Abstract
Background
The prognostic value of left ventricular dyssynchrony measured by gated single-photon emission computed tomography (GSPECT) myocardial perfusion imaging (MPI) and its relationship to electrical dyssynchrony measured by QRS duration are incompletely understood. The aim of this study was therefore to examine the independent and incremental prognostic value of dyssynchrony in yet the largest group of patients with coronary artery disease (CAD).
Methods and Results
Patients presenting for GSPECT- MPI between July 1993 and May 1999 in normal sinus rhythm were identified from the Duke Nuclear Cardiology Databank and the Duke Databank for Cardiovascular Disease (N = 1244). After a median of 4.2 years, 336 deaths occurred. At 8 years, the Kaplan-Meier estimates of the probability of death were 34.0% among patients with a phase bandwidth <100° and 56.8% among those with a bandwidth ≥100°. After adjustment for standard clinical variables, QRS dyssynchrony was independently associated with death (Hazard Ratio (HR), per 10°: 1.092, 95% Confidence Interval (CI) 1.048,1.139, P < .0001). Phase bandwidth was similarly associated with death after clinical adjustment (HR per 10°: 1.056, 95% CI 1.041,1.072, P < .0001). In clinically adjusted models examining QRS duration in addition to phase bandwidth, phase bandwidth had a stronger association with mortality. After accounting for left ventricular ejection fraction (LVEF), neither QRS duration nor phase bandwidth were statistically significant. Among patients with EF >35%, QRS duration and phase bandwidth together provided value above that provided by LVEF alone (P = 0.0181). When examining cardiovascular death, results were consistent with all-cause death.
Conclusions
Among patients with CAD, mechanical left ventricular dyssynchrony measured by GSPECT MPI has a stronger relationship with outcomes than electrical dyssynchrony measured by QRS duration. After adjustment for baseline characteristics and LVEF, neither mechanical nor electrical dyssynchrony is independently associated with all-cause death or cardiac death. Among patients with EF >35%, mechanical and electrical dyssynchrony together provided prognostic value above that afforded by LVEF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
More than 16 million American adults have been diagnosed with coronary artery disease (CAD), which underlies 1 in 6 deaths.1 Over $11 billion was paid to Medicare beneficiaries for in-hospital admissions where the primary diagnosis was CAD, and costs are projected to increase twofold over the next 20 years.1 Prognostication may help better target preventive therapies, improve outcomes, and reduce cost. In the current era, gated single-photon emission computed tomography (GSPECT) myocardial perfusion imaging (MPI) is commonly used in this capacity, largely to ascertain the presence and severity of epicardial CAD.
In addition to myocardial perfusion, GSPECT MPI also allows for assessment of left ventricular (LV) dyssynchrony due to recent software developments.2 A series of three-dimensional images of the LV are produced and correspond to sequential time points in the cardiac cycle. Wall motion and wall thickening and are measured by a change in the location of each myocardial segment and a change in signal intensity, respectively. Phase analysis assesses regional LV count changes throughout the cardiac cycle. The time when each segment starts to contract is recorded. The degree of heterogeneity in the distribution of these time intervals (phase standard deviation (SD)) and the range of time during which 95% of the LV is initiating contraction (phase bandwidth (BW)) serve as measures of LV dyssynchrony.
Cardiac resynchronization is a natural therapy to consider in the setting of mechanical dyssynchrony. It is most effective in the setting of electrical dyssynchrony, namely, left bundle branch block and a wide QRS.3 Use is currently restricted to patients with LV ejection fraction (EF) ≤35% but has been considered in patients with an EF >35% (clinicaltrials.gov identifier NCT01735916). Unfortunately, 1 in 3 patients receiving cardiac resynchronization therapy do not benefit from it.4,5 Whether mechanical LV dyssynchrony could aid in the selection of cardiac resynchronization therapy candidates by providing a more prognostic therapeutic target than electrical dyssynchrony or is independently associated with death are unknown. Accordingly, we sought to examine (1) the prognostic value of mechanical LV dyssynchrony measured by GSPECT MPI compared with electrical dyssynchrony measured by QRS duration and left bundle branch block and (2) the association between mechanical LV dyssynchrony measured by GSPECT MPI and all-cause and cardiovascular death among patients with CAD. This to our knowledge is the largest study exploring the prognostic implications of mechanical LV dyssynchrony by a nuclear technique.
