an unpleasant taste to water, which may be because of carbonates reactions on basic materials in the soil (BIS 2012). Dissolved carbon dioxide, bicarbonate, and carbonates produce alkalinity in water. HCO3− plus CO32− ions are being estimated from the alkalinity. Most dissolved inorganic substances present in the water are in ionized form and contribute to electrical conductivity. Electrical conductivity of water is considered to be an indication of the total dissolved salt content (Hem 1985). In the study area, EC ranges between 220 and 1214 μS/cm (Fig. 4a) and TDS varies from 114 to 862 mg/l. Out of 24 water samples, only one sample shows TDS concentration more than 500 mg/l, indicating that it is not suitable for drinking purpose (BIS 2012). TDS variation in natural waters largely depends on rainfall, type of soil and rock, land use, and vegetative cover of the area.
The concentration of calcium and magnesium as CaCO3 present in the water can be expressed as total hardness. The concentration of TH of the study area ranges between 54 and 232 mg/l. Groundwater classified based on TH concentration (Todd and Mays 2005) reveals that the majority of samples (79.2%) are falling under moderately hard to hard water category (Fig. 4b and Table 5). However, as per BIS, less than 600 mg/l concentration in groundwater is acceptable for drinking and domestic needs when alternate source of water is not available. Hardness results from reactions of the cations such as Ca2+ and Mg2+. The range of Ca2+ and Mg2+ concentrations in the study area is 19–82 mg/l and 0.7–13.3 mg/l, respectively. Calcium ions generally exceed the magnesium ions in the groundwater. High calcium content in the groundwater causes encrustation, scaling, and abdominal disorders and is not desirable for domestic use. All samples of Mg2+ concentration are within permissible limits of BIS (Table 4). Na+ and K+ concentrations in the groundwater samples varied from 9.8 to 51 mg/l and 1 to 12.1 mg/l, respectively. The high Na+ ion may be attributed to cation exchange and anthropogenic activities.
Iron (Fe) is biologically an important element which is essential to all organisms and present in hemoglobin system. High concentration of iron causes slight toxicity. The concentration of total iron ranges between 0.1 and 2.2 mg/l indicating that some of the samples exceed the limits. Based on iron concentration prescribed for drinking water, the study area is classified into three categories such as low (< 0.3 mg/l), moderate (0.3–1 mg/l), and high iron (> 1 mg/l) (Fig. 4c). As per BIS, out of 24 water samples, 21 samples showing high concentration of Fe (> 0.3 mg/l) which are not suitable for drinking. The main cause for the excess concentration of iron in deep aquifer may be due to rock interaction with the aquifer. The reduction of iron increases with increase of the depth of groundwaters, because of less availability of oxygen in deep aquifers. In general, iron exists in water in soluble ferrous state, and can remain in the solution only in the absence of oxygen (Hem 1962). High Fe in groundwater can also be expected from weathering of Fe-bearing minerals such as feldspars, pyroxenes, epidote, and hornblende present in the aquifer matrix, and dissolution of iron pipes and its components. Fe2+ will oxidize to insoluble ferric iron (Fe3+) and precipitate as ferric hydroxide after exposure to the atmosphere. The occurrence of iron concentration in the groundwater of the study area may be due to leaching, corrosion of casing pipe in the bore wells fitted with handpumps, as they are not in regular use, and also from the chemical decomposition of ferruginous laterite, sediments, and crystalline rocks of the region. High concentration of Fe water may be treated by simply exposing the water to surface or by installation of iron treatment plants in the region.
Among the anions, HCO3−+CO32− collectively are the dominated ions and are estimated from the total alkalinity of groundwater in the study area. Bicarbonates range between 47.2 and 207.3 mg/l. High values of bicarbonates result from CO2 which might be released from the decay of organic matter and root respiration in soil zone. This CO2 combines with H2O to form HCO3−, which in turn converts to CO32− in rock weathering during infiltration of recharge water (Berner and Berner 1987).
