Abstract
Background
The aim of this study was to determine whether right ventricle (RV) 18F-fluorodeoxyglucose (FDG) uptake can predict positive findings of endomyocardial biopsy (EMB) in patients with cardiac sarcoidosis (CS).
Methods
70 consecutive patients with clinically diagnosed CS who had undergone FDG PET were registered in the present study. Patients without EMB (n = 42) were excluded. Ultimately, 28 patients were studied. EMB samples were obtained from the RV septum. We evaluated the FDG uptake on six segments (RV, left ventricle anterior, septal, lateral, inferior, and apex).
Results
Positive EMB was found in six patients (21%). Patients were divided into two groups according to positive (n = 12 [43%]) or negative (n = 16 [57%]) RV FDG uptake. Patients with positive RV FDG uptake had a significantly higher frequency of positive EMB than those without (42% vs. 6%, P = 0.024). On the other hand, there was no EMB-predictive value for the FDG uptakes in the other five segments, the cardiac metabolic volume, total lesion glycolysis, left ventricular ejection fraction, or any electrocardiogram findings.
Conclusions
FDG uptake of the RV but no other heart segment was associated with positive EMB in CS patients. The presence of RV FDG uptake could improve the rate of positive EMB up to 42% in patients with CS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcoidosis is a systemic granulomatous disease that affects multiple organs, including the heart, lung, lymph node, skin, eyes, and central nervous system.1 The presence of cardiac sarcoidosis (CS) has been recognized as a determinant of worse clinical outcomes such as atrioventricular block, ventricular arrhythmias, congestive heart failure, and sudden death, either alone or those combinations.2,3 Therefore, cardiac involvement should be detected earlier and treated as soon as possible because corticosteroid therapy is effective for CS patients with a preserved left ventricular ejection fraction (LVEF) before left ventricle (LV) dysfunction develops.4,5 However, it is often difficult to diagnose CS accurately because the sampling error of endomyocardial biopsy (EMB) is associated with a low sensitivity in the detection of epithelioid granuloma.6,7
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) is a useful modality for detecting active inflammatory lesions associated with sarcoidosis with a high sensitivity.8,9,10 Especially, the presence of right ventricle (RV) FDG uptake may reflect the spread of disease activity. In fact, suspected CS patients who had RV FDG uptake showed a greater number of LV-involved segments and met the Japan Ministry of Health and Welfare (JMHW) diagnostic criteria more frequently.11 Moreover, RV FDG uptake has been known to be a predictor of death or ventricular tachycardia in CS patients.2 Therefore, FDG PET has been used worldwide for diagnosis, assessment of disease activity, and even a prediction of adverse events for patients with CS. A recent study demonstrated that FDG PET-guided sampling of mediastinal lymph nodes is useful for diagnosing pathohistology in CS patients,12 suggesting that FDG PET-guided sampling of EMB may also be useful.
Accordingly, the aim of this study was to determine whether RV FDG uptake could predict positive EMB in CS patients.
Materials and Methods
Study Design
This was a single-center, observational, retrospective study that included consecutive patients with a diagnosis of CS between March 2011 and April 2018. CS was defined as meeting the Guidelines for Diagnosis and Treatment of Cardiac Sarcoidosis (Japanese Circulation Society 2016).13 The study protocol was approved by the Ethics Committee of Hokkaido University Hospital (IRB No. 017-0446). The investigation conformed with the principles outlined in the Declaration of Helsinki.
Study Population
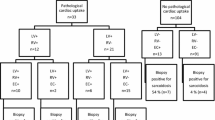
We retrospectively registered 70 consecutive patients, who underwent 18F-FDG PET between March 2011 and April 2018, diagnosed as CS. Patients without EMB (n = 42) were excluded. Ultimately, 28 patients (histological diagnosis group [n = 5], clinical diagnosis group [n = 19], isolated CS group [n = 4]), which was defined as meeting the Guidelines for Diagnosis and Treatment of Cardiac Sarcoidosis [Japanese Circulation Society 2016]13) who underwent EMB were included in this study (Figure 1).
EMB Procedure
All EMB procedures were performed via the femoral approach under fluoroscopic image guidance and echocardiography support. In some cases, right ventriculography support was used. A 5.5-Fr long curved sheath (95 cm, MEDIKIT) was placed in the RV, and the head of the bioptome was placed in the middle of the interventricular septum. All EMB procedures were performed by two experienced operators. Harvested myocardial tissue specimens were fixed in 10% buffered formalin. Histologic evaluation was performed by two expert pathologists. We defined as “positive EMB” if non-caseating epithelioid granulomas was confirmed in the myocardial tissue.
