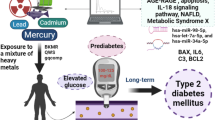
Abstract
Scarce information exists about the link between mixed heavy metals and metabolic syndrome (MetS) and its components, as well as its molecular mechanism. Thus, we identified the associations of serum cadmium, lead, and mercury with MetS and its components using linear regression models, weighted quantile sum (WQS) regression, quantile g-computation (qgcomp), and Bayesian kernel machine regression (BKMR). Of the 5581 subjects included, 30.8% had MetS. In the logistic regression model, serum mercury was associated with MetS and its components, and significant trends were observed for these heavy metal quantiles (p < 0.001). Serum mercury levels were also linked with MetS and its components in the WQS and qgcomp models. In BKMR analysis, the overall effect of the mixture was significantly associated with MetS and its components. Serum mercury showed positive trends and was observed as the most important factor associated with MetS, along with elevated waist circumference and elevated blood pressure. In in-silico toxicogenomic data mining, we found several pathways (insulin resistance, IL6 signaling pathway, and adipogenesis), regulation of lipid localization, and metabolic syndrome X as key molecular mechanisms that may be affected by heavy metals and involved in the development of MetS. We identified hsa-miR-124-3p as the highest interaction and expression implicated in the MetS process. We also used miRNAsong to create and test a miRNA sponge sequence for these miRNAs, which may be promising for being used in MetS therapy. In particular, the cutoff levels for exposure levels related to MetS and its components were also reported.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid social and economic growth, as well as lifestyle changes, have resulted in a dramatic increase in adult metabolic syndrome (MetS) prevalence in South Korea, and this trend is expected to continue (Duc et al. 2021a, b, c; Nguyen and Kim 2021). MetS is a significant public health issue that contributes to the risk of cardiovascular diseases, diabetes, non-alcoholic fatty liver disease, cancer, gout, sleep apnea syndrome, dementia, polycystic ovary syndrome, and other clinical consequences (Beck-Nielsen 2013). Genetics, an imbalance of energy expenditure and consumption, high-calorie food intake, lack of physical fitness, a sedentary lifestyle, stress, and health issues are all known to have a role in MetS development. However, emerging evidence suggests that these characteristics are insufficient to adequately explain the MetS problem (Lopomo et al. 2016; Park et al. 2017; Nguyen and Kim 2021; Nguyen et al. 2021a, b, c, d). Environmental factors, in addition to these, are a risk factor for MetS (Valera et al. 2012; Angeli et al. 2013; Poursafa et al. 2014; Arbi et al. 2017). Therefore, risk factors as well as MetS should be controlled to reduce the societal disease burden and to improve quality of life.
Humans can be easily exposed to heavy metals through the air, food, water, or industrial environments, especially in the industrialization and urbanization eras, because heavy metals are long-lasting environmental contaminants (Poursafa et al. 2014; Ali et al. 2019; Nguyen et al. 2021a, b, c, d). The most harmful heavy metals researched are mercury (Hg), cadmium (Cd), and lead (Pb) (Duc et al. 2021a, b, c; Nguyen et al. 2021a, b, c, d). The most common sources of cd exposure are cigarette smoking and contaminated food (Satarug et al. 2017). Cosmetics, fossil fuels, air, polluted waste, and food, particularly contaminated fish and seafood, are all potential sources of Hg exposure (Wolkin et al. 2012; Garí et al. 2013; Çamur et al. 2016). Pb exposure was caused by industrial operations, fuel, cigarette smoke, contaminated food, soil, water, and air, and residential Pb-based paints (Aelion et al. 2012; Hrubá et al. 2012). Thus, humans can be exposed to a variety of heavy metals at the same time because of interactions between co-administered heavy metals (Duc et al. 2021a, b, c; Nguyen et al. 2021a, b, c, d).
Cd, Hg, and Pb have long been recorded as environmental risk factors for multi-organ dysfunction. Converging evidence finds that Cd can play an important role in the pathogenesis of MetS in the general adult population (Tinkov et al. 2017; Duc et al. 2021a, b, c; Nguyen and Kim 2021; Nguyen et al. 2021a, b, c, d). Several studies have also found that Pb and Hg exposure is linked to MetS in children and non-pregnant adults (Valera et al. 2012; Zhang et al. 2012; Rothenberg et al. 2015). Despite the fact that numerous researchers have attempted to investigate the effects of Cd, Hg, and Pb on MetS, these studies on MetS have largely focused on just one heavy metal. Somewhat surprisingly, the effects of mixing these heavy metals have not been sufficiently investigated. Until now, when studying chemical exposure, most scientists have used a combination of effects and different methods to arrive at more reliable conclusions (Duc et al. 2021a, b, c; Nguyen et al. 2021a, b, c, d). A recent literature review reported the role of Cd, Pb, and Hg on MetS and also recommended evaluating the interaction between these heavy metals and MetS to gain a better understanding of the mixed harmful effects of these heavy metals on MetS (Xu et al. 2021).
As a result, it is critical that we comprehend the consequences of interactions between heavy metals found in the environment and MetS, especially in terms of genes, pathways, and miRNA interaction. We hypothesized that interactions between heavy metals like Cd, Hg, and Pb are inextricably linked to MetS in adults, so we conducted this study to determine the effects of interactions between serum Cd, Hg, and Pb levels on MetS in Korean adults aged ≥ 18 years. Through the Comparative Toxicogenomics Database data mining analysis (CTD), MicroRNA ENrichment TURned NETwork (MIENTURNET), we explored the genes (gene interactions, networks, molecular functions, biological processes, cellular components, pathways, and diseases) and miRNAs (gene and miRNA interaction, network, pathways, and diseases) associated with mixed heavy metals and the development of MetS. Furthermore, we created and tested in-silico miRNA sponge sequences for miRNA-induced MetS using the microRNA sponge generator and tester (miRNAsong).
Materials and Methods
Study Population
The heavy metal dataset from the Korean National Health and Nutrition Examination Survey (KNHANES) IV (2009), KNHANES V (2010–2012), KNHANES VI (2013), and KNHANES VII (2016–2017) were used to investigate the link between a mixture of serum Hg, Pb, and Cd levels and MetS in Korean individuals aged ≥ 18 years (Duc et al. 2021a, b, c; Ministry-of-Health-and-Welfare 2021). These investigations are representative yearly assessments of the civilian, non-institutionalized Korean general population’s serum heavy metal concentrations, health, and nutritional status. The KNHANES included a total of 10,533 (2009), 8958 (2010), 8518 (2011), 8058 (2012), 8018 (2013), 8150 (2016), and 8127 (2017) individuals. We removed 13,281 subjects under the age of 18; 32,013 records lacking serum Cd, Hg, and Pb; 5124 records lacking urine cotinine; and the other 9944 records missing covariates from the 60,362 participants who took part in the survey between 2009 and 2013 (Fig. 1). As a result, a total of 5581 participants were considered for data analysis. The KNHANES website (http://knhanes.cdc.go.kr/) included a thorough explanation of the plan, standardized protocol, and survey license (Nguyen et al. 2021a, b, c, d).
Serum Cd, Hg, and Pb Determinations
Cd, Hg, and Pb analyses have been previously described (Duc et al. 2021a, b, c; Nguyen and Kim 2021; Nguyen et al. 2021a, b, c, d). Briefly, serum Cd, Hg, and Pb levels were analyzed by the NEODIN Medical Institute, which is accredited by the Ministry of Health and Welfare of Korea. These tests met the criteria of the Korean Occupational Safety and Health Administration program, the German External Quality Assessment Scheme, and the U.S. CDC. Serum Cd and Pb levels were calculated by graphite furnace atomic absorption spectrometry (model AAnalyst 600; PerkinElmer, Turku, Finland) using Zeeman background correction. The serum total Hg levels were measured by a direct-mercury analyzer (model DMA-80 Analyzer; Bergamo, Italy) and gold amalgam (KCDC). For internal quality assurance and control, commercial standards (Lyphochek Whole Blood Metals, Bio-Rad, CA, USA) were employed as reference materials. The limits of detection (LODs) for Pb, Hg, and Cd were 0.223 µg/dL, 0.05 µg/L, 0.087 µg/L, respectively.