Dissolved carbon dioxide (CO2) is the main source of acidity in unpolluted waters. The excess content of bicarbonates indicates an intense weathering of rocks, which favors an active mineral dissolution (Stumm and Morgan 1996). The range of chloride concentrations in the study area is 12–168 mg/l. Cl− concentration is directly related to mineral concentration of the water possibly due to weathering of shales and phyllites. Cl− may be mostly derived from domestic effluents, fertilizers, septic tanks, and natural sources such as rainfall and weathering of chloride bearing minerals (Hem 1985). The concentration of Cl− in the study area is less than 250 mg/l indicating that all water samples fall under permissible limits of BIS. The range of SO42−, NO3−, and F− is 0.2–6.4 mg/l, 0.2–9.6 mg/l, and 0.1–0.6 mg/l, respectively. The concentrations of Cl− and SO42− in groundwater are less as compared to HCO3− ions, indicating that the water has more temporary hardness and can be removed by boiling the water. SO42−, NO3−, and F− concentrations in the water samples are found to be within the limits of BIS. The concentration of nitrogen in groundwater is derived from the biosphere. Total nitrogen is originally predetermined from the atmosphere and it undergoes a series of reversible oxidation-reduction reactions that convert it from nitrogenous organic molecules to ammonia (NH3), nitrite (NO2−), and nitrate (NO3−).
Organic nitrogen compounds enter the environment from sewage, livestock manure, and animal waste. The primary source of NO3 is from manufactured fertilizers which contains ammonium and potassium nitrate. Contamination of groundwater by nitrate leaching is a major concern as these are not evaporated and remain in water until they are consumed by plants and microorganisms. Generally, the high concentration of SO42− and NO3− in groundwater is due to the intensive urban and industrialization. The study area is free from nitrate pollution owing to zero usage of nitrogen rich inorganic fertilizers by tribal communities and also due to absence of urban conglomerates and industrial clusters. But the use of cow dung and vegetative waste for agriculture in excess quantities also contributes to nitrate pollution in groundwater (Reddy et al. 2011). Fluoride concentration between 0.6 and 1 mg/l in potable water is essential for human survival and higher concentration of this element causes toxic effects (BIS 2012).
Water quality index
WQI provides a rating for overall water quality of a location by considering multiple water quality parameters of their individual characteristics. WQIA was computed for drinking water quality by using BIS (2012) water quality specifications. The unit weight (wi) has been assigned for each selected parameter according to the expert’s knowledge and the relative importance of parameter in the overall quality of water for drinking purposes (Horton 1965; Dunnette 1979; Srinivasamoorthy et al. 2008; Vasanthavigar et al. 2010). The weight of the parameters varies from 1 to 5 indicating that the category of water ranges from excellent to unfit for drinking. The maximum weight was given for pH, Fe, and NO3− due to their importance in water quality assessment as BIS prescribed no relaxation under the category of permissible limit in the absence of alternate source. Whereas the lowest weight was assigned for Mg2+ as it is not harmful for potability. Unit weights (wi) and the calculated relative weights (Wi) for selected parameters are given in Table 6.
The groundwater water quality is categorized based on WQIA classification, as (a) excellent, when WQIA is less than 25; (b) good, if it is in between 25.1 and 50; (c) poor, if it is between 50.1 and 75; (d) very poor, if it is between 75.1 and 100; and (e) unfit, when it is more than 100 for drinking. The computed values of WQI from the groundwater of the study area vary from 20.5 to 122.7 (Table 1). The water quality classification based on WQI values is shown in Table 7. It is observed that about 60% samples are found in poor to unfit and nearly 40% samples falls under excellent to good water for drinking. Out of 24, three samples are unfit for drinking (Fig. 4d). Overall, pH, TDS, TH, Ca2+, and Fe concentrations are high in number for groundwater samples of 4, 1, 2, 2, and 21, respectively (Table 4). Fe plays a major role in overall WQI rating, indicating that 60% of samples are not good for human health. High concentration of Fe is objectionable for taste and esthetic reasons. Excess Fe in groundwater will not create any adverse physiological effect on human health and can easily be removed by aeration (Born et al. 1987).
Groundwater use for irrigation purposes
The suitability of groundwater for irrigation mainly depends on the relative concentrations of salinity and sodium in relation to other cations and anions. In the present study the groundwater is assessed for irrigation suitability using salinity hazard, percent sodium (%Na), sodium adsorption ratio (SAR), residual sodium carbonate (RSC), permeability index (PI), and Kelly’s ratio (KR).
