Abstract
Background
The ability of adenosine stress myocardial contrast echocardiography (AS-MCE) to reveal decreased coronary blood flow or perfusion defects (PDs) has not been explored for clinical implications after coronary revascularization. This study sought to identify the prognostic value of PDs in asymptomatic patients following percutaneous coronary intervention (PCI).
Methods
We retrospectively analyzed 342 asymptomatic patients (67 years of mean age, 72% male) who underwent PCI with stents at least 9 months before AS-MCE between May 2019 and December 2020. Resting regional wall motion abnormality (rRWMA) and the patterns of PDs were assessed, and further PDs were classified as ischemic or fixed type. The primary endpoint was the composite of hospitalization for worsening heart failure, coronary revascularization, and cardiac death.
Results
In AS-MCE (median time interval following PCI: 17.4 months), PDs were present in 93 (27.2%) out of 342 patients; 70 of ischemic PD (75.3%), 58 of fixed PD (62.4%). Those with PD showed a higher frequency of rRWMA than those without PD (53.8 vs. 15.7%, p < 0.001). During the median follow-up of 22.6 months, 26 (7.6%) patients experienced more associated clinical outcomes with PD than rRWMA. Cox analysis revealed that the combined findings of rRWMA and PD, and specifically, ischemic PD of ≥ 2 segments were associated with a high increase in adverse outcomes.
Conclusions
AS-MCE provided prognostic value in asymptomatic patients with prior PCI. PD might be complementary to rRWMA in risk stratification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid advancement of pharmacology and percutaneous coronary intervention (PCI) techniques, more and more coronary stents have been employed in many patients with complex coronary artery disease (CAD). Although there is room for controversy, the current guidelines recommend the revascularization of only critical infarct-related arteries and suggest further performing functional tests in case of other non-critical stenosed arteries at an appropriate time during the follow-up period [1,2,3]. In addition, in asymptomatic patients with recent revascularization or > 1 year after revascularization, the non-invasive stress test can be considered to evaluate residual ischemia or the patient’s clinical status just in case of incomplete revascularization [4, 5]. More specifically, invasive coronary angiography (CAG) or coronary anatomic imaging may be useful in the patients at high risk based on these non-invasive ischemic stress tests.
Nevertheless, in real-world practice, the incidence of 1- or 2-year major adverse cardiac events (up to 10%/year) is not negligible [6], and the rate of adverse events, including recurrent CAD or progressive worsening left ventricular (LV) dysfunction, is reported to reach around 7.0% following PCI during long-term follow-up [1,2,3]. Among unexpected adverse events, abnormal myocardial contractility (hypokinesia or akinesia) is frequently observed in asymptomatic patients even after successful PCI. This LV mechanical dysfunction can be caused by stent re-stenosis, de novo stenosis of coronary artery, microvascular ischemia, or progressive myocardial fibrosis, all of which can be explained by the inflammatory processes or episodes of coronary artery occlusion [7,8,9]. In contrast, normal contractility with well-preserved systolic function can be occasionally encountered with coronary arteries non-critically stenosed or with chronic total occlusion with the collateral flow [10].
In an effort to address these issues, adenosine stress myocardial contrast echocardiography (AS-MCE), which allows visualization of myocardial perfusion imaging (MPI), has been used to evaluate myocardial perfusion defect (PD) of ischemia or scar formation and CV prognosis in patients with CAD. Recent research has shown a sensitivity of 90% and a specificity of 85% for AS-MCE in detecting ischemia [11,12,13]. Accordingly, abnormal stress MPI could indicate the need for coronary revascularization or specifically reveal microvascular dysfunction in myocardial infarction or non-ischemic diseases [14,15,16].
However, the prognostic usefulness of AS-MCE has been still unclear in the case of successful revascularization. Although the need for functional studies after PCI is recognized, there is no consensus on the preferred tests of asymptomatic patients. Considering the gap between the guideline and clinical practice, an appropriate evaluation of the risk-stratification, not for the invasive revascularization, may be required in clinical practice, especially using functional stress imaging modality. Thus, we sought to evaluate the clinical implications of MPI in asymptomatic patients with prior PCI and to determine the relationship between LV mechanical dysfunction and MPI during the AS-MCE study.
