Abstract
The physical phenomenon underlying the electrohydrodynamic atomization also known as electrospinning or electrospraying was first discovered in the seventeenth century by William Gilbert. Although the technology has been applied in the plastic and textile industries, its application in the food industry has been relatively new. Not all biopolymers are electrospinnable; however, cellulose acetate, chitosan, zein, alginate, gelatin, and soy protein have been investigated for the development of edible nanofibers using electrospinning. The factors that govern the electrospinning ability of a polymer are the molecular weight of the polymer, viscosity, conductivity, concentration, and the type of solvent used to dissolve the polymer. Electrospun fibers exhibit high porosity that is deemed suitable as a carrier and an encapsulation method for drugs and therapeutic agents such as antioxidants and other bioactive compounds. This chapter provides a detailed description of the preparation of polymer solutions for electrospinning and control of fiber morphology and diameter, loading of bioactive compounds to polymer solutions, and the resulting fiber properties and stability tests of the loaded nanofibers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Key words
1 Introduction
1.1 Historical Background
Electrospinning is a method to produce ultrafine fibers from a polymer solution with diameters ranging from a few nanometers to micrometers using a high-voltage electric field.
In the electrospinning process, a polymer solution is pumped across a high-voltage electric field using a capillary pump. Once the electric field reaches a critical value at which the repulsive electric force overcomes the surface tension of polymer solution, the polymer solution is ejected from the tip to a collector. The polymer jet solidifies due to the fast evaporation of the solvent while traveling to the collector and is deposited on it.
Electrospinning and electrospraying take place under a phenomenon known as electrohydrodynamic atomization (EHDA). The importance of EHDA is that its ability to produce fine, near-monodisperse particles and fibers in the micro to nano range, with high precision and efficiency of mass transfer control. The physical phenomenon underlying EHDA was first noted in the seventeenth century by William Gilbert, who observed that a droplet would adopt a cone shape and a spray of liquid would eject from the droplet when a piece of charged amber was placed close to it [1].
In 1882, Lord Rayleigh theoretically estimated the maximum number of charges that a liquid droplet could carry before liquid jets would be ejected from the surface. The physics behind the phenomenon can be summarized as: “the tendency of a capillary force to form a spherical form of equilibrium can be overcome by electrifying the drop” [2]. “Rayleigh instability” was later often denoted as an occurrence of instability of electrospinning fiber jets, which minimizes the surface energy of the liquid jet by breaking it up into droplets [3].
Not all polymers are electrospinnable. The factors that govern the electrospinning ability of a polymer include viscosity, conductivity, molecular weight, and the type of solvent used. The concentration of the polymer used in the solvent determines whether it can be electrospun into nanofibers or not. Concentration also plays a crucial part in the morphology of the fabricated nanofibers [4].
The production of nanofibers from polymer solution/melt under an electrical field has been investigated to produce edible nanofibers for the food industry, biomedical, and cosmetic applications [5]. The low rate of production and the restrictions on the use of solvent that have to comply with food-grade materials are some of the main limitations of electrospinning for the fabrication of edible fibers [6]. Various biopolymers, including cellulose acetate/egg albumen blends [7], chitosan [8], pullulan/β-cyclodextrin blends [9], zein [10], alginate [11], gelatin [12], soy protein [13], etc., have been investigated for the development of edible nanofibers using electrospinning.
1.2 Theory of Electrospinning
During the electrospinning process, the polymer solution is subjected to a strong electric field that causes the buildup of electrical charges within the liquid to form repulsive forces. When the charged electrical forces overcome the surface tension of the polymer liquid, the meniscus of the liquid clinging to the tip of the spinneret will elongate into a conical shape (“Taylor cone”) and draws towards the grounded collector [14].
Before reaching the collector, the solvent in the polymer jet evaporates during the flight and the fibers are deposited on the surface of the collector (Fig. 1a). Scanning electron microscopy (SEM) image of electrospun fibers is shown in Fig. 1b. Under certain set of operating conditions, the solution jet breaks down into droplets rather than fibers resulting in electrospraying as shown in Fig. 1c.
Image of electrospun fiber mat (a); SEM images of electrospun fibers (b); electrosprayed droplets (c) [15]
2 Materials
2.1 Polymers for Electrospinning
The carriers (polymers) that have been regularly used in the food industry are “generally recognized as safe” (GRAS) materials, which can be natural biopolymers, like polysaccharides, proteins, lipids, and gums, or synthetic polymers, like polyvinyl alcohol (PVOH); polyethylene oxide (PEO); and polyvinylpyrrolidone (PVP) [16]. It is imperative to determine the polymer solution properties such as rheology, viscosity, surface tension, and electrical conductivity to achieve successful electrospinning process. While this information will give a profound insight into the electrospinnability of the selected polymer, it is also important to note that solution properties may vary with the choice of solvent. As a result, each polymer will require certain adjustments together with specific processing conditions in order to be electrospun. Natural biopolymers generally will exhibit limited success in electrospinning due to bio-variations and molecular weight disparity. Consequently, the addition of synthetic polymers into the biopolymers is frequently performed to improve its electrospinnability. Table 1 summarizes various GRAS polymers that have been reported to be electrospinnable in their native forms.
