Abstract
Needleless electrospinning, an electrohydrodynamic process, is an emerging approach to producing nanofiber mats from an open liquid surface. Importantly, the approach offers 3–250 times higher production rates than needle-based electrospinning systems and has the potential to develop biocompatible and biodegradable nanofibers that have numerous applications in the food industry. The electrospinning potential of various biomaterials (from plant and animal sources) in needleless configurations is highlighted in this review. Also, the factors influencing the production rate and quality of needleless electrospun nanofibers are emphasized. Further, the reported uses of needleless electrospun nanofiber mats in food applications like packaging, filtration, bioactive encapsulation, enzyme immobilization, and food quality sensing are presented. Finally, challenges and areas to be explored further are summarized, considering prospects. Electrospun nanofibers are valued for their characteristics and unique capabilities. However, often, scale-up production is challenging, limiting its usage in multiple commercial applications. Overcoming this concern, needleless electrospinning is a viable approach for scaling up the production of nanofibers. Offering properties on par with conventional electrospinning, the needleless approach is finding expanding avenues in different sectors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanofibers are the non-woven filamentous mats produced at the nanoscale (below 100 nm) with unique functionality as a result of their higher surface area to volume ratios [1], and electrospinning is a popular approach for producing nanofibers [2]. Electrospinning is an electrohydrodynamic approach in which the electrostatic forces are used for the rapid (in milliseconds) production of nanofibers. The approach can be effectively used to make complex assemblies like porous fibers, core-shell fibers, nano-nets, and sandwiched membranes [3].
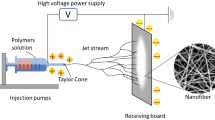
During electrospinning, the polymer solution is exposed to an external electric field and electrical stress. As a result of the liquid’s subsequent surface tension, the spinneret (spinning nozzle through which the polymer solution flows to form fibers) tip develops a cone-jet configuration. Small droplet atomization takes place when the electrical stress overcomes the surface tension of the liquid. Also, as the atomized droplets travel toward the collector, the solvent evaporates, resulting in the production of submicron-sized solid particles or fibers [4].
The scalability of the conventional needle electrospinning process is a concern, with a typical output of 0.1 to 5 ml/h from a single capillary and produced fibers with a mass flow of one order smaller. This is because of the rate of solvent evaporation from the polymer solution, resulting in fiber productivity of typically less than 0.3 g/h per needle [5]. The fiber orientation in needle electrospinning is also inhomogeneous and random [6]. Needle electrospinning can also have clogging issues, which reduces the diameter of the formed fibers [7]. The use of multiple jet spinnerets is one viable solution for increasing productivity. However, this method may also result in needle clogging due to the inhomogeneity in the applied electric field across the needles and repulsion among jets, in turn, reducing fiber quality and enhancing the complexity of cleaning the spinnerets. Thus, it is suggested to produce nanofibers with less reliance on the number of fluidic channels that can improve fiber productivity.
Given this, needleless electrospinning (NE) has the potential to produce nanofibers on a large scale in a compact space from an open liquid surface. The needleless fiber generator produces numerous jets at the same time, without the requirement of capillary flow, which is normally associated with needle-based systems. As an emerging concept for food-based applications, this review presents the concepts of NE and its role in the production of nanofibers at a higher production capacity with uniformly distributed fiber formation. The review also discusses the factors influencing nanofiber properties, food applications of nanofibers spun using needleless configurations, limitations in the challenges involved, and the future scope.
Principle of Needleless Electrospinning
NE, also known as free surface electrohydrodynamic jetting, is a self-organized process that produces nanofibers by triggering liquid polymer solutions by destabilizing the polymer droplets under the applied electrostatic forces (electrohydrodynamic destabilization). As a result, the stretching and thinning of immersed droplets to form jets. A cone structure (Taylor cone) is produced at the tip of the polymer solution, from which charged particle jets are initiated. Needle electrospinning can only form one Taylor cone per needle; whereas, NE can result in the formation of multiple Taylor cones (Fig. 1A). This increases the number of formed polymer jets, allowing scaled-up production of nanofibers [3]. In a typical NE system, nanofibers are generated in four steps:
-
1.
When the spinneret (Fig. 2) is partly immersed in the polymer solution and rotates, a thin layer of polymer solution is formed on the spinneret surface
-
2.
The rotation also results in the formation of initial perturbations in the solution layer. This leads to a localized increase in solution concentration to generate active sites. It further roots the growth of perturbation causing the formation of conical spikes on the solution surface
-
3.
Then, when high voltage is applied, the free surface of the polymer solution experiences instability in electric field distribution at irregular intervals, and the spikes concentrate electric charges. This causes the jets to turn transverse to the direction of the electric field and thus the jets are decelerated by the constantly increasing drag force of the gas which results in “Taylor cones” formation
-
4.
Finally, polymer jets are expanded out from the “Taylor cones”; a cloud of charges is expanded toward the collector during which solvent vaporization also takes place, solidifying the jet and the resultant fibrous cloud drifts onto the surface of collector (Fig. 3)
If the polymer concentration and molecular weight are lower, electrospraying takes place instead of spinning [8]. When the liquid possesses sufficient surface tension and relatively low viscosity, the efflux will disassemble downstream and subsequently split into numerous charged micro-droplets. The lack of sufficient molecular cohesion for the polymer mixtures at low concentrations results in numerous breaks and resultant droplet formation, which is defined as electrospraying [9]. As the solvent evaporates from the droplet, the droplet size decreases, causing an increase in charge density inside the droplet, resulting in the Coulombic explosion, which aids in the production of sprayed micro or nanoparticles. The electrospraying setup is placed inside a chamber and circulated with conditioned air to ensure the flow of nanoparticles toward the collector (Fig. 1B).
Needleless Electrospinning System Configurations
The efficiency of nanofiber formation in NE is strongly dependent on the stability of Taylor cones. Taylor cones often tend to move along the perimetric wall of the rotating roller to form a solution jet in the presence of a strong electric field. Thus, inter-molecular interactions between the polymer molecules in the solution will influence the stability of the formed Taylor cone. Also, the rheological properties of the solution (viscosity and flow behavior) must be considered, as these would directly impact the take-up capacity of the solution by the fiber generator [10]. NE also requires a higher voltage to initiate the electrospinning process as compared to needle electrospinning.
Spinneret geometry parameters such as the dimensions and shape have a significant impact on the rate of production of nanofibers in NE. Typically, spinnerets are classified based on the path of the jet from the spinneret to the collector and based on the motion of the spinneret [11]. Various spinneret geometries explored for needleless electrospinning are presented in Fig. 2. There are several types of NE spinnerets, including linear-rotary, static bubble, magnetic field assisted, and coil. The spinneret design and configuration directly impact the distributed electric field intensity which leads to the variations in the distribution of charge density on the polymer surface that affects the Taylor cone formation [12]. For example, a coil spinneret provides higher productivity as compared to a cylinder spinneret which has the same diameter and length [13]. Increasing the rim radius increases the surface area, which decreases the electric field intensity and distribution in the spinneret. As a result, the rate of fiber production is lowered [5]. The amount of available spinneret surface area has a direct impact on fiber production rate and other fiber properties.
The spinneret assembly must be made of the appropriate material because some solvents can be corrosive. The resistance of Teflon, stainless steel, and polyether ether ketone (PEEK) to typical solvents is well-known [14]. The choice of spinneret material is thus identified as a key factor in ensuring the safety of nanofibers used in food processing systems.