Salinity hazard
The level of salinity hazard depends on sodium ion and total salt content of the water. A high salt content (high EC) in irrigation waters leads to formation of saline soil and affects plants growth. Concentrations of soluble salts in irrigation water can be expressed as low (EC < 250 μS/cm), medium (250–750 μS/cm), high (750–2250 μS/cm), and very high (EC > 2250 μS/cm) salinity zones. By considering the salinity hazard, it is found that more than 85% of samples fall under moderate salinity category (Table 8). High salinity waters (sample nos. S6 and S18) are not recommended for usage on soil with restricted drainage.
Percent sodium
Excess sodium combining with carbonates will lead to formation of alkaline soils, while sodium with chlorides forms the saline soils which results in reduced permeability of soil and supports little or no plant growth (Hem 1985). Sodium content is usually expressed in terms of percentage sodium calculated by the following formula using values in meq/l:
Generally, the percent sodium should not exceed 60 in waters used for irrigation purpose. The calculated values of percent sodium vary from 16.7 to 49.6 meq/l (Table 3), thus the groundwater is suitable for irrigation. The high percent Na in the rainy period might be due to long residence time of water, dissolution of minerals from lithological composition and chemical fertilizers (Swarna Latha and Nageswara Rao 2012). Groundwaters are classified on the basis of %Na and EC according to Wilcox (1948) as shown in Fig. 5a. The diagram revels that all samples are under excellent to permissible category.
Sodium adsorption ratio
SAR is an important parameter for determining the suitability of groundwater for irrigation purpose as it is responsible for sodium hazard (Richards 1954). The SAR is also called sodium hazard or alkali hazard. It is calculated by the following formula using values in meq/l:
SAR values of groundwater samples in the study area varies from 0.4 to 1.8 indicating that the groundwater can be used for irrigation purpose, since the values fall in the permissible range of 0 to 6 (Bouwer 1978). High SAR value of the water makes soil unfit for plant growth due to loss of soil permeability. Sodium reduces soil permeability and encourages hardening of the soil (Davis and De-Wiest 1966). The water observed in the study area is of an excellent category and could be used for irrigation on almost all types of soils without any water salinity controlling measures. The rating of groundwater samples in relation to salinity hazard and sodium hazard can be explained by plotting the chemical data on U.S. Salinity Laboratory Diagram (USDA 1955). Out of 24 samples, one sample falls in low (C1S1) category, and 21 samples fall in medium to low (C2S1) category and remaining 2 are in high salinity to low sodium (C3S1) categories (Fig. 5b). The groundwater of the study area can be used for irrigation purpose in almost all types of soil with little danger of development of exchangeable sodium and salinity.
Residual sodium carbonate
A relation between weak acids and alkaline earths explains the suitability of water for irrigation (Richards 1954). This can be expressed as residual sodium carbonate, which is calculated by the following formula using the values in meq/l:
The range of RSC in groundwater of the study area varies from − 1.34 to 0.52 meq/l. All the samples fall in safe category, as RSC < 1.25 meq/l. RSC > 2.5 meq/l is unsafe for irrigation and excessive RSC affects the yield of crops (Richards 1954).
Permeability index
Excess presence of Na+, Ca2+, Mg2+, and HCO3− ions damage the soil structure and affect the permeability. Doneen (1964) has assessed the suitability of water quality for irrigation based on the cations in relation to the alkalies as permeability index:
where the concentration of ions are in meq/l.
On the basis of permeability index, the groundwater can be classified as Class I, II, and III to find out the suitability of groundwater for irrigation. The computed values of PI from the groundwater of the study area vary between 52 and 94.8 (Table 3). Majority of groundwater samples fall under Class I and Class II (75% or more of maximum permeability) type illustrating that the groundwater of the study area is suitable for irrigation purposes (Doneen 1964).
Kelley’s ratio
Kelly ratio value in groundwater is computed to evaluate the suitability of groundwater for irrigation purpose. The formula suggested by Kelley (1951) is used to estimate the ratio expressed as:
where the concentration of cations are in mg/l.
The calculated values of KR vary from 0.22 to 1.15 mg/l (Table 3). Twenty-two groundwater samples fall in good quality water category as KR < 1. And only two samples show KR > 1 indicating that they are not suitable for irrigation (Kelley, 1951).