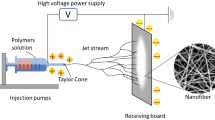
2.2 Electrospinning Setup
A typical electrospinning setup includes a syringe with a metal capillary as the spinneret (e.g., hypodermic syringe needle), syringe pump, high-voltage power supply, and collector that can be grounded or oppositely charged (Fig. 2). The arrangement of the electrospinning setup is generally horizontal, but vertical setups that are controlled by gravitational forces have also been reported. The syringe serves as a reservoir to contain a polymer solution, whereas the pump is used to feed the polymer solution into the spinneret. The spinneret will be charged using a direct current (DC) high-voltage power supply that has either positive or negative outputs. The spinneret can be configured as single, coaxial, or multiaxial to meet different needs. Similarly, the syringe pump can be either single or multichannel that holds more than one syringe. DC high-voltage power supply is the most important component during the electrospinning process. The maximum output of voltage for a DC high-voltage power supply may vary (typically around 20–35 kV). Electrospun fibers will be collected onto a stationary or rotating collector, where the type of collector determines morphology and alignment of the fabricated fibers. The electrospun fiber mats obtained are generally dried in a vacuum oven overnight to remove the residual solvent.
Schematic illustrations of electrospinning setup. (a) Horizontal, (b) vertical. (Adapted from Neo [15])
3 Methods
3.1 Preparation of Polymer Solution for Electrospinning
-
1.
When preparing a polymer solution for electrospinning process, it is imperative to make sure that the polymer is fully dissolved in the solvent. In addition, the selected solvent needs to be able to dissolve the targeted bioactive compound for encapsulation without affecting its functional properties. Certain polymers (e.g., chitosan in acetic acid; PVOH in water) might require stirring overnight or heating to yield a uniform solution.
-
2.
The choice of solvent will determine the surface tension, conductivity, and volatility of the solution. Selection of a desirable solvent is generally based on empirical and solubility models. Interestingly, a solvent with high solubility or volatility does not warrant a good electrospinning process. Some studies have proposed the use of ternary solubility diagram (Teas graph) to systematically explore the appropriate electrospinnable binary solvent systems for various polymers [17, 18].
-
3.
Spinning solutions also can be obtained by mixing two polymer solutions prepared separately. Gentle mixing is required to reduce the number of bubbles that may be formed and to obtain a homogeneous solution at a desired ratio.
-
4.
A bare minimum polymer concentration is necessary for fiber formation and varies according to a polymer’s molecular weight as well as the type solvent used. The polymer molecular weight will influence the bare minimum polymer concentration for fiber formation. It will also have an effect on viscosity, conductivity, as well as surface tension of the solution.
-
5.
Electrospraying (formation of droplets, a sister technology of electrospinning) will take place when the solution viscosity is too low to sustain a stable electrified polymer jet due to Rayleigh instability. Bead-on-string structure will form at an intermediate viscosity, which is considered undesirable in electrospinning as it compromises the high surface-area-to-volume ratio. It is useful to know that uniform fibers can be fabricated when the polymer solution is above the entangle concentration (Ce). Hence, the rheological behavior of a polymer solution is deemed important for a successful electrospinning process. Rheological measurements allow the determination of the intrinsic viscosity and molecular organization of the polymer in a solution.
-
6.
A polymer’s intrinsic viscosity in a specific solvent can be measured using rotational or microfluidic rheometer at a range of concentrations (C). When the polymer concentration C is less than its entanglement concentration Ce, the polymer solution can be specified as dilute (C < Ce), when C is equal to Ce (C = Ce), it is considered semi-dilute, and when C > Ce, the polymer solution is considered concentrated. Ce can be estimated from a logarithmic plot of specific viscosity against concentration (Fig. 3). A polymer concentration in solution higher than Ce is known to exhibit sufficient chain entanglement for stable electrospinning process to fabricate bead-free fibers [20].
-
7.
Biopolymers with polyelectrolyte character such as alginate and pectin and gums like Arabic gum and Karaya gum are commonly unable to form stable polymer jets during the electrospinning process due to their high surface tension, limited viscoelasticity, and insufficient molecular entanglement [21, 22]. Electrospinning of these biopolymers is frequently performed with another synthetic polymer such as PEO and PVA that serve as carriers to provide necessary entanglement for electrospinning.
-
8.
Salts and surfactants can be added to modulate the solution conductivity and surface tension.
Logarithmic plot of specific viscosity (ηsp) and concentration of zein powder (1–35 wt%) in 80 wt% ethanol aqueous solutions [19]. (Reprinted from Journal of Food Engineering, 109, 645–651, with permission from Elsevier)
3.2 Control of Electrospun Fiber Morphology and Diameter
-
1.