The fiber production rate is largely affected by the electric field intensity which needs to be strong and narrowly distributed in the fiber generation area [5]. Metal electrode with a sharp edge, also called corona electrode, helps in the formation of the highest electrical charge density [15]. Xiong et al. simulated the electric field distribution of mushroom-like spinnerets [3] and reported that the strongest charge density was toward the edge of the free surface in the uncovered mushroom spinneret. Furthermore, the electrical charge density was observed to be uniformly distributed and to have great spinning capacity with concentrating charges near the edge, significantly lowering the required critical voltage.
Apart from the uniformity of charge density distribution, modifications in the spinneret configurations are also known to form nanofibers with various structures and functionalities. For instance, Zhou et al. used a dual-wire spinneret system in which the electrohydrodynamic destabilization and electrocapillary force resulted in the formation of a chain of droplets between the two wires [16]. The complex morphology of droplets in the channel can facilitate surface jetting and result in the formation of core-shell nanofibers. Also, a threaded rod spinneret was designed by Zheng et al. [17]; it could clean the residual polymer solution on the spinneret (self-cleaning) enabling efficient spinning with a production rate of 5-6 g/h. Several novel spinnerets, such as mushroom spinneret [18], multi-unit spinning to derive 3D nanofibrous structures [19], sprocket wheel spinneret [20], and linear flume spinneret [21], are presently being investigated for nanofiber production for NE. Furthermore, a needleless electrospinning device has recently been patented, which consists of an enclosure that collects the vapor formed during the spinning process and recovers the solvents [22].
Polymers Explored for Needleless Electrospinning Applications
Just like conventional electrospinning, NE also can spin a wide variety of synthetic, bio-based, and composite polymers. However, the polymer-solvent combinations for efficient spinning and the toxicity of solvents are the key factors contributing to the wide applicability of the spun polymers.
Synthetic Polymers
Various water-soluble and water-insoluble polymers have been explored in NE. Poly(vinyl alcohol) [23], poly(2ethyl2oxazolene), poly(vinylpyrrolidone), etc. as water-soluble polymers and poly(styrene-co-acrylonitrile), acrylonitrile butadiene styrene, and poly (vinyl alcohol-co-ethylene) [24], etc. as water-insoluble polymers were found to be suitable to produce nanofibers.
Biopolymers
Biological materials derived from plants and animals can be spun in their natural state or as blends. The properties of biobased materials and their availability have sparked interest in their use in the production of electrospun nanofibers [25]. Various polymers derived from plant sources, such as cellulose derivatives, zein, and lignin, animal sources, such as collagen and gelatin, and marine sources, such as chitosan, chitin, hyaluronic acid, etc., have been investigated for use in food and pharmaceutical fields. Owing to their biocompatibility and biofunctionality, naturally derived products have gained attention in electrospinning. A majority of these electrospun polymers are proteins or polysaccharides. The spinning conditions of various biomaterials in NE are given in Table 1.
Biopolymers from Plant Sources
Cellulose, being insoluble in water, requires bio-compatible green solvents (such as glycerin, sodium hydroxide [26], quaternary onium hydroxides, ionic liquids, and deep eutectic solvents [27]) for the preparation of spinning solutions and for their use in food applications [28]. NE efficiently regenerates cellulose into micro/nanofibers from its soluble derivatives. Similarly, carboxymethyl cellulose, a water-soluble cellulose derivative, has been used for plant extract encapsulation [29]. As fillers, cellulose derivatives are also used in improving the mechanical strength of nanofibers. For example, microcrystalline cellulose was incorporated into polyvinylpyrrolidone nanofiber to enhance its mechanical properties [30]. In the context of alginate, the high viscosity of sodium alginate necessitates a combination with carrier polymers or solvents with good fiber-forming capacity. The incorporation of carrier polymers and the addition of trace components such as Ca2+ can assist in an increase in intermolecular interactions between the alginate polymers, which further results in smooth, ultrafine fibers. The spinnability of lignin in needleless configuration has also been explored with a wire electrode [31].
Biopolymers from Animal Sources
The spinnability of chitosan is challenging as it has a polycationic property [32] with a rigid chemical structure and strong intramolecular hydrogen bonds resulting in enhanced viscosity and decreased chain movements. Chitosan electrospun fibers are often formed in a Trifluoroacetic acid solution or a co-solvent system of Trifluoroacetic acid and dichloromethane. Also, obtaining a homogeneous fiber matrix and the formation of electrical sparks are challenges, often resulting in the formation of spindle or bead-like structures in spun fiber mats. Thus, the spinning of chitosan as a blend of other polymers such as poly (ethylene oxide) and poly (vinyl alcohol) has been investigated to provide homogeneous fiber formation.
Silk fibroin is soluble in concentrated aqueous acidic solutions and in aqueous salt solutions with high ionic strength. Calcium chloride has been reported to improve silk fibroin solubility in the formic acid solvent [33]. Gelatin, obtained by thermal degradation of collagen, results in a 3D macromolecular network due to the presence of ionizable groups and strong hydrogen bonding. This results in reducing the mobility of gelatin and, therefore, the spinnability of gelatin is poor. This can be improved by spinning gelatin with a blend of polymers. NE has also proven to produce composite nanofibers of poorly miscible polymers fluid mixing electrospinning method. For example, chitosan oligosaccharide and sodium alginate are poorly miscible. However, the composite nanofibers were obtained by mixing chitosan oligosaccharide/gelatin and sodium alginate/gelatin solutions [34]. Various studies have reported NE of gelatin nanofibers with production rates up to 100 g/h [35]. Further, casein has been explored for electrospinning by using a mixed polymeric solution containing poly (ethylene oxide) and poly (vinyl alcohol). Due to its low tensile strength and water solubility, it requires crosslinking post-fiber formation. The presence of glycerol and paraffin oil in the spinning solution also results in overcoming the brittle structure of formed nanofibers [36]. Whey protein isolate contributes as an emulsifier and helps in chain entanglements during emulsion electrospinning. Thus, it is used as an ideal carrier for encapsulation of bioactive components [37].
Composite Polymers
Composite nanofibers exhibit enhanced mechanical strength and performance, as well as the ability to provide active and/or smart properties that are useful [38]. A study on NE to produce double-layered composites of chitosan and bacterial cellulose showed that the electrospun bacterial pigment-incorporated composites had an improved antimicrobial activity as well as the ability to prevent external contamination compared to bacterial cellulose layers without pigment incorporation [39]. In another study, potato protein-maltodextrin conjugates were electrospun using a rotating cylinder spinneret in a NE system, and a high fiber production rate (5.8 ± 0.4 g/h) was reported. An increase in protein content resulted in a decrease in the production rate and an increase in fiber diameter [40]. Earlier, whey protein and soy protein mixed with maltodextrin were electrospun in a roller electrospinning setup, and the latter resulted in decreased surface tension and increased electrical conductivity [41].
Composite structures of nanofibers consisting of spirulina protein concentrates and gelatin have been developed by Mosayebi et al. [42]; gelatin and soy protein isolate were combined in a 40: 60 ratio to create uniform bead-free nanofibers that had a smaller average diameter (208 ± 46 nm). In another study, composite structures of PVA and gelatin were spun in NE using dimethyl sulfoxide. These nanofibers were suggested for potential food applications [24]. A blend of gelatin and silk fibroin was also explored; an increase in gelatin content resulted in thicker fibers with diameters ranging from 200 to 660 nm [43].