Echocardiography
Echocardiography was performed using either an Aplio Artida® SSH-88-CV or Aplio® SSA-770A (Toshiba Medical Systems, Tochigi, Japan). The LVEF was calculated from apical four- and two-chamber views using the biplane method of disks.14 Abnormal wall motion, regional wall thinning or thickening, and ventricular aneurysm were considered abnormal for ultrasound cardiography based on the Guidelines for Diagnosis and Treatment of Cardiac Sarcoidosis (Japanese Circulation Society 2016).13 Echocardiography data were evaluated by two experienced cardiologists.
18F-FDG PET Imaging
All patients fasted for a median of 20 (inter quartile range [IQR] 18 to 21) hours before FDG PET studies to reduce physiological myocardial uptake of FDG.15,16 The previous study showed that Group A (6 hours fast) showed a higher percentage of diffuse LV uptake than did group B (18 hours) (27.6 vs 0.0%, P = 0.0041).17 In this study, there were seven patients (25%) taking low-carbohydrate diet preparation on suppressed physiological myocardial 18F-FDG uptake. All these patients showed reduced physiological FDG uptake. However, there were 21 (75%) patients who did not take low-carbohydrate diet. The patients with RV FDG uptake had perfusion defect on SPECT and/or delayed enhancement of magnetic resonance imaging. Accordingly, there would be not physiological RV FDG uptake in this cohort. PET imaging was performed using a Biograph® 64 TruePoint with TrueV PET/computed tomography (CT) scanner (Siemens Japan, Tokyo). Low-dose CT for PET/CT was performed for attenuation correction. Scans were performed 60 min after the administration of 242.6 ± 52.3 MBq of FDG. Fasting plasma glucose level was measured before 18F-FDG injection (mean 92.7 ± 14.7 mg/dl).
Acquired images were resliced into a series of short-axis, horizontal long-axis, and vertical long-axis images. The LV and RV FDG uptake were defined as being positive when it was detected visually by two nuclear medicine physicians independently. We use the program in a public website (www.metavol.org), which has been use for performing SUV analysis.18,19 Cardiac metabolic volume (CMV) was defined as the volume within the boundary determined by the threshold (SUV mean of blood pool × 1.5).19 Total lesion glycolysis (TLG) was calculated by multiplying CMV by mean SUV.20 We evaluated the FDG uptake on 6 segments (RV, LV anterior, septal, lateral, inferior, and apex). Two nuclear medicine physicians independently evaluated the LV and RV myocardial FDG images blinded to EMB result. Discordant interpretations were evaluated by a third observer (N.M.).
To evaluated with a second FDG PET after initiation of immunosuppressive therapy, we assessed SUV %change which was calculated as follows: (SUV [baseline]-SUV [after treatment]) × 100/SUV [baseline]. Furthermore, we assessed LV and RV SUVmax change between baseline and after treatment in cases of FDG RV uptake or not.
Statistical Analysis
Continuous variables are presented as means ± SDs when normally distributed, and as medians and IQRs when non-normally distributed. Parameters were compared between groups with and without events using an unpaired t test or Mann–Whitney U test for continuous variables and a Chi-squared test or Fisher’s exact test for dichotomous variables, as appropriate. The changes of SUVmax between baseline and after treatment were evaluated by Wilcoxon rank sum test.
The cumulative incidence of the composite of adverse events including advanced atrioventricular block, ventricular tachycardia or ventricular fibrillation, heart failure hospitalization, and cardiac death was estimated by Kaplan–Meier analysis, and log-rank test was performed to assess significance according to the presence of RV FDG uptake. To evaluate the influence of FDG uptake on the result of EMB, we constructed a univariate logistic regression model.
All tests were two tailed, and a value of P < 0.05 was considered statistically significant. All analyses were performed with JMP Pro® 13.0 (SAS Institute, Cary, NC, USA).