In addition to solution parameters, the processing and ambient parameters will also influence morphology of electrospun fibers. Processing parameters involve applied voltage, solution feed rate, and distance between spinneret and collector. Ambient parameters are environmental conditions like relative humidity and temperature where the electrospinning takes place. These parameters are subjected to the electrospinning setup and design.
-
2.
The applied voltage will influence the surface charges on the spinneret and the subsequent polymer jet formation. Additionally, the threshold voltage to initiate cone jet formation is also determined by surface tension and viscosity of the polymer solution. A higher voltage can lead to smaller fiber diameter due to higher repulsive forces that promote stretching and whipping of the jet. On the contrary, higher applied voltage can also lead to larger fiber diameter caused by greater mass flow of the polymer solution.
-
3.
Solution feed rate varies with the inner diameter of the spinneret. A simple hypodermic needle with blunted tip and internal diameter below 2 mm is generally used in conventional electrospinning setup. Thinner fibers may be fabricated at lower feed rate as the solvent has more time to be evaporated, which favors the jet elongation.
-
4.
Similarly, a longer distance between the spinneret and the collector will produce thinner fibers as it will allow longer times for the jet to be stretched and elongated. The distance between spinneret and collector is generally suggested to be in the range of 10–20 cm. This is because the electrostatic forces between two can be reduced by the square of the distance between them.
-
5.
Higher environmental temperatures generally promote thinner fibers due to reduction in surface tension and solution viscosity. Furthermore, provision of higher energy to the polymer molecule at higher temperature will also promote electrical conductivity of the solution.
-
6.
The effect of humidity on electrospinning is often overlooked despite relative humidity has been confirmed to influence morphology and structure of the fabricated fibers. A high relative humidity normally will allow the formation of porous, wrinkled, or rough fibers by the polymer jet through moisture condensation or charge dissipation [23]. This wide range of obtained morphologies has offered several possibilities that are beneficial in encapsulation applications.
-
7.
The effect of processing and ambient parameter on electrospinning may vary from case to case. Modeling the relationship and optimization of these parameters using response surface methodology (RSM) can be considered useful in obtaining the desired fiber morphology and diameter.
3.3 Loading of Bioactive Compounds into Fibers
3.3.1 Blended Electrospinning
Encapsulation of bioactive compounds is often achieved by mixing the active component and polymer in a common solvent as a blend prior to the electrospinning process. Blended electrospinning is a simple method, in which the bioactive compound can be dissolved directly into the polymer solution. The solution jet will solidify and dry up during its flight towards the electrospinning collector that results in entrapment of the active component within the polymeric matrix.
Blended electrospinning is not suitable for the loading of sensitive biological compounds. Examples like proteins, enzymes, or cells may be denatured in the organic solvent used or destroyed during the blending process. Nevertheless, these limitations can be overcome by first encapsulating the sensitive biological compound as small particles followed by blending with polymer solution before electrospinning.
Non-electrospinnable natural polymers such as keratin and lignin could be made into nanofibers by blending them with suitable copolymers, for example, blending lignin with dendrimers such as polyamidoamine (PAMAM). The blending helps lignin to become a more spinnable fiber due to its good miscibility [24].
3.3.2 Coaxial Electrospinning
Coaxial electrospinning involves a concentric spinneret that will form a core-sheath fiber structure. The inner jet or core fluid contains the bioactive compounds, while the outer shell will be an electrospinnable polymer solution.
The bioactive compound and the polymer will be divided in a core-sheath structure through electrospinning of two coaxial capillaries concurrently. This structure governs the release kinetics of the incorporated bioactive component as the release mechanism is led by polymeric shell degradation.
The core and shell solutions are prepared individually in an appropriate solvent followed by loading them into two different syringes. Next, two separate syringe pumps will drive both solutions to a coaxial concentric spinneret that connects both syringes for electrospinning. The electrospinning could be done horizontally or vertically [25].
3.3.3 Emulsion Electrospinning
Emulsion electrospinning is another approach that allows fabrication of core-shell fiber structure. Unlike coaxial electrospinning, this approach involves single feeding capillary that is similar to the blended electrospinning. The bioactive compound and the selected polymer will be dissolved in two immiscible solvents followed by emulsification through the addition of emulsifier or surfactant. By incorporating emulsion droplets, various lipophilic components are allowed to be loaded into the fibers’ hydrophilic polymer matrix [26].
For a water-in-oil (w/o) emulsion system, a hydrophilic bioactive compound will be dissolved in aqueous solution as dispersed phase, whereas a hydrophobic polymer together with an emulsifier or surfactant will be dissolved in an organic solvent as continuous phase. The two solutions are homogenized to form a uniform emulsion before the electrospinning process.