Factors Influencing the Spinnability of Polymers in Needleless Configurations
The spinnability of polymers is influenced by solution parameters and process conditions. This section discusses the factors that require consideration during the spinning of polymer solutions in NE.
Properties of Spinning Solutions
NE requires suitable spinning solution properties such as polymer concentration in the solution mixture, electrical conductivity, viscosity, and surface tension, all of which are considered in conventional electrospinning processes as well. These would vary for different polymers and different configurations of NE systems. In general, NE requires a solution with higher viscosity (and polymer concentration) as compared with needle electrospinning. This is required for the formation of a thicker solution layer on the spinneret surface. As a result, boundary layer resistance is reduced and the mass flow rate is increased [44].
Electrospinning Process Conditions
Apart from solution mixture properties, the spinning efficiency in NE is also influenced by the system properties such as applied voltage, collector to spinneret distance, temperature, and relative humidity inside the spinning chamber. In NE, the surface of the drum requires more charges to be applied to the polymer solution to form spherical droplets, and this results in the entanglement of the polymer chains. Therefore, NE requires a higher applied voltage [45]. The critical voltage required to start electrospinning is highly dependent on material properties, environmental conditions (humidity and temperature), and collecting distance. A large collector-to-spinneret distance allows adequate jet stretching and complete solvent evaporation, resulting in a decrease in the diameter of the formed fibers [46].
The mechanical motion of the spinnerets also affects the fiber production rate. The rotating spinnerets produce uniform bead-free fibers at a faster spinning rate than stationary nozzle-free spinnerets. At lower spinneret speeds, uneven coverage of the spinneret surface restricts continuous jet formation [44]. When the rotation speed of the moving spinnerets is increased, fiber diameter is decreased as a result of jet ejection from different directions [17]. Thus, suitable rotation speeds must be established for different polymer solutions for the continuous generation of fiber jets. However, these rotation speeds depend on the type and properties of the polymer solution and the design of the spinneret assembly.
Properties of Electrospun Nanofibers Prepared Using Needleless Configurations
Nanofibers produced through NE have recently been investigated for their suitability as packaging materials, carriers for encapsulation, quality sensing strips, and other applications. However, the characteristics of nanofibers decide their suitability for different applications.
Surface Morphology and Porosity
NE produces coarser fibers with a wider diameter distribution than needle electrospinning because the Taylor cone diameter is not controlled. In a study conducted by Huang et al. disk-electrospun nanofibers resembled uniform fiber morphology with no beads, similar to those spun from needled electrospinning systems [47]. However, the disk electrospinning process produced coarser fibers with a wider distribution in fiber diameter (581 ± 276 nm). This was attributed to the widely distributed electrical field intensity on the disk edge. Further, disk electrospun membranes had larger pore diameters explaining lower fiber densities. NE has also been reported to generate more uniform nanofibers as compared to single-needle electrospinning carried out under the same conditions as a result of the uniformity of the applied electric field around the flat spinneret [48]. At higher concentrations of polymers, NE can produce defect and bead-free nanofibers as compared to needle spinning, as needle spinning requires frequent clearing of the needle tip to inhibit solution clogging at higher polymer concentrations [49]. Also, the spinneret’s configurations tend to directly impact the fiber morphology. At the same applied voltage, nanofibers generated in a disk nozzle were finer as compared to the cylinder nozzle [44]. Figure 4 presents the morphology and fiber diameters of NE nanofibers.
Thermal Properties
NE nanofibers can offer higher thermal stabilities, and this is due to the alignment of polymeric materials during whipping and stretching, producing nanofibers with highly ordered structures. Additionally, during electrospinning, atoms of polymeric molecules are physically linked with one another to form lengthy polymeric chains wherein intermolecular interactive forces increase the temperature at which degradation takes place. Zein nanofibers showed higher temperatures of initiating second weight loss (260 °C) as compared to pure zein (240 °C) [50]. Being a low-temperature fiber formation process, the process of electrospinning did not have any negative impact on the glass transition temperature of zein nanofibers. In a study involving the incorporation of chitosan, the formation of crystal regions in the nanofibers was hindered, resulting in a decrease in endothermic peaks with an increase in the chitosan concentration [51]. Similarly, in another study conducted on cinnamic aldehyde encapsulation in zein nanofibers, the incorporation of cinnamic aldehyde resulted in a decrease in the thermal stability of zein nanofibers [52].
Mechanical Properties
NE can impact the mechanical strength and associated stability of nanofibers. This is due to an increase in polymeric crystallinity during the process as compared to needle-spun nanofibers [53]. Improved mechanical strength is an important phenomenon in explaining the suitability of nanofibers for filtration applications. A decrease in fiber diameter is expected to improve the mechanical properties of the spun nanofibers. However, in a study conducted by Zahran et al. when fiber diameter approached lower values (~ 34 nm), a weak mechanical performance was observed [30]. Therefore, fiber morphology and diameter should be optimal to achieve the required mechanical performances.
The addition of a significant amount of NE fibers can result in a notable increase in the tensile strength of films. For example, an increase in zein nanofiber concentration in a casted gelatin film increased Young’s modulus as a result of increased stiffness and density. Also, an inverse relationship was observed between the nanofiber concentration and elongation at breaking due to the increased brittleness of films resulting in a reduction in direct interactions between the available protein chain networks [50]. The addition of microcrystalline cellulose has been reported to increase the storage modulus of electrospun poly(lactic) acid (PLA) fibers by 279% [30]. Further, the application of various crosslinking strategies in spun nanofibers is reported to increase the fiber’s mechanical strength [35].
Applications of Needleless Electrospun Nanofibers in the Food Industry
This section presents insights from recent research works that involve NE applications for the food sector.
Encapsulation of Bioactive Components
Electrospun nanofibers have the potential to be used in the food industry as the encapsulation carrier of various food additives and bioactive components. They have been explored for their feasibility, stability, and robustness for appropriate drug delivery profiles. Electrospun drug-loaded nanofibers have been prepared by treating the co-dissolved solution of a guest bioactive ingredient and the host polymer excipient in a single fluid process. NE is a novel approach for scaled-up production of bioactive components encapsulated nanofibers with controlled release.
In a study conducted by Karim et al. NE was used for the synthesis of zein nanofibers loaded with cinnamic aldehyde for the reduction of nitrite in sausages [52]. They concluded that nanofibers had no negative effects on the color, texture, and sensory properties of sausages when compared to samples with 120 ppm nitrite. In another study, zein nanofibers were used to encapsulate capsaicin, and encapsulation efficiencies of up to 93% were achievable [54]. Ramakrishnan et al. have studied the efficiency of curcumin encapsulation in polycaprolactone fibers in an NE process and reported 93% entrapment efficiency and a controlled release behavior for a period of 48 h [55]. In another study, tea tree essential oil was encapsulated in polyamidoamine dendritic polymers using NE, and a high air filtration efficiency and controlled release of volatile fragrance components from encapsulated tea tree essential oil were achieved [56].
An earlier study used a wire electrospinning setup to encapsulate neem seed oil in cellulose acetate, reporting improved mycelial growth inhibition against A. flavus and A. alternate [57]. Additionally, it was demonstrated that encapsulation of bioactives in spun nanofibers can improve their thermal stability by more than 50% as compared to their free counterparts. The application of NE can thus result in fiber mats that can deliver multiple drugs/bioactive ingredients at varied rates, and different fiber populations can also influence the mechanical and physical properties. Also, the encapsulation of nanoparticles into nanofibers is another novel approach that has shown a higher production rate (1.690 g/h average) with uniform fiber diameters [58]. In addition to its time-regulated release kinetics and preservation of bioactivity, the needleless emulsion electrospinning method improves cell metabolic activity and viability [59].
