Abstract
Background
Left ventricular mechanical dyssynchrony has been shown to provide significant clinical values for chronic heart failure (HF) and cardiac resynchronization therapy (CRT). The purpose of this study was to evaluate whether electrical dyssynchrony combined with mechanical dyssynchrony has an incremental benefit over electrical dyssynchrony or mechanical dyssynchrony alone to predict clinical events in patients with acute heart failure (AHF).
Methods
Ninety-six AHF patients who received standard 12-lead ECG, gated single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI), and echocardiography were enrolled. Thirty-two normal subjects were collected as the control group to get the normal database of mechanical dyssynchrony. The end point is the composite of all-cause death and heart transplantation. Electrical dyssynchrony was defined as QRS duration > 120 ms. Mechanical dyssynchrony was defined as > mean + 2 × SD phase standard deviation (PSD) or phase bandwidth (PBW) based on our normal database.
Results
During the follow-up of 28 ± 10 months, complete data were obtained in 92 patients. 26 (28.3%) Patients who reached the end point were classified into the event group. There were no significant differences in PSD or PBW between the event and non-event groups. However, PBW > 77.76° was independently associated with the end point in the univariate and multivariate analysis (hazard ratio 2.92, 95% confidence interval 1.00-8.47, P = .049; hazard ratio 3.89, 95% confidence interval 1.01-14.97, P = .048). The Kaplan-Meier curve with a log-rank test showed that the end point rate was significantly higher in the patients with PBW > 77.76° (log-rank P = .039). Moreover, the ROC curve analysis showed that the area under the curve (AUC) for predicting end point events by the integrative analysis of QRS > 120 ms and PBW > 77.76° was significantly improved compared to QRS duration > 120 ms (AUC: 0.75 vs 0.68, P = .001) or PBW > 77.76° (AUC: 0.75 vs 0.62, P = .049), respectively. The model of combined electrical and mechanical dyssynchrony yielded a further significantly improved risk prediction for adverse events in the global χ2.
Conclusions
The combination of QRS duration > 120 ms and PBW > 77.76° was an independent predictor of all-cause death and heart transplantation in AHF patients. The integrative analysis of electrical and mechanical dyssynchrony provides incremental prognostic value for clinical use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute heart failure (AHF) refers to a rapid onset or worsening of symptoms and/or signs of heart failure (HF).1 AHF may present as a first occurrence, or as a consequence of acute decompensation of chronic HF. Previous studies have shown that left ventricular ejection fraction (LVEF) and QRS duration are well-established predictors in patients with chronic heart failure.2 A wide QRS complex on the surface ECG is a marker of poor prognosis in patients with HF.3
Left ventricular mechanical dyssynchrony (LVMD) plays an important role in the pathogenesis of HF. However, because patients with AHF may have transient episodes of LVMD resulting in acute elevation of left ventricle (LV) fulfilling pressure and pulmonary edema, assessment of arrhythmias usually needs to be performed under stable clinical conditions.4,5 Lee et al found that AHF patients had worse LVMD than chronic HF patients.6 Therefore, LVMD may be a prognostic factor for the patients with AHF, even though it has recovered in patients with chronic HF.7,8
Noteworthy, electrical dyssynchrony and mechanical dyssynchrony are not always interchangeable: only 60% of patients with left bundle branch block selected according to the Strauss criteria had mechanical dyssynchrony measured by phase analysis on gated MPI.9 Moreover, studies have found that intraventricular rather than interventricular dyssynchrony measured by tissue Doppler has increased prognostic value over QRS duration in patients with chronic HF.4,5
In this study, we seek to determine whether the combination of QRS duration on ECG and mechanical dyssynchrony measured on gated SPECT MPI has an incremental prognostic value over QRS duration or mechanical dyssynchrony alone in AHF patients.
Methods
Study Population
From May 2012 to September 2016, 96 AHF patients who met one of the following inclusion criteria were enrolled in our study: (1) pulmonary congestion; (2) heart dysfunction, which is physically examined for signs or symptoms of HF, including exertional dyspnea and paroxysmal nocturnal dyspnea/orthopnea, edema of the lower extremities; (3) structural heart disease, including ischemic cardiomyopathy, myocardial infarction, dilated cardiomyopathy, hypertrophic cardiomyopathy in dilation stage, hypertensive heart disease. Pulmonary congestion was defined as “congestion” on a chest x-ray and/or as rales on physical examination. Exclusion criteria included age more than 80 years, pacemaker, implantable cardioverter defibrillator, significant structural valvar disease, coexisting malignant disease, or severe illness. All subjects underwent standard 12-lead ECG, echocardiography, and gated rest SPECT MPI.