Methods
Study population
This study was a single-center retrospective observational registry. Through a thorough review of the medical records from May 2019 to December 2020, all consecutive patients who underwent AS-MCE at the Keimyung University Dongsan Cardiovascular Imaging Center (Daegu, South Korea) were screened for inclusion in the current study: the performance of AS-MCE was at the physician’s discretion and patient’s agreement because of the recommendation against routine cardiac stress testing for asymptomatic patients after PCI within two years. We identified the patients (1) who underwent PCI with coronary stents at least 9 months before AS-MCE, (2) who underwent conventional echocardiography at or near time of index PCI before hospital discharge, and (3) who were free from typical angina pain or equivalent since the index PCI. However, we excluded patients (1) who showed poor imaging quality of conventional echocardiography or AS-MCE, (2) who underwent coronary artery bypass graft surgery, or (3) who exhibited valvular heart disease of more than moderate grade.
Echocardiography
Immediately before or after the index PCI, comprehensive baseline echocardiography was performed in all study patients according to the current recommended guidelines [17]: quantification of LV chamber and wall thickness, LV ejection fraction (LVEF), mitral inflow study, and tissue Doppler velocity imaging (TDI). During follow-up periods at outpatient clinics, AS-MCE was done using a Phillips Epic Console (Phillips Health Care, Amsterdam, Netherlands) equipped with a 3.5-MHz transducer according to the guidelines [18]. The Definity contrast agent (Lantheus Medical Imaging Inc., North Billerica, MA, USA) was infused using a perfusion pump machine from the resting to the recovery phase through the stress phase. During the stress phase, adenosine was infused (140–200 ug/kg/min) for 8 min. Real-time MCE imaging was captured with a very low mechanical index (0.13–0.15) and performed with flash-replenishment using a high-flash mechanical index (0.75-1.0). Imagings were captured at rest and until 8 min after adenosine administration at 2-minute intervals. During the recovery phase, myocardial contrast flash-replenishment continued to be captured. The image was stored for 10 cardiac cycles of cardiac beats. Using a visual assessment of MPI during AS-MCE, resting regional wall motion abnormality (rRWMA) and PDs were semi-quantified (Fig. 1). PD was defined as a delay or absence of myocardial perfusion lasting for more than 2 beats after flash (Fig. 2). A fixed pattern was defined as a PD appearing throughout the rest and stress phases. An ischemic pattern was defined as PD appearing only at the stress phase with worsening myocardial contractility. Written informed consents regarding AS-MCE were obtained from all patients at the time of echocardiography.
Adenosine stress echocardiography. In case of ischemic perfusion defect, perfusion defect (red arrow) was observed lasting for more than 2 beats after plash during just stress phase at basal inferior segment of wall motion abnormality (A). However, the normal perfusion (red arrow) could be observed during both rest and stress phases even in the akinetic segment of basal inferior wall (B)
Clinical outcomes
The outcomes in this study were determined with review of patient medical records. Patients whose follow-up records were unavailable were censored. The primary endpoint was the composite outcome consisting of the first occurrence of hospitalization for worsening heart failure (HF), coronary revascularization for stable angina or acute coronary syndrome (ACS), and cardiac death. Worsening HF was defined according to the Framingham Heart Study criteria, requiring hospitalization for intravenous diuretic use to relieve dyspnea caused by pulmonary edema which was seen on a chest radiograph. Cardiac death was identified as death caused by acute myocardial infarction (AMI), ventricular arrhythmias, or congestive HF. All echocardiographic parameters were reviewed and adjudicated by two expert cardiologists (H. Kim and I-C. Kim). This study was approved by the Institutional Review Board (IRB) of the Keimyung University Dongsan Medical Center. The study conformed to the ethical guidelines of the Declaration of Helsinki, and the IRB waived the consent because of the retrospective nature of the study.
Statistics
Continuous variables are expressed as mean ± standard deviation when normally distributed or as median (interquartile range [IQR]) otherwise. Discrete variables are expressed as frequency (proportion). The Student’s t-test, Mann-Whitney U-test, or chi-square test were performed to compare groups as appropriate. The effects of predictors on clinical outcomes were determined using Cox regression analysis; variables with statistical significance in the univariable Cox models were entered in the multivariable models, excluding variables with significant correlations between each other. The first model adjusted for the segment numbers of ischemic PD, and the second model further adjusted for the combined findings of rRWMA and PD. Kaplan-Meier analysis according to the risk-stratification was used to construct event-free survival curves and compare them using the log-rank test. Inter-observer and intra-observer reproducibility were performed on 15 randomly selected MCE images and the reliabilities for the presence of PD were assessed by Cohen’s k analysis. All statistical analyses were performed using the Statistical Package for Social Science (version 13.0, SPSS Inc., Chicago, Illinois, USA). Statistical significance was set at p-value < 0.05.