Covariates
Laboratory measurements, as well as demographic and socioeconomic variables (e.g., urine cotinine, HDL-C, age, education level, etc.), have been described in detail elsewhere (Duc et al. 2021a, b, c; Yun et al. 2021). Potential covariates were first recorded in the literature, or subjective previous knowledge plus variables with p values of less than 0.25 in univariate analysis, and then added into the entire model. Continuous variables were energy consumption (Kcal) and ln2-transformed creatinine levels. Other covariates were classified as follows: sex (males, females), age group (18–29, 30–39, 40–49, 50–59, 60–69, ≥ 70), residential areas (urban, rural), occupation (blue-collar, white-collar, and unemployed), educational level (≤ middle school, high school, ≥ college), BMI group (> 18.5, 18.5–25, 25–30, ≥ 30), monthly household income (< 2000, ≥ 2000 and < 4000, ≥ 4000 and < 6000, ≥ 6000), family history of hyperlipidemia, diabetes or cardiovascular diseases (yes, no), drinking status (often, occasionally, never or rarely), smoking (non/ex-smoker, current smoker), and physical activity (not regular, regular).
Outcomes
Elevated waist circumference, elevated triglycerides, decreased HDL-C, elevated blood pressure, and elevated serum fasting glucose were used to define MetS, according to the American Heart Association/National Heart, Lung, and Blood Institute’s clinical diagnostic criteria. MetS was classified as having three or more of the five risk factors listed below. (1) Elevated waist circumference (WC ≥ 80 cm for women, and ≥ 90 cm for men), (2) elevated triglycerides (TG ≥ 150 mg/dL or receiving medication to reduce triglycerides), (3) decreased high-density lipoprotein cholesterol (HDL-C < 50 mg/dL in women or the receipt of medication for increasing HDL-C), (4) elevated blood pressure (systolic blood pressure ≥ 130 mmHg and/or ≥ 85 mmHg diastolic blood pressure, a history of hypertension or receipt of antihypertensive drug treatment), (5) elevated fasting glucose (≥ 100 mg/dL or receipt of drug treatment for elevated fasting glucose) (Duc et al. 2021a, b, c; Nguyen and Kim 2021).
Statistical Analysis
The analysis was carried out using STATA (version 16.0; StataCorp, Texas, USA) and R (version 4.1.0). Frequencies and proportions were utilized for categorical variables, while means and standard deviations, or median and interquartile range, were used for continuous variables. To compare differences in continuous and categorical variables, the Student’s t test or Wilcoxon rank-sum test, as well as χ2 tests, were utilized.
Heavy levels were ln2 transformed due to their right-skewed distribution. Heavy levels were described using the geometric mean (GM) and a 95% confidence interval. In order to determine their relationships with MetS and its components, the logistic regression model used the median of heavy level quartiles as categorical and continuous variables. We also looked at the Pearson correlation coefficients between the three heavy metals’ ln2-transformed values and cardiometabolic risk factors.
The impacts of mixing these heavy metals were studied using generalized linear regression, weighted quantile sum (WQS) regression, quantile g-computation (qgcomp), and Bayesian kernel machine regression (BKMR) models.
Logistic and Linear Regression Models
First, we compared the second, third, and fourth quartiles of a heavy metal’s levels to the first quartile of a heavy metal’s levels to analyze the link between each heavy metal and MetS and its components using multivariate logistic regression. Second, we examined linear regression with each heavy metal’s ln2-transformed levels as continuous variables and MetS as a continuous outcome variable. Third, we explored the interaction between these heavy metals and MetS and its components.
Secondary Analysis
We used three approaches to further evaluate the effects of heavy metal mixtures on MetS and its components, including WQS, qgcomp, and BKMR (Duc et al. 2021a, b, c; Nguyen et al. 2022).
Weighted Quantile Sum (WQS) Regression Model
This method has been described in detail elsewhere (Duc et al. 2021a, b, c; Nguyen et al. 2021a, b, c, d). In brief, the study population was randomly divided into a training dataset (40%, n = 2232) and a validation dataset (60%, n = 3349) as part of the approach. Using the training dataset, bootstrapping was used to determine empirical weights for each heavy metal in the combination. In the present study, heavy metals with estimated weights greater than 0.333 (1/3) were judged to have a substantial impact on the WQS score. We created and analyzed both a positive and negative WQS score since the WQS approach implies that all mixture components act in the same directionality on MetS and its components (Nguyen et al. 2022). The analysis was carried out using the R package gWQS.
Quantile G-Computation (qgcomp)
This approach’s aim and process have been provided elsewhere (Duc et al. 2021a, b, c; Nguyen et al. 2021a, b, c, d). In brief, the qgcomp.noboot function was used to estimate exposure effects, which divides all heavy metals into quintiles, assigns a positive or negative weight to each heavy metal, and fits a linear model for continuous outcomes using Bayesian variable penalization. Heavy metals with estimated weights greater than 0.05 were considered to have a substantial impact on the qgcomp score in the current investigation. In addition, qgcomp.boot was utilized to test the total exposure effect’s linearity (Duc Nguyen et al. 2022a, b). The plot was made using g-computation and bootstrap variance with B up to 200 to depict the joint intervention levels of heavy metal exposure to MetS and its components. The analysis was performed using the R package qgcomp.
Bayesian Kernel Machine Regression (BKMR) Model
The objective and process of this approach have been described elsewhere (Duc et al. 2021a, b, c; Nguyen et al. 2021a, b, c, d). In brief, we used the following equation in the BKMR model:
The exposure–response function h in Eq. (1) compensates for non-linearity and/or interaction between the various heavy metal components in the mixture, whereas Z = Z1, Z2,…, Zq accounts for q possible confounders. All heavy metals were ln2-transformed and normalized in this research, and a Gaussian kernel function was applied with a component-wise variable approach. The posterior inclusion probabilities (PIPs) for each heavy metal were calculated, and estimates of the exposure–outcome function were derived after fitting the final model with the Markov Chain Monte Carlo (MCMC) sampler for 10,000 iterations. To evaluate whether a heavy metal is relevant, a PIP threshold of 0.5 is frequently utilized. We compared the results when all heavy metals were set to their 25th, 30th, 35th, 40th, 45th, 55th, 60th, 65th, 70th, or 75th percentiles to when they were all set to their 50th percentile to assess the overall effect of heavy metal mixtures on MetS and its components. Second, by plotting the exposure–outcome function of a single heavy metal while fixing the second metal at the 25th, 50th, and 75th percentiles and setting all other heavy metals to their median values, potential interactions between each pair of heavy metals were explored pairwise. In addition, we visualized the bivariate exposure–response function with a third exposure fixed at different quantiles to look for potential 3-way interactions. The bkmr R package was used to do the analysis.
Threshold Estimation
We used threshold regression to calculate the cutoff thresholds for exposure levels that are relevant to MetS and its components in order to estimate the threshold for heavy metals affecting MetS and its components (Duc Nguyen et al. 2022a, b).
Detecting Common Genes for Heavy Metal Exposure and Metabolic Syndrome
In the current study, the link between MetS and heavy metals was recognized by investigative heavy metal–gene/protein interactions attained from the CTD (http://CTD.mdibl.org). The analysis reported in the present study was used from the data downloaded in November 2021. We then found the genes linked to heavy metals and MetS development. A network of overlapping genes induced by the three heavy metals, along with four related genes linked with MetS, were analzyed using GeneMANIA (http://geneMANIA.org/plug-in/). The ToppGeneSuite portal (https://toppgene.cchmc.org and its ToppFun function (https://toppgene.cchmc.org/enrichment.jsp) were used to link biological processes, pathways, and diseases associated with MetS to the genes induced by the heavy metal mixture (Duc Nguyen et al. 2022a, b).
