Abstract
Background
We evaluated the prognostic value of changes in perfusion defect size (PDS) on serial MPS in patients treated with primary percutaneous coronary intervention (PCI) after acute myocardial infarction (AMI).
Methods
We enrolled 112 patients treated with primary PCI after AMI who underwent two stress MPS within 1 month and after 6 months. Improvement in PDS was defined as a reduction ≥5%. Remodeling was defined as an increase in left ventricular (LV) end-diastolic volume index ≥20%. Cardiac events included cardiac death, nonfatal MI, unstable angina, repeated revascularization, and heart failure.
Results
During a median follow-up of 86 months, 22 events occurred. Event rate was higher (P < .01) in patients with worsening of PDS compared to those with unchanged or improved PDS. Moreover, patients with remodeling had a higher (P < .001) event rate compared to those without. At Cox analysis, worsening of PDS and remodeling resulted independent predictors of events (both P < .01). Patients with both worsening of PDS and remodeling had the worst event-free survival (P <.001).
Conclusion
In patients treated with primary PCI after AMI, worsening of PDS and remodeling are associated to higher risk of events at long-term follow-up. Gated stress MPS improves risk stratification in these patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, primary percutaneous intervention (PCI) is the treatment of choice for acute myocardial infarction (AMI) to improve outcome by limiting the extent and severity of perfusion defects.1 Nevertheless, additional myocardial damage and cardiac remodeling of the left ventricle can develop during the first months after an acute event, with a negative influence on long-term prognosis.2,3 Single-photon emission computed tomography myocardial perfusion imaging (MPS) has been widely used in patients treated with primary PCI, to confirm the success of revascularization and to estimate the risk of cardiac death or reinfarction.4,5 Serial evaluation of left ventricular (LV) myocardial perfusion and function by gated MPS has also been proposed to guide management after revascularization procedures.6 Prior studies found a significant correlation between perfusion parameters measured in the subacute phase after AMI and functional outcome.7,8,9 In particular, infarct size and severity, evaluated by gated MPS one week after AMI, predict cardiac remodeling during follow-up.9 Moreover, infarct size is independently associated with cardiac death and reinfarction beyond clinical variables during long-term follow-up.10 The variations in the extent and severity of myocardial perfusion defects and functional impairment may also have an impact in the long-term risk of AMI patients treated with primary PCI. The aim of the present study was to evaluate the prognostic value of changes in perfusion defect size (PDS) on serial MPS in patients treated with primary PCI after AMI.
Methods
Study Population
The study population consisted of 121 patients treated with primary PCI for AMI at the University of Naples Federico II from February 2009 to December 2011, who underwent two stress MPS within 1 month (MPS-1) and 6 months (MPS-2) after PCI. AMI was diagnosed according to American College of Cardiology/American Heart Association guidelines.11 The exclusion criteria were previous MI or revascularization procedure (based on history, electrocardiogram, and echocardiographic pattern); persistence or recurrence of typical or atypical angina; repeated coronary revascularization within 6 months from MPS-2, severe ventricular arrhythmias, second- or third-degree atrioventricular block; valvular disease requiring surgery; pericarditis; severe renal dysfunction (i.e., creatinine plasma levels >2.5 mg/dl); severe concomitant noncardiac diseases, dementia, and inability to participate in a prospective study for any reason. The Ethics Committee of our Institution approved the protocol and all subject provided informed consent.