Thirty-two subjects who were free of hypertension, diabetes mellitus, heart failure, coronary heart disease, congenital heart disease, valvular heart disease, and other cardiac diseases were enrolled in the database of normal subjects from 2009 to 2012 and were used as the control group. Both the AHF patients and the control subjects were enrolled from the First Affiliated Hospital of Nanjing Medical University. The study was approved by the Institutional Ethical Committee of the First Affiliated Hospital of Nanjing Medical University, and written informed consent forms were obtained from all participants.
Myocardial 99mTc-Sestamibi Scintigraphy
First, all AHF patients in this study were evaluated to see if they could lie down for more than 4 hours before receiving gated rest SPECT MPI scan to ensure that they could complete the scan successfully. Then, the gated rest SPECT MPI was performed around 60 minutes post injection using 20-30 mCi of Tc-99m sestamibi. SPECT planar images were acquired with a dual-headed camera (Philips Medical Systems, Milpitas, CA, USA) using a standard resting protocol. The imaging parameters were 20% energy window around 140 keV, 180° orbit, and 32 steps with 25 seconds per step, 8-bin gating, and 64 projections per gate. The total acquisition time was 14 minutes for each patient. Image reconstruction and reorientation were completed with Emory Reconstruction Toolbox (ERToolbox; Atlanta, GA). SPECT images were reconstructed by ordered subset expectation maximization (OSEM) with 3 iterations and 10 subsets and then filtered by a Butterworth low-pass filter with a cut off frequency of 0.4 cycles·cm−1 and an order of 10.
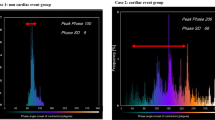
The resulting short-axis images were input into Emory Cardiac Toolbox (ECToolbox V4.0; Syntermed, Atlanta, GA) to identify the LV parameters. These parameters were then submitted to an automatic myocardial sampling algorithm, which searched in 3D for maximal count circumferential profiles in each cardiac frame. Subsequently, the LV dyssynchrony phase angles were calculated by the 1-harmonic Fourier approximation, which measures the change of counts in the left ventricular myocardium in a cardiac cycle.10,11 The mechanical dyssynchrony was characterized by the phase standard deviation (PSD) and phase histogram bandwidth (PBW), which represent the standard deviation of phase distribution and the phase bandwidth that includes 95% of the samples over the left ventricle, respectively.
Electrocardiography and Echocardiography
A 12-lead surface continuous ECG was recorded at rest after admission. The QRS width was automatically measured using Fukuda Denshi FX-8322 (Fukuda Denshi, Tokyo, Japan).
Echocardiography was performed using the Vivid E9 ultrasound system (GE HealthCare, Wauwatosa, WI, USA). LV parameters were acquired by M-mode echocardiography according to the guidelines of the American Society of Echocardiography.12 The LV end-systolic volumes, end-diastolic volumes, and LVEF were measured using the modified biplane Simpson rule.
Study End Point
The composite event of all-cause death or heart transplantation was defined as the study end point. Patients were followed up to 3 years after inclusion. End events were ascertained every 6 months by phone contact with patients or their relatives, as well as checking patient hospital records and government records of death.
Statistics
Differences between the event and non-event groups were compared by the Student t-test and nonparametric Mann-Whitney U-test for continuous variables and Pearson χ2 test for categorical variables. The univariate Cox regression analysis was applied to estimate potential predictors for events at the long-term follow-up. The stepwise Cox regression was performed to analyze the independent predictors of mortality/heart transplantation. Variables with P < .10 in the univariate Cox regression were included, and variables with P > .05 in the stepwise Cox regression were excluded. Differences in events were compared across subgroups by Kaplan-Meier analysis using the log-rank test. The predictive powers of electrical dyssynchrony, mechanical dyssynchrony, and their combination were evaluated using the receiver operator characteristic (ROC) curve analysis. P < 0.05 was considered to be statistically significant. χ2 Statistic by a likelihood ratio test was performed to calculate the incremental value of electrical and mechanical dyssynchrony. Statistical analysis was performed by R ver.3.6.0 software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
During the follow-up of 28 ± 10 months, complete data were obtained in 92 AHF patients. The baseline characteristics are listed in Table 1. Twenty-four patients with all-cause death and 2 patients with heart-transplant were included in the event group. The rest 66 patients were classified as the non-event group. The average age was 54.6 years, and 70 (76.1%) patients were male. Ischemic heart failure was found in 19 (20.7%) patients, and 58 (63%) patients were classified as NYHA functional class III. Systolic blood pressure, diastolic blood pressure, proBNP, the width of QRS, and rest scar detected from SPECT MPI were significantly different between the event and non-event groups. In the quantitative analysis of PSD and PBW, there was no significant difference between the groups with and without events. However, after dichotomizing PSD and PBW, there was a significant difference in PBW between groups with and without events.