Methods
Data Sources
The Duke Nuclear Cardiology Databank and the Duke Databank for Cardiovascular Disease (DDCD) are single center, observational registries of patients undergoing cardiac imaging and cardiovascular procedures for cardiac disease. A longitudinal record is established for each patient with significant disease of at least one major epicardial CAD. Demographic and clinical information are collected at the time of procedures and data are linked electronically across these registries.
Study Population
In the current analysis, the study population consisted of serial patients presenting for GSPECT MPI between July 1993 and May 1999. LV dyssynchrony data were available only for patients in normal sinus rhythm. Additional inclusion criteria were the availability of electrocardiographic results within a 60-day window period before or after GSPECT MPI and LV dysfunction measured by GSPECT MPI.
Stress Testing, GSPECT MPI, and Nuclear Dyssynchrony Measurement
Patients able to exercise underwent treadmill stress testing. The Bruce protocol was used unless the referring physician requested an alternative protocol. Patients incapable of exercise underwent pharmacologic stress testing. The Duke protocol for SPECT has been described previously.6,7 Images were obtained with multi-head detectors. The time of acquisition was 30 seconds per projection at rest and 20 seconds per projection during stress. The majority of patients received high-dose 99mTc (21-30 mCi). The raw stress SPECT data were post-processed to obtain the dyssynchrony measurements of phase standard deviation (SD) and phase bandwidth (BW) using standard, commercially available software (Emory Toolbox, Emory University, Atlanta, GA) as described in a previous publication.2
Follow-up and Outcome Measures
Patients were contacted at regular annual intervals by the DDCD follow-up staff to determine vital status.8,9 Deaths and their causes are adjudicated by two independent data abstractors and a faculty cardiologist using data from telephone interviews with family members, death certificates, hospital discharge summaries, and/or matches from a search of the National Death Index with International Classification of Diseases, Tenth Revision, (ICD-10) coded cause of death. For the current analysis, all-cause death and cardiovascular (CV) death were the primary and secondary outcomes, respectively.
Statistical Analysis
Distributions of baseline clinical characteristics are described using medians (25th and 75th percentiles) for continuous variables and percentages for categorical and ordinal variables. Estimates for time to CV and all-cause death stratified by QRS duration and phase BW were obtained using the Kaplan-Meier methods. Associations between phase BW and CV and all-cause death were examined using Cox proportional hazards modeling. The linear relationships between continuous measures and outcomes were examined using cubic spline testing and transformations were implemented if necessary to satisfy this assumption.10 In the initial model, adjustment covariates included baseline characteristics thought to be associated with prognosis. A series of additional models sequentially included electrical dyssynchrony measured by QRS duration, mechanical LV dyssynchrony measured by phase BW, and LVEF. Subgroup analysis of among patients with an EF >35% was performed. In sensitivity analyses, the same models assessed phase SD in lieu of phase BW.
Tests were two-sided, and significance was present if P < 0.05. Analyses were performed using SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA). The Duke University Health System institutional review board approved the study and granted a waiver of consent.
Results
Baseline Characteristics
Table 1 shows baseline characteristics of the final study population stratified by the degree of mechanical LV dyssynchrony. Among 1244 patients, the median age was 64 (interquartile range 55-72) years, 68.7% were male, and 72.8% were white. Compared with patients with lower phase BW, patients with higher phase BW had a higher proportion of males (73.6% vs 67.0%, P = 0.027), more commonly had heart failure (38.9% vs 19.6%, P < 0.001), and more frequently had advanced heart failure symptoms (P < 0.001). They also more often had a history of myocardial infarction (45.4% vs 27.7%, P < 0.001). Figure 1 shows the distribution of phase BW, which is right-skewed. Low phase BW predominates, but the degree of mechanical LV dyssynchrony varies considerably.
Unadjusted Outcomes
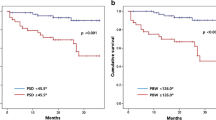
A total of 336 deaths, of which 203 were CV, occurred during a median follow of 4.2 (interquartile range 2.8-6.2) years. Figure 2 and Table 2 provide Kaplan-Meier time to event estimates for all-cause and CV mortality stratified according to the severity of LV dyssynchrony measured by phase BW. At 8 years, the mortality estimate was 34.0% among patients with BW <100° and 56.8% among those with a BW ≥100°. For CV death, 8-year event estimates were 22.7% and 43.6% for the 2 strata, respectively.