Gated SPECT
All patients underwent single-day stress/rest 99mTc sestamibi gated MPS according to the recommendations of the European Association of Nuclear Medicine.12 Gated MPS studies were acquired using a dual-head rotating gamma camera (E.CAM, Siemens Medical Systems, Hoffman Estates, IL, USA) equipped with a low-energy, high-resolution collimator and connected with a dedicated computer system. Imaging was started 60 min after the tracer injection at rest and 45 min after pharmacological stress with dipyridamole (0.142 mg/kg per min intravenous over 4 min). The image interpretation was performed in the absence of attenuation or scatter correction. An automated software program (e-soft, 2.5, QGS/QPS, Cedars-Sinai Medical Center, Los Angeles, CA) was used to calculate end-diastolic volume (EDV), end-systolic volume (ESV), LV ejection fraction (EF), and the scores incorporating both the extent and severity of perfusion defects, using standardized segmentation of 17 myocardial regions.13,14 Volumetric data were corrected for body surface area and expressed as indexes (I). Perfusion defects were quantitated as % of LV myocardium and expressed as PDS (total perfusion defect), ischemia (reversible perfusion defect), and scar (fixed perfusion defect). Abnormal perfusion was defined as PDS ≥5% LV mass.15,16
Variations in perfusion pattern between MPS-1 and MPS-2 were categorized as improvement, with a decrease of ≥5% in PDS; no change; and worsening with an increase of ≥5% in PDS. Remodeling was defined as an increase in LVEDVI at MPS-2 ≥20% from MPS-1.9
Follow-Up
Patient follow-up was prospectively obtained by use of a questionnaire that was assessed by a phone call to all patients and/or general practitioners or cardiologists and by review of hospital or physicians’ records by individuals blinded to the patient’s test results. The end point was the occurrence of cardiac death, nonfatal MI, unstable angina, repeated late coronary revascularization, and heart failure whichever occurred first. Cardiac death, defined as due to new AMI, ventricular arrhythmias, refractory heart failure, or cardiogenic shock, was confirmed by review of death certificate, hospital chart, or physician’s records. Nonfatal MI was defined based on the criteria of typical chest pain, elevated cardiac enzyme levels, and typical alterations of the ECG. Heart failure was considered as event as worsening signs and symptoms, signifying failure of the primary therapeutic management strategy, and resulting in escalation of therapy as an outpatient or requiring hospital admission.17 Unstable angina is defined as myocardial ischemia at rest or on minimal exertion in the absence of acute cardiomyocyte injury/necrosis.18 Patients experiencing noncardiac death during follow-up were censored at the time of death. The date of the last examination or consultation was used to determine follow-up.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation and categorical data as percentage. Differences between groups were analyzed by unpaired t test and χ2 analysis as appropriate. A P value <0.05 was considered statistically significant. Annualized event rates (AER) expressed as % person-years were calculated as the cumulative number of events divided by person-time. Univariable and multivariable Cox regression analyses for the composite endpoint of cardiac death, nonfatal MI, unstable angina, repeated coronary revascularization, and heart failure were performed. The incremental prognostic value of clinical data and imaging findings considering variables in hierarchical order was assessed by the likelihood ratio χ2. Event-free survival curves were obtained by the Kaplan-Meier method and compared with the log-rank test. As the analysis focused on the prognostic value of serial changes in perfusion pattern, landmark analyses of outcomes starting at 6 months were performed. Statistical analysis was performed with STATA 15.0 for Windows (StataCorp LP, College Station, TX).
Results
Follow-up was 97% complete, leaving 112 patients for the final analysis. During a median follow-up of 86 months (range 13-170), 22 events occurred in 22 patients (20% cumulative event rate, annual event rate 2.2% person-years). The events were cardiac death in 4 (18%), nonfatal MI in 6 (27%) patients, typical angina in 11 (50%) patients requiring coronary revascularization in 9 subjects, and heart failure in 1 (5%) patient. Clinical characteristics at MPS-1 in patients with and without events are presented in Table 1. Patients with events were slightly older compared to those without. Of the 9 patients with chest pain at MPS-1, 4 had ischemia and no one experienced a cardiac event during follow-up.
Imaging findings at MPS-1 and MPS-2 in the overall patient population are reported in Table 2. All perfusion defects were in the territory of the infarct-related coronary artery. In Fig. 1, MPS-1 and MPS-2 perfusion findings in patients with and without events are reported. Changes in stress and rest LVEF from MPS-1 (1 month) to MPS-2 (6 months) according to occurrence of cardiac events are depicted in Fig. 2.
Changes in PDS and LV Function According to Events
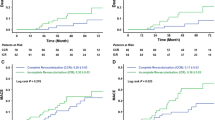
PDS improved in 35 (31%) patients, remained unchanged in 64 (57%), and worsened in 13 (12%) patients. The AER was higher (P < .001) in patients with PDS worsening compared to those with unchanged or improved PDS (Fig. 3). Cardiac remodeling was observed in 13 (12%) patients, of whom 5 (38%) showed PDS worsening. The AER was significantly higher in patients with remodeling as compared to those without (9.3% vs. 1.4%, P < .001).