Results
Baseline characteristics of the study population
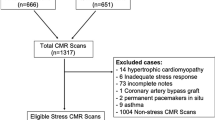
Among the 390 screened patients who underwent PCI, 48 were excluded (25 for poor echocardiographic imaging, 17 for follow-up loss in outpatient clinics, and 6 for lack of follow-up data). Eventually, the study included a total of 342 patients. Table 1 shows their baseline characteristics according to the presence of PDs on AS-MCE. Patients with PDs displayed a more frequency of diabetes mellitus and higher creatine kinase-MB levels than those without PD. They had higher frequency of ST segment elevation AMI (22.6 vs. 6.4%, p < 0.001), but lower frequency of chronic stable angina (41.9 vs. 56.2%, p = 0.021). However, PCI-related variables, such as disease-vessel, the number of employed stents, and coronary arteries, were not different between the two groups. As anticipated, the use of beta-blockers and diuretics was more frequently observed in patients with PD. Regarding echocardiography during index PCI, patients with PD had increased LV chamber, a greater LV volume index, lower LVEF, and lower TDI compared with those without PD.
Patients underwent the AS-MCE test at a median time of 17.4 months [12.5, 55.9] from the index PCI. During AS-MCE, rRWMA was more likely to be observed in patients with PDs (53.8 vs. 15.7%, p < 0.001). In particular, among the patients with PDs, those with ischemic PD and fixed PD were 75.3% and 62.4%, respectively. Furthermore, more than half of patients with ischemic PDs showed ischemic PD of ≥ 2 segments (75.7%).
Among a total of 93 patients of PD segments, the subsequent treatment responses of physicians to AS-MCE findings were as follows: 85 (91.4%) patients had the intensified modification of medication therapy, 19 (20.4%) had coronary computed tomographic angiography, but 2 (2.1%) had PCI. Basically, all of the patients with > 2 PD segments had changes in medication intensification. There was good agreement in inter-observer and intra-observer variability in the assessment of PD: Cohen’s k coefficient = 0.89 [95% confidence interval (CI): 0.80–0.94] and 0.92 (95% CI: 0.88–0.94), respectively.
Clinical outcomes
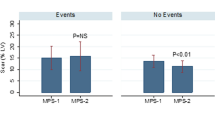
During the median follow-up of 22.6 months [13.2, 29.0] after AS-MCE, a total of 26 patients experienced clinical outcomes. From the composite of clinical outcomes, HF worsening and revascularization of stable angina or ACS occurred more commonly in patients with PDs, who also showed two cardiac deaths. Clinical outcomes frequently developed in patients with both PDs and rRWMA; more specifically, PDs contributed to the event rate more significantly than rRWMA (p < 0.001) (Fig. 3).
According to the Kaplan-Meier analysis, it was found that the rate of clinical events varied among patients with PD. In all patients, ischemic PD of ≥ 2 segments showed the largest between-group difference (Fig. 4). Furthermore, the subgroup of patients with PD, regardless of rRWMA, showed the most unfavorable prognosis. In univariable Cox hazard regression analysis, diabetes mellitus, rRWMA of AS-MCE, fixed PD, and ischemic PD were significant predictors of CV outcomes in both models (model 1 and 2) (Table 2). Notably, in multivariable analysis, rRWMA, the presence of PDs, and their combination displayed a high increase in clinical outcomes.
Discussion
Assessment following percutaneous coronary intervention
Recently, the chances to treat patients with coronary stents in daily clinical practice are increasing. Established patients with CAD who received stents have continued to get much attention because of concerns regarding stent restenosis or de novo CAD during long-term follow-up. However, regarding post-PCI surveillance, it is not mandatory to evaluate the patency of coronary stents or the presence of ischemia in asymptomatic patients [1]. In addition to the lack of demonstratable benefit of cardiac stress test following PCI, the discrepancies between the guidelines and reality could give rise to a point worth reconsidering the usefulness of AS-MCE. This argument is because these recommendations, initially based on the modalities of radionuclide imaging or dobutamine echocardiography, have not been updated, whereas the rapid advances in MCE techniques have increased the clinical usefulness of cardiac imaging. In the current study, a significant number of patients with PD were identified and, it would be better to have a chance to take steps for proper management of the disease, including unnecessary invasive CAG or recommendation of only the medication changes.