Corrosivity ratio
The corrosivity ratio is the ratio of alkaline earths to saline salts present in the groundwater which can be assessed by using the following equation:
where the concentration of ions are in mg/l.
From Table 3, it is observed that out of 24 samples, only 5 are showing the CR > 1, indicating the corrosive nature (Ryner 1944). The effects of corrosion are losses in hydraulic capacity of metallic pipes. Excessive corrosive water cannot be transported through the metal pipes and only be transported through non-corrosive (polyvinyl chloride-PVC) pipes.
Conclusions
The study area is dominated by hard rock aquifer which is influenced by rock–water interaction. Ca-Na-HCO3-Cl type is the dominant facies in almost all the aquifers except a few representations of Ca-HCO3-Cl type is mainly due to the recharge of groundwater. Groundwater in the study area are acidic to alkaline (pH 6.2–8.7) with a total alkalinity of 36–158 mg/l, fresh water types (as TDS < 1000 mg/l), and soft to hard water as TH between 54 and 232 mg/l. As TDS concentrations are low (114–862 mg/l) with a mean of 277 mg/l suggesting that the concentrations of anions and cations are very low. The low concentrations of various chemical ions in the groundwater are mainly by intensive soil erosion due to runoff. The area is characterized by structural hills exhibiting conical tops and moderate to very steep slopes resulting in the water running down quickly without giving enough time for leaching. Very low abundance of various minerals in groundwater caused dental caries, osteoporosis, gastro-intestinal irritation, liver and kidney problems, toxicity, etc. where people are suffering from a long period in the study area. According to water quality standards, some groundwater samples are not suitable for drinking due to the presence of excess concentrations of pH, TDS, TH, Ca2+, and Fe. WQI analysis revealed only 40% of samples are suitable for potability. Some groundwater samples showing high salinity are recommended for irrigation with proper drainage. On the other hand, the concentrations of iron in the groundwaters are very high. The inhabitants of vulnerable tribal populations are forced to use iron affected groundwater for domestic purposes due to the lack of safe and other alternative sources of water supply. Children are more susceptible to the effect of pollution compared with adults. Continuous utilization of high amount of iron in drinking water in the human body causes hemochromatosis resulting in tissues being damaged. The findings reveal that 87.5% of samples have an overload of iron content possibly due to the influence of regional geological and hydrogeological conditions on the groundwater regime of the study area. The chief sources of iron in groundwaters are iron-bearing minerals like hematite, goethite, limonite, feldspar, pyroxene, epidote, and hornblende in the rocks and sediments. Geological Survey of India found that the bauxite lateritic cappings/deposits consist of 10–25% rich of iron oxide with the thickness of about 54 m occurring at higher altitudes (1100 to 1400 m amsl) over the garnet-sillimanite-gneisses (Khondalite) rocks of the present study region. A significant percentage of iron is abundant in rocks and soils, since the source rocks contain minerals and soil is derived from them. The weathering and leaching processes, mainly by moving and percolating water, play a key role in the occurrence of iron in groundwater. The incidence of iron in groundwaters may be governed by the iron-bearing minerals through the contact between the source mineral and water.
It is the right time that an affordable solution needs to be found to minimize the problem of high iron contamination in groundwaters for improving the quality of health of the large native tribal population in the study area. The solution to minimize the iron concentrations in drinking water could be the utilization of iron removal plants and filters. The soluble iron can be removed by a reverse osmosis process. However, it is a difficult process and costly in large-scale community water supply and economically not feasible. The simple method to remove iron from water is aeration where the iron reacts with oxygen to form compounds which get precipitated and this can be separated through filtration. Also, prominent methods like domestic iron removal unit (DIRU/NEERI) and UNICEF/Bangladesh design have been developed for removing iron from water are proved satisfactory and effective, those may be recommended. In conclusion, the long periods of monitoring and extensive investigations are required for finding potential groundwater environmental problems caused by the pollution conditions in the area. In the study area, to restrict the runoff for recharging water table to augment groundwater through artificial recharge is the alternative method to cater the needs of domestic and irrigation requirements.