Predictors of Events
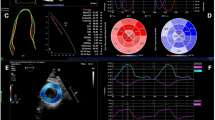
The results of Cox univariable and multivariable analyses are shown in Table 3. At multivariable analysis, worsening in PDS and remodeling resulted independent predictors of events. The difference in PDS values between MPS-1 and MPS-2 was a significant predictor of outcome (hazard ratio 1.06, 95% confidence interval 1.02-1.10; P < .01). The addition of remodeling to a model including clinical variables and categories of PDS changes increased the global χ2 from 3.68 to 45.91 (P < .05). The AER of patients with or without PDF worsening and with or without LV remodeling is reported in Figure 4. The worst outcome was observed in patients with PDS worsening and remodeling (log-rank 51, P for trend <.001) (Fig. 5). Two representative examples of patients with worsened (A) and unchanged (B) perfusion patterns are reported in Fig. 6.
Discussion
In this prospective study, prognostic significance of the changes in perfusion and functional variables by gated MPS were assessed during a long-term follow-up in a cohort of patients with AMI treated with primary PCI. From our data, it emerged that worsening of the perfusion defects from MPS-1 (1 month) to MPS-2 (6 months) imaging and the presence of LV remodeling are associated with a poor prognosis. In particular, patients with both PDS worsening and cardiac remodeling had the worst outcome.
Primary PCI is the preferred reperfusion strategy in patients presenting with AMI. However, additional myocardial damage and progressive enlargement or remodeling of the LV can occur during the first months after AMI, with a negative influence on long-term prognosis.2,3 Cardiac remodeling, defined as the progressive dilatation and change in shape, size, and function of the left ventricle, represents an important cause of heart failure in patients after AMI.19 The changes in perfusion findings, as well as of LV function over time after PCI, could give additional insight into the mechanisms of myocardial recovery used in predicting future events.20
After AMI, cardiac imaging by gated MPS has been used to quantify the infarct size and severity, as well as the presence of LV functional impairment.21,22 Moreover, the availability of software for automated reproducible quantitative assessment made gated MPS to be particularly effective in serial evaluation6. From published data, it emerged a significant late reduction in infarct size in patients after AMI probably related to the vascular stunning and gradual recovery of microvascular function.23,24 Berti et al.9 confirmed that in patients submitted to successful PCI, a spontaneous reduction in infarct size occurs beyond the 1-month evaluation and it has been associated with improvement in LV function. They also found that infarct size measured in the subacute phase after AMI, but not late infarct resorption, is a strong predictor of LV remodeling.9 Those findings suggest that perfusion defect is strongly related to LV functional recovery. To take into account the amount of myocardial tissues subject to recovery after AMI, myocardial salvage area (defined as the difference between the initial area of myocardium at risk and the final area of necrosis) by gated MPS has been proposed8 and it resulted to be associated to the functional recovery 6 months after AMI. The amount of perfusion impairment has been also correlated with survival after medical therapy or PCI in patients with AMI. Stone et al.25 evaluated the relationship between infarct size assessed early after PCI in ST-elevated MI and all-cause mortality, reinfarction, and hospitalization for heart failure. For this purpose, it was considered a pooled patient-level analysis from 10 randomized primary PCI trials, in a total of 2,632 patients.25 A strong correlation between infarct size assessed within 1 month by either cardiac magnetic resonance or MPS imaging and all-cause mortality and hospitalization for heart failure during a short-term follow-up (1 year) was found. However, the stress parameters and functional impairments were not considered.25 Smith et al.10 evaluated the long-term prognostic value of infarct size and myocardial ischemia on MPS in a large cohort of 1092 patients after PCI for ST-segment elevation MI. In patients with reduced LVEF, infarct size was independently associated with cardiac death or reinfarction, whereas myocardial ischemia was not. Conversely, in patients with LVEF >45%, only ischemia was independently associated with cardiac death or reinfarction.
In our study, we considered a smaller cohort of 112 patients evaluated with stress MPS one month and 6 months after PCI for AMI to assess the prognostic value of perfusion and functional changes in a long-term follow-up. From our data, it emerged that the worsening of PDS, but not infarct size or ischemia at 6 months (MPS-2), was predictive of prognosis. Previous studies reported that the extent and severity of perfusion defects measured within 1 week26 or 6 months10 after AMI had high prognostic impact leading to an accurate risk stratification. It should be noted that differently from those previous investigations, we considered only patients who underwent salvage reperfusion therapy within 6 hours from acute event, with a small amount of perfusion defects and relatively preserved LV function.
Our data also confirm that cardiac remodeling is a strong predictor of events.27 However, despite a significant association between infarct size and cardiac remodeling has been reported,9 in our population, we did not identify any predictor of cardiac remodeling. This is probably due to the small number of patients (n = 13) who showed LV remodeling. Despite this, at survival analysis, the worst prognosis has been observed in patients with both perfusion worsening and remodeling, suggesting a synergic effect of perfusion and functional impairment on prognostication.