Results
Patient Characteristics
Baseline characteristics are shown in Supplementary Table S1, which differed between the study cohort and exclude patients. The clinical characteristics of all 28 subjects are shown in Table 1. Patients were divided into two groups according to positive (n = 12 [43%]) or negative (n = 16 [57%]) RV uptake on FDG PET. Positive EMB was found in 6 patients (21%). Patients with positive RV FDG uptake had significantly higher frequency of positive EMB than those without (Figure 2). The group with positive RV FDG uptake had significantly higher levels of serum brain-type natriuretic peptide and angiotensin-converting enzyme. There was no difference in age, sex, heart rate, systolic blood pressure, comorbidities, cardiac sign of symptoms, electrocardiogram abnormalities, or echocardiography findings. There was no relation between extracardiac FDG uptake and the results of EMB (Supplementary Tables S2, S3) and/or the presence of RV FDG uptake in this cohort. Twenty-three (82%) patients underwent myocardial perfusion SPECT with 99mTc-MIBI. Perfusion defects on SPECT were found in 18 in these patients. There was no significant difference between patients with RV FDG uptake group and those without (82% vs 75%, P = 0.69). There were no complications associated with EMB in the present study.
PET Variables of the Patients with RV FDG Uptake
PET variables of the patients with RV FDG uptake are shown in Table 2. CMV, TLG, LV SUVmax, and frequency of LV lateral or septal FDG uptake were significantly higher in patients with RV FDG uptake than in those without. Furthermore, the frequency of LV anterior and apex FDG uptake tended to be higher in patients with RV FDG uptake than in those without, but the difference did not reach statistical significance.
FDG Uptake and the Result of EMB
Positive RV FDG uptake was associated with positive EMB in our CS patient series (Table 3, odds ratio 10.7, 95% confidence interval 1.39 to 227, P = 0.046). On the other hand, there was no predictive value regarding positive EMB for FDG uptakes in the other five segments, CMV, TLG, LVEF, electrocardiogram findings, or any blood tests.
RV FDG Uptake and Clinical Outcome (The Study Cohort Plus the Excluded Patients [n = 70])
During a median follow-up period after the first PET (median, 42 months; IQR 27 to 60 months), adverse events occurred in 13 patients (20%), including 2 advanced atrioventricular block, 5 ventricular tachycardia or ventricular fibrillation, 5 heart failure hospitalization, and 1 cardiac death in the study group and the excluded group (n = 70). Kaplan–Meier analysis revealed that composite adverse those events more frequently occurred in patients with RV FDG uptake compared to those without (36% vs 7%, log-rank; P = 0.006, Supplementary Figure 1).
The Evaluation with a Second FDG PET After Initiation of Immunosuppressive Therapy
There were 21 (75%) patients evaluated with a second FDG PET after initiation of immunosuppressive therapy. There was no difference in response assessed by SUV %change to immunosuppressive therapy in both patients with RV FDG uptake and those without (77% vs 76%, P = 0.93). In addition, there was also no difference in both patients with positive EMB and those without (89% vs 73%, P = 0.40). There would be no relation of the response to immunosuppressive therapy in patients with FDG uptake in the RV and/or positive EMB in this cohort. Furthermore, we assessed LV SUVmax (Figure 3A, B) and RV SUVmax (Figure 3C) changes between baseline and after treatment in cases of FDG RV uptake or not. There were significant improvements after initiation of immunosuppressive therapy in all the three groups. Representative cases from the groups with and without RV FDG uptake are shown in Figure 4.
Changes in LV and RV SUVmax after initiation of immunosuppressive therapy. (A) LV SUVmax in case of patients without RV FDG uptake. (B) LV SUVmax in case of patients with RV FDG uptake. (C) RV SUVmax in case of patients with RV FDG uptake. Vertical bars represent medians with interquartile ranges. Open circle; EMB positive, close circle; EMB negative
Representative case from the group with RV FDG uptake. Maximum intensity projection view of the 18F-FDG PET (A), axial views at cardiac level (B), short-axis view at mid-ventricle level (C), horizontal long-axis view at upper ventricle level (D) are presented. FDG PET shows significant focal accumulation in the RV wall (SUVmax 5.1). In addition, EMB identified the presence of granulomas consistent with the diagnosis of sarcoidosis, Hematoxylin–Eosin (E), Elastica-Masson (F). Representative case from the group without RV FDG uptake. Maximum intensity projection view of the 18F-FDG PET (G), axial views at cardiac level (H), short-axis view at mid-ventricle level (I), horizontal long-axis view at upper ventricle level (J) are presented. FDG PET shows significant focal accumulation in the LV septum (SUVmax 10.0). In addition, EMB did not identify the presence of granulomas consistent with the diagnosis of sarcoidosis, Hematoxylin–Eosin (K), Elastica-Masson (L)
Discussion
The major findings of the present study were that patients with RV FDG uptake had significantly greater CMV, TLG, LV SUVmax, and LV involvement than those without, suggesting that RV involvement indicates the spread of disease activity into the whole heart. Accordingly, RV FDG uptake could predict positive EMB in CS patients.