Adjusted Outcomes
Clinical variables used in all multivariable Cox models include age, gender, race, history of hypertension, CHF, peripheral vascular disease, cerebrovascular disease, smoking, diabetes, prior myocardial infarction, chronic obstructive pulmonary disease, and chronic renal disease (defined as creatinine >3.0, dialysis, or transplant). After multivariable adjustment for clinical characteristics, sequential models indicated BW was more strongly associated with all-cause death than SD (global Chi square of 192.8 vs 188.1). Model comparison between BW and SD suggested that BW provided a stronger model than SD after adjusting for clinical characteristics. The decision to stratify patients at a value of 100° (degrees) for graphical display was based on a visual examination of the splines and distribution of BW in the population. After adjustment for clinical variables, QRS was independently associated with all-cause death (HR per 10°: 1.092, 95% CI 1.048, 1.139, P < .0001). Phase BW was similarly associated with death after clinical adjustment (HR per 10°: 1.056, 95% CI 1.041, 1.072). In the models examining QRS duration in addition to phase BW, phase BW exhibited a stronger association with outcome. Figure 3 displays global LR Chi squares in order to provide an additive approach to variable contribution to overall models. After accounting for LVEF, neither QRS duration nor phase BW were statistically significant. Among patients with EF >35%, BW + QRS significantly added to EF for death (P = 0.0181). When examining CV death, results were comparable to all-cause death. P values for each sequential model were <0.0001 for both outcomes. In sensitivity analyses using phase SD in lieu of phase BW, results were comparable in magnitude and directionality. Left bundle branch block was assessed in all models along with QRS and was not significant once QRS was included.
A and B Incremental association between clinical variables, QRS duration and left bundle branch block, phase bandwidth (BW), and all-cause (A) or cardiovascular death (B) as indicated by the global χ 2 in serial multivariable models (P values <0.0001 for each sequential model for both outcomes). Clinical variables include age, sex, race, hypertension, history of heart failure, history of peripheral vascular disease, cerebrovascular disease smoking, diabetes, history of myocardial infarction, chronic obstructive pulmonary disease, and renal disease. EF left ventricular ejection fraction
Discussion
The current analysis has three main findings. First, among patients with angiographically significant CAD, mechanical LV dyssynchrony measured by GSPECT MPI adds prognostic information above that provided by standard clinical covariates and electrical dyssynchrony measured by QRS duration. Second, after adjustment for continuously valued LVEF, neither mechanical nor electrical dyssynchrony are independently associated with all-cause death or CV death. Third, among patients with EF >35%, mechanical and electrical dyssynchrony together provide value above that provided by LVEF alone.
Candidacy for cardiac resynchronization therapy is based on QRS duration and the presence or absence of a left bundle branch block in addition to LVEF ≤35% and New York Heart Association functional class II-IV.12 Investigators have considered expanding criteria to those with an LVEF >35% (clinicaltrials.gov identifier NCT01735916). Professional guidelines have issued a class I indication for patients with a QRS duration ≥150 ms and a left bundle branch block, a class IIa indication for patients with a QRS duration ≥ 150 without a left bundle branch block, and a class IIa indication for patients with a QRS duration 120 to 149 ms and a left bundle branch block.12 If amenable to intervention with resynchronization therapy, it may be a worthwhile therapeutic target. The current analysis indicates neither mechanical nor electrical dyssynchrony are independently associated with death after adjusting for LVEF in the total population. Nonetheless, among patients with EF >35%, mechanical and electrical dyssynchrony together add incremental value. These data suggest mechanical LV dyssynchrony measured by GSPECT could complement electrocardiographic dyssynchrony data with regard to cardiac resynchronization therapy patient selection. Notably, the EF of the current study cohort is largely >35%, whereas an ejection ≤35% is required for eligibility for cardiac resynchronization therapy in current clinical practice. Future studies will need to investigate the prognostic value of LV dyssynchrony in populations in whom cardiac resynchronization therapy is currently indicated. This requires adequately powered studies with subgroups with an EF of ≤35%. Whereas dyssynchrony data may prove useful in identifying candidates likely to respond to cardiac resynchronization therapy, simultaneously collected GSPECT MPI data regarding scar location may aid in LV lead placement13 and also impact the likelihood of therapeutic response.