Mechanical Dyssynchrony
The baseline characteristics and parameters of rest SPECT MPI of controls and AHF patients are summarized in Table 2. Compared with the controls, the AHF patients had significantly elevated rest scar, PSD, and PBW. We defined 21.25° of PSD and 77.76° of PBW, which were mean + 2 × SD from the control group, as the threshold of LVMD.
Prediction of End Point Events
In the univariate analysis, as shown in Table 3, ESV (HR 1.04; 95% CI 1.00-1.08; P = .039), EDV (HR 1.05; 95% CI 1.00-1.09; P = .033), and rest scar (HR 1.04; 95% CI 1.02-1.06; P < .001) were associated with adverse events. Both electrical dyssynchrony defined by QRS duration (HR 1.02; 95% CI 1.01-1.03; P = 0.001), and mechanical dyssynchrony defined by PBW > 77.76° (HR 2.92; 95% CI 1.00-8.47; P = .049) were associated with adverse events. However, PSD > 21.25° and LVEF were not significantly associated with clinical events (HR 1.98; 95% CI 0.79-4.93; P = .143). In the multivariate Cox proportional hazard analysis, both QRS duration > 120 ms (HR 1.03; 95% CI 1.01-1.04; P < .001) and PBW > 77.76° (HR 5.05; 95% CI 1.44-14.69; P = .011) were independent predictors of adverse events. PSD > 21.25° was still negative to predict clinical events.
Using both electrical and mechanical dyssynchrony parameters, four subgroups were formed for Kaplan-Meier analysis: (i) Group 1: QRS duration ≤ 120 ms and PBW ≤ 77.76°; (ii) Group 2: QRS duration ≤ 120 ms and PBW > 77.76°; (iii) Group 3: QRS duration > 120 ms and PBW ≤ 77.76°; (iv) Group 4: QRS duration > 120 ms and PBW > 77.76°. The Kaplan-Meier survival probability curve showed that at 36 months, the PBW > 77.76° group had a higher end point rate than the PBW ≤ 77.76° group (log-rank P = .039; Figure 1). Adverse events during follow-up were 0% (0/19) in Group 1, 25.0% (10/40) in Group 2, 36.4% (4/11) in Group 3, and 54.5% (12/22) in Group 4 (long rank P < .001, Figure 2).
As shown in Figure 3, QRS duration combined with PBW showed the highest accuracy to predict end point events compared to either QRS duration > 120 ms (AUC: 0.747 vs 0.679, P = .001) or PBW > 77.76° (AUC: 0.747 vs 0.620, P = .049) alone, respectively. Furthermore, Figure 4A shows that the value of χ2 values with the addition of electrical and mechanical dyssynchrony above and beyond electrical dyssynchrony or mechanical dyssynchrony alone, and the incremental value of χ2 values with the addition of electrical and mechanical dyssynchrony above and beyond clinical/laboratory values (4B). Clinical/laboratory values include systolic blood pressure, NT-proBNP and body mass index.
Receiver-operating characteristic curves to predict clinical events occurring from enrollment to the end of the follow-up period for QRS duration and PBW. AUC1= AUC of QRS duration combined with PBW (AUC 0.75; sensitivity 62%; specificity 74%); AUC2= AUC of QRS duration (AUC 0.68; sensitivity 62%; specificity 74%); AUC3= AUC of PBW (AUC 0.62; sensitivity 85%; specificity 39%)
The incremental value of χ2 values with the addition of electrical and mechanical dyssynchrony above and beyond electrical dyssynchrony or mechanical dyssynchrony alone (A), and the incremental value of χ2 values with the addition of electrical and mechanical dyssynchrony above and beyond clinical/laboratory values (B). Clinical/laboratory values include systolic blood pressure, NT-proBNP and body mass index. #P < .05, ##P < .01, ###P < .001
An example of an AHF patient with electrical and mechanical dyssynchrony is shown in Figure 5.