A previous study found that five of six patients with focal RV FDG uptake had positive EMB results for sarcoidosis.2 Our study was consistent with this and extended to new findings that RV was the only predictor of positive EMB. FDG uptake of the other five heart segments, as well as CMV, TLG, LVEF, electrocardiogram findings, and any blood tests, had no predictive value in our larger cohort. These results would be based on the fact that patients with positive RV FDG uptake had significantly high levels of CMV, TLG, LV SUVmax, and high frequency of LV FDG uptake in other regions, suggesting that epithelioid granuloma might be widely distributed in cases of positive RV FDG uptake. In previous autopsy cases, patients who died of CS more frequently had RV regional involvement than those who died of another cause.21 Another study showed that RV FDG uptake was more common in patients who also have findings suggestive of suspected systemic sarcoidosis.22 It would imply that RV involvement occurs in the advanced stages of sarcoidosis. In the current study, all 12 patients with RV FDG uptake also showed similar LV wall uptake. Furthermore, composite adverse cardiac events were more frequently occurred in patients with RV FDG uptake compared to those without.
In the EMB for diagnosis CS, sampling errors are associated with a low sensitivity in the detection of epithelioid granuloma.23,24,25 In previous studies, the CS-diagnostic value of EMB showed a sensitivity no better than 20% to 30%.26 On the other hand, accumulation of FDG in mediastinal lymph nodes on PET in patients with CS was shown to provide a reliable guide for diagnostic biopsies.12 In that study, 24 patients underwent mediastinoscopy for sampling of PET-positive mediastinal lymph nodes, and microscopy revealed diagnostic non-caseating granulomatous inflammation in 24 of the 24 cases (sensitivity 100%). Another case study reported that abnormal FDG uptake corresponded to active granulomatous sarcoid lesions on autopsy.27 The distribution of sarcoid lesions in the heart corresponds with that of FDG uptake. These findings might indicate that lesions showing FDG uptake in patients with CS might provide a reliable source for diagnostic biopsies.
There are some complications resulting from the EMB procedure. Major complications have included death, pericardial tamponade, advanced atrioventricular block requiring permanent pacemaker implantation, and severe tricuspid valve damage. There are also minor complications including chest pain, access site hematomas, arrhythmias, and small pericardial effusions.28,29 A previous study reviewed EMB-related complications, and reported rates varying from 0.71% to 9.2%.30 Thus, the sensitivity of EMB for diagnosis CS is low but many serious complications have been reported. Because negative RV uptake was highly associated with very low rate of positive EMB (only one patient out of sixteen), we might be able to select other organs than the heart for biopsy site as a first choice in negative RV FDG uptake patients. In the Guidelines for Diagnosis and Treatment of Cardiac Sarcoidosis (Japanese Circulation Society 2016),13 either myocardial or extracardiac histology of non-caseating granulomatous inflammation has been mandatory in addition to clinical manifestations and findings at cardiac imaging compatible with CS. In brief, EMB is not always necessary to diagnosis CS when histological diagnosis is done in other organs. Although a previous case study reported that the use of electroanatomic mapping-guided EMB can be decisive in achieving a CS diagnosis,31 there has been no report that FDG PET-guided EMB has been shown to be useful for biopsy-proven CS.