References
Alharbi TG (2018) Identification of hydrogeochemical processes and their influence on groundwater quality for drinking and agricultural usage in Wadi Nisah, Central Saudi Arabia. Arab J Geosci 11(359). https://doi.org/10.1007/s12517-018-3679-z
Apambire WB, Boyle DF, Michel FA (1997) Geochemistry, genesis, and health implications of fluoriferous groundwaters in the upper regions of Ghana. Environ Geol 33:13–24
APHA (1998) Standard methods for the examination of water and wastewater. American Public Health Association, Washington. D.C
Belkhiri L, Narany TS (2015) Using multivariate statistical analysis, geostatistical techniques and structural equation modeling to identify spatial variability of groundwater quality. Water Resour Manag 29:2073–2089. https://doi.org/10.1007/s11269-015-0929-7
Berner EK, Berner RA (1987) The global water cycle: geochemistry and environment. Prentice-Hall, New Jersey
Bhargava DS (1985) Expression for drinking water supply standards. J Environ Engg ASCE 106(4):757–771
BIS (2012) Drinking water-specification. Bureau of Indian Standards, New Delhi IS: 10500
Bo Z, Mei H, Yongsheng Z, Xueyu L, Xuelin Z, Jun D (2003) Distribution and risk assessment of fluoride in drinking water in the west plain region of Jilin Province, China. Environ Geochem Health 25:421–431. https://doi.org/10.1023/B:EGAH.0000004560.47697.91
Born SM, Yanggen DA, Zaporozec A (1987) A guide to groundwater quality planning and management for local governments. Wisconsin Geological and Natural History Survey
Bouwer H (1978) Groundwater hydrology. McGraw-Hill, New Delhi
Brown RM, McClelland NI, Deininger RA, Tozer RG (1970) A water quality index-do we dare? Water Sewage Works 11:339–343
CGWB (2010) Ground water quality in shallow aquifers in India. Central Ground Water Board, Govt. of India
Chadha DK (1999) A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol J 7:431–439. https://doi.org/10.1007/s100400050216
Davis SN, De Wiest RJM (1966) Hydrogeology. John Wiley and Sons Inc, New York
Doneen LD (1964) Notes on water quality in agriculture. Published as a water sciences and engineering paper-4001. Department of Water Sciences and Engineering, University of California
Dunnette DA (1979) A geographically variable water quality index used in Oregon. Water Poll Cont Fed 51(1):53–61
Durov SA (1948) Natural waters and graphic representation of their compositions. Dokl Akad Nauk SSSR 59:87–90
Dwivedi SL, Pathak V (2007) A preliminary assignment of water quality index to Manadakini Rivier, Chitrakoot. Indian J Environ Prot 27(11):1036–1038
Fendorf S, Michael HA, Alexander G (2010) Spatial and temporal variations of groundwater arsenic in south and southeast Asia. Science 328(5982):1123–1127
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall, Englewood Cliffs, NJ
Gibbs RJ (1970) Mechanism controlling world’s water chemistry. Science 170:1088–1090
Glynn PD, Plummer LN (2005) Geochemistry and the understanding of ground-water systems. Hydrogeol J 13(1):263–287. https://doi.org/10.1007/s10040-004-0429-y
Goyal M, Dhar DN, Singh SK (2010) Physicochemical methods for monitoring seasonal variations in Cl−, F−, and Fe++ in underground water in Unnao district in Uttar Pradesh (India). Environ Monit Assess 171:425. https://doi.org/10.1007/s10661-009-1288-8
Gulgundi MS, Shetty A (2018) Groundwater quality assessment of urban Bengaluru using multivariate statistical techniques. Appl Water Sci 8(43). https://doi.org/10.1007/s13201-018-0684-z
Hem JD (1962) Chemistry of iron in natural water. U.S. Geological Survey. U.S. Govt. Print. Off, Washington
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water. USGS Water Supply Paper 2254
Hill RA (1940) Geochemical patterns in the Coachella valley. California Trans Am Geophys Union 21:46–49
Horton RK (1965) An index number system for rating water quality. J Water Poll Cont Fed 37(3):300–306