Discussion
The main finding of this study is that the QRS duration > 120 ms and PBW > 77.76° are independent predictors of all-cause death and heart transplantation in AHF patients. The combination of QRS duration and PBW provides an incremental prognostic value to each measure separately. LVMD measured by gated SPECT MPI has been suggested useful for prognosis in the chronic HF population13 and particularly in patient selection and prediction of response and prognosis for cardiac resynchronization therapy (CRT).14,15 Henneman et al found that a cut off value of 43° for PSD (sensitivity 0.7; specificity 0.7) and 135° for PBW (sensitivity 0.74; specificity 0.74) could improve the CRT patient selection.16 Furthermore, the phase analysis of gated SPECT MPI was used to recommend the optimal CRT LV lead positions, which could improve volumetric response and long-term prognosis.13
Noteworthy, AHF patients have worse LV mechanical dyssynchrony than chronic HF patients.7,8 A study in 201 patients with acute onset cardiomyopathy and LV dysfunction (LVEF < 40%) showed that LV dyssynchrony was observed in 54% patients, even with a narrow QRS duration; and LV dyssynchrony was resolved within 6 months in 42% of all patients after conventional medical therapy.8 Another study in 50 patients with acute decompensated heart failure also showed that LV dyssynchrony was present in about half of the patients, whose hemodynamics recovered after intensive medical therapy.7 The mechanism of worse dyssynchrony in AHF could be that the activation of renin-angiotensin and sympathetic systems leads to tachycardia and an elevation in vascular resistance, which may result in exacerbation of dyssynchrony. Moreover, patients with baseline LV dyssynchrony had more hemodynamic recovery after intensive medical therapy than patients without baseline LV dyssynchrony,7 suggesting that the presence of LV dyssynchrony in the patients with acute decompensated heart failure, LV dysfunction, and wide QRS duration, although often associated with the propensity to have CRT,17 could identify the patients with better hemodynamic responses to intensive medical therapy as an alternative to CRT.
In our study, no significant differences in PSD or PBW were found between the patients with or without clinical events. However, LVMD (PBW > 77.76°), a cut off value derived from our normal database, had a clinically significant prognostic value for patients with AHF. PBW > 77.76° achieved an AUC of 0.62, whereas PSD > 21.25° did not show clinical significance. A possible explanation is that PSD may be inappropriate to characterize the widely distributed and sometimes multi-peak distributions in phase histograms.18
According to the current guidelines, the selection criteria of CRT implantation heavily relied on electrical dyssynchrony on ECG without the use of mechanical dyssynchrony.1 Mechanical dyssynchrony is a significant prognosticator and independent of electrical dyssynchrony in predicting adverse events in patients with heart failure.19,20 However, electrical dyssynchrony is not always accompanied by mechanical dyssynchrony.9,21 The integrative analysis of electrical and mechanical dyssynchrony has been demonstrated to improve CRT patient selection.22,23 Our investigation in this study demonstrated that the integrative analysis of electrical and mechanical dyssynchrony had a significant improvement over either QRS duration > 120 ms (AUC: 0.75 vs 0.68, P = .001) or PBW > 77.76° (AUC: 0.75 vs 0.62, P = .049) alone. Based on our prognostic model, intensive medical therapy or early device therapy may be considered for AHF patients with both electrical and mechanical dyssynchrony. First, although the clinical symptoms are not severe in AHF patients with both electrical and mechanical dyssynchrony, intensive treatment should be given to improve prognosis. Second, regardless of LV dyssynchrony in AHF patients, medical therapy instead of CRT should be the first choice because LV dyssynchrony resolved within 6 months in almost half of all patients after medical therapy.7,8 Third, early CRT should be considered for AHF patients with both electrical and mechanical dyssynchrony who do not respond to medical therapy.
Study Limitations
This study has several limitations. First, both ischemic cardiomyopathy (ICM, n = 19, 20.7%) and non-ICM patients were included in this study. Due to the different etiology, patients with ICM and non-ICM have different electrical activation and mechanical contraction patterns.24,25 Therefore, a single cut off value may affect the accuracy to predict adverse events in AHF patients. Second, this study enrolled a relatively small number of patients from a single center with the inherent limitation of such study design. Additional prospective multicenter studies in a larger population are needed.