This study has some limitations that must be considered. The patient population and the number of events were relatively small and selected according to the availability of the gated MPS studies. Subjects with a history of prior MI and patients with suboptimal PCI results were not considered. Therefore, our observations might not apply to those patients. It should be also considered that in the present study, patients were referred to stress MPS as part of a research protocol. Radionuclide imaging following PCI is not recommended in asymptomatic patients <2 years after revascularization procedures.28 Despite our result may contribute to elucidate the prognostic implications of perfusion and functional changes after PCI, this method cannot be applied in clinical routine.
New Knowledge Gained
This study first demonstrated that worsening of gated MPS parameters at 6 months in patients treated with primary PCI after AMI is associated to an unfavorable outcome at long-term follow-up. Serial imaging with gated MPS might be used to assess the changes in perfusion and functional parameters able to identify the patients at higher risk of cardiac events during long-term follow-up.
Conclusion
Patients treated with primary PCI after AMI with PDS worsening and cardiac remodeling are at higher risk of events. The identification of patients with both perfusion and functional impairment at gated MPS may have a role in risk stratification of patients after AMI in a long-term follow-up.
Abbreviations
- AMI:
-
Acute myocardial infarction
- PCI:
-
Percutaneous coronary intervention
- MPS:
-
Single-photon emission computed tomography myocardial perfusion imaging
- LV:
-
Left ventricular
- PDS:
-
Perfusion defect size
- EDV:
-
End-diastolic volume
- ESV:
-
End-systolic volume
- EF:
-
Ejection fraction
- AER:
-
Annual event rate
References
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, ESC Scientific Document Group, et al (2018) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39:119–177
Zhao Z-Q, Nakamura M, Wang N-P (2000) Reperfusion induces myocardial apoptotic cell death. Cardiovasc Res 45:651–660
Bolognese L, Neskovic AN, Parodi G, Cerisano G, Buonamici P, Santoro GM et al (2002) Left ventricular remodeling after primary coronary angioplasty. Patterns of left ventricular dilation and long-term prognostic implications. Circulation 106:2351–2357
Gibbons RJ, Miller TD, Christian TF (2000) Infarct size measured by single photon emission computed tomographic imaging with (99m)Tc-sestamibi: a measure of the efficacy of therapy in acute myocardial infarction. Circulation 101:101–108
Petretta M, Acampa W, Daniele S, Zampella E, Assante R, Nappi C et al (2016) Long-term survival benefit of coronary revascularization in patients undergoing stress myocardial perfusion imaging. Circ J 80:485–493
Iskandrian AE, Hage FG, Shaw LJ, Mahmarian JJ, Berman DS (2014) Serial myocardial perfusion imaging: defining a significant change and targeting management decisions. JACC Cardiovasc Imaging 7:79–96
Romero-Farina G, Aguadé-Bruix S, Candell-Riera J, Pizzi MN, Pineda V, Figueras J et al (2013) Acute myocardial infarction: estimation of at-risk and salvaged myocardium at myocardial perfusion SPECT 1 month after infarction. Radiology 269:577–584
Calabretta R, Castello A, Linguanti F, Tutino F, Ciaccio A, Giglioli C et al (2018) Prediction of functional recovery after primary PCI using the estimate of myocardial salvage in gated SPECT early after acute myocardial infarction. Eur J Nucl Med Mol Imaging 45:530–537
Berti V, Sciagrà R, Acampa W, Ricci F, Cerisano G, Gallicchio R et al (2011) Relationship between infarct size and severity measured by gated SPECT and long-term left ventricular remodelling after acute myocardial infarction. Eur J Nucl Med Mol Imaging 38:1124–1131
Smit JM, Hermans MP, Dimitriu-Leen AC, van Rosendael AR, Dibbets-Schneider P, de Geus-Oei LF et al (2018) Long-term prognostic value of single-photon emission computed tomography myocardial perfusion imaging after primary PCI for STEMI. Eur Heart J Cardiovasc Imaging 19:1287–1293
Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M et al (2004) ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). J Am Coll Cardiol 44:E1-211
Verberne HJ, Acampa W, Anagnostopoulos C, Ballinger J, Bengel F, De Bondt P et al (2015) EANM procedural guidelines for radionuclide myocardial perfusion imaging with SPECT and SPECT/CT: 2015 revision. Eur J Nucl Med Mol Imaging 42:1929–1940