Food Packaging Systems
Organic acids, antimicrobial peptides, and essential oils are examples of natural antimicrobial substances that are frequently used in active food packaging systems. Biobased polymeric materials have unique ion binding and aroma barrier properties, with increased antimicrobial activity. Their reactive functional groups make them easily modifiable. Biopolymer-based nanofibers capture antimicrobial agents, and their notable functionality and biodegradability make them versatile choices for the creation of novel packaging systems [60]. The layer-by-layer customization of nanofiber mats by NE can help create products with beneficial properties for potential uses in the food packaging industry. One such product is the bacterial cellulose and PVA-chitosan produced by NE and functionalized with bacterial pigment prodigiosin, which has shown promising results not only for the packaging interior but also prevents the packaging exterior from contamination due to its chitosan antibacterial properties and multifunctional external surface with antibacterial activity [39].
In another recent study, Zataria multiflora essential oil was encapsulated into polyvinyl alcohol via electrospinning for the active packaging of strawberries [61]. Results indicated that the strawberries treated with PVA/Zataria multiflora essential oil preserved the anthocyanin, total phenols, and antioxidants during storage. The fibers also delayed the biochemical and physiological changes in fruit and extended the shelf life. Another study reported the development of nanofiber mats of PLA/polyethylene glycol blends incorporated with peppermint essential oil by solution-blow-spinning for potential packaging applications. Results confirmed extended shelf life, reduced weight loss, and increased firmness [62].
A recent study used gluten/zein nanofibers to encapsulate star anise essential oil, which was then further cross-linked by xylose Maillard reactions. The obtained nanofibers with essential oil/cyclodextrin inclusions demonstrated exceptional antimicrobial effectiveness against both E. coli and S. aureus, indicating a promising application potential in food-active packaging [63]. Also, antioxidant nanofibers were developed using gelatin and spirulina protein concentrate using NE (Fig. 5), having wide applicability in high-humidity food packaging applications along with the potential for nutraceutical delivery [42]. Overall, findings confirm that NE mats are feasible, effective, and environmentally friendly options for active packaging applications.
Generation of needleless electrospun antioxidant nanofibers based on gelatin and spirulina protein concentrate. Source: [42]
Enzyme Immobilization
Enzyme-immobilized nanofibers support advances in bioprocessing efficiency and bioactive packaging. Using NE to create nanofibers has also been investigated for the biofunctionalization and immobilization of enzymes. For example, biofunctionalization using the one-step carbodiimide method was used to immobilize trypsin on the surface of biocompatible chitosan nanofibers. This resulted in activity of up to 209.8 ± 0.6 IU/cm2 [64]. Immobilization in nanofiber matrices also supports long-term storage stability and improved reusability. In another study, chymotrypsin was immobilized in nylon 6,6 nanofibers, reporting enhanced thermos-stability as compared to native enzymes and enzymes immobilized on planar films [65], and these nanofibers retained activity over a wider range of temperatures. Similarly, poly (acrylonitrile co-methyl methacrylate) nanofibers were spun to immobilize β-galactosidase using nano spider NE, and improved temperature and pH stability were observed as compared to the free form [66]. Nylon 6 nanofiber carriers produced using NE have shown higher protein loading (71%) upon laccase immobilization with higher residual activity (29%) even after 7 cycles of usage. Such findings explain the potential of NE as a cheap and effective immobilization approach for enzymes on a large scale [67].
Further, steady-state kinetics of immobilized enzymes were assayed in different studies and fitted with the Michaelis–Menten equation to find the kinetic parameters (Michaelis constant and maximum activity). The Michaelis constant increased during immobilization by NE, representing a decrease in the affinity of the enzyme (Table 2) to the substrate. This results in the quick dissociation of an enzyme-substrate complex for the biocatalysis to take place. Enzymes immobilized in nanofibers have been reported to experience more diffusion-oriented collisions with the substrate to create the enzyme-substrate complex for biocatalysis over time [65].
Filtration
Fibrous materials such as nonwoven fabrics are extensively used as filtration media due to their flexibility, low initial resistance to flow, and large retentate holding capacity. The removal efficiency is typically higher in fibers with smaller pore diameters, but this is often assisted by high filtration resistance. Reducing filtration resistance while maintaining high filtration efficiency is challenging. Electrospun fibers serve as a relatively mature filter medium in many application fields [68]. Typically, they have high permeability and a larger surface area which makes them suitable for separation and filtration applications.
Xiong et al. developed a sandwiched air filter assembly by controlled accumulation of nanofibers using NE with improved air filtration efficiency [69]. Also, NE can support improved water filtration efficiency. The arrangement of oriented fibers can affect the permeability and the separation efficiency of the membrane [70]. Needleless electrospun konjac glucomannan incorporated polyacrylonitrile has also been reported to have potential applications in pollutant removal in food industry wastewater [71]. In another study, chitin nanowhiskers were reinforced on the needleless electrospun poly(vinylidene fluoride) membrane, and improvements in thermal stability, mechanical properties, pure water flux, and oil-water filtration performance of the membranes were achieved [72]. Overall, the scope of NE membranes in food and beverage filtration applications remains underexplored.
Food Quality Monitoring
The higher specific surface area with predictable pore geometries helps NE fibers offer improved material reactivities and fastens the absorption and release rate kinetics by increasing the number of interactive sites [73]. Colorimetric freshness sensor films developed using the electrospinning method showed potential for food quality sensing applications [4]. In a study conducted by Liu et al. a novel colorimetric film was developed using NE to monitor shrimp spoilage during storage [74]. The film consisted of polycaprolactone fiber in which anthocyanin dye was immobilized; a reversible color change could happen, widening prospects for repeated usage. NE has also been explored for the preparation of cellulose acetate-based halochromic nanofibers wherein a clear change in color at different pH conditions occurs, extending applications in food quality monitoring systems [75]. NE-based porous structures have shown high sensitivity and elevated performance in the production of low-cost optical sensors wherein polyamide 6 has been electrospun for the development of Fabry-Pérot optical sensing structures [76].
Further, electrospun nanofibers have the potential to be used as effective sorbents for solid-phase extraction [77]. The microextraction efficiency of NE nanofibers has also been assessed for the analytical quantification of trace components present in food samples. Chen et al. reported that the electrospun polyacrylonitrile/covalent organic framework nanofibers spun using a wire electrospinning system exhibited good stability and extraction efficiency for trace sulfonamide residues in food [78]. In short, there is ample research potential in the use of electrospun nanofibers for food quality sensing applications.
Challenges and Directions for Future Research
Though NE offers advantages, particularly in terms of its scalability, these systems are also associated with certain challenges that need to be addressed. Major considerations and avenues for future research are presented in this section.
Process Control
NE is a multivariate process in which the quality of the formed fiber is associated with various factors, making the process complex for precise flow rate control. This results in the lack of uniformity and homogeneity of the formed fibers. These process control limitations can be addressed by the application of modeling and simulation tools that can better explain the underlying mechanisms. Simulation studies can help in understanding the behavior of polymer solutions when subjected to high voltage electric potential and how electric field distribution takes place in NE, also providing insights into the mechanism of Taylor cone formation. When it comes to the NE process, the distribution of electrostatic field intensity has a significant impact on the quality of obtained fibers and overall spinning performance. An inhomogeneity in the electric field distribution along the surface of the collector results in a decrease in the efficiency of the process. Various numerical simulation models [79] have been explored to simulate the NE and to understand the effect of different parameters such as spinneret configuration [12], electric field intensity on the collector [80], and electrode geometries on the productivity, fiber homogeneity, and efficiency of fiber formation in NE.