Ivanova IS, Lepokurova OE, Pokrovskii OS, Shvartsev SL (2014) Iron containing groundwater in the upper hydrodynamic zone in the central part of West Siberian, Artesian basin. Water Res 41(2):163–177. https://doi.org/10.1134/S0097807814020080
Jagannadha Sarma VV, Swamy AN (1981) Groundwater quality in Visakhapatnam basin, India. Water Air Soil Pollut 16:317–329. https://doi.org/10.1007/BF01046912
Jalali M (2005) Nitrates leaching from agricultural land in Hamadan, western Iran. Agric Ecosyst Environ 110:210–218. https://doi.org/10.1016/j.agee.2005.04.011
Kanoua W, Merkel B (2017) Hydrochemical evolution and arsenic release in shallow aquifer in the Titas Upazila, Eastern Bangladesh. Arab J Geosci 10(290). https://doi.org/10.1007/s12517-017-3063-4
Kelley (1951) Alkali soils-their information properties and reclamation. Reinhold Publishing Corp, New York
Khan F, Husain T, Lumb A (2003) Water quality evaluation and trend analysis in selected watersheds of the Atlantic region of Canada. Environ Monit Assess 88:221–242. https://doi.org/10.1023/A:1025573108513
Langelier WF, Ludwig HF (1942) Graphic methods for indicating the mineral character of natural waters. J Am Water Works Assoc 34:335–352
Lloyd JW (1965) The hydrochemistry of the aquifers of North-Eastern Jordan. J Hydrol 3:319–330
Mahapatra SS, Sahu M, Patel RK, Panda BN (2012) Prediction of water quality using principal component analysis. Water Qual Expo Health 4(2):93–104. https://doi.org/10.1007/s12403-012-0068-9
Mandal BK, Chodhury TR, Samanta G, Basu GK et al (1996) Arsenic in groundwater in seven districts of West Bengal, India-the biggest arsenic calamity in the world. Curr Sci 70(11):976–986
Masoud AA (2014) Groundwater quality assessment of the shallow aquifer west of the Nile Delta (Egypt) using multivariate statistical and geostatistical techniques. J Afr Earth Sci 95:123–137
Nageswara Rao K, Swarna Latha P (2017) Socio-economic conditions of vulnerable tribal population in forested habitations of Eastern Ghats area, Visakhapatnam district, Andhra Pradesh, In Socio-Economic History of Tribal India. Avon Publications, New Delhi
Oborn ET, Hem JD (1962) Some effects of the larger types of aquatic vegetation on iron content of water. US Geological Survey Water Supply Paper 1459-L
Piper AM (1944) A graphic procedure in geochemical interpretation of water analyses. Trans Am Geophys Union 25:914–923
Promma K, Zheng C, Asnachinda P (2007) Groundwater and surface-water interactions in a confined alluvial aquifer between two rivers: effects of groundwater flow dynamics on high iron anomaly. Hydrogeol J 15:495–513. https://doi.org/10.1007/s10040-006-0110-8
Rao NS, Rao PS, Reddy GV et al (2012) Chemical characteristics of groundwater and assessment of groundwater quality in Varaha River basin, Visakhapatnam district, Andhra Pradesh, India. Environ Monit Assess 184:5189. https://doi.org/10.1007/s10661-011-2333-y
Reddy DV, Nagabhushanam P, Peters E (2011) Village environs as source of nitrate contamination in groundwater: a case study in basaltic geo-environment in central India. Environ Monit Assess 174:481–492. https://doi.org/10.1007/s10661-010-1472-x
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. US. Department of Agriculture Handbook 60, Washington
Romani S (1981) A new diagram for classification of natural waters and interpretation of chemical analysis data. Stud Environ Sci 17:743–748
Routroy S, Harichandan R, Mohanty JK, Panda CRA (2013) Statistical appraisal to hydrogeochemistry of fluoride contaminated ground water in Nayagarh district, Odisha. J Geol Soc India 81:350–360. https://doi.org/10.1007/s12594-013-0045-3
Ruiz RL, Zapata EP, Parra R, Harter T, Mahlknecht J (2015) Investigation of the geochemical evolution of groundwater under agricultural land: a case study in northeastern Mexico. J Hydrol 521:410–423. https://doi.org/10.1016/j.jhydrol.2014.12.026
Ryner JW (1944) A new index for determining amount of calcium carbonate scale formed by water. J Am Water Assn 36:472–486
Satyanarayana M, Periakali P (2003) Geochemistry of ground water in ultrabasic peninsular gneissic rocks, Salem district, Tamil Nadu. J Geol Soc India 62:63–73