The organic continuous phase will evaporate faster than the aqueous dispersed phase during electrospinning process, which resulted in viscosity gradient of the system. The gradient will cause an ongoing development of the aqueous dispersed phase to stay in the fiber interior. Consequently, the hydrophilic bioactive compound can be encapsulated in hydrophobic polymer fiber in core-sheath structure with emulsifier/surfactant at the interface. Due to the high specific surface area, interconnected pore structures, and capability to incorporate active components at nanoscale, electrospun nanofibrous membranes can be used to segregate oil and water at high permeability and low energy cost [27].
3.4 Characterization of Electrospun Fibers
Electrospun fiber mats will exhibit high surface-area-to-volume ratios and quantum confinement effects as compared to its bulk counterparts. Hence, it is essential to perform physicochemical characterization of the fabricated system and to determine the system efficacy. The characterization is usually performed using microscopy (e.g., scanning electron microscopy, transmission electron microscopy), spectroscopy (e.g., X-ray diffraction, infrared spectroscopy), and thermal methods (e.g., differential scanning calorimetry, thermogravimetric analysis). Cross-linking of the electrospun fiber mat will be done to improve their mechanical properties based on the intended application. The bioactivity properties and controlled release performance of the encapsulated bioactive compounds are usually investigated by dissolution of the fibers in a suitable medium followed by spectrophotometric assays.
The electrospun fiber mats exhibit high porosity that is deemed suitable as a drug or therapeutic agent carrier [28]. Electrospinning has been a promising technique for encapsulation of antioxidants and other bioactive compounds [29]. Research on the incorporation of antioxidants and other functional ingredients into electrospun fiber matrices have been reported by several researchers [30,31,32].
Zein (Ze), a prolamin fraction obtained from maize, is known for its good thermal resistance and oxygen barrier properties, which finds many applications in the packaging sector [33]. Gallic acid is a naturally occurring phenolic acid that is widely present in the plant kingdom. Studies have shown that gallic acid and its derivatives are antioxidants that exhibit anti-inflammatory and antimicrobial abilities [34, 35]. The physicochemical changes of gallic acid-loaded zein fibers (Ze-GA) fabricated by using electrospinning as an encapsulation technique have been studied by Neo et al. [30, 31] and Neo [15].
3.4.1 Solution Properties of Zein and Gallic Acid
Zein solutions at a concentration of 25 wt% and gallic acid at different concentrations were prepared by dissolving them in 80 wt% ethanol aqueous solutions.
The viscosity was found to increase with the increment of gallic acid, but the conductivity decreased with the increase in concentration of gallic acid in the polymer solution (Table 2). The conductivity of weak electrolytes such as gallic acid is highly concentration dependent, and the dissociation of weak electrolytes in solution to produce ions is even lower at higher concentrations.
3.4.2 Fiber Properties
The Ze-Ga composite fiber mats were stained with osmium tetroxide and observed using high-resolution TEM (Fig. 4). The neat Ze fibers demonstrated smooth surfaces without any visible aggregates. Additionally, Ze-GA electrospun fibers were found to show dark patches throughout the fibers. A possible explanation for this might be due to staining of the phenol ring of gallic acid with osmium tetroxide that led to formation of black stained areas [36]. This observation is encouraging and implies successful encapsulation of gallic acid within the zein fibers.
TEM images of Ze and Ze-GA electrospun fibers at 100 nm scale bar. (a) Ze, (b) Ze-GA 5%, (c) Ze-GA 10%, (d) Ze-GA 20% [15]
3.4.3 Fiber Diameter
There was an increase in average fiber diameter with the increment of incorporated gallic acid content. Such observation is not surprising as the number of molecular entanglement is directly proportional to the gallic acid concentration, which will increase the viscosity and the resultant fiber diameter [19]. Figure 5 shows the distribution of fiber diameters of the Ze-GA composite fibers as the concentration of GA increased from 0% to 20%.
Distribution of fiber diameter of Ze-GA electrospun fibers at applied voltage of 16 kV, feed rate of 0.8 mL/h, and distance between needle tip and collector of 13 cm. (a) Ze, (b) Ze-GA 5%, (c) Ze-GA 10%, (d) Ze-GA 20% [15]
3.4.4 Thermal Properties of the Electrospun Fibers
Figure 6 shows the melting endotherms of the neat zein and Ze-GA composite electrospun fibers. A sharp endothermic peak corresponded to the melting point of highly crystalline gallic acid was recorded at 260 °C as shown in Fig. 6e. Interestingly, the neat Ze and Ze-GA composite fibers had demonstrated different thermal profile from pure gallic acid, and a broad endotherm in the range of 40–100 °C with a peak at 68 °C was observed in these electrospun fibers (Fig. 6a–d).