Prediction of Transcription Factors, miRNA, miRNA–Target Interactions, Networks, Pathways, Diseases, and Sponge
To identify the transcription factors that regulated the MetS-related genes, ChIP-X Enrichment Analysis version 3 (CHEA3) was used (https://maayanlab.cloud/chea3/) (Nguyen and Kim 2022a, b, c, d). Cytoscape version 3.9.1 was used to visualize the integrated regulatory network, which encompasses the top 10 transcription factors. The miRNA–target interaction networks were generated and investigated using MIENTURNET (Licursi et al. 2019). To obtain possible miRNA data, we submitted lists of genes connected to MetS development to MIENTURNET. We used miRTarBase to create miRNA networks based on experimentally validated and/or computationally predicted genes from heavy metal exposure and MetS development (Duc Nguyen et al. 2022a, b). Using bioinformatics and evolutionary genomics software, a Venn diagram was created to illustrate the expression of heavy metal-induced miRNA (https://bioinformatics.psb.ugent.be/webtools/Venn/) (Nguyen and Kim 2022a, b, c, d). The MIENTURNET web tool was used to query the WikiPathways and disease ontology databases for the functional enrichment analysis (Licursi et al. 2019). p values were adjusted using the Benjamini–Hochberg approach, and a threshold of 0.05 was used to identify functional annotations that were significantly enriched over the whole gene list in the input list. We used a web-based application, miRNA sponge generator, and tester (miRNAsong, http://www.med.muni.cz/histology/miRNAsong), to develop and test miRNA sponge sequences specific to target miRNAs induced by examined heavy metals (Nguyen and Kim 2022a, b, c, d).
Results
Characteristics of the Study Population
The present study included 1717 participants aged ≥ 18 years who had MetS and 3864 without MetS. Participants with MetS were more likely to be elderly, females, married, city dwellers, less educated, unemployed, from low-income families, heavy drinkers, physically inactive, and have a family history of CVDs and diabetes. Participants with MetS had higher BMI, WC, lipid profiles (total cholesterol, LDL-C, triglyceride), fasting glucose, HbA1c, hs-CRP, blood pressure (systolic and diastolic blood pressure), liver function biomarkers (AST and ALT), and urine cotinine. Table 1 shows demographic information stratified by the presence or absence of MetS.
Characteristics of Heavy Metal Exposure
Table 2 shows the mean and geometric mean levels stratified by the presence or absence of MetS in both males and females of three heavy metals. Serum levels of Cd, Hg, and Pb were more likely to be higher in males or females with MetS compared with those without MetS. Males with MetS had higher serum Hg and Pb levels than females with MetS. In contrast, females with MetS had higher serum Cd levels than males with MetS. There was a significant difference in serum heavy metals between males and females.
Figure 2 provided the Pearson correlation coefficients (r) among serum heavy metals and cardiometabolic risk factors (p value < 0.001, r ranging from – 0.36 to 0.86). There was a strong correlation between fasting glucose and HbA1c (r = 0.83), BMI and WC (r = 0.86), and diastolic blood pressure and systolic blood pressure (r = 0.63). The other correlations were relatively moderate or weak. For example, the correlation between ln2-transformed serum Pb and Cd (r = 0.09) and ln2-transformed serum Hg and Pb (r = 0.28).
Pairwise Pearson correlations among ln2-transformed levels of heavy metals and cardiometabolic risk factors in the population (n = 5581), KNHANES, Korean, 2009–2017. BMI body mass index; Chol cholesterol; DBP diastolic blood pressure; EN energy intake; Glu serum glucose; HDL-C high-density lipoprotein cholesterol; HbA1c hemoglobin A1c; TG triglycerides; SBP systolic blood pressure; WC waist circumference; ln2CO ln2-transformed levels of urine cotinine; ln2Cd, ln2Hg, ln2Pb ln2-transformed levels of cadmium, mercury, and lead
The Link Between Serum Heavy Metal Levels and Metabolic Syndrome and Its Components was Assessed Using a Multivariate Logistic and Linear Regression Model
Tables 3A, B show the results of the single heavy metals and their interactions using the multivariate logistic and linear regression models. First, we identify the association between a single heavy metal and both MetS and its components when considering heavy metals as categorical variables.
In the multivariable logistic regression analysis, serum Cd showed significant associations with MetS and its components (elevated WC, elevated triglycerides, and elevated blood pressure) in the upper two quartiles, a significant trend (p values for trend < 0.001). There were significant links between serum Hg and both MetS and its components (reduced HDL-C, elevated blood pressure, and elevated fasting glucose), with a significant trend (p values for trend < 0.001). Unsurprisingly, in the multivariable linear regression models, serum Cd and Hg were found to be linked with MetS treated as a continuous variable. Furthermore, we found significant links between serum Pb and both elevated triglycerides and elevated blood pressure, a significant trend (p values for trend < 0.001).
Next, we assessed the link between heavy metals and both MetS and its components when treating heavy metals as continuous variables. In the multivariable linear regression models, serum Cd was found to be related to MetS, elevated WC, elevated triglycerides, and elevated blood pressure. Serum Hg was found to be linked with MetS, elevated WC, reduced HDL-C, elevated blood pressure, and elevated fasting glucose, while there were significant associations between serum Pb, elevated triglycerides, and elevated blood pressure. As expected, serum Cd and Hg were also found to be related to MetS treated as a continuous variable. These findings were also consistent with the results from the logistic regression model.
On the other hand, we assessed the interaction of heavy metals on MetS. After adjusting for potential confounders, we found an interaction between serum Cd and Hg levels on elevated triglycerides. Furthermore, there was an interaction between serum Cd, Hg, and Pb levels on MetS (including when MetS was treated as categorical and continuous variables) and elevated triglycerides (Table 3B).
The Link Between Heavy Metals and Metabolic Syndrome and Its Components were Assessed Using the WQS Model
In the current study, the WQS indices were linked with MetS and its components (elevated WC, elevated blood pressure, and elevated fasting glucose (Table 4A, B). In the fully adjusted models, the WQS indexes were significantly linked with MetS (OR 1.66, 95% CI 1.19–2.32), elevated WC (OR 1.62, 95% CI 1.19–2.20), elevated blood pressure (OR 2.03, 95% CI 1.29–2.19), and fasting glucose (OR 1.31, 95% CI 1.02–1.73). Table S1A-B and Fig. 3A–F show the projected weights of serum heavy metals for each WQS index. Serum Hg was the highest weight in almost all models, except for the reduced HDL-C model. Following that, serum Cd was given a medium weight, and serum Pb was given the lightest.
WQS model regression index weights for MetS (A), elevated WC (B), elevated triglycerides (C), reduced HDL-C (D), elevated blood pressure (E), elevated fasting glucose (F), and MetS components (G). Models were adjusted for sex, age group, BMI group, occupation, family history of hyperlipidemia, family history of CVD, family history of diabetes, physical activity, drinking status, residential areas, smoking, educational level, monthly household income, energy intake, and ln2-cotinine. ln2Cd, ln2Hg, ln2Pb among ln2-transformed levels of cadmium, mercury, and lead
To investigate further the effects of mixture exposure-induced MetS changes, we treated MetS as a continuous outcome and fitted a WQS model to measure the effects of mixed three heavy metals on MetS. After adjusting for possible covariates, a quartile increase in the WQS index was related to a 0.12 unit increase in MetS (95% CI 0.07–0.17). Table S1B and Fig. 3G provide the weights of heavy metals. Serum Pb was quantified as the lightest weight, and serum Hg was the most weighted. We also evaluated weights derived from bootstrap models with negative mixture effects for MetS and its components, but no significant association was found (data not shown).
The Link Between Heavy Metals and Metabolic Syndrome and Its Components was Assessed Using the qgcomp Model
Like the WQS model, the qgcomp indices were linked with MetS and its components (elevated WC, elevated triglycerides, and elevated blood pressure). In the entirely adjusted models (Table 5A, B), a quartile increase in the qgcomp index was significantly linked with MetS (OR = 1.35, 95% CI 1.06–1.69), elevated WC (OR = 1.38, 95% CI 1.12–1.70), elevated triglycerides (OR = 1.38, 95% CI 1.11–1.70), and elevated blood pressure (OR: 1.98, 95% CI 1.57–2.48). Table S2A and B and Fig. 4A–F show the projected weights of heavy metals for each qgcomp index and the joint effect of mixed three heavy metals on MetS. Serum Hg was the highest positive weight in almost all models, including MetS, elevated WC, elevated triglycerides, elevated blood pressure, and elevated fasting glucose. Following that, serum Cd was given a moderate positive weight, whereas serum Pb was given the lowest.