Compared with prior studies examining potential associations between LV mechanical dyssynchrony and death14-18 or a composite outcome including death,19,20 ours has the largest sample size and corresponding event rate. Whereas prior studies found associations between mechanical dyssynchrony and outcomes after accounting for LVEF, the current analysis did not for the total study population. A major reason for divergent conclusions is likely adjustment for continuously valued LVEF in the current study compared with dichotomized LVEF in many of the prior reports. Use of continuously valued adjustment variables is preferred, as all potentially explanatory information is incorporated. Notably, differing study populations may also underlie the variable conclusions. LV dyssynchrony may portend a worse prognosis among patients with end-stage renal disease,16,17 non-ischemic cardiomyopathy,18 or a lower LVEF,15,20 compared with the current study population.
In addition to examining whether GSPECT MPI LV dyssynchrony data can and in the identification of patients likely to respond to cardiac resynchronization therapy, future studies should examine other patient populations in need of accurate prognostication or elucidation of underlying pathophysiology. A patient population in whom dyssynchrony data are promising is those with cancer undergoing chemotherapy.21 A change in echocardiographic myocardial deformation imaging reportedly precedes a decline in EF and may allow for a prompt change in chemotherapeutic regimen. Echocardiographic strain has also been implicated in the mechanism of heart failure with preserved EF.22 Whether mechanical dyssynchrony measured by GSPECT MPI could be used in analogous fashions or others is unknown.
Limitations
Limitations of the current analysis are several and should be acknowledged. First, the majority of the study cohort had an EF >35%. Future studies should examine patients with a reduced EF. Second, the registry is based at a single center. Replication at other sites is required to ascertain the generalizability of our findings. Third, use of cardiovascular implantable electronic devices such as cardiac resynchronization therapy, implantable cardioverter-defibrillators, or pacemakers was not captured. The absence of these data may have led to unmeasured confounding. Residual confounding may exist and impact the validity of our results.
Conclusions
Among patients with significant CAD, mechanical LV dyssynchrony measured by GSPECT MPI has a stronger association with all-cause mortality and CV mortality than electrical dyssynchrony measured QRS duration and left bundle branch after accounting for clinical characteristics. After adjustment for LVEF, neither form of dyssynchrony has a significant independent relationship with all-cause or CV death. Among patients with an LVEF exceeding 35%, mechanical and electrical dyssynchrony together provide information above that afforded by LVEF alone. Whether mechanical dyssynchrony could complement electrical dyssynchrony in the selection of cardiac resynchronization therapy candidates is unknown and warrants further investigation.
New Knowledge Gained
In the current analysis of patients with angiographically significant CAD, mechanical LV dyssynchrony measured by GSPECT MPI has a stronger relationship with outcomes than electrical dyssynchrony measured by QRS duration. After adjustment for baseline characteristics and LVEF, neither mechanical nor electrical dyssynchrony are independently associated with all-cause death or CV death. Among patients with an LVEF >35%, mechanical and electrical dyssynchrony together provide value above that afforded by LVEF alone.
Abbreviations
- BW:
-
Bandwidth
- CAD:
-
Coronary artery disease
- CV:
-
Cardiovascular
- DDCD:
-
Duke databank for cardiovascular disease
- EF:
-
Ejection fraction
- GSPECT:
-
Gated single-photon emission computed tomography
- LV:
-
Left ventricular/ventricle
- MPI:
-
Myocardial perfusion imaging
- SD:
-
Standard deviation
References
Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics-2011 update: A report from the American Heart Association. Circulation 2011;123:e18-209.
Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, et al. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: Development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol 2005;12:687-95.
Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, Brown M, et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT). Circulation 2011;123:1061-72.
Linde C, Leclercq C, Rex S, Garrigue S, Lavergne T, Cazeau S, et al. Long-term benefits of biventricular pacing in congestive heart failure: Results from the multisite stimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 2002;40:111-8.
Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845-53.
Borges-Neto S, Tuttle RH, Shaw LK, Smith WT, Jain D, Coleman RE, et al. Outcome prediction in patients at high risk for coronary artery disease: Comparison between 99mTc tetrofosmin and 99mTc sestamibi. Radiology 2004;232:58-65.