DSC thermograms of different samples. (a) Ze, (b) Ze-GA 5%, (c) Ze-GA 10%, (d) Ze-GA 20%, (e) gallic acid powder [30]. (Reprinted from Food Chemistry, 136, 1013–1021, with permission from Elsevier)
Several studies described these characteristic endotherms of Ze fibers as the “dehydration temperature” (TD) that is due to the evaporation of bound water or volatile components with elevated temperature [37, 38]. DSC thermograms of the Ze-GA composite fibers (Fig. 6b–d) did not show any melting peaks of gallic acid as seen in Fig. 6e, which suggested a change of physical properties in the loaded gallic acid from highly crystalline to amorphous structure. Glass transition temperature (Tg) of the neat Ze fibers without GA was at 156 °C (Table 3), and degradation of Ze and all the Ze-GA composite fibers took place at approximately 210 °C (Fig. 6). All the Ze-GA composite fibers also exhibited Tg values lower than that of neat Ze in the range of 125–145 °C as shown in Table 3.
Xie et al. [39] suggested that the reduction of Tg could be due to the plasticizing effect contributed by the incorporated component into the polymer solution [39]. The results obtained also implied the presence of molecular interactions between GA and Ze, where GA has been well integrated within the zein matrices modifying the mobility of zein molecular chains.
3.4.5 X-Ray Diffraction (XRD) Pattern of Electrospun Fibers
Figure 7 is a schematic of X-ray diffraction (XRD) of gallic acid and the electrospun fibers. The XRD studies revealed a high degree of crystallinity of gallic acid, where peaks at 2θ values of 16.1°, 25.3°, and 27.6° were being observed (Fig. 7f). Physical mixture of zein and gallic acid powder (ratio 4:1) was found to exhibit diffraction peaks at around 9.3°, 16.4°, 20.3°, 25.3°, and 27.5° 2θ as shown in Fig. 7b. Peaks at 16.4°, 25.3°, and 27.5° 2θ are characteristics of gallic acid powder in crystalline state. However, a typical amorphous broad peak at around 12.0° 2θ was observed in XRD of the Ze and Ze-GA composite fibers (Fig. 7a, c–e). Interestingly, Ze fibers had lost the two diffraction peaks (Fig. 7a) of raw zein powder as physical mixture at 9.3° and 20.3° 2θ as shown in Fig. 7b, which suggested transition on its physical structure and order after electrospinning. The loss of the signature diffraction peaks of gallic acid powder in the XRD pattern of all Ze-GA electrospun fibers is indicative of difference between the physical structure of gallic acid before and after the incorporation into zein electrospun fibers, which is in line with the findings from DSC studies as highlighted earlier. Shape and position of the amorphous haloes as observed in the Ze-GA composite electrospun fibers were similar to the neat Ze. In general, the composite electrospun fibers had showed amorphous properties similar to neat Ze electrospun fibers even after the incorporation of gallic acid. Diminished crystalline intensity of the gallic acid also may reflect the degree to which it has been encapsulated within the polymeric network. Rostamabadi et al. [40] suggested that the crystallinity of the fabricated encapsulation system will influence its relative release performance, which signifies the importance of performing X-ray crystallography analysis [40].
XRD patterns of different samples. (a) Ze, (b) physical mixture of Ze-GA powder (4:1 w/w), (c) Ze-GA 5%, (d) Ze-GA 10%, (e) Ze-GA 20%, (f) gallic acid powder [30]. (Reprinted from Food Chemistry, 136, 1013–1021, with permission from Elsevier)
3.4.6 Stability Testing Using Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
ATR-FTIR spectroscopy is a good method to measure stability of the nanofiber matrices at high temperatures and storage. In the case of zein and gallic acid nanofiber mats that were described earlier, ATR-FTIR spectra were recorded on a Thermo Electron NICOLET 8700 FTIR spectrophotometer using the Smart Orbit ATR accessory with diamond crystal, single bounce at 45° (Thermo Electron Corporation, Waltham, MA, USA) over the wave number region of 600–3600 cm−1. Interferograms were averaged for 32 scans at 4 cm−1 resolution. The measurements were carried out in triplicate, and the signals were processed using the OMNIC 5.0 spectroscopic software (Nicolet Instrument Corp., Madison, WI). The stability test was repeated after storing a new batch of Ze and Ze-GA fiber mats individually at 60 °C for 30 days. The ATR-FTIR spectra were recorded, and the measurements were carried out in duplicate and are shown in Fig. 8. There were no changes in the spectra after storage at 60 °C for 30 days, showing good stability of the zein-gallic acid nanofibers.
ATR-FTIR spectra of Ze and Ze-GA electrospun fibers after 30 days of storage at 60 °C [15]
4 Notes
-
1.
In general, prepare a range of biopolymer concentrations to be investigated (e.g., 0.1–30%, w/v). Take note on the moisture content of the biopolymer powder when performing the calculations. For each concentration, weigh the biopolymer powder into a right vessel. Add the solvent with constant stirring on a magnetic stirrer until the biopolymer powder is fully dissolved. Depending on the polymer and its solubility in the solvent, the samples may need to be heated for about 1 h or more to facilitate the process. Turn off heat and allow the solution to cool to room temperature. The solution is then ready for characterization such as rheological testing and electrospinning.