Studies on computational modeling of NE have also explored predicting the conditions for controlled fiber deposition [81]. Recently, a computational model was developed by Domaschke et al. to predict the 3D structure and macroscopic mechanical responses of the electrospun membranes based on geometry (diameter distribution, persistence length, and porosity) and mechanical properties [82]. The need for simulation tools to comprehend the spinning behavior in NE is also becoming a possibility. However, to date, no studies report the simulation of bio nanofibers production through NE.
Apart from these aspects, the high excitation voltage required to form Taylor cones, poor free liquid surface stability, and the difficulties in regulating the spatial motion of the numerous jets limits the applicability of NE on the industrial scale. Though there are recent studies that are emerging studies the characteristics of the needleless electrospun nanofibers through biopolymers, the comparison between properties of the polymeric/composite nanofibers and their spinnability can help in determining the most feasible spinning method to produce nanofibers with enhanced fiber characteristics.
The machine learning approach also has the potential to develop electrospinning systems with integrated decision-making algorithms to control the process. In a study conducted by Hwang et al. an adaptive electrospinning system was developed with a reinforcement learning algorithm that interacts with the formed fiber in real time to measure its thickness [83]. Based on the pre-trained algorithm, the system controls the movement of the collector for the uniform deposition of the fiber. These types of data-driven modeling approaches can also be explored in NE to improve fiber productivity as well as quality.
Safety
NE operates at a higher applied voltage which may sometimes result in spark generation in the system. Thus, it is important to understand the flash point of solvents before being used as in NE; solvents such as chloroform, tetrahydrofuran, and toluene cannot be used as base solvents [45]. A disadvantage of this technology when utilized on an industrial scale is the greater amount of undesired residual solvents and monomers remaining in the nanofibrous material, with possible cytotoxicity effects. These chemicals may cause health and environmental issues during production, handling, and usage [84]. Nevertheless, these toxic residuals can be effectively removed by a washing-out procedure in distilled water [85]. Thus, the technology should further explore the effective toxic residual removal systems and protocols for efficient and safe industrial production of nanofibers.
Improving Productivity and Fiber Quality Using Novel Technologies That Assist Needleless Electrospinning
Various novel approaches can aid NE in improving productivity and fiber quality. For example, a high-intensity focused ultrasound was used in ultrasound-assisted NE, resulting in the formation of an ultrasound fountain on the free surface and aided in jet initiation and bead-free fiber generation [86]. However, when compared to conventional electrospinning, the ultrasound-assisted process produced thicker fibers, which could be attributed to the increased number of variations in the ultrasound-assisted NE. It has also been reported that changing the pulse rate (cycles per second) and intensity changes the fiber diameter. A novel shear-aided NE method, in which the spinneret manipulates solution properties based on rotational speed and orifice gap size, was also reported [87].
Another study found that the electric field and aerodynamic field have a synergistic effect on the NE process, increasing the rate of fiber production [88]. Another novel approach is the use of a magnetic field to aid in free surface perturbation and the subsequent formation of steady vertical polymer spikes, which increases the fiber production rate [13]. Also, the construction of stable and annular pre-Taylor cones with high curvature can assist in promoting the production rate of nanofibers with superior quality [3]. Though these assisted technologies help to improve the NE performances in thermoplastic polymers, studies on assisted technologies for bioderived materials are still limited, which should garner significant attention.
Conclusion
Given the rising focus on bio-based materials, studies on their spinning potential and associated parameters require a detailed investigation, particularly in needleless configurations, recognized for enhanced production rates. Overall, the approach will have takers from different segments of the food industry, ranging from encapsulation to enzyme immobilization to food packaging to food quality sensing. Understanding the underlying mechanisms, such as the diffusion kinetics of encapsulated bioactives/enzymes, and optimizing spinning conditions, spinneret configurations, and polymer solution properties are the major aspects that require research attention. These would assist in optimizing fiber geometry and functionality to meet the requirements of specific applications. The inhomogeneity of fiber distribution in needleless configurations remains a limitation and modeling, and simulation tools can assist in improved process control and system performance. To conclude, needleless electrospinning is a promising area with significant research and commercial potential for a range of food applications.
Availability of Data and Materials
Not applicable.
References
MacDiarmid AG (2001) Nobel lecture: “synthetic metals”: a novel role for organic polymers. Rev Mod Phys 73:701. https://doi.org/10.1103/RevModPhys.73.701
De Oliveira Mori CLS, Dos Passos NA, Oliveira JE et al (2014) Electrospinning of zein/tannin bio-nanofibers. Ind Crops Prod 52:298–304. https://doi.org/10.1016/j.indcrop.2013.10.047
Xiong J, Liu Y, Li A et al (2021) Mass production of high-quality nanofibers via constructing pre-Taylor cones with high curvature on needleless electrospinning. Mater Des 197:109247. https://doi.org/10.1016/j.matdes.2020.109247
Amal Nath V, Vijayakumar R, Leena MM et al (2022) Co-electrospun-electrosprayed ethyl cellulose-gelatin nanocomposite pH-sensitive membrane for food quality applications. Food Chem 394:133420. https://doi.org/10.1016/j.foodchem.2022.133420
Niu H, Wang X, Lin T (2012) Needleless electrospinning: influences of fibre generator geometry. J Text Inst 103:787–794. https://doi.org/10.1080/00405000.2011.608498
Yuan H, Zhou Q, Zhang Y (2017) Improving fiber alignment during electrospinning. In: Electrospun Nanofibers. Woodhead Publishing, pp 125–147
Kanjanapongkul K, Wongsasulak S, Yoovidhya T (2010) Investigation and prevention of clogging during electrospinning of zein solution. J Appl Polym Sci 118:1821–1829. https://doi.org/10.1002/APP.32499
Bö Ttjer R, Grothe T, Wehlage D, Ehrmann A (2018) Electrospraying poloxamer/(bio-) polymer blends using a needleless electrospinning machine. J Text Fibrous Mater 1:1–7. https://doi.org/10.1177/2515221117743079