Borges-Neto S, Pagnanelli RA, Shaw LK, Honeycutt E, Shwartz SC, Adams GL, et al. Clinical results of a novel wide beam reconstruction method for shortening scan time of Tc-99m cardiac SPECT perfusion studies. J Nucl Cardiol 2007;14:555-65.
Harris PJ, Harrell FE Jr, Lee KL, Behar VS, Rosati RA. Survival in medically treated coronary artery disease. Circulation 1979;60:1259-69.
Mark DB, Nelson CL, Califf RM, Harrell FE Jr, Lee KL, Jones RH, et al. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation 1994;89:2015-25.
Stone C, Koo C. Additive splines in statistics. Proc Stat Comp Sect Am Statist Assoc 1985:45-8.
Stone CJK, C-Y. Additive splines in statistics. Additive splines Statistical Computing Section. 1986:45-8.
Tracy CM, Epstein AE, Darbar D, Dimarco JP, Dunbar SB, Estes NA 3rd, et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Heart Rhythm 2012;9:1737-53.
Zhou W, Hou X, Piccinelli M, Tang X, Tang L, Cao K, et al. 3D fusion of LV venous anatomy on fluoroscopy venograms with epicardial surface on SPECT myocardial perfusion images for guiding CRT LV lead placement. JACC Cardiovasc Imaging 2014;7:1239-48.
Pazhenkottil AP, Buechel RR, Husmann L, Nkoulou RN, Wolfrum M, Ghadri JR, et al. Long-term prognostic value of left ventricular dyssynchrony assessment by phase analysis from myocardial perfusion imaging. Heart 2011;97:33-7.
Uebleis C, Hellweger S, Laubender RP, Becker A, Sohn HY, Lehner S, et al. Left ventricular dyssynchrony assessed by gated SPECT phase analysis is an independent predictor of death in patients with advanced coronary artery disease and reduced left ventricular function not undergoing cardiac resynchronization therapy. Eur J Nucl Med Mol Imaging 2012;39:1561-9.
AlJaroudi W, Aggarwal H, Venkataraman R, Heo J, Iskandrian AE, Hage FG. Impact of left ventricular dyssynchrony by phase analysis on cardiovascular outcomes in patients with end-stage renal disease. J Nucl Cardiol 2010;17:1058-64.
Aggarwal H, AlJaroudi WA, Mehta S, Mannon R, Heo J, Iskandrian AE, et al. The prognostic value of left ventricular mechanical dyssynchrony using gated myocardial perfusion imaging in patients with end-stage renal disease. J Nucl Cardiol 2014;21:739-46.
Goldberg AS, Alraies MC, Cerqueira MD, Jaber WA, Aljaroudi WA. Prognostic value of left ventricular mechanical dyssynchrony by phase analysis in patients with non-ischemic cardiomyopathy with ejection fraction 35-50% and QRS < 150 ms. J Nucl Cardiol 2014;21:57-66.
Aljaroudi WA, Hage FG, Hermann D, Doppalapudi H, Venkataraman R, Heo J, et al. Relation of left-ventricular dyssynchrony by phase analysis of gated SPECT images and cardiovascular events in patients with implantable cardiac defibrillators. J Nucl Cardiol 2010;17:398-404.
Zafrir N, Nevzorov R, Bental T, Strasberg B, Gutstein A, Mats I, et al. Prognostic value of left ventricular dyssynchrony by myocardial perfusion-gated SPECT in patients with normal and abnormal left ventricular functions. J Nucl Cardiol 2014;21:532-40.
Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J Am Coll Cardiol 2014;63:2751-68.
Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014;63:447-56.
Acknowledgements
SB received research Grant support from GE Health. AEI has received research grants from Astellas Pharma USA.
Funding
Funding was provided by GE Healthcare.
Disclosure
MF, LKS, and PLH report no relevant conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
See related editorial, doi:10.1007/s12350-016-0416-4.
Rights and permissions
About this article
Cite this article
Hess, P.L., Shaw, L.K., Fudim, M. et al. The prognostic value of mechanical left ventricular dyssynchrony defined by phase analysis from gated single-photon emission computed tomography myocardial perfusion imaging among patients with coronary heart disease. J. Nucl. Cardiol. 24, 482–490 (2017). https://doi.org/10.1007/s12350-015-0388-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-015-0388-9