-
2.
In preliminary experiments, it was found that Ze solutions of over 25 wt% concentration gave uniform nanofibers without blemishes [15]. Zein solutions used for electrospinning were prepared at a concentration of 25 wt% by dissolving Ze powder in 80 wt% ethanol aqueous solutions under constant stirring at room temperature. Electrospun Ze fibers containing various amounts of gallic acid were prepared by first dissolving gallic acid in 80 wt% ethanol aqueous solutions, followed by dissolving Ze powder to obtain 25 wt% Ze solutions.
-
3.
The specific viscosity ηsp of the polymer was calculated from Eq. 1.
$$ {\eta}_{\mathrm{s}\mathrm{p}}=\left({\eta}_0\hbox{--} {\eta}_{\mathrm{s}}\right)/{\eta}_{\mathrm{s}} $$(1)where ηs is the solvent viscosity (2.58 mPa s), η0 is the zero shear rate viscosity of the zein solutions at different concentrations.
-
4.
The entanglement concentration (Ce) is the concentration at which a logarithmic plot of zero shear viscosity against concentration intersects. It is important to note that Ce is dependent on the polymer chain length, macromolecular structure, and the solvent used. Therefore, for each polymer in a given solvent, the Ce has to be determined first in order to produce uniform nanofibers with the desired morphological properties.
-
5.
Instead of DC high-voltage power supply, AC potential can also be used as the driving force in electrospinning. A study by Kessick et al. [41] suggested that the electrospun fibrous matrices fabricated using an AC potential would exhibit a greater degree of fiber alignment and more residual solvent as compared to DC potential [41].
-
6.
The major drawback of blended electrospinning is migration of the charged bioactive molecules onto the jet surface during electrospinning process. The bioactive compounds are randomly dispersed in the fiber matrix and generally observed on the surface of the fibers. Localization of the bioactive compounds on the fiber surface will lead to unwanted burst of release. Burst release may be resolved by inducing chemical bonding between the bioactive compounds with the polymeric carrier at molecular level. Additionally, the release performance can also be improved by blending cyclodextrin inclusion complexes of the bioactive compound with the polymer solution before electrospinning process [42].
-
7.
Coaxial electrospinning is a complex technique that requires careful control of processing parameters. A common solvent is suggested to be added into the core and shell fluids to attain the best electrospinning results [43]. The flow rate of both inner and outer fluids has to be precisely tailored in order to achieve stable coaxial jet formation. Successful fabrication of core-sheath structure using an inner needle diameter of 0.21–0.35 mm and an outer needle of 0.82–1.2 mm with core-to-shell flow ratios of 1:3 and 1:10 was reported by William et al. [44].
-
8.
Viscosity of the dispersed and continuous phase; electrospinnability of the polymer; type of emulsifier/surfactant; and processing conditions need to be carefully tuned for successful emulsion electrospinning. Viscosity of the dispersed phase is required to be higher than continuous phase in order to form a continuous core along the fiber axis [45] as suggested by Angeles et al. [45]. Emulsion electrospinning is more likely to destruct the sensitive bioactive compounds as compared to coaxial electrospinning due to the mixing and interface tension between aqueous and organic phase of the emulsion [46].
References
Luo CJ, Loh S, Stride E, Edirisinghe M (2012) Electrospraying and electrospinning of chocolate suspensions. Food Bioprocess Technol 5:2285–2300
Rayleigh L (1882) XX. On the equilibrium of liquid conducting masses charged with electricity. Philos Mag 14:184–186
Miao J, Miyauchi M, Simmons TJ, Dordick JS, Linhardt RJ (2010) Electrospinning of nanomaterials and applications in electronic components and devices. J Nanosci Nanotechnol 10:5507–5519
Muthu Kumar TS, Senthil Kumar K, Rajini N, Siengchin S, Ayrilmis N, Rajulu AV (2019) A comprehensive review of electrospun nanofibers: food and packaging perspective. Compos Part B 175(2019):107074. https://doi.org/10.1016/j.compositesb.2019.107074
Kouhi M, Prabhakaran MP, Ramakrishna S (2020) Edible polymers: an insight into its application in food, biomedicine and cosmetics. Trends Food Sci Technol 103(2020):248–263