Shaid A, Wang L, Padhye R, Jadhav A (2018) Needleless electrospinning and electrospraying of mixture of polymer and aerogel particles on textile. Adv Mater Sci Eng. https://doi.org/10.1155/2018/1781930
Cengiz F, Dao TA, Jirsak O (2010) Influence of solution properties on the roller electrospinning of poly(vinyl alcohol). Polym Eng Sci 50:936–943. https://doi.org/10.1002/pen.21599
Kouhi M, Mobasheri M, Valipouri A (2023) Needleless electrospinning. Electrospun Nanofibrous Membr Princ Appl 145–171. https://doi.org/10.1016/B978-0-12-823032-9.00011-8
Qin Z, Yan G, Zhang X et al (2022) Finite element method assisted design of needleless electrospinning systems for mass production of polymer nanofibers. Chem Eng Sci 259:117817. https://doi.org/10.1016/j.ces.2022.117817
Yarin AL, Zussman E (2004) Upward needleless electrospinning of multiple nanofibers. Polymer (Guildf) 45:2977–2980. https://doi.org/10.1016/j.polymer.2004.02.066
Zhou W, Zhang W, Liu Y et al (2017) Polydopamine-functionalized poly(ether ether ketone) tube for capillary electrophoresis-mass spectrometry. Anal Chim Acta 987:64–71. https://doi.org/10.1016/j.aca.2017.08.033
Molnar K, Nagy ZK (2016) Corona-electrospinning: needleless method for high-throughput continuous nanofiber production. Eur Polym J 74:279–286. https://doi.org/10.1016/j.eurpolymj.2015.11.028
Zhou Z, Wu XF, Ding Y et al (2014) Needleless emulsion electrospinning for scalable fabrication of core–shell nanofibers. J Appl Polym Sci 131. https://doi.org/10.1002/app.40896
Zheng G, Jiang J, Wang X et al (2018) Self-cleaning threaded rod spinneret for high-efficiency needleless electrospinning. Appl Phys A Mater Sci Process 124:1–8. https://doi.org/10.1007/S00339-018-1892-y
Latiffah E, Agung BH, Hapidin DA, Khairurrijal K (2022) Fabrication of polyvinylpyrrolidone (PVP) nanofibrous membranes using mushroom-spinneret needleless electrospinning. J Phys Conf Ser 2243:012101. https://doi.org/10.1088/1742-6596/2243/1/012101
Yan G, Yang Z, Li J et al (2022) Multi-unit needleless electrospinning for one-step construction of 3D waterproof MF-PVA nanofibrous membranes as high-performance air filters. Small 2206403. https://doi.org/10.1002/smll.202206403
Ali U, Niu H, Aslam S et al (2017) Needleless electrospinning using sprocket wheel disk spinneret. J Mater Sci 52:7567–7577. https://doi.org/10.1007/S10853-017-0989-6
Wei L, Liu C, Mao X et al (2019) Multiple-jet needleless electrospinning approach via a linear flume spinneret. Polymers (Basel) 11:2052. https://doi.org/10.3390/polym11122052
Jin X, Peters R, Pearson E (2021) Apparatus for continuous needleless electrospinning a nanoscale or submicron scale polymer fiber web onto a substrate
Ng JJ, Supaphol P (2018) Rotating-disk electrospinning: needleless electrospinning of poly(caprolactone), poly(lactic acid) and poly(vinyl alcohol) nanofiber mats with controlled morphology. J Polym Res 25:1–9. https://doi.org/10.1007/S10965-018-1540-4
Wortmann M, Frese N, Sabantina L et al (2019) New polymers for needleless electrospinning from low-toxic solvents. Nanomaterials 9:52. https://doi.org/10.3390/nano9010052
Angel N, Li S, Yan F, Kong L (2022) Recent advances in electrospinning of nanofibers from bio-based carbohydrate polymers and their applications. Trends Food Sci Technol 120:308–324. https://doi.org/10.1016/j.tifs.2022.01.003
K Li H Yang L Jiang (2020) Glycerin, NaOH aqueous solution as a green solvent system for dissolution of cellulose. Polym et al (2020) 12(1735):12–1735 https://doi.org/10.3390/POLYM12081735
Ge W, Shuai J, Wang Y et al (2022) Progress on chemical modification of cellulose in “green” solvents. Polym Chem 13:359–372. https://doi.org/10.1039/D1PY00879J
Onwukamike KN, Grelier S, Grau E et al (2019) Critical review on sustainable homogeneous cellulose modification: why renewability is not enough. ACS Sustain Chem Eng 7:1826–1840. https://doi.org/10.1021/acssuschemeng.8B04990
Maver T, Kurečič M, Pivec T et al (2020) Needleless electrospun carboxymethyl cellulose/polyethylene oxide mats with medicinal plant extracts for advanced wound care applications. Cellulose 27:4487–4508. https://doi.org/10.1007/S10570-020-03079-9
Zahran SME, Abdel-Halim AH, Nassar K, Nada AA (2020) Fabrication of nanofiltration membrane based on non-biofouling PVP/lecithin nanofibers reinforced with microcrystalline cellulose via needle and needle-less electrospinning techniques. Int J Biol Macromol 157:530–543. https://doi.org/10.1016/j.ijbiomac.2020.04.152
Mikeš P, Baker DA, Uhlin A et al (2021) The mass production of lignin fibres by means of needleless electrospinning. J Polym Environ 29:2164–2173. https://doi.org/10.1007/S10924-020-02029-7
Azmana M, Mahmood S, Hilles AR et al (2021) A review on chitosan and chitosan-based bionanocomposites: promising material for combatting global issues and its applications. Int J Biol Macromol 185:832–848. https://doi.org/10.1016/j.ijbiomac.2021.07.023
Sasithorn N, Mongkholrattanasit R, Martinová L (2016) Preparation of silk fibroin nanofibres by needleless electrospinning using formic acid-calcium chloride as the solvent. Appl Mech Mater 848:203–206. https://doi.org/10.4028/www.scientific.net/amm.848.203
Lu W, Xu H, Zhang B et al (2016) The preparation of chitosan oligosaccharide/alginate sodium/gelatin nanofibers by spiral-electrospinning. J Nanosci Nanotechnol 16:2360–2364. https://doi.org/10.1166/jnn.2016.10910
Lu W, Ma M, Xu H et al (2015) Gelatin nanofibers prepared by spiral-electrospinning and cross-linked by vapor and liquid-phase glutaraldehyde. Mater Lett 140:1–4. https://doi.org/10.1016/j.matlet.2014.10.146
Minaei F, Ravandi SAH, Hejazi SM, Alihosseini F (2019) The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning. E-Polymers 19:154–167. https://doi.org/10.1515/epoly-2019-0017
Chen L, Xiang M, Wu F et al (2023) Encapsulation of lycopene into electrospun nanofibers from whey protein isolate-Tricholoma lobayense polysaccharide complex stabilized emulsions: structural characterization, storage stability, in vitro release, and cellular evaluation. Int J Biol Macromol 238:123993. https://doi.org/10.1016/j.ijbiomac.2023.123993
Rhim JW, Park HM, Ha CS (2013) Bio-nanocomposites for food packaging applications. Prog Polym Sci 38:1629–1652. https://doi.org/10.1016/j.progpolymsci.2013.05.008
Amorim LFA, Mouro C, Riool M, Gouveia IC (2022) Antimicrobial food packaging based on prodigiosin-incorporated double-layered bacterial cellulose and chitosan composites. Polymers (Basel) 14:315. https://doi.org/10.3390/polym14020315
Gibis M, Pribek F, Kutzli I, Weiss J (2021) Influence of the protein content on fiber morphology and heat treatment of electrospun potato protein–maltodextrin fibers. Appl Sci 11:7896. https://doi.org/10.3390/APP11177896/S1