Missed opportunities to detect CAD recurrence because of the vague symptoms in the elderly eventually lead to ACS or its relevant CV events. Moreover, among the functional tests to detect or predict ischemia after initial PCI, the exercise-electrocardiography test for angina symptoms or electrocardiographic abnormalities seem to have low sensitivity and specificity [19]. Furthermore, radionuclide imaging techniques are expensive and not often feasible. Unfortunately, the role of functional tests for ischemia after PCI is not yet recognized; revascularization of epicardial coronary arteries cannot sufficiently resolve ischemia, making it challenging to discriminate the remnant ischemia following PCI from that due to new lesion [20]. Although the patency of coronary stents is frequently observed even in the hypokinetic lesion, LV remodeling can continue and progress to advanced cardiomyopathy [18]. In patients with rRWMA without obstructive epicardial vessels, microvascular dysfunction, not an uncommon disease entity, can account for this LV mechanical dysfunction [9, 10]. They frequently have metabolic abnormalities or many CV risk factors and microvascular dysfunction can have a high incidence in patients with prior PCI [8,9,10, 16]. For this reason, an MPI study is necessary to evaluate the substantial implications of ischemia following PCI. In addition to residual ischemia, further evaluation of non-culprit arteries remaining after index PCI is also required during the follow-up of patients with PCI at the outpatient clinic.
Myocardial perfusion defect in epicardial revascularization
Ischemic heart disease is not synonymous with obstructive CAD. Accordingly, defining the role of AS-MCE to help identify patients with unresolved CAD is invaluable because most centers do not routinely examine expensive and infeasible cardiac magnetic resonance (CMR) or radionuclide tests. In clinical practice, rRWMA can be seen with or without stent restenosis during the long-term period following PCI. This is because impairment to the microvascular level of the myocardium continues even after epicardial revascularization, such as hibernating phenomenon [21, 22]. These findings may confer the mandate to visualize ischemic lesions and perfusion levels in the myocardium on MPI [18].
In the current study, AS-MCE simultaneously provided information on both PDs and rRWMA. For example, in cases of hypokinetic or akinetic segments even after successful PCI, MPI can guide the strategies of CAG. Nearly a third of patients (43.8%) with rRWMA showed a normal MPI, which may indicate appropriate myocardial perfusion that does not require invasive CAG. In contrast, more aggressive treatment may be necessary in the case of myocardial PDs despite normal myocardial contractility. Thus, these can be in line with the usefulness of MCE in evaluating microvascular obstruction [23].
Nevertheless, in some cases, it may be difficult to determine the need for revascularization in a dysfunctional myocardium with a normal MPI. Limited data are available regarding the implication of normal MPI during both the resting and stress phases in the akinetic or hypokinetic segments [24]. These discrepancies between MPI and contractility can be commonly demonstrated in peri-infarct lesions such as the “Takotsubo effect” in patients with AMI [25], in which this mismatch could be induced by a microvascular obstruction, hemorrhage, edema, or suppressed metabolic status in the early phase of AMI [26, 27]. However, in contrast to the Takotsubo mismatch, AS-MCE was performed in the long-term follow-up period after PCI (median time elapsed from index PCI to MPI: 17 months) in the current study. As such, rather than acute changes such as the Takotsubo mismatch, chronic interstitial fibrotic changes around the injured myocardium may occur. This mismatch between normal MPI and mechanical dysfunction can be also observed, particularly in non-ischemic dilated cardiomyopathy (DCM) with myocardial fibrosis in CMR studies. In these patients with non-ischemic DCM, myocardial fibrosis or decreased myocardial blood volume (MBV) was suggested as the cause of myocardial mechanical dysfunction: MBV was found to have decreased without the presence of PDs during both the resting and stress phases [28, 29].
Prognostic value of adenosine stress myocardial contrast echocardiography
Despite the many current studies on the usefulness of PCI in patients with stable angina, it remains uncertain whether coronary revascularization affects clinical outcomes. Currently, no study has used AS-MCE to evaluate the prognosis of clinical events in asymptomatic patients following PCI. The ability of AS-MCE to predict functional recovery or viability since revascularization was comparable to that of dobutamine stress echocardiography or radionuclide studies [30, 31]. We also observed that MPI, together with rRWMA, was informative for outcomes, including ischemic CAD and HF worsening.