Kouhi M, Jayarama Reddy V, Ramakrishna S (2019) GPTMS-modified bredigite/PHBV nanofibrous bone scaffolds with enhanced mechanical and biological properties. Appl Biochem Biotechnol 188:357–368
Wongsasulak S, Patapeejumruswong M, Weiss J, Supaphol P, Yoovidhya T (2010) Electrospinning of food-grade nanofibers from cellulose acetate and egg albumen blends. J Food Eng 98:370–376
Yue T-T, Li X, Wang X-X, Yan X, Yu M, Ma J-W et al (2018) Electrospinning of carboxymethyl chitosan/polyoxyethylene oxide nanofibers for fruit fresh keeping. Nanoscale Res Lett 13:239. https://doi.org/10.1186/s11671-018-2642-y
Fuenmayora CA, Mascheronia E, Cosioa MS, Piergiovannia L, Benedettia S, Ortenzic M, Schiraldia A, Manninoa S (2013) Encapsulation of R-(+)-limonene in edible electrospun nanofibers. Chem Eng Trans 32:1771–1776. https://doi.org/10.3303/CET1332296
de Oliveira Mori CLS, dos Passos NA, Oliveira JE, Mattoso LHC, Mori FA, Carvalho AG et al (2014) Electrospinning of zein/tannin bio-nanofibers. Ind Crop Prod 52:298–304
Rezaei S, Valipouri A, Ravandi SAH, Kouhi M, Mobarakeh LG (2019) Fabrication, characterization, and drug release study of vitamin C–loaded alginate/polyethylene oxide nanofibers for the treatment of a skin disorder. Polym Adv Technol 30:2447–2457
Tang Y, Zhou Y, Lan X, Huang D, Luo T, Ji J et al (2019) Electrospun gelatin nanofibers encapsulated with peppermint and chamomile essential oils as potential edible packaging. J Agric Food Chem 67(8):2227–2234
Shankar A, Seyam A-F, Hudson S (2013) Electrospinning of soy protein fibers and their compatibility with synthetic polymers. J Textile Apparel Technol Manag 8(1):1–14. Spring 2013
Hohman MH, Shin M, Brenner MP (2001) Electrospinning and electrically forced jets. I. Stability theory. Phys Fluids 13(8):2201–2220
Neo YP (2014) Electrospinning as a novel encapsulation method for food applications. PhD thesis, The University of Auckland, New Zealand
Zagho MM, Elzatahry A (2016) Recent trends in electrospinning of polymer nanofibers and their applications as templates for metal oxide nanofiber preparations. In: Electrospinning - material, techniques, and biomedical applications. IntechOpen, London. https://doi.org/10.5772/65900
Lasprilla-Botero J, Álvarez-Láinez M, Lagaron JM (2018) The influence of electrospinning parameters and solvent selection on the morphology and diameter of polyimide nanofibers. Mater Today Commun 14:1–9
Luo CJ, Nangrejo M, Edirisinghe M (2010) A novel method of selecting solvents for polymer electrospinning. Polymer 51(7):1654–1662
Neo YP, Ray S, Easteal AJ, Nikolaidis MG, Quek SY (2012) Influence of solution and processing parameters towards the fabrication of electrospun zein fibers with sub-micron diameter. J Food Eng 109:645–651
Kong L, Ziegle GR (2014) Molecular entanglement and electrospinnability of biopolymers. J Vis Exp (91):e51933. https://doi.org/10.3791/51933. http://www.jove.com/video/51933
Padil VV, Cheong JY, AkshayKumar KP, Makvandi P, Zare EN, Torres-Mendieta R, Wacławek S, Černík M, Kim ID, Varma RS (2020) Electrospun fibers based on carbohydrate gum polymers and their multifaceted applications. Carbohydr Polym 247(1):116705
Xiao Y, Lin L, Shen M, Shi X (2020) Design of DNA aptamer-functionalized magnetic short nanofibers for efficient capture and release of circulating tumor cells. Bioconjug Chem 31(1):130–138
Szewczyk PK, Stachewicz U (2020) The impact of relative humidity on electrospun polymer fibers: from structural changes to fiber morphology. Adv Colloid Interf Sci 286:102315. https://doi.org/10.1016/j.cis.2020.102315
Akbari S, Bahi A, Farahani A, Milani AS, Ko F (2021) Fabrication of lignin/dendrimer electrospun blended fiber mats. Molecules 26(3):518. https://doi.org/10.3390/molecules26030518
Baykara T, Taylan G (2021) Coaxial electrospinning of PVA/Nigella seed oil nanofibers: processing and morphological characterization. Mater Sci Eng B 265:115012. https://doi.org/10.1016/j.mseb.2020.115012
Arecchi A, Mannino S, Weiss J (2010) Electrospinning of poly(vinyl alcohol) nanofibers loaded with hexadecane nanodroplets. J Food Sci 75:N80–N88
Su R, Li S, Wu W, Song C, Liu G, Yu Y (2021) Recent progress in electrospun nanofibrous membranes for oil/water separation. Sep Purif Technol 256(2021):117790. https://doi.org/10.1016/j.seppur.2020.117790
Deng Y-F, Zhang D, Zhang N, Huang T, Lei Y-Z, Wang Y (2021) Electrospun stereocomplex polylactide porous fibers toward highly efficient oil/water separation. J Hazard Mater 407:124787. https://doi.org/10.1016/j.jhazmat.2020.124787