Conclusions
The combination of QRS duration > 120 ms and PBW > 77.76° was an independent predictor of all-cause death and heart transplantation in AHF patients. The integrative analysis of electrical and mechanical dyssynchrony provides incremental prognostic value for clinical use.
New knowledge gained
Electrical dyssynchrony combined with mechanical dyssynchrony provides incremental prognostic value for AHF patients.
Abbreviations
- AHF:
-
Acute heart failure
- HF:
-
Heart failure
- LV:
-
Left ventricle
- LVEF:
-
Left ventricular ejection fraction
- LVMD:
-
Left ventricular mechanical dyssynchrony
- NYHA:
-
New York Heart Association
- PBW:
-
Phase bandwidth
- PSD:
-
Phase standard deviation
- SPECT:
-
Single-photon emission computed tomography
References
Ponikowski A, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol 2016;69:1167.e1-e85. https://doi.org/10.1016/j.recesp.2016.10.014.
Bruch C, Gotzmann M, Stypmann J, Wenzelburger F, Rothenburger M, Grude M, et al. Electrocardiography and Doppler echocardiography for risk stratification in patients with chronic heart failure: Incremental prognostic value of QRS duration and a restrictive mitral filling pattern. J Am Coll Cardiol 2005;45:1072-5. https://doi.org/10.1016/j.jacc.2004.12.064.
Lund LH, Jurga J, Edner M, Benson L, Dahlström U, Linde C, et al. Prevalence, correlates, and prognostic significance of QRS prolongation in heart failure with reduced and preserved ejection fraction. Eur Heart J 2013;34:529-39. https://doi.org/10.1093/eurheartj/ehs305.
Cho GY, Song JK, Park WJ, Han SW, Choi SH, Doo YC, et al. Mechanical dyssynchrony assessed by tissue doppler imaging is a powerful predictor of mortality in congestive heart failure with normal QRS duration. J Am Coll Cardiol 2005;46:2237-43. https://doi.org/10.1016/j.jacc.2004.11.074.
Bader H, Garrigue S, Lafitte S, Reuter S, Jaïs P, Haïssaguerre M, et al. Intra-left ventricular electromechanical asynchrony: A new independent predictor of severe cardiac events in heart failure patients. J Am Coll Cardiol 2004;43:248-56. https://doi.org/10.1016/j.jacc.2003.08.038.
Lee APW, Zhang Q, Looi JL, Sun JP, Fang F, Liu YT, et al. Left ventricular systolic dyssynchrony in acute decompensated heart failure. Int J Cardiol 2013;168:4285-6. https://doi.org/10.1016/j.ijcard.2013.04.200.
Mullens W, Borowski AG, Curtin R, Grimm RA, Thomas JD, Tang WHW. Mechanical dyssynchrony in advanced decompensated heart failure: Relation to hemodynamic responses to intensive medical therapy. Heart Rhythm 2008;5:1105-10. https://doi.org/10.1016/j.hrthm.2008.04.004.
Tanaka H, Tanabe M, Simon MA, Starling RC, Markham D, Thohan V, et al. Left ventricular mechanical dyssynchrony in acute onset cardiomyopathy: Association of its resolution with improvements in ventricular function. JACC Cardiovasc Imaging 2011;4:445-56. https://doi.org/10.1016/j.jcmg.2011.02.012.
Sillanmäki S, Lipponen JA, Tarvainen MP, Laitinen TT, Hedman M, Hedman A, et al. Relationships between electrical and mechanical dyssynchrony in patients with left bundle branch block and healthy controls. J Nucl Cardiol 2018. https://doi.org/10.1007/s12350-018-1204-0.
Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, et al. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: Development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol 2005;12:687-95. https://doi.org/10.1016/j.nuclcard.2005.06.088.
Henneman MM, Chen J, Ypenburg C, Dibbets P, Bleeker GB, Boersma E, et al. Phase analysis of gated myocardial perfusion single-photon emission computed tomography compared with tissue Doppler imaging for the assessment of left ventricular dyssynchrony. J Am Coll Cardiol 2007;49:1708-14. https://doi.org/10.1016/j.jacc.2007.01.063.
Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guide. Circulation 2003;108:1146-62. https://doi.org/10.1161/01.CIR.0000073597.57414.A9.