Ischemic PD of ≥ 2 segments in the stress phase appears to influence adverse outcomes. These findings would justify an explorative CAG to relieve the myocardium in patients at high risk, and extend to cases in which the myocardium shows normal contractility in conventional echocardiography. More specifically, contrast agents enable physicians to visualize the myocardium to assess small or large ischemic lesions and recognize whether ischemia is related to prior PCI. Based on these strengths, AS-MCE could guide physicians in deciding the treatment strategy more rapidly and efficiently than other stress test modalities.
In the real world, abnormal contractility is commonly observed on daily echocardiography, from which it is not clear whether additional CAG with PCI could be valuable for the prognosis of asymptomatic patients [18, 19]. Abnormal contractility can be related to the chronic fibrosis with MBV loss similar to idiopathic DCM, a fixed scar lesion without viability, or a subacute stunned lesion in addition to a well-known hibernating or ischemic lesion [19]. Given the viability and long-term CV prognosis, not all mechanical dysfunctions require revascularization without evaluation of risk stratification. In this respect, the further functional evaluation of MPI shown in the current study appears to be more important than the assessment of mechanical dysfunction alone. AS-MCE can reveal the consistency between revascularized coronary artery territories and mechanical dysfunction, inform decisions about PCI follow-up, and provide further information about myocardial viability. With these scientific approaches using AS-MCE, the simultaneous analysis of bot mechanical and functional dysfunction could enhance the risk stratification of CV outcomes.
Study limitations
This study has some limitations. First, the elapsed time between AS-MCE and PCI was not the same between the subjects because the study was a retrospective review of the cohort’s medical records. Second, We did not measure the contrast velocity nor the slope of its intensity ascending curve from the plots of myocardial contrast intensity versus pulsing intervals. Although such post-hoc analysis may be required to support the results, it is a time-consuming process. Hence, the visual estimation of MPI appears to be a pragmatic approach that could be sufficient to guide clinicians in the treatment strategy. Third, we did not aim to assess the relationship between PD and coronary anatomy but to emphasize the importance of PD in risk stratification and the further prognostic impact, which the physicians should incorporate into diagnostic strategies of coronary imaging. Last, this was a retrospective study, and thus the analyses for the different context of CV treatment response to the AS-MCE findings were limited. We need a further prospective study to confirm the predictive ability of the PD for prognosis in AS-MCE. Unfortunately, little is known about the role of the non-invasive functional stress test of AS-MCE in revascularized patients. However, as recently mentioned in the current guidelines, we believed that clinical status and, if any, remaining ischemia can be assessed in asymptomatic patients with revascularization, based on which the treatment strategies, including invasive CAG, could be carefully determined [5]. Therefore, the risk stratification based on PD of ≥ 2 segments in AS-MCE rather than ischemia itself can be beneficial for patients who might have been considered high risk.
Conclusions
In this clinical follow-up study, AS-MCE, as a surveillance test, provided information on MPI in asymptomatic patients following PCI. In particular, combined with LV mechanical dysfunction (rRWMA), MPI could enhance the risk stratification of CV outcomes; patients who underwent PCI and had more than two ischemic PD segments on MPI were at high risk. Therefore, AS-MCE will increase awareness of MPI and contribute a lot to the study for the viability of normal-looking myocardial muscles, thereby facilitating the use of this test in the surveillance of complex patients with previous coronary revascularization.
Availability of data and material
Not applicable.
Code Availability
Not applicable.