Gqcomp model regression index weights and Joint effect (95% CI) of the mixture on for MetS (A), elevated WC (B), elevated triglycerides (C), reduced HDL-C (D), elevated blood pressure (E), elevated fasting glucose (F), and MetS components (G). Models were adjusted for Models were adjusted for sex, age group, BMI group, occupation, family history of hyperlipidemia, family history of CVD, family history of diabetes, physical activity, drinking status, residential areas, smoking, educational level, monthly household income, energy intake, and ln2-cotinine. ln2Cd, ln2Hg, ln2Pb ln2-transformed levels of cadmium, mercury, and lead
We considered MetS as a continuous outcome and fitted a qgcomp model to assess the effects of mixed three heavy metals on MetS (Table 5B). Expectedly, after adjusting for all covariates, a quartile increase in the qgcomp index was linked with a 0.10-unit increase in MetS (95% CI 0.04–0.15). Table S2B and Fig. 4G show the weights of each heavy metal and the joint effect of three heavy metals on MetS. Serum Hg was found to be the most positively weighted (weighted at 0.768).
The Link Between Heavy Metals and Metabolic Syndrome and Its Components Using the BKMR Model
We employed the BKMR method to identify the impacts of mixed three heavy metals further, taking into consideration the constraints of linearity and interactions in the prior methods. The PIPs derived from the BKMR model for three heavy metals are summarized in Table S3. As a variable significance metric, PIPs were used as a higher value (closer to 1), indicating more relevance. In the current investigation, serum Hg PIPs were shown to be higher than other heavy metals in all models except for the HDL-C model.
The overall links between the mixed three heavy metals, MetS, and its components are described in Fig. 5A–G. MetS (including categorical and continuous variables), elevated WC, elevated triglycerides, and elevated blood pressure increased significantly when mixed heavy metals were at or above the 60th percentile versus the 50th percentile, implying substantial, positive links with MetS and its components. Despite the fact that there were no statistically significant differences between the elevated fasting glucose and reduced HDL-C models, there was an increased tendency.
Cumulative effect (95% CI) of the heavy metal exposure on MetS (A), elevated WC (B), elevated triglycerides (C), reduced HDL-C (D), elevated blood pressure (E), elevated fasting glucose (F), and MetS components (G) when all the heavy metals at particular percentiles were compared to all the heavy metals at their 50th percentile. The results were analyzed by the BKMR model, adjusted for sex, age group, BMI group, occupation, family history of hyperlipidemia, family history of CVD, family history of diabetes, physical activity, drinking status, residential areas, smoking, educational level, monthly household income, energy intake, and ln2-cotinine. ln2Cd, ln2Hg, ln2Pb ln2-transformed levels of cadmium, mercury, and lead
On the other hand, we explored the univariate (individually heavy metal) exposure–response functions of exposure to heavy metal on MetS and its components (Fig. 6A–D). When three heavy metals were at their median levels, serum Cd, Hg, and Pb showed increasing links with MetS and its components at the highest levels. We found that three heavy metals had a positive association with MetS and its components.
Univariate exposure–response function (95% CI) between heavy metal exposure and MetS (A), elevated WC (B), elevated triglycerides (C), reduced HDL-C (D), elevated blood pressure (E), elevated fasting glucose (F), and MetS components (G) while fixing the levels of other heavy metal at median values. The results were analyzed by the BKMR model adjusted for sex, age group, BMI group, occupation, family history of hyperlipidemia, family history of CVD, family history of diabetes, physical activity, drinking status, residential areas, smoking, educational level, monthly household income, energy intake, and ln2-cotinine. ln2Cd, ln2Hg, ln2Pb ln2-transformed levels of cadmium, mercury, and lead
We also examined how heavy metal genres interact with others. We estimated the exposure–response function of a unique heavy metal (serum Cd) for the second heavy metal fixed at its tenth, fifty-fifth, and ninetieth percentages, respectively. Furthermore, we predicted probable 3-way interactions by displaying the bivariate exposure–response function with a third exposure fixed at different quantiles (Fig. 7A–G). According to our findings, three investigated heavy metals (serum Cd, Hg, and Pb) all have the potential to interact. At different quantiles of another heavy metal, the slopes of one heavy metal’s exposure–response function were elevated or declined, showing interactions. The scale shows the level of interaction in 3-way interaction models, with higher values suggesting greater interaction.
Bi-variate exposure–response functions of three heavy metal mixtures in MetS (A), elevated WC (B), elevated triglycerides (C), reduced HDL-C (D), elevated blood pressure (E), elevated fasting glucose (F), and MetS components (G), when bivariate intake–response functions for each of the exposure1 heavy metals when exposure2 heavy metals were at their 10%, 50%, and 90% levels, and other nutrients were fixed at their median levels. The results were examined by the BKMR model, Models were adjusted for sex, age group, BMI group, occupation, family history of hyperlipidemia, family history of CVD, family history of diabetes, physical activity, drinking status, residential areas, smoking, educational level, monthly household income, energy intake, and ln2-cotinine. “est” can be understood as the association between nutrient intakes, MetS and MetS components. ln2Cd, ln2Hg, ln2Pb ln2-transformed levels of cadmium, mercury, and lead
Genes, miRNAs, Pathways, and Biological Process
Through the CTD data mining analysis, we explored the genes associated with each heavy metal and the development of MetS (Table 6). For each metal, gene sets were selected directly from the “disease” CTD data-tabs. Following that, the MyVenn CTD tool was used to identify shared genes in the mixed heavy metals. We observed Cd, Hg, and Pb altered 13, 09, and 4 genes involved in MetS development. Mixed heavy metals interacted with four genes (CRP, IL6, PON1, TRIB3) and were found to be associated with MetS. Physical interactions (77.6% of interactions) and co-expression (8.0%) were discovered to be the most common interactions among MetS-related genes, while predicted (5.3%), co-localization (3.6%), genetic interactions (2.8%), pathway (1.8%), and shared protein domains were found to be less common (Fig. 8). Further steps of our study were targeted at identifying the molecular functions, cellular components, molecular pathways, biological processes, and diseases associated with the genes altered by mixed heavy metals in order to investigate the biological importance of the examined genes. Genes associated to mixed heavy metals and MetS can be classified into four molecular pathways: the IL6 signaling pathway, IL6-mediated signaling events, insulin resistance, and adipogenesis. Our gene ontology analysis highlighted the most essential biological processes influenced by mixed heavy metals and linked to the development of MetS. The most critical processes contributing to MetS were classified as regulation of lipid localization, negative regulation of lipid storage, acute-phase response, lipoprotein particle binding, protein-lipid complex binding, and high-density lipoprotein particle. The most common diseases associated with mixed heavy metals were metabolic syndrome X, carotid atherosclerosis, hyperhomocysteinemia, and acute coronary syndrome.
Generated network of overlapping genes induced by mixed heavy metals (Pb, Hg, and Cd), along with four metabolic syndrome-related genes. GeneMANIA (http://geneMANIA.org/plug-in/) was used to create the network. Physical interactions (two genes are connected if they are observed to interact in a protein–protein interaction research); Co-expression (two genes are related if the levels of their expression are similar across situations in gene expression research); Colocalization (proteins observed in a similar location or genes expressed in the similar tissue); Genetic Interactions (two genes are functionally related if the effects of perturbing one gene were observed to be altered by perturbations to another gene); Pathway (two genes are connected if they contribute to the similar reaction within a pathway); Shared protein domains (protein domain data)
In terms of miRNAs, we used MIENTURNET’s network analysis to assess the relationships between target genes and miRNAs. The most significant genes related to mixed heavy metals-induced MetS development (e.g., IL6, TRIB3, and PON1) were target genes specifically linked with miRNAs. We found that 91 miRNAs matched 03 genes (IL6, TRIB3, and PON1) related to MetS development caused by mixed heavy metals. hsa-miR-124-3p were the miRNAs with the highest expression and interaction, respectively. A network of the miRNA–target interactions was constructed from the 19 selected miRNAs that were linked with three genes induced by mixed heavy metals. Furthermore, the functional enrichment analysis of the Wiki pathways and disease ontology in which their targets were linked was provided. Among the most enriched pathways in which the targets of these 19 miRNAs were involved, we found insulin pathway, transcription factor regulation in adipogenesis, adipogenesis, and cytokines and inflammatory response in the KEGG pathway enrichment analysis, and the Disease Ontology database observed atherosclerosis, arteriosclerotic cardiovascular disease, and arteriosclerosis, which were related to MetS (Fig. 9A–F). On the other hand, we observed that several transcription factors (SNAI1, CEBPG, NR1H4, DDIT3, CREB3L3, and MLXIPL) regulated MetS-related genes (CRP, IL6, PON1, and TRIB3) (Fig. 9G).