Schoeller H (1967) Geochemistry of groundwater. In: An international guide for research and practice. UNESCO, Paris, pp 1–18
Singaraja C, Chidambaram S, Prasanna MV, Thivya C, Thilagavathi R (2014) Statistical analysis of the hydrogeochemical evolution of groundwater in hard rock coastal aquifers of Thoothukudi district in Tamil Nadu, India. Environ Earth Sci 71(1):451–464. https://doi.org/10.1007/s12665-013-2453-5
Singh KP, Malik A, Mohan D, Sinha S (2004) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India): a case study. Water Res 38:3980–3992. https://doi.org/10.1016/j.watres.2004.06.011
Smith DG (1990) A better water quality indexing system for rivers and streams. Water Res 24(10):1237–1244. https://doi.org/10.1016/0043-1354(90)90047-A
Srinivasamoorthy K, Chidambaram M, Prasanna MV, Vasanthavigar M, John PA, Anandhan P (2008) Identification of major sources controlling groundwater chemistry from a hard rock terrain-a case study from Mettur taluk, Salem district, Tamilnadu, India. J Earth Sys Sci 117:49–58. https://doi.org/10.1007/s12040-008-0012-3
Stumm W, Morgan JJ (1996) Aquatic chemistry. Wiley, New York
Subramani T, Rajmohan L, Elango L (2010) Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, Southern India. Environ Monit Assess 162:123–137
Sundaray SK, Panda UC, Nayak BB, Bhatta D (2006) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of the Mahanadi river-estuarine system (India) - a case study. Environ Geochem Health 28:317–330. https://doi.org/10.1007/s10653-005-9001-5
Swamee PK, Tyagi A (2000) Describing water quality with aggregate index. J Environ Engg ASCE 26(5):451–455
Swarna Latha P, Nageswara Rao K (2012) An integrated approach to assess the quality of groundwater in a coastal aquifer of Andhra Pradesh, India. Environ Earth Sci 66:2143–2169
Thakur DS, Thakur DC, Saini AS (1991) Socio-economic impact of tribal development programmes in Himachal Pradesh. J Rural Dev 10:823–830
Todd DK, Mays LW (2005) Groundwater Hydrology. Wiley, New York
UNICEF, FAO and SaciWATERs (2013) Water in India: situation and prospects. UNICEF India, New Delhi
USDA (1955) Water, the 1955 year book of agriculture. US Department of Agriculture 175
Valenzuela VL, Ramırez HJ, Reyes LJ, Sol UA, Lazaro MO (2006) The origin of fluoride in groundwater supply to Hermosillo City, Sonora, Mexico. Environ Geol 51:17–27
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K et al (2010) Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environ Monit Assess 171:595–609. https://doi.org/10.1007/s10661-009-1302-1
Weiner ER (2000) Applications of environmental chemistry-a practical guide for environmental professionals. Lewis Publishers, New York
WHO (2011) Guidelines for drinking water quality. World Health Organization Press, Geneva
WHO-UNICEF (2015) Progress on sanitation and drinking water-2015 update and MDG assessment. World Health Organization Press, Geneva, pp 1–80
Wilcox LV (1948) The quality of water for irrigation use. No. 170282. United States Department of Agriculture, Economic Research Service
Acknowledgments
The authors would like to thank Prof. K. Narendra, Dept. of Civil Engg., GITAM University, Visakhapatnam, for his support and encouragement. We also thank the anonymous reviewers and editor for their perceptive comments and suggestions.
Funding
The first author received assistance from Science and Engineering Research Board (SERB), Department of Science and Technology, New Delhi, in the form of Young Scientist Fellowship No. SR/FTP/ES-143/2010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: Antonio Pulido-Bosch.
Rights and permissions
About this article
Cite this article
Rao, K.N., Latha, P.S. Groundwater quality assessment using water quality index with a special focus on vulnerable tribal region of Eastern Ghats hard rock terrain, Southern India. Arab J Geosci 12, 267 (2019). https://doi.org/10.1007/s12517-019-4440-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-019-4440-y