Study Limitations
There are several potential limitations of the present study which should be acknowledged. First, this was a single-center study with a relatively small sample size, thereby limiting our ability to generalize the findings and reducing the statistical power for detecting differences in negative data. However, a similar sample size was used in previous studies evaluating active inflammatory lesions associated with sarcoidosis using FDG PET.8 This is reasonable because FDG PET measurements can detect active inflammatory lesions associated with sarcoidosis with a high sensitivity.8,9,10 Despite the relatively small sample size, positive RV FDG uptake was associated with positive EMB in our CS patient series. In this regard, a larger-scale multi-center study is warranted to confirm the relationship between FDG uptake of RV and the result of EMB in patients with CS. Second, there was unavoidable selection bias in our study because many patients in the study population had to be excluded. In fact, baseline characteristics differed between patients who underwent EBM and those without (Supplementary Table S1). The rate of extracardiac positive biopsy of patients without EMB was significantly higher than study patients (90% vs 61%, P = 0.029). Furthermore, patients with EMB had higher rate of intraventricular septum wall thinning, lower LVEF, higher BNP, higher CMV, and higher TLG compared to patients without EMB, which indicates that there is the significant selection bias for the choice of EMB in this study. These results reflect that cardiologists might tend to perform EMB in patients with advance stage of CS. Nonetheless, high rate of sampling error is still existed in patients with CS, especially CS without RV involvement.
Conclusions
FDG uptake of RV, but not of other regions, was associated with positive EMB in CS patients. The presence of RV FDG uptake could improve the rate of positive EMB up to 42% in patients with CS.
New Knowledge Gained
The present study showed that CS patients with RV FDG uptake had significantly greater CMV, TLG, LV SUVmax, and LV involvement than those without. RV FDG uptake would indicate the spread of disease activity into the whole heart and the advanced stages of sarcoidosis. Accordingly, in CS patients with RV involvement, EMB could tend to be positive.
Abbreviations
- CMV:
-
Cardiac metabolic volume
- CS:
-
Cardiac sarcoidosis
- EMB:
-
Endomyocardial biopsy
- FDG:
-
18F-fluorodeoxyglucose
- LV:
-
Left ventricle
- LVEF:
-
Left ventricular ejection fraction
- PET:
-
Positron emission tomography
- RV:
-
Right ventricle
- SUV:
-
Standardized uptake value
- TLG:
-
Total lesion glycolysis
References
Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007;357:2153-65.
Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014;63:329-36.
Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller-Quernheim J. Sarcoidosis. Lancet 2014;383:1155-67.
Chiu CZ, Nakatani S, Zhang G, Tachibana T, Ohmori F, Yamagishi M, et al. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol 2005;95:143-6.
Nagai T, Nagano N, Sugano Y, Asaumi Y, Aiba T, Kanzaki H, et al. Effect of Corticosteroid Therapy on Long-Term Clinical Outcome and Left Ventricular Function in Patients With Cardiac Sarcoidosis. Circ J 2015;79:1593-600.
Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014;11:1305-23.
Uemura A, Morimoto S, Hiramitsu S, Kato Y, Ito T, Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J 1999;138:299-302.
Ishimaru S, Tsujino I, Takei T, Tsukamoto E, Sakaue S, Kamigaki M, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J 2005;26:1538-43.
Manabe O, Oyama-Manabe N, Ohira H, Tsutsui H, Tamaki N. Multimodality evaluation of cardiac sarcoidosis. J Nucl Cardiol 2012;19:621-4.
Mc Ardle BA, Leung E, Ohira H, Cocker MS, deKemp RA, DaSilva J, et al. The role of F18-fluorodeoxyglucose positron emission tomography in guiding diagnosis and management in patients with known or suspected cardiac sarcoidosis. J Nucl Cardiol 2013;20:297-306.
Manabe O, Yoshinaga K, Ohira H, Sato T, Tsujino I, Yamada A, et al. Right ventricular 18F-FDG uptake is an important indicator for cardiac involvement in patients with suspected cardiac sarcoidosis. Ann Nucl Med 2014;28:656-63.
Simonen P, Lehtonen J, Kandolin R, Schildt J, Marjasuo S, Miettinen H, et al. F-18-fluorodeoxyglucose positron emission tomography-guided sampling of mediastinal lymph nodes in the diagnosis of cardiac sarcoidosis. Am J Cardiol 2015;116:1581-5.
Terasaki F, Yoshinaga K. New guidelines for diagnosis of cardiac sarcoidosis in Japan. Ann Nucl Cardiol 2017;3:42-5.
Schiller NB, Acquatella H, Ports TA, Drew D, Goerke J, Ringertz H, et al. Left ventricular volume from paired biplane two-dimensional echocardiography. Circulation 1979;60:547-55.
Langah R, Spicer K, Gebregziabher M, Gordon L. Effectiveness of prolonged fasting 18F-FDG PET-CT in the detection of cardiac sarcoidosis. J Nucl Cardiol 2009;16:801-10.