Kutzli I, Gibis M, Baier SK, Weiss J (2019) Electrospinning of whey and soy protein mixed with maltodextrin – influence of protein type and ratio on the production and morphology of fibers. Food Hydrocoll 93:206–214. https://doi.org/10.1016/j.foodhyd.2019.02.028
Mosayebi V, Fathi M, Shahedi M et al (2022) Fast-dissolving antioxidant nanofibers based on Spirulina protein concentrate and gelatin developed using needleless electrospinning. Food Biosci 47:101759. https://doi.org/10.1016/j.fbio.2022.101759
Sasithorn N, Martinová L (2015) Needleless electrospinning of silk fibroin/gelatin blend nanofibres. Appl Mech Mater 804:213–216. https://doi.org/10.4028/www.scientific.net/amm.804.213
Niu H, Lin T, Wang X (2009) Needleless electrospinning. I. A comparison of cylinder and disk nozzles. J Appl Polym Sci 114:3524–3530. https://doi.org/10.1002/APP.30891
Moon S, Gil M, Lee KJ (2017) Syringeless electrospinning toward versatile fabrication of nanofiber web. Sci Rep 7. https://doi.org/10.1038/srep41424
Wei L, Sun R, Liu C et al (2019) Mass production of nanofibers from needleless electrospinning by a novel annular spinneret. Mater Des 179:107885. https://doi.org/10.1016/j.matdes.2019.107885
Huang C, Niu H, Wu C et al (2013) Disc-electrospun cellulose acetate butyrate nanofibers show enhanced cellular growth performances. J Biomed Mater Res Part A 101A:115–122. https://doi.org/10.1002/jbm.a.34306
Wang L, Zhang C, Gao F, Pan G (2016) Needleless electrospinning for scaled-up production of ultrafine chitosan hybrid nanofibers used for air filtration. RSC Adv 6:105988–105995. https://doi.org/10.1039/C6RA24557A
Hwang M, Karenson MO, Elabd YA (2019) High production rate of high purity, high fidelity nafion nanofibers via needleless electrospinning. ACS Appl Polym Mater 1:2731–2740. https://doi.org/10.1021/acsapm.9b00681
Karim M, Fathi M, Soleimanian-Zad S (2020) Incorporation of zein nanofibers produced by needle-less electrospinning within the casted gelatin film for improvement of its physical properties. Food Bioprod Process 122:193–204. https://doi.org/10.1016/j.fbp.2020.04.006
Li TT, Yan M, Zhong Y et al (2019) Processing and characterizations of rotary linear needleless electrospun polyvinyl alcohol (PVA)/chitosan (CS)/graphene (Gr) nanofibrous membranes. J Mater Res Technol 8:5124–5132. https://doi.org/10.1016/j.jmrt.2019.08.035
Karim M, Fathi M, Soleimanian-Zad S (2021) Nanoencapsulation of cinnamic aldehyde using zein nanofibers by novel needle-less electrospinning: production, characterization and their application to reduce nitrite in sausages. J Food Eng 288:110140. https://doi.org/10.1016/j.jfoodeng.2020.110140
Wang X, Niu H, Wang X, Lin T (2012) Needleless electrospinning of uniform nanofibers using spiral coil spinnerets. J Nanomater 2012:1–9. https://doi.org/10.1155/2012/785920
Rezazadeh A, Moghaddas Kia E, Hamishehkar H et al (2022) Capsaicin-incorporated zein electrospun nanofibers: characterization and release behavior. Food Biosci 49:101843. https://doi.org/10.1016/j.fbio.2022.101843
Ramakrishnan R, Ramakrishnan P, Ranganathan B et al (2019) Effect of humidity on formation of electrospun polycaprolactone nanofiber embedded with curcumin using needdleless electrospinning. Mater Today Proc 19:1241–1246. https://doi.org/10.1016/j.matpr.2019.11.128
Mounesan M, Akbari S, Brycki BE (2022) Needleless electrospun mats based on polyamidoamine dendritic polymers for encapsulation of essential oils in personal respiratory equipment. J Ind Text 51:6333–6352. https://doi.org/10.1177/15280837211048155
Ai-Tang R, Utarak H, Yoovidhya T et al (2013) Fabrication and antifungal activity of cellulose acetate-based fibers encapsulating natural neem seed oil. Adv Mater Res 747:166–169. https://doi.org/10.4028/www.scientific.net/amr.747.166
Prabu GTV, Dhurai B (2020) A novel profiled multi-pin electrospinning system for nanofiber production and encapsulation of nanoparticles into nanofibers. Sci Rep 10:1–11. https://doi.org/10.1038/s41598-020-60752-6
Buzgo M, Filova E, Staffa AM et al (2018) Needleless emulsion electrospinning for the regulated delivery of susceptible proteins. J Tissue Eng Regen Med 12:583–597. https://doi.org/10.1002/TERM.2474
Fabra MJ, López-Rubio A, Lagaron JM (2014) Biopolymers for food packaging applications. Smart Polym their Appl 476–509. https://doi.org/10.1533/9780857097026.2.476
Yuan M, Zhang Z-H, Roy S et al (2023) Assessment of Zataria multiflora essential oil- incorporated electrospun polyvinyl alcohol fiber mat as active packaging. Polymers (Basel) 15:1048. https://doi.org/10.3390/polym15041048
Mendes JF, Norcino LB, Corrêa TQ et al (2023) Obtaining poly (lactic acid) nanofibers encapsulated with peppermint essential oil as potential packaging via solution-blow-spinning. Int J Biol Macromol 230:123424. https://doi.org/10.1016/j.ijbiomac.2023.123424
Zhang Y, Yang K, Qin Z et al (2022) Cross-linked gluten/zein nanofibers via Maillard reaction with the loading of star anise essential oil/β-cyclodextrin inclusions for food-active packaging. Food Packag Shelf Life 34:100950. https://doi.org/10.1016/j.fpsl.2022.100950
Srbová J, Slováková M, Křípalová Z et al (2016) Covalent biofunctionalization of chitosan nanofibers with trypsin for high enzyme stability. React Funct Polym 104:38–44. https://doi.org/10.1016/j.reactfunctpolym.2016.05.009
Wong DE, Senecal KJ, Goddard JM (2017) Immobilization of chymotrypsin on hierarchical nylon 6,6 nanofiber improves enzyme performance. Colloids Surfaces B Biointerfaces 154:270–278. https://doi.org/10.1016/j.colsurfb.2017.03.033
El-Aassar MR (2013) Functionalized electrospun nanofibers from poly (AN-co-MMA) for enzyme immobilization. J Mol Catal B Enzym 85–86:140–148. https://doi.org/10.1016/j.molcatb.2012.09.002
Fatarella E, Spinelli D, Ruzzante M, Pogni R (2014) Nylon 6 film and nanofiber carriers: preparation and laccase immobilization performance. J Mol Catal B Enzym 102:41–47. https://doi.org/10.1016/j.molcatb.2014.01.012
Daels N, De Vrieze S, Decostere B et al (2010) The use of electrospun flat sheet nanofibre membranes in MBR applications. Desalination 257:170–176. https://doi.org/10.1016/j.desal.2010.02.027
Xiong J, Zhou M, Zhang H et al (2018) Sandwich-structured fibrous membranes with low filtration resistance for effective PM2.5 capture via one-step needleless electrospinning. Mater Res Express 6:035027. https://doi.org/10.1088/2053-1591/aaf760
Li X, Zhang Y, Li H et al (2014) Effect of oriented fiber membrane fabricated via needleless melt electrospinning on water filtration efficiency. Desalination 344:266–273. https://doi.org/10.1016/j.desal.2014.04.003
Mamun A, Trabelsi M, Klöcker M et al (2020) Needleless electrospun polyacrylonitrile/konjac glucomannan nanofiber mats. J Eng Fiber Fabr 15. https://doi.org/10.1177/1558925020964806
Gopi S, Kargl R, Kleinschek KS et al (2018) Chitin nanowhisker – inspired electrospun PVDF membrane for enhanced oil-water separation. J Environ Manage 228:249–259. https://doi.org/10.1016/j.jenvman.2018.09.039