miRNA–target interaction network for miRNAs derived from the list of genes linked with metabolic syndrome induced by the mixture of three heavy metals (CTD Database (http://CTD.mdibl.org). The bar plot represents each gene resulting in the enrichment along with the number of its miRNA (A–C). An analysis of network and Wiki pathway enrichment shows that the network provides the miRNA–target interactions retrieved from MIENTURNET (D). Orange dots represent miRNA targets, blue dots represent target genes. The main enrichment results for the targets of the miRNAs appearing in the network are shown as dot plots, with the Y-axis reporting the annotation categories [i.e., Wiki pathways (E) and disease ontology (F)] and the X-axis reporting the miRNAs, with the number of recognized targets (i.e., targets with at least one annotation) in round brackets. The colored dots represent adjusted p values, and the size of the dots represents gene ratios (i.e., the number of miRNA targets observed annotated in each category over the total number of recognized targets indicated in round brackets). The transcription factor-gene regulatory networks related to metabolic syndrome induced by mixed heavy metals were assessed using CHEA3. The transcription factor is denoted in bright blue, whereas the gene target is presented in pink (G). Venn diagram and prediction of miRNA Sponges. Venn diagram for the differentially expressed miRNAs induced by studied heavy metals prediction of miRNA Sponges (H). Results of generating and testing a miRNA sponge sequence for one selected miRNA using miRNAsong (I). FDR false discovery rate
A Venn diagram analysis revealed that the heavy metals investigated induced miRNA expression. Three miRNAs were observed in a mixture of three heavy metals, including hsa-miR-124-3p, hsa-miR-1273 g-3p, and hsa-miR-335-5p (Fig. 9H and Table S4). As previously stated, the miRNA with the highest expression and interaction was hsa-miR-124-3p, so we used miRNAsong to create and test a miRNA sponge sequence for this miRNA (Fig. 9I). The sponge sequence contained two multiple miRNA-binding sites; a bulge at approximately nucleotide position 22–28; and an AUGG spacer sequence between specific multiple miRNA-binding sites. The generated sequence binds miRNA with a free energy of duplex of – 89.4 kcal/mol in in-silico tests for off-targets (settings: – 25 kcal/mol cutoff and canonical 6-mer seed). Furthermore, 34 miRNAs can interact with this sponge in Homo sapiens at a cutoff of – 25 kcal/mol and the seed region features: 6-mer seed (2–7) (Table S5).
Discussion
The effects of heavy metal mixtures on MetS and its components in Korean adults aged ≥ 18 years were investigated using four distinct statistical models. On the one hand, generalized linear regression demonstrated that serum Hg was the strongest predictor of MetS and its components (elevated WC, reduced HDL-C, elevated blood pressure, and elevated fasting glucose). Serum Cd, Hg, and Pb levels were found to interact with MetS and elevated triglycerides. On the other hand, MetS and its components were also shown to be affected by mixed heavy metals, especially serum Hg, in the WQS and qgcomp models. In the BKMR model, the univariate exposure–response function demonstrated a positive association between MetS and its components and serum levels of three investigated heavy metals. Furthermore, MetS and its components were shown to be significantly linked to overall mixed exposure. There was no statistically significant link between overall mixed exposure and elevated triglycerides, reduced HDL-C, and higher fasting glucose, but there was a growing trend. Different statistical approaches reported the mixed effects of heavy metals on MetS and its components in a comparable way. Our findings suggest that long-term exposure to heavy metals, particularly Hg, may play a key role in the development of MetS.
In the current work, we used an in-silico toxicogenomic data mining approach to explore the key molecular pathways and biological processes of MetS associated with heavy metals and their mixes, as well as their relationship to the development of MetS. The IL6 signaling pathway, IL6-mediated signaling events, insulin pathway, transcription factor regulation in adipogenesis, adipogenesis and cytokines, and the inflammatory response pathway have all been identified as key pathways that may be affected by heavy metals and involved in the development of MetS.
We identified multiple genes and miRNAs with high expression and interaction induced by the heavy metals investigated, including CRP, IL6, PON1, and hsa-miR-124-3p, all of which are implicated in the MetS process. We also used miRNAsong to create and test a miRNA sponge sequence for these miRNAs. Because miRNA sponges have the characteristic of inhibiting all seed family members, and when more miRNA-binding sites are added, which can be used to suppress a full miRNA cluster, they may be promising for being used in MetS therapy.
Due to the potential negative effects of heavy metals on human health, exposure to heavy metals has become a global public health hazard in recent decades (WHO 2017). Remarkably, human exposure to heavy metals has increased as a result of worldwide urbanization and industrialization (Wang et al. 2018a, b; Duc et al. 2021a, b, c). In our analysis, males had considerably greater Hg and Pb levels than females in our analysis, but not Cd. These findings concur with the previous studies (You et al. 2011; Cho et al. 2014; Eom et al. 2014; Kim et al. 2014; Duc et al. 2021a, b, c). It has been known that Hg and Pb levels have been shown to be strongly linked with smoking status and alcohol consumption (Duc et al. 2021a, b, c; Nguyen and Kim 2021). It suggests there were significant gender variations in alcohol use and smoking status.
In the present study, we observed that a mixture of heavy metals, particularly serum Hg, was linked to the risk of MetS in Korean adults. A recent study found that a combination of heavy metals such as Cd, Hg, and Pb may contribute to the risk of obesity, hypertension, and diabetes development (Wang et al. 2018). Heavy metals are known causative factors in a variety of diseases, including CVDs, and numerous mechanisms have been proposed to explain their association with MetS (Duc et al. 2021a, b, c; Nguyen and Kim 2021). First, heavy metals (Cd, Hg, and Pb) can cause inflammatory cytokines and the production of antithrombotic substances as well as destroy blood clots (Angeli et al. 2013; Arbi et al. 2017). Second, they also increase the levels of reactive nitrogen and oxygen species, causing oxidative stress, which can damage DNA and oxidize protein thiol groups (Jomova and Valko 2011). Third, Cd, Hg, and Pb can have an effect on lipid metabolism, especially total cholesterol and LDL-C (Nguyen et al. 2021a, b, c, d). More specifically, Hg, Cd, and Pb have been shown to play a role in the development of MetS by causing adipose tissue endocrine dysfunction, as well as glucose metabolism and lipid dysregulation (Iavicoli et al. 2009; Chang et al. 2011; Regnier and Sargis 2014). Cd, Hg, and Pb can induce damage to vascular endothelial cells and worsen hypertension by catalyzing the production of antithrombotic and inflammatory mediators (Yamamoto et al. 1993; Angeli et al. 2013; Nguyen et al. 2021a, b, c, d). Hg can affect nitric oxide bioavailability, activation of antioxidant defenses, and endothelial function (Houston 2011). Hg has been linked to an increase in serum oxidized LDL, lipid peroxidation, and LDL oxidation. These activities make LDL metabolism harder, resulting in its aggregation (Nguyen et al. 2021a, b, c, d ). As a result, Hg has been shown to increase the progression of carotid atherosclerosis (Salonen et al. 2000; Arbi et al. 2017). Through the oxidative stress cascade, Hg can also impact the function and survival of islet b-cells (Chen et al. 2009), whereas, Cd can deplete glutathione and protein-bound sulfhydryl groups, increasing the production of reactive oxygen species like superoxide ions, hydrogen peroxide, and hydroxyl radicals. These ROS are known to increase urinary lipid metabolite excretion and lipid peroxidation. On the other hand, Pb-mediated hypercholesterolemia has been linked to the inhibition of cholesterol synthesis enzymes (e.g., 3-hydroxyl-3-methylglutaryl-CoA reductase, farnesyl diphosphate synthase, squalene synthase) and catabolic enzymes (e.g., 7 alpha-hydroxylase) (Nguyen, et al. 2021a, b, c, d). Pb may also increase the hepatic gene expression of lanosterol 14-demethylase (CYP51), a cytochrome P450 isoform, resulting in an increase in cellular and total cholesterol levels (Kojima et al. 2002). These effects are known to play an important role in the pathology of MetS development.