Germano G, Kiat H, Kavanagh PB, Moriel M, Mazzanti M, Su HT et al (1995) Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med 36:2138–2147
Berman DS, Abidov A, Kang X, Hayes SW, Friedman JD, Sciammarella MG et al (2004) Prognostic validation of a 17-segment score derived from a 20-segment score for myocardial perfusion SPECT interpretation. J Nucl Cardiol 11:414–423
Slomka PJ, Nishina H, Berman DS, Akincioglu C, Abidov A, Friedman JD et al (2005) Automated quantification of myocardial perfusion SPECT using simplified normal limits. J Nucl Cardiol 12:66–77
Otaki Y, Betancur J, Sharir T, Hu LH, Gransar H, Liang JX et al (2020) 5-Year prognostic value of quantitative versus visual MPI in subtle perfusion defects: results from REFINE SPECT. JACC Cardiovasc Imaging 13:774–785
Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A et al (2015) 2014 ACC/AHA Key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 28(66):403–469
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, ESC Scientific Document Group, et al (2019) ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 41:407–477
French BA, Kramer CM (2007) Mechanisms of post-infarct left ventricular remodeling. Drug Discov Today Dis Mech. 4:185–196
Acampa W, Evangelista L, Petretta M, Liuzzi R, Cuocolo A (2007) Usefulness of stress cardiac single-photon emission computed tomographic imaging late after percutaneous coronary intervention for assessing cardiac events and time to such events. Am J Cardiol 100:436–441
Spinelli L, Petretta M, Cuocolo A, Nicolai E, Acampa W, Vicario L et al (1999) Prediction of recovery of left ventricular dysfunction after acute myocardial infarction: comparison between 99mTc-sestamibi cardiac tomography and low-dose dobutamine echocardiography. J Nucl Med 40:1683–1692
Spinelli L, Petretta M, Acampa W, He W, Petretta A, Bonaduce D et al (2003) Prognostic value of combined assessment of regional left ventricular function and myocardial perfusion by dobutamine and rest gated SPECT in patients with uncomplicated acute myocardial infarction. J Nucl Med 44:1023–1029
Uren NG, Crake T, Lefroy DC, de Silva R, Davies GJ, Maseri A (1993) Delayed recovery of coronary resistive vessel function after coronary angioplasty. J Am Coll Cardiol 21:612–621
Lipiecki J, Cachin F, Durel N, de Tauriac O, Ponsonnaille J, Maublant J (2004) Influence of infarct-zone viability detected by rest Tc-99m sestamibi gated SPECT on left ventricular remodeling after acute myocardial infarction treated by percutaneous transluminal coronary angioplasty in the acute phase. J Nucl Cardiol 11:673–681
Stone GW, Selker HP, Thiele H, Patel MR, Udelson JE, Ohman EM et al (2016) Relationship between infarct size and outcomes following primary PCI: patient level analysis from 10 randomized trials. J Am Coll Cardiol 67:1674–1683
Dakik HA, Wendt JA, Kimball K, Pratt CM, Mahmarian JJ (2005) Prognostic value of adenosine Tl-201 myocardial perfusion imaging after acute myocardial infarction: results of a prospective clinical trial. J Nucl Cardiol 12:276–283
Bax JJ, Schinkel AF, Boersma E, Elhendy A, Rizzello V, Maat A et al (2004) Extensive left ventricular remodeling does not allow viable myocardium to improve in left ventricular ejection fraction after revascularization and is associated with worse long-term prognosis. Circulation. 110(11 Suppl 1):II18–II22
Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA et al (2009) 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation 119:e561–e587
Disclosure
Emilia Zampella, Teresa Mannarino, Valeria Gaudieri, Adriana D’Antonio, Francesco Giallauria, Roberta Assante, Valeria Cantoni, Roberta Green, Ciro Gabriele Mainolfi, Carmela Nappi, Andrea Genova, Mario Petretta, Alberto Cuocolo, and Wanda Acampa declare that they have no financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 3 (M4A 3502 KB)
Rights and permissions
About this article
Cite this article
Zampella, E., Mannarino, T., Gaudieri, V. et al. Effect of changes in perfusion defect size during serial stress myocardial perfusion imaging on cardiovascular outcomes in patients treated with primary percutaneous coronary intervention after myocardial infarction. J. Nucl. Cardiol. 29, 2624–2632 (2022). https://doi.org/10.1007/s12350-021-02770-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-021-02770-z