References
Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV, ACCF/AHA/ACP/AATS (2012) /PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 60:2564 – 603
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Ting HH, O’Gara PT, Kushner FG, Ascheim DD, Brindis RG, Casey DE Jr, Chung MK, de Lemos JA, Diercks DB, Fang JC, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX (2016) 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. J Am Coll Cardiol 67:1235–1250
Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ (2014) 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 64:e139–228
Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, Min JK, Patel MR, Rosenbaum L, Shaw LJ, Stainback RF, Allen JM, American College of Cardiology Foundation Appropriate Use Criteria Task Force, ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR /STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons.J Am Coll Cardiol63:380–406
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ, ESC Scientific Document Group (2020) 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 41:407–477
Kuramitsu S, Sonoda S, Ando K, Otake H, Natsuaki M, Anai R, Honda Y, Kadota K, Kobayashi Y, Kimura T (2021) Drug-eluting stent thrombosis: current and future perspectives. Cardiovasc Interv Ther 36:158–168
Weil BR, Suzuki G, Canty JM Jr (2020) Transmural variation in microvascular remodeling following percutaneous revascularization of a chronic coronary stenosis in swine. Am J Physiol Heart Circ Physiol 318:H696–705
Mangiacapra F, Bressi E, Di Gioia G, Pellicano M, Di Serafino L, Peace AJ, Bartunek J, Morisco C, Wijns W, De Bruyne B, Barbato E (2020) Coronary microcirculation and peri-procedural myocardial injury during elective percutaneous coronary intervention. Int J Cardiol 306:42–46
Geshi T, Nakano A, Uzui H, Okazawa H, Yonekura Y, Ueda T, Lee JD (2008) Relationship between impaired microvascular function in the non-infarct-related area and left-ventricular remodeling in patients with myocardial infarction. Int J Cardiol 126:366–373
Díez-Delhoyo F, Gutiérrez-Ibañes E, Sanz-Ruiz R, Vázquez-Álvarez ME, González Saldívar H, Rivera Juárez A, Sarnago F, Martínez-Sellés M, Bermejo J, Soriano J, Elízaga J, Fernández-Avilés F (2019) Prevalence of Microvascular and Endothelial Dysfunction in the Nonculprit Territory in Patients With Acute Myocardial Infarction. Circ Cardiovasc Interv 12:e007257
Ito H (2012) Myocardial contrast echocardiography after myocardial infarction. Curr Cardiol Rep 14:350–358
Karogiannis N, Senior R (2017) Contrast echocardiography for detection of myocardial perfusion abnormalities: A clinical perspective. Herz 42:287–294
Monakier D, Woo A, Vannan MA, Rakowski H (2004) Myocardial contrast echocardiography in chronic ischemic and nonischemic cardiomyopathies. Cardiol Clin 22:269–282
Qian L, Xie F, Xu D, Porter TR (2021) Long-term prognostic value of stress myocardial perfusion echocardiography in patients with coronary artery disease: a meta-analysis. Eur Heart J Cardiovasc Imaging 22:553–562
Lin Y, Guan X, Ren K, Zhu Y, Lu Y, Shang Y (2020) Low-dose dobutamine stress myocardial contrast echocardiography for the evaluation of myocardial microcirculation and prediction of overall cardiac function recovery. Exp Ther Med 20:1315–1320
Taqui S, Ferencik M, Davidson BP, Belcik JT, Moccetti F, Layoun M, Raber J, Turker M, Tavori H, Fazio S, Lindner JR (2019) Coronary Microvascular Dysfunction by Myocardial Contrast Echocardiography in Nonelderly Patients Referred for Computed Tomographic Coronary Angiography. J Am Soc Echocardiogr 32:817–825
Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, Horton K, Ogunyankin KO, Palma RA, Velazquez EJ (2019) Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 32:1–64
Pellikka PA, Arruda-Olson A, Chaudhry FA, Chen MH, Marshall JE, Porter TR, Sawada SG (2020) Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. J Am Soc Echocardiogr 33:1–41
Dori G, Denekamp Y, Fishman S, Bitterman H (2003) Exercise stress testing, myocardial perfusion imaging and stress echocardiography for detecting restenosis after successful percutaneous transluminal coronary angioplasty: a review of performance. J Intern Med 253:253–262
Gabaldon-Perez A, Marcos-Garces V, Gavara J, Rios-Navarro C, Miñana G, Bayes-Genis A, Husser O, Sanchis J, Nunez J, Chorro FJ, Bodi V (2021) Coronary Revascularization and Long-Term Survivorship in Chronic Coronary Syndrome. J Clin Med 10:610