To our knowledge, only a small amount of research has looked at the link between mixed chemical exposure and MetS and its components, and there is only a small amount of evidence that describes cutoff values for clinically significant exposure levels. When compared to prior studies of adults in Canada, Germany, and the United States, Korean adults showed higher chemical exposures (including Cd, Hg, and Pb) (Becker et al. 2002; Canada 2010; Zhang et al. 2019; Duc et al. 2021a, b, c; Nguyen and Kim 2021; Nguyen et al. 2021a, b, c, d). The disparities in studied heavy metal exposure levels could be explained by significant heterogeneity across the reported study samples, such as diet, residential location (urban, rural, or industrial), and the primary source of exposure (drinking water and food, etc.). Furthermore, cross-study comparisons are difficult due to a lack of criteria for acceptable exposure levels based on serum or urine chemical levels. Lanphear et al. observed that even at blood Pb levels of less than 10 µg/dL, it can cause cognitive deficits in children and adolescents in the United States (Lanphear et al. 2000). The effects of heavy metals on human health are undeniable. Thus, it is necessary to estimate the cutoff thresholds for exposure levels that are clinically relevant. In the current study, we estimated the cutoff thresholds for exposure levels that are relevant to MetS and its components (Table 7).
Our in-silico investigation has found that CRP, IL6, PON1, and TRIB3 were common genes for MetS development induced by a mixture of three heavy metals. The IL6 signaling pathway and IL6-mediated signaling events, insulin resistance, and adipogenesis are four biochemical pathways that are linked to combined heavy metals and MetS. The most important biological processes impacted by combined heavy metals and connected to the development of MetS were “control of lipid localization,” “negative regulation of lipid storage,” and “acute-phase response,” “lipoprotein particle binding,” “protein-lipid complex binding,” and “high-density lipoprotein particle.” Furthermore, metabolic syndrome “X,” “carotid atherosclerosis,” “hyperhomocysteinemia,” and acute coronary syndrome were among the most common disorders related to heavy metals combined. Several studies supported our findings of the link between these genes and MetS development (Choi et al. 2004; Nishida et al. 2007; Martín-Cordero et al. 2011; Todendi et al. 2015; Nguyen et al. 2021a, b, c, d). For example, a cross-sectional study showed a significant link between IL6 and CRP gene polymorphisms and metabolic disorders in children and adolescents (Todendi et al. 2015). PON1 levels and activity are significantly lower in people with cardiovascular and hepatic disorders, as well as diabetes and obesity (Meneses et al. 2019). A case–control study of Chinese adults aged 30–77 years observed that in those with metabolic syndrome, a decrease in serum obestatin caused by the TRIB3 Q84R polymorphism exacerbates carotid atherosclerosis (Cui et al. 2012).
In terms of miRNAs, the most significant genes related to mixed heavy metals-induced MetS development (e.g., IL6, TRIB3, and PON1) were target genes specifically linked with miRNAs. We found several pathways (insulin pathway, transcription factor regulation in adipogenesis, adipogenesis, and cytokines and inflammatory response), and diseases (“atherosclerosis,” “arteriosclerotic cardiovascular disease,” and “arteriosclerosis”), which were related to MetS. We also observed hsa-miR-124-3p were the miRNAs with the highest expression and interaction related to MetS development. These findings were supported by previous studies. For instance, an in vitro study reported that miR-124 can repress genes linked with triglyceride and fatty acid breakdown as well as promote the accumulation of triglycerides in hepatoma cells (Shaw et al. 2018). Other in vitro also found miR1243p expression levels were considerably higher than in the control groups in the hindlimb ischemia model’s ischemic tissue and hypoxic human umbilical vein endothelial cells, implying miR‑124‑3p was a crucial regulator of angiogenesis in peripheral arterial disease (Shi et al. 2020). In a cross-sectional study of 12 Chinese type 2 diabetes patients aged ≤ 65 years, it was observed that hsa-miR-124-3p expression levels were downregulated, suggesting that this miRNA can contribute to the pathology of diabetes (Zhu et al. 2017). Three of the most common approaches for miRNA loss-of-function research are genetic knockouts, antisense oligonucleotide inhibitors, and sponges. The “sponge” method for inducing continuous miRNA loss of function in cell lines and transgenic animals was first described. Thus, we constructed and in-silico analyzed a miRNA sponge sequence for this miRNA that was the most frequent miRNA with high expression and interaction with studied heavy metals, as well as connected to MetS development, utilizing miRNAsong. On the other hand, we also observed six transcription factors (SNAI1, CEBPG, NR1H4, DDIT3, CREB3L3, and MLXIPL) that regulated four genes related to MetS induced by mixed heavy metals. These transcription factors were found to be related to MetS and its components in the previous studies (Pan et al. 2009; Yang et al. 2012; Delgado-Lista et al. 2013; Heni et al. 2013; McCann et al. 2021; Yong et al. 2021). These findings could be useful in future studies looking at the effects of heavy metals, miRNAs, transcription factors, and MetS development.
To our knowledge, this is the first large-scale investigation in Korea to report the combined effects of heavy metals on MetS and its components in individuals aged 18 years or older. Secondary analyses using three innovative mixture modeling approaches (WQS, qpcomp, and BKMR) found that our findings were mainly robust. This study, however, has several limitations. First, the cross-sectional approach was unable to determine whether heavy metals and MetS are causally linked. Second, heavy metal exposure was determined by taking a single serum sample. Third, MetS is a chronic condition. The assessments may not have adequately reflected chronic exposure circumstances because serum samples of heavy metals were used to determine heavy metal exposure levels. Fourth, although three common heavy metals were considered in this study, other chemicals (e.g., perfluorooctanoic acid) were not assessed.
Fifth, the miRNA sponges created in this study may not be suitable for a variety of models in practice, so these results should be viewed primarily as a precursor to more in-depth in-vitro and in-vivo laboratory testing.
Conclusion
MetS, and its components, were significantly associated with the combined effect of three heavy metals. Along with elevated waist circumference and blood pressure, serum Hg showed positive trends and was identified as the most important factor associated with MetS. In-silico toxicogenomic data mining revealed that mixed heavy metals interacted with four genes (CRP, IL6, PON1, and TRIB3) and were associated with MetS. Physical interactions were found to be the most common (77.6% of interactions) among MetS-related genes. Several pathways (for example, insulin pathway, transcription factor regulation in adipogenesis, adipogenesis, and cytokines and inflammatory response), regulation of lipid localization, and metabolic syndrome X were identified as key molecular mechanisms that may be affected by heavy metals and involved in the development of MetS. SNAI1, CEBPG, NR1H4, DDIT3, CREB3L3, and MLXIPL were key transcription factors related to pathogenesis of MetS induced by mixed heavy metals. The highest interaction and expression implicated in the MetS process was identified as hsa-miR-124-3p. In particular, the cutoff levels for exposure levels related to MetS and its components were also described.
Data Availability
Data from KNHANES is available online at https://knhanes.kdca.go.kr/knhanes/main.do.