Zhang X, Qian Z, Tang H, Hua W, Su Y, Xu G, et al. A new method to recommend left ventricular lead positions for improved CRT volumetric response and long-term prognosis. J Nucl Cardiol. 2019. https://doi.org/10.1007/s12350-019-01735-7.
Zhang Q, Yu CM. Clinical implication of mechanical dyssynchrony in heart failure. J Cardiovasc Ultrasound 2012;20:117-23. https://doi.org/10.4250/jcu.2012.20.3.117.
Zhou W, Garcia EV. Nuclear image-guided approaches for cardiac resynchronization therapy (CRT). Curr Cardiol Rep 2016;18:7. https://doi.org/10.1007/s11886-015-0687-4.
Henneman MM, Chen J, Dibbets-Schneider P, Stokkel MP, Bleeker GB, Ypenburg C, Van Der Wall EE, Schalij MJ, Garcia EV, Bax JJ. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT preferably predict response to CRT? J Nucl Med 2007;48:1104-11. https://doi.org/10.2967/jnumed.107.049700.
Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol 2004;44:1834-40. https://doi.org/10.1016/j.jacc.2004.08.016.
Zhou W, Hung G-U. Left-ventricular mechanical dyssynchrony in the prognosis of dilated cardiomyopathy: Which parameter is more useful? J Nucl Cardiol 2018;25:1688-91. https://doi.org/10.1007/s12350-017-0863-6.
Cho GY, Kim HK, Kim YJ, Choi DJ, Sohn DW, Oh BH, et al. Electrical and mechanical dyssynchrony for prediction of cardiac events in patients with systolic heart failure. Heart 2010;96:1029-32. https://doi.org/10.1136/heartjnl-2017-311813.
Hess PL, Shaw LK, Fudim M, Iskandrian AE, Borges-Neto S. The prognostic value of mechanical left ventricular dyssynchrony defined by phase analysis from gated single-photon emission computed tomography myocardial perfusion imaging among patients with coronary heart disease. J Nucl Cardiol 2017;24:482-90. https://doi.org/10.1007/s12350-015-0388-9.
Yu CM, Lin H, Zhang Q, Sanderson JE. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart 2003;89:54-60. https://doi.org/10.1136/heart.89.1.54.
van’t Sant J, ter Horst IAH, Wijers SC, Mast TP, Leenders GE, Doevendans PA, et al. Measurements of electrical and mechanical dyssynchrony are both essential to improve prediction of CRT response. J Electrocardiol 2015;48:601-8. https://doi.org/10.1016/J.JELECTROCARD.2015.01.015.
Cheng C-M, Su C-S, Chou P, Liao Y-C, Wang C-Y, Zhang J-R, et al. Prediction of both electrical and mechanical reverse remodeling on acute electrocardiogram changes after cardiac resynchronization therapy. Circ J 2017;81:1322-8. https://doi.org/10.1253/circj.cj-16-1181.
Peichl P, Kautzner J, Čihák R, Bytešník J. The spectrum of inter- and intraventricular conduction abnormalities in patients eligible for cardiac resynchronization therapy. Pacing Clin Electrophysiol 2004;27:1105-12. https://doi.org/10.1111/j.1540-8159.2004.00592.x.
Lin X, Xu H, Zhao X, Chen J. Sites of latest mechanical activation as assessed by SPECT myocardial perfusion imaging in ischemic and dilated cardiomyopathy patients with LBBB. Eur J Nucl Med Mol Imaging 2014;41:1232-9. https://doi.org/10.1007/s00259-014-2718-6.
Acknowledgments
This study was funded by Grants from the Six Talents Peak Project of Jiangsu Province (Project Number 2014-WSN-008, PI: Yanli Zhou). It was also supported by a Grant from the American Heart Association (Project Number 17AIREA33700016, PI: Weihua Zhou) and a New Faculty Grant from Michigan Technological University Institute of Computing and Cybersystems (PI: Weihua Zhou).
Disclosures
None of the authors have any relevant conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors have also provided an audio summary of the article, which is available to download as ESM, or to listen to via the JNC/ASNC Podcast.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, Y., He, Z., Liao, S. et al. Prognostic value of integrative analysis of electrical and mechanical dyssynchrony in patients with acute heart failure. J. Nucl. Cardiol. 28, 140–149 (2021). https://doi.org/10.1007/s12350-020-02429-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-020-02429-1