Parikh K, Choy-Shan A, Ghesani M, Donnino R (2021) Multimodality Imaging of Myocardial Viability. Curr Cardiol Rep 23:5
Garcia MJ, Kwong RY, Scherrer-Crosbie M, Taub CC, Blankstein R, Lima J, Bonow RO, Eshtehardi P, Bois JP (2020) State of the Art: Imaging for Myocardial Viability: A Scientific Statement From the American Heart Association. Circ Cardiovasc Imaging 13:e000053
Janardhanan R, Swinburn JM, Greaves K, Senior R (2003) Usefulness of myocardial contrast echocardiography using low-power continuous imaging early after acute myocardial infarction to predict late functional left ventricular recovery. Am J Cardiol 92:493–497
Senior R, Becher H, Monaghan M, Agati L, Zamorano J, Vanoverschelde JL, Nihoyannopoulos P (2009) Contrast echocardiography: evidence-based recommendations by European Association of Echocardiography. Eur J Echocardiogr 10:194–212
Qiu Q, Abdelghany M, Subedi R, Scalzetti E, Feiglin D, Wang J, Liu K (2019) Discrepant myocardial microvascular perfusion and mechanics after acute myocardial infarction: Characterization of the “Tako-tsubo effect” with real-time myocardial perfusion contrast echocardiograph. Int J Cardiol 276:1–7
Ortiz-Pérez JT, Meyers SN, Lee DC, Kansal P, Klocke FJ, Holly TA, Davidson CJ, Bonow RO, Wu E (2007) Angiographic estimates of myocardium at risk during acute myocardial infarction: validation study using cardiac magnetic resonance imaging. Eur Heart J 28:1750–1758
White SK, Hausenloy DJ, Moon JC (2012) Imaging the myocardial microcirculation post-myocardial infarction. Curr Heart Fail Rep 9:282–292
Miyata-Fukuoka Y, Kawai H, Iseki O, Yamanaka Y, Ueda Y, Yokoyama M, Hirata KI (2016) Myocardial blood volume reserve by intravenous contrast echocardiography predicts improvement in left ventricular function in patients with nonischemic dilated cardiomyopathy. J Echocardiogr 14:163–170
Neglia D, Parodi O, Gallopin M, Sambuceti G, Giorgetti A, Pratali L, Salvadori P, Michelassi C, Lunardi M, Pelosi G, Marzilli M, L’Abbate A (1995) Myocardial blood flow response to pacing tachycardia and to dipyridamole infusion in patients with dilated cardiomyopathy without overt heart failure. A quantitative assessment by positron emission tomography. Circulation 92:796–804
deFilippi CR, Willett DL, Irani WN, Eichhorn EJ, Velasco CE, Grayburn PA (1995) Comparison of myocardial contrast echocardiography and low-dose dobutamine stress echocardiography in predicting recovery of left ventricular function after coronary revascularization in chronic ischemic heart disease. Circulation 92:2863–2868
Shimoni S, Frangogiannis NG, Aggeli CJ, Shan K, Verani MS, Quinones MA, Espada R, Letsou GV, Lawrie GM, Winters WL, Reardon MJ, Zoghbi WA (2003) Identification of hibernating myocardium with quantitative intravenous myocardial contrast echocardiography: comparison with dobutamine echocardiography and thallium-201 scintigraphy. Circulation 107:538–544
Acknowledgements
This work was supported by the research promoting grant from the Keimyung University Dongsan Medical Center in 2020.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors approved the final article and declared their contributions to the article as following, Hyungseop Kim, In-Cheol Kim: Conceptualization, Methodology, Writing-Original Draft, Writing-Review & Editing. Hyuck-Jun Yoon, Jongmin Hwang: Software, Validation, Investigation, Data Curation, Writing-Review & Editing. Hyoung-Seob Park: Conceptualization, Methodology, Writing-Original Draft, Writing-Review & Editing. Cheol Hyun Lee, Yun-Kyeong Cho: Software, Formal analysis. Chang-Wook Nam, Seongwook Han: Resources, Visualization. Seung-Ho Hur: Writing-Review & Editing, Supervision.
Corresponding author
Ethics declarations
Declarations of interest
none.
Conflict of interest
No potential conflicts of interest to disclose.
Ethics approval:
This study was approved by the institutional review board of Keimyung University Dongsan Medical Center (Reference No. 2019-06-031) and conformed to the ethical guidelines of the Declaration of Helsinki.
Consent to participate:
Not applicable.
Consent to publication:
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, H., Kim, IC., Hwang, J. et al. Surveillance of adenosine stress myocardial contrast echocardiography following percutaneous coronary intervention. Int J Cardiovasc Imaging 38, 1909–1918 (2022). https://doi.org/10.1007/s10554-022-02583-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-022-02583-2