References
Aelion CM, Davis HT, Lawson AB, Cai B, McDermott S (2012) Associations of estimated residential soil arsenic and lead concentrations and community-level environmental measures with mother–child health conditions in South Carolina. Health Place 18:774–781
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J Chem. https://doi.org/10.1155/2019/6730305
Angeli JK, Pereira CAC, de Oliveira FT, Stefanon I, Padilha AS, Vassallo DV (2013) Cadmium exposure induces vascular injury due to endothelial oxidative stress: the role of local angiotensin II and COX-2. Free Radic Biol Med 65:838–848
Arbi S, Oberholzer HM, Van Rooy MJ, Venter C, Bester MJ (2017) Effects of chronic exposure to mercury and cadmium alone and in combination on the coagulation system of Sprague-Dawley rats. Ultrastruct Pathol 41:275–283
Becker K, Kaus S, Krause C, Lepom P, Schulz C, Seiwert M, Seifert B (2002) German Environmental Survey 1998 (GerES III): environmental pollutants in blood of the German population. Int J Hyg Environ Health 205:297–308
Beck-Nielsen H (2013) The metabolic syndrome. Springer, Wien
Çamur D, Güler Ç, Vaizoğlu SA, Özdilek B (2016) Determining mercury levels in anchovy and in individuals with different fish consumption habits, together with their neurological effects. Toxicol Ind Health 32:1215–1223
Canada H (2010) Report on human biomonitoring of environmental chemicals in Canada. Results of the Canadian Health Measures Survey Cycle 1 (2007–2009). Book Report on human biomonitoring of environmental chemicals in Canada. Results of the Canadian Health Measures Survey Cycle 1 (2007–2009). Health Canada Ottawa, Ontario
Chang J-W, Chen H-L, Su H-J, Liao P-C, Guo H-R, Lee C-C (2011) Simultaneous exposure of non-diabetics to high levels of dioxins and mercury increases their risk of insulin resistance. J Hazard Mater 185:749–755
Chen YW, Yang CY, Huang CF, Hung DZ, Leung YM, Liu SH (2009) Heavy metals, islet function and diabetes development. Islets 1:169–176
Cho S, Jacobs DR, Park K (2014) Population correlates of circulating mercury levels in Korean adults: the Korea National Health and Nutrition Examination Survey IV. BMC Public Health 14:1–10
Choi KM, Lee J, Lee KW, Seo JA, Oh JH, Kim SG, Kim NH, Choi DS, Baik SH (2004) Comparison of serum concentrations of C-reactive protein, TNF-alpha, and interleukin 6 between elderly Korean women with normal and impaired glucose tolerance. Diabetes Res Clin Pract 64:99–106
Cui A-d, Gai N-n, Zhang X-h, Jia K-z, Yang Y-l, Song Z-j (2012) Decreased serum obestatin consequent upon TRIB3 Q84R polymorphism exacerbates carotid atherosclerosis in subjects with metabolic syndrome. Diabetol Metab Syndr 4:52
Delgado-Lista J, Perez-Martinez P, Garcia-Rios A, Phillips CM, Hall W, Gjelstad IMF, Lairon D, Saris W, Kieć-Wilk B, Karlström B et al (2013) A gene variation (rs12691) in the CCAT/enhancer binding protein α modulates glucose metabolism in metabolic syndrome. Nutr Metab Cardiovasc Dis 23:417–423
Duc HN, Oh H, Kim M-S (2021a) The effect of mixture of heavy metals on obesity in individuals ≥50 years of age. Biol Trace Elem Res. https://doi.org/10.1007/s12011-021-02972-z
Duc HN, Oh H, Kim MS (2021b) Effects of antioxidant vitamins, curry consumption, and heavy metal levels on metabolic syndrome with comorbidities: a Korean community-based cross-sectional study. Antioxidants (basel) 10(5):8
Duc HN, Oh H, Yoon IM, Kim M-S (2021c) Association between levels of thiamine intake, diabetes, cardiovascular diseases and depression in Korea: a national cross-sectional study. J Nutr Sci 10:e31
Duc Nguyen H, Hee Jo W, Hong Minh Hoang N, Kim M-S (2022a) Anti-inflammatory effects of B vitamins protect against tau hyperphosphorylation and cognitive impairment induced by 1,2 diacetyl benzene: an in vitro and in silico study. Int Immunopharmacol 108:108736
Duc Nguyen H, Oh H, Kim M-S (2022b) Association between exposure to chemical mixtures in relation to serum total IgE among adults 19–86 years old. Int Immunopharmacol 102:108428
Eom S-Y, Choi S-H, Ahn S-J, Kim D-K, Kim D-W, Lim J-A, Choi B-S, Shin H-J, Yun S-W, Yoon H-J (2014) Reference levels of blood mercury and association with metabolic syndrome in Korean adults. Int Arch Occup Environ Health 87:501–513
Garí M, Grimalt JO, Torrent M, Sunyer J (2013) Influence of socio-demographic and diet determinants on the levels of mercury in preschool children from a Mediterranean island. Environ Pollut 182:291–298
Heni M, Wagner R, Ketterer C, Böhm A, Linder K, Machicao F, Machann J, Schick F, Hennige AM, Stefan N et al (2013) Genetic variation in NR1H4 encoding the bile acid receptor FXR determines fasting glucose and free fatty acid levels in humans. J Clin Endocrinol Metab 98:E1224–E1229
Houston MC (2011) Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J Clin Hypertens 13:621–627
Hrubá F, Strömberg U, Černá M, Chen C, Harari F, Harari R, Horvat M, Koppová K, Kos A, Krsková A (2012) Blood cadmium, mercury, and lead in children: an international comparison of cities in six European countries, and China, Ecuador, and Morocco. Environ Int 41:29–34
Iavicoli I, Fontana L, Bergamaschi A (2009) The effects of metals as endocrine disruptors. J Toxicol Environ Health B 12:206–223
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283:65–87
Kim Y-N, Kim YA, Yang A-R, Lee B-H (2014) Relationship between blood mercury level and risk of cardiovascular diseases: results from the fourth Korea National Health and Nutrition Examination Survey (KNHANES IV) 2008–2009. Prev Nutri Food Sci 19:333
Kojima M, Nemoto K, Murai U, Yoshimura N, Ayabe Y, Degawa M (2002) Altered gene expression of hepatic lanosterol 14alpha-demethylase (CYP51) in lead nitrate-treated rats. Arch Toxicol 76:398–403
Lanphear BP, Dietrich K, Auinger P, Cox C (2000) Cognitive deficits associated with blood lead concentrations <10 microg/dL in US children and adolescents. Public Health Rep 115:521–529
Licursi V, Conte F, Fiscon G, Paci P (2019) MIENTURNET: an interactive web tool for microRNA-target enrichment and network-based analysis. BMC Bioinform 20:545
Lopomo A, Burgio E, Migliore L (2016) Epigenetics of obesity. Prog Mol Biol Transl Sci 140:151–184
Martín-Cordero L, García JJ, Hinchado MD, Ortega E (2011) The interleukin-6 and noradrenaline mediated inflammation-stress feedback mechanism is dysregulated in metabolic syndrome: effect of exercise. Cardiovasc Diabetol 10:42
McCann MA, Li Y, Muñoz M, Gil V, Qiang G, Cordoba-Chacon J, Blüher M, Duncan S, Liew CW (2021) Adipose expression of CREB3L3 modulates body weight during obesity. Sci Rep 11:19400
Meneses MJ, Silvestre R, Sousa-Lima I, Macedo MP (2019) Paraoxonase-1 as a regulator of glucose and lipid homeostasis: impact on the onset and progression of metabolic disorders. Int J Mol Sci 20:4049
Nguyen HD, Kim M-S (2021) Effects of heavy metal, vitamin, and curry consumption on metabolic syndrome during menopause: a Korean community-based cross-sectional study. J Menopause 28:1
Nguyen HD, Kim M-S (2022a) Effects of heavy metals on cardiovascular diseases in pre and post-menopausal women: from big data to molecular mechanism involved. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-21208-8
Nguyen HD, Kim M-S (2022b) Exposure to a mixture of heavy metals induces cognitive impairment: genes and microRNAs involved. Toxicology. https://doi.org/10.1016/j.tox.2022.153164
Nguyen HD, Kim M-S (2022c) The protective effects of curcumin on metabolic syndrome and its components: in-silico analysis for genes, transcription factors, and microRNAs involved. Arch Biochem Biophys. https://doi.org/10.1016/j.abb.2022.109326
Nguyen HD, Kim M-S (2022d) Mixtures modeling identifies vitamin B1 and B3 intakes associated with depression. J Affect Disord. https://doi.org/10.1016/j.jad.2021.12.133