Celebioglu A, Uyar T (2021) Electrohydrodynamic encapsulation of eugenol-cyclodextrin complexes in pullulan nanofibers. Food Hydrocoll 111:106264. https://doi.org/10.1016/j.foodhyd.2020.106264
Neo YP, Ray S, Jin J, Gizdavic-Nikolaidis M, Nieuwoudt MK, Liu D, Quek SY (2013a) Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: a physicochemical study based on zein–gallic acid system. Food Chem 136:1013–1021
Neo YP, Swift S, Ray S, Gizdavic-Nikolaidis M, Jin J, Perera CO (2013b) Evaluation of gallic acid loaded zein sub-micron electrospun fiber mats as novel active packaging materials. Food Chem 141:3192–3200
Hu X, Wang X, Han L, Li S, Zhou W (2021) Antioxidant and antimicrobial polyvinyl alcohol electrospun nanofibers containing baicalein-hydroxypropyl-β-cyclodextrin inclusion complex. Colloids Surf A Physicochem Eng Asp 614:126135. https://doi.org/10.1016/j.colsurfa.2021.126135
Zhou L, Wang Y (2021) Physical and antimicrobial properties of zein and methylcellulose composite films with plasticizers of oleic acid and polyethylene glycol. LWT Food Sci Technol 140:110811. https://doi.org/10.1016/j.lwt.2020.110811
Ahmad B, Hafeez N, Rauf A, Bashir S, Linfang H, Rehman M, Mubarak MS, Uddin MS, Bawazeer S, Shariati MA, Daglia M, Wan C, Rengasamy KRR (2021) Phyllanthus emblica: a comprehensive review of its therapeutic benefits. S Afr J Bot 138(202):278–310. https://doi.org/10.1016/j.sajb.2020.12.028
Rodrigues L, Donado-Pestana CM, Moura MHC, Rossi e Silva R, Pessoa EVM, Genovese MI (2021) Phenolic compounds from jaboticaba (Plinia jaboticaba (Vell.) Berg) ameliorate intestinal inflammation and associated endotoxemia in obesity. Food Res Int 141:110139. https://doi.org/10.1016/j.foodres.2021.110139
Kriegel C, Kit KM, McClements DJ, Weiss J (2009) Nanofibers as carrier systems for antimicrobial microemulsions. Part I: Fabrication and characterization. Langmuir 25:1154–1161
Luo Y, Teng Z, Wang Q (2011) Development of zein nanoparticles coated with carboxymethyl chitosan for encapsulation and controlled release of vitamin D3. J Agric Food Chem 60:836–843
Muller V, Piai JF, Fajardo AR, Favaro SL, Rubira AF, Muniz EC (2011) Preparation and characterization of zein and zein-chitosan microspheres with great prospective of application in controlled drug release. J Nanomater 2011:928728. https://doi.org/10.1155/2011/928728
Xie C, Li X, Luo X, Yang Y, Cui W, Zou J, Zhou S (2010) Release modulation and cytotoxicity of hydroxycamptothecin-loaded electrospun fibers with 2-hydroxypropyl-β-cyclodextrin inoculations. Int J Pharm 391:55–64
Rostamabadi H, Falsafi SR, Assadpour E, Jafari SM (2020) Evaluating the structural properties of bioactive-loaded nanocarriers with modern analytical tools. Compr Rev Food Sci Food Saf 19(6):3266–3322
Kessick R, Fenn J, Tepper G (2004) The use of AC potentials in electrospraying and electrospinning processes. Polymer 45(9):2981–2984
Oliveira MF, Suarez D, Rocha JCB, Teixeira AVND, Cortes ME, De Sousa FB, Sinisterra RD (2015) Electrospun nanofibers of polyCD/PMAA polymers and their potential application as drug delivery system. Mater Sci Eng C Mater 54:252–261
Nikmaram N, Roohinejad S, Hashemi S, Koubaa M, Barba FJ, Abbaspourrad A, Greiner R (2017) Emulsion-based systems for fabrication of electrospun nanofibers: food, pharmaceutical and biomedical applications. RSC Adv 7(46):28951–28964
Williams GR, Raimi-Abraham BT, Luo CJ (2018) Nanofibers in drug delivery. UCL Press, London. https://doi.org/10.14324/111.9781787350182. 212 p
Angeles M, Cheng HL, Velankar SS (2008) Emulsion electrospinning: composite fibers from drop breakup during electrospinning. Polym Adv Technol 19(7):728–733
Zamani M, Prabhakaran MP, Ramakrishna S (2013) Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int J Nanomedicine 8:2997–3017. https://doi.org/10.2147/IJN.S43575
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this chapter
Cite this chapter
Perera, C.O., Neo, Y.P. (2022). Electrospinning in Food Processing. In: Gavahian, M. (eds) Emerging Food Processing Technologies. Methods and Protocols in Food Science . Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2136-3_10
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2136-3_10
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2135-6
Online ISBN: 978-1-0716-2136-3
eBook Packages: Springer Protocols