Machac J, Bacharach SL, Bateman TM, Bax JJ, Beanlands R, Bengel F, et al. Positron emission tomography myocardial perfusion and glucose metabolism imaging. J Nucl Cardiol 2006;13:e121-51.
Manabe O, Yoshinaga K, Ohira H, Masuda A, Sato T, Tsujino I, et al. The effects of 18-h fasting with low-carbohydrate diet preparation on suppressed physiological myocardial (18)F-fluorodeoxyglucose (FDG) uptake and possible minimal effects of unfractionated heparin use in patients with suspected cardiac involvement sarcoidosis. J Nucl Cardiol 2016;23:244-52.
Hirata K, Kobayashi K, Wong KP, Manabe O, Surmak A, Tamaki N, et al. A semi-automated technique determining the liver standardized uptake value reference for tumor delineation in FDG PET-CT. PLoS ONE 2014;9:e105682.
Manabe O, Kroenke M, Aikawa T, Murayama A, Naya M, Masuda A et al. Volume-based glucose metabolic analysis of FDG PET/CT: The optimum threshold and conditions to suppress physiological myocardial uptake. J Nucl Cardiol 2017.
Ishiyama M, Soine LA, Vesselle HJ. Semi-quantitative metabolic values on FDG PET/CT including extracardiac sites of disease as a predictor of treatment course in patients with cardiac sarcoidosis. EJNMMI Res 2017;7:67.
Tavora F, Cresswell N, Li L, Ripple M, Solomon C, Burke A. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Cardiol 2009;104:571-7.
Tuominen H, Haarala A, Tikkakoski A, Kahonen M, Nikus K, Sipila K. 18-FDG-PET in a patient cohort suspected for cardiac sarcoidosis: Right ventricular uptake is associated with pathological uptake in mediastinal lymph nodes. J Nucl Cardiol 2018.
Kim JS, Judson MA, Donnino R, Gold M, Cooper LT Jr, Prystowsky EN, et al. Cardiac sarcoidosis. Am Heart J 2009;157:9-21.
Doughan AR, Williams BR. Cardiac sarcoidosis. Heart. 2006;92:282-8.
Kandolin R, Lehtonen J, Graner M, Schildt J, Salmenkivi K, Kivisto SM, et al. Diagnosing isolated cardiac sarcoidosis. J Intern Med 2011;270:461-8.
Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology Eur Heart J. 2007;28:3076-93.
Koiwa H, Tsujino I, Ohira H, Yoshinaga K, Otsuka N, Nishimura M. Images in cardiovascular medicine: imaging of cardiac sarcoid lesions using fasting cardiac 18F-fluorodeoxyglucose positron emission tomography: an autopsy case. Circulation 2010;122:535-6.
Holzmann M, Nicko A, Kuhl U, Noutsias M, Poller W, Hoffmann W, et al. Complication rate of right ventricular endomyocardial biopsy via the femoral approach: a retrospective and prospective study analyzing 3048 diagnostic procedures over an 11-year period. Circulation 2008;118:1722-8.
Yilmaz A, Kindermann I, Kindermann M, Mahfoud F, Ukena C, Athanasiadis A, et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation 2010;122:900-9.
Slawek S, Araszkiewicz A, Gaczkowska A, Koszarska J, Celinski D, Grygier M, et al. Endomyocardial biopsy via the femoral access - still safe and valuable diagnostic tool. BMC Cardiovasc Disord 2016;16:222.
Nery PB, Keren A, Healey J, Leug E, Beanlands RS, Birnie DH. Isolated cardiac sarcoidosis: establishing the diagnosis with electroanatomic mapping-guided endomyocardial biopsy. Can J Cardiol 2013;29:e1-3.
Acknowledgements
The authors are grateful to Dr Tomoko Mitsuhashi and Dr Ken Kuwahara for their support with the histologic evaluation. The authors are also grateful to Kota Ohno, PhD, for statistical support.
Disclosure
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Funding
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Omote, K., Naya, M., Koyanagawa, K. et al. 18F-FDG uptake of the right ventricle is an important predictor of histopathologic diagnosis by endomyocardial biopsy in patients with cardiac sarcoidosis. J. Nucl. Cardiol. 27, 2135–2143 (2020). https://doi.org/10.1007/s12350-018-01541-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-018-01541-7