Nguyen HD, Oh H, Hoang NHM, Jo WH, Kim MS (2021a) Environmental science and pollution research role of heavy metal concentrations and vitamin intake from food in depression: a national cross-sectional study (2009–2017). Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-021-15986-w
Nguyen HD, Oh H, Hoang NHM, Kim M-S (2021b) Association between heavy metals, high-sensitivity C-reaction protein and 10-year risk of cardiovascular diseases among adult Korean population. Sci Rep 11:14664
Nguyen HD, Oh H, Jo WH, Hoang NHM, Kim MS (2021c) Mixtures modeling identifies heavy metals and pyrethroid insecticide metabolites associated with obesity. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-021-16936-2
Nguyen HD, Oh H, Kim MS (2021d) Effects of heavy metals on hypertension during menopause: a Korean community-based cross-sectional study. Menopause 28(12):1400–1409
Nguyen HD, Oh H, Kim M-S (2022) The effects of chemical mixtures on lipid profiles in the Korean adult population: threshold and molecular mechanisms for dyslipidemia involved. Environ Sci Pollut Res 29(26):39182–39208
Nishida M, Moriyama T, Ishii K, Takashima S, Yoshizaki K, Sugita Y, Yamauchi-Takihara K (2007) Effects of IL-6, adiponectin, CRP and metabolic syndrome on subclinical atherosclerosis. Clin Chim Acta 384:99–104
Pan LA, Chen YC, Huang H, Zhang L, Liu R, Li X, Qiang O, Zeng Z (2009) G771C polymorphism in the MLXIPL gene is associated with a risk of coronary artery disease in the Chinese: a case-control study. Cardiology 114:174–178
Park SS, Skaar DA, Jirtle RL, Hoyo C (2017) Epigenetics, obesity and early-life cadmium or lead exposure. Epigenomics 9:57–75
Poursafa P, Ataee E, Motlagh ME, Ardalan G, Tajadini MH, Yazdi M, Kelishadi R (2014) Association of serum lead and mercury level with cardiometabolic risk factors and liver enzymes in a nationally representative sample of adolescents: the CASPIAN-III study. Environ Sci Pollut Res 21:13496–13502
Regnier SM, Sargis RM (2014) Adipocytes under assault: environmental disruption of adipose physiology. Biochim Biophys Acta 1842:520–533
Rothenberg SE, Korrick SA, Fayad R (2015) The influence of obesity on blood mercury levels for US non-pregnant adults and children: NHANES 2007–2010. Environ Res 138:173–180
Salonen JT, Seppänen K, Lakka TA, Salonen R, Kaplan GA (2000) Mercury accumulation and accelerated progression of carotid atherosclerosis: a population-based prospective 4-year follow-up study in men in eastern Finland. Atherosclerosis 148:265–273
Satarug S, Vesey DA, Gobe GC (2017) Current health risk assessment practice for dietary cadmium: data from different countries. Food Chem Toxicol 106:430–445
Shaw TA, Singaravelu R, Powdrill MH, Nhan J, Ahmed N, Özcelik D, Pezacki JP (2018) MicroRNA-124 regulates fatty acid and triglyceride homeostasis. iScience 10:149–157
Shi Y, Xu X, Luan P, Kou W, Li M, Yu Q, Zhuang J, Xu Y, Peng W, Jian W (2020) miR-124-3p regulates angiogenesis in peripheral arterial disease by targeting STAT3. Mol Med Rep 22:4890–4898
Tinkov AA, Filippini T, Ajsuvakova OP, Aaseth J, Gluhcheva YG, Ivanova JM, Bjørklund G, Skalnaya MG, Gatiatulina ER, Popova EV (2017) The role of cadmium in obesity and diabetes. Sci Total Environ 601:741–755
Todendi PF, Klinger EI, Ferreira MB, Reuter CP, Burgos MS, Possuelo LG, Valim AR (2015) Association of IL-6 and CRP gene polymorphisms with obesity and metabolic disorders in children and adolescents. An Acad Bras Cienc 87:915–924
Valera B, Muckle G, Poirier P, Jacobson SW, Jacobson JL, Dewailly E (2012) Cardiac autonomic activity and blood pressure among Inuit children exposed to mercury. Neurotoxicology 33:1067–1074
Wang M, Liu R, Chen W, Peng C, Markert B (2018a) Effects of urbanization on heavy metal accumulation in surface soils, Beijing. J Environ Sci 64:328–334
Wang X, Mukherjee B, Park SK (2018b) Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003–2014. Environ Int 121:683–694
Ministry-of-Health-and-Welfare. (2021) Korea National Health & Nutrition Examination Survey. Book Korea National Health & Nutrition Examination Survey
WHO J (2017) Preventing noncommunicable diseases (NCDs) by reducing environmental risk factors. Book Preventing noncommunicable diseases (NCDs) by reducing environmental risk factors World Health Organization, Geneva (WHO/FWC/EPE/17.1)
Wolkin A, Hunt D, Martin C, Caldwell KL, McGeehin MA (2012) Blood mercury levels among fish consumers residing in areas with high environmental burden. Chemosphere 86:967–971
Xu P, Liu A, Li F, Tinkov AA, Liu L, Zhou JC (2021) Associations between metabolic syndrome and four heavy metals: a systematic review and meta-analysis. Environ Pollut 273:116480
Yamamoto C, Kaji T, Sakamoto M, Kozuka H (1993) Cadmium stimulation of plasminogen activator inhibitor-1 release from human vascular endothelial cells in culture. Toxicology 83:215–223
Yang Z, Norwood KA, Smith JE, Kerl JG, Wood JR (2012) Genes involved in the immediate early response and epithelial-mesenchymal transition are regulated by adipocytokines in the female reproductive tract. Mol Reprod Dev 79:128–137
Yong J, Parekh VS, Reilly SM, Nayak J, Chen Z, Lebeaupin C, Jang I, Zhang J, Prakash TP, Sun H et al (2021) Chop/Ddit3 depletion in β cells alleviates ER stress and corrects hepatic steatosis in mice. Sci Transl Med 139(604):eaba9796
You C-H, Kim B-G, Kim J-M, Yu S-D, Kim Y-M, Kim R-B, Hong Y-S (2011) Relationship between blood mercury concentration and waist-to-hip ratio in elderly Korean individuals living in coastal areas. J Prev Med Public Health 44(5):218–225
Yun S, Nguyen HD, Park JS, Oh C, Kim MS (2021) The association between the metabolic syndrome and iron status in pre- and postmenopausal women: Korean National Health and Nutrition Examination Survey (KNHANES) in 2012. Br J Nutr. https://doi.org/10.1017/S0007114521001331
Zhang A, Hu H, Sánchez BN, Ettinger AS, Park SK, Cantonwine D, Schnaas L, Wright RO, Lamadrid-Figueroa H, Tellez-Rojo MM (2012) Association between prenatal lead exposure and blood pressure in children. Environ Health Perspect 120:445–450
Zhang Y, Dong T, Hu W, Wang X, Xu B, Lin Z, Hofer T, Stefanoff P, Chen Y, Wang X et al (2019) Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: comparison of three statistical models. Environ Int 123:325–336
Zhu Z, Yin J, Li DC, Mao ZQ (2017) Role of microRNAs in the treatment of type 2 diabetes mellitus with Roux-en-Y gastric bypass. Braz J Med Biol Res 50:e5817–e5917
Acknowledgements
The authors are grateful to all research staff for their excellent contributions in data collection in the survey.
Funding
This study supported by National Research Foundation of Korea (NRF) (Grant No. 2022R1A2C1005643).
Author information
Authors and Affiliations
Contributions
HDN: Conceptualization, methodology, formal analysis, investigation, resources, data curation, writing—original draft, writing—review & editing, visualization. MSK: visualization.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to Participate
Before investigations, all participants in KNHANES provided written informed consent, which was carried out by the Health and Nutrition Examination Department of the Korea Centers for Disease Control and Prevention. This study was approved by the KNHANES inquiry commission (IRB Approval numbers: 2009-01CON-03-2C, 2010-02CON-21-C, 2011-02CON-06-C, 2012-01EXP-01-2C, 2013-07CON-03-4C, 2013-12EXP-03-5C). From 2016 to 2017, KNHANES was exempt from review regarding research ethics under the Bioethics and Safety Act.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, H.D., Oh, H. & Kim, MS. Effect of Mixture of Heavy Metals on Metabolic Syndrome and Its Components in Individuals ≥ 18 Years of Age: From Big Data to Molecular Mechanisms Involved. Expo Health 15, 773–805 (2023). https://doi.org/10.1007/s12403-022-00523-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-022-00523-y