Mercante LA, Scagion VP, Migliorini FL et al (2017) Electrospinning-based (bio)sensors for food and agricultural applications: a review. TrAC Trends Anal Chem 91:91–103. https://doi.org/10.1016/j.trac.2017.04.004
Liu L, Zhang J, Zou X et al (2022) A high-stable and sensitive colorimetric nanofiber sensor based on PCL incorporating anthocyanins for shrimp freshness. Food Chem 377:131909. https://doi.org/10.1016/j.foodchem.2021.131909
Elveren B, Hribernik S, Kurečič M (2022) Fabrication of polysaccharide-based halochromic nanofibers via needle-less electrospinning and their characterization: a study of the leaching effect. Polymers (Basel) 14:4239. https://doi.org/10.3390/polym14194239
Ponce-alcántara S, Martín-sánchez D, Pérez-márquez A et al (2018) Optical sensors based on polymeric nanofibers layers created by electrospinning. Opt Mater Express 8:3163–3175. https://doi.org/10.1364/ome.8.003163
Háková M, Raabová H, Havlíková LC et al (2018) Testing of nylon 6 nanofibers with different surface densities as sorbents for solid phase extraction and their selectivity comparison with commercial sorbent. Talanta 181:326–332. https://doi.org/10.1016/j.talanta.2018.01.043
Chen A, Guo H, Luan J et al (2022) The electrospun polyacrylonitrile/covalent organic framework nanofibers for efficient enrichment of trace sulfonamides residues in food samples. J Chromatogr A 1668:462917. https://doi.org/10.1016/j.chroma.2022.462917
Iranshahi K, Schoeller J, Luisier N et al (2022) Improving needleless electrospinning throughput by tailoring polyurethane solution properties with polysiloxane additives. ACS Appl Polym Mater 4:2205–2215. https://doi.org/10.1021/acsapm.2C00263
Smółka K, Firych-Nowacka A, Wiak S (2022) Analysis of the electrostatic field distribution to improve the electrospinning process—practical tips. J Comput Sci 59:101542. https://doi.org/10.1016/j.jocs.2021.101542
İçoğlu Hİ, Yıldırım B, Kılıç A et al (2023) Controlled fiber deposition via modeling the auxiliary electrodes of the needleless electrospinning to produce continuous nanofiber bundles. Mater Today Commun 34:104966. https://doi.org/10.1016/j.mtcomm.2022.104966
Domaschke S, Morel A, Kaufmann R et al (2020) Predicting the macroscopic response of electrospun membranes based on microstructure and single fibre properties. J Mech Behav Biomed Mater 104:103634. https://doi.org/10.1016/j.jmbbm.2020.103634
Hwang SH, Song JY, Il RH et al (2023) Adaptive electrospinning system based on reinforcement learning for uniform-thickness nanofiber air filters. Adv Fiber Mater 5:617–631. https://doi.org/10.1007/S42765-022-00247-3
Dodero A, Castellano M, Vicini S et al (2022) Eco-friendly needleless electrospinning and tannic acid functionalization of polyurethane nanofibers with tunable wettability and mechanical performances. Macromol Mater Eng 307:2100823. https://doi.org/10.1002/mame.202100823
Dubský M, Kubinová Š, Širc J et al (2012) Nanofibers prepared by needleless electrospinning technology as scaffolds for wound healing. J Mater Sci Mater Med 23:931–941. https://doi.org/10.1007/S10856-012-4577-7
Nieminen HJ, Laidmäe I, Salmi A et al (2018) (2018) Ultrasound-enhanced electrospinning. Sci Reports 81(8):1–6. https://doi.org/10.1038/s41598-018-22124-z
Kara Y, He H, Molnár K (2020) Shear-aided high-throughput electrospinning: a needleless method with enhanced jet formation. J Appl Polym Sci 137:49104. https://doi.org/10.1002/APP.49104
Yan G, Niu H, Zhou H et al (2018) Electro-aerodynamic field aided needleless electrospinning. Nanotechnology 29:235302. https://doi.org/10.1088/1361-6528/aab830
Esmaeilzadeh I, Mottaghitalab V, Tousifar B et al (2015) A feasibility study on semi industrial nozzleless electrospinning of cellulose nanofiber. Int J Ind Chem 6:193–211. https://doi.org/10.1007/S40090-015-0043-y
Roemhild K, Niemz F, Mohan T et al (2016) The cellulose source matters—hollow semi spheres or fibers by needleless electrospinning. Macromol Mater Eng 301:42–47. https://doi.org/10.1002/MAME.201500191
Neibolts N, Platnieks O, Gaidukovs S et al (2020) Needle-free electrospinning of nanofibrillated cellulose and graphene nanoplatelets based sustainable poly (butylene succinate) nanofibers. Mater Today Chem 17:100301. https://doi.org/10.1016/j.mtchem.2020.100301
Grimmelsmann N, Homburg SV, Ehrmann A (2017) Needleless electrospinning of pure and blended chitosan. IOP Conf Ser Mater Sci Eng 225:012098. https://doi.org/10.1088/1757-899X/225/1/012098
Lou CW, Lin MC, Huang CH et al (2022) Preparation of needleless electrospinning polyvinyl alcohol/water-soluble chitosan nanofibrous membranes: antibacterial property and filter efficiency. Polymers (Basel) 14:1054. https://doi.org/10.3390/polym14051054
Poshina DN, Khadyko IA, Sukhova AA et al (2019) Needleless electrospinning of a chitosan lactate aqueous solution: influence of solution composition and spinning parameters. Technologies 8:2. https://doi.org/10.3390/technologies8010002
Turan D, Gibis M, Gunes G et al (2018) The impact of the molecular weight of dextran on formation of whey protein isolate (WPI)–dextran conjugates in fibers produced by needleless electrospinning after annealing. Food Funct 9:2193–2200. https://doi.org/10.1039/C7FO02041D
Kutzli I, Gibis M, Baier SK, Weiss J (2018) Fabrication and characterization of food-grade fibers from mixtures of maltodextrin and whey protein isolate using needleless electrospinning. J Appl Polym Sci 135:46328. https://doi.org/10.1002/APP.46328
Cengiz-Ca̧llioǧlu F (2014) Dextran nanofiber production by needleless electrospinning process. E-Polymers 14:5–13. https://doi.org/10.1515/epoly-2013-0021
El-Aassar MR, Al-Deyab SS, Kenawy ER (2013) Covalent immobilization of β-galactosidase onto electrospun nanofibers of poly (AN-co-MMA) copolymer. J Appl Polym Sci 127:1873–1884. https://doi.org/10.1002/APP.37922
Keirouz A, Zakharova M, Kwon J et al (2020) High-throughput production of silk fibroin-based electrospun fibers as biomaterial for skin tissue engineering applications. Mater Sci Eng C 112:110939. https://doi.org/10.1016/j.msec.2020.110939
Vadodaria K, Stylios G (2016) A study of bubble electrospinning of ethylcellulose ultrafine fibres. Polym Polym Compos 24:265–272. https://doi.org/10.1177/096739111602400405
Kutzli I, Gibis M, Baier SK, Weiss J (2018) Formation of whey protein isolate (WPI)-maltodextrin conjugates in fibers produced by needleless electrospinning. J Agric Food Chem 66:10283–10291. https://doi.org/10.1021/acs.jafc.8B02104
Author information
Authors and Affiliations
Contributions
Literature survey and writing—original draft: V.R.; writing—review and editing: L.M., M.M.L., J.A.M., and C.A.; conceptualization, review, and supervision: J.A.M. and C.A.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raja, V., Mahalakshmi, L., Leena, M.M. et al. Needleless Electrospinning: Concepts and Applications in the Food Industry. Food Eng Rev 16, 252–269 (2024). https://doi.org/10.1007/s12393-023-09362-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-023-09362-2