Abstract
Objective
The identification of cardiac sarcoidosis is challenging as there is no gold standard consensually admitted for its diagnosis. The aim of this study was to evaluate the diagnostic value of the assessment of cardiac dynamic 18F-fluoro-2-deoxyglucose positron emission tomography (18F-FDG PET/CT) and net influx constant (Ki) in patients suspected of cardiac sarcoidosis.
Methods
Data obtained from 30 biopsy-proven sarcoidosis patients suspected of cardiac sarcoidosis who underwent a 50-min list-mode cardiac dynamic 18F-FDG PET/CT after a 24 h high-fat and low-carbohydrate diet were analyzed. A normalized coefficient of variation of quantitative glucose influx constant, calculated as the ratio: standard deviation of the segmental Ki (min−1)/global Ki (min−1) was determined using a validated software (Carimas® 2.4, Turku PET Centre). Cardiac sarcoidosis was diagnosed according to the Japanese Ministry of Health and Welfare criteria. Receiving operating curve analysis was performed to determine sensitivity and specificity of cardiac dynamic 18F-FDG PET/CT analysis to diagnose cardiac sarcoidosis.
Results
Six out of 30 patients (20%) were diagnosed as having cardiac sarcoidosis. Myocardial glucose metabolism was significantly heterogeneous in patients with cardiac sarcoidosis who showed significantly higher normalized coefficient of variation values compared to patients without cardiac sarcoidosis (0.513 ± 0.175 vs. 0.205 ± 0.081; p = 0.0007). Using ROC curve analysis, we found a cut-off value of 0.38 for the diagnosis of cardiac sarcoidosis with a sensitivity of 100% and a specificity of 91%.
Conclusions
Our results suggest that quantitative analysis of cardiac dynamic 18F-FDG PET/CT could be a useful tool for the diagnosis of cardiac sarcoidosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcoidosis is a multisystem disease of unknown etiology characterized by the formation of non-caseating granulomas in many organs [1]. Cardiac involvement in sarcoidosis is a cause of morbidity and mortality. Indeed, cardiac granulomas may lead to conduction and rhythm abnormalities, sudden death and congestive heart failure. The prognosis of sarcoidosis is commonly considered as favorable but is significantly changed by the presence of a cardiac localization [2]. Cardiac involvement is symptomatic in only 5% of patients with sarcoidosis, whereas it occurs in 27% of sarcoidosis cases, according to autopsy findings [3]. Identification of cardiac sarcoidosis (CS) is challenging and early diagnosis is needed to initiate a corticosteroid treatment, which is effective against most active clinical manifestations of CS and may improve the prognosis. However, there is no gold standard consensually admitted for the diagnosis of CS, and a lack of agreement on key aspects of patient management [4]. The 1993 guidelines of the Japanese Ministry of Health and Welfare (JMHW), revised in 2006 by the Japanese Society of Sarcoidosis and other Granulomatous Disorders [5], is frequently used as a clinical gold standard for the diagnosis of CS [6]. The Heart Rhythm Society recently published a consensus statement on the diagnosis of cardiac sarcoidosis as well [7].
18F-fluoro-2-deoxyglucose (18F-FDG) is known to accumulate in macrophages and reactive lymphocytes of inflammatory tissues allowing the detection of both inflammation and infection using 18F-FDG PET/CT [8]. Myocardial physiological 18F-FDG uptake can be suppressed using prolonged fasting, heparin load and dietary carbohydrate restriction before the examination [9, 10]. When patient preparation is performed correctly, 18F-FDG PET/CT may be helpful for detecting active lesions of CS.
In most studies, a focal myocardial 18F-FDG uptake using a visual analysis was considered as positive for active CS lesions. Several studies aimed to quantify the myocardial 18F-FDG uptake using the standard uptake value (SUV). Tahara et al. suggested that an increased heterogeneity of myocardial 18F-FDG uptake may be a characteristic feature of CS [11]. In their study, the heterogeneity of 18F-FDG uptake was assessed using the coefficient of variation (COV), calculated as the ratio of the standard deviation of segmental uptake, divided by the mean left ventricular SUV, using a 17-segment model of the heart.
Qualitative or semi-quantitative analysis of the myocardial 18F-FDG uptake appears helpful in this context, but does not fully benefit from the ability of dynamic 18F-FDG PET/CT to provide a quantitative assessment of the myocardial glucose metabolism, based on compartmental analysis [12]. To date, no previous study evaluated the value of quantitative analysis of the myocardial glucose metabolic rate in suspected CS. Therefore, the aim of our study was to evaluate the diagnostic value of cardiac dynamic 18F-FDG PET/CT in patients suspected of CS.
Materials and methods
Study population
We retrospectively analyzed the data from 30 consecutive biopsy-proven sarcoidosis patients suspected of CS who underwent a cardiac dynamic 18F-FDG PET/CT from September 2012 to June 2014 as part of clinical routine evaluation for radiological and clinical sarcoidosis suspicion of definite proven sarcoidosis at Caen University Hospital. Data from 4 patients were excluded because of non-compliance to the diet (2 cases) or because of a history of prior myocardial infarction (2 cases). Clinical evaluation, assessment of disease duration, 12-lead electrocardiogram (ECG) and transthoracic echocardiography were performed in all patients. Serum calcium, C-reactive protein (CRP), angiotensin-converting enzyme (ACE) were measured as well. In all patients, the diagnosis of sarcoidosis was established using the following criteria: consistent clinical presentation, histological evidence of non-caseous granulomas on biopsy specimens and the absence of any infection, occupational factors, or medical treatment known to cause granulomatous disease [1]. According to French regulatory rules for observational studies, our regional review committee (CPP Nord-Ouest 3) waived the requirement for additional approval.
Transthoracic echocardiography
All patients underwent echocardiography using a 1.5 MHz echo-Doppler probe with a iE33 imaging system (Phillips, Eindhoven, Netherlands) as part of routine evaluation for newly diagnosed sarcoidosis [13]. LV ejection fraction was obtained using the modified biplane Simpson’s method.
Myocardial perfusion SPECT imaging
According to our institutional procedures, rest myocardial perfusion SPECT imaging (MPI) was performed in sarcoidosis patients 1 h after the injection of 99mTc sestamibi (7 MBq/kg). When the rest MPI was considered abnormal, MPI was repeated within 1 week after a pharmacological stress (dipyridamole, 0.56 mg/kg). MPI was analysed by an experienced reader, blinded of any clinical data, and was considered as positive for CS in case of perfusion abnormality at rest reversible after dipyridamole, or in case of non-reversible perfusion abnormality after dipyridamole injection associated with an abnormal wall motion within the same segments.
18F-FDG PET/CT: pretest preparation
The patients received a menu indicating allowed and prohibited foods. Patients were asked to take a high-fat and low-carbohydrate (HFLC) dinner the day before the 18F-FDG PET/CT. Oral instructions were also given by a telephone call to ensure about proper understanding of the diet. A physician checked the patient compliance to diet the day of scanning. All patients fasted for at least 12 h before the examination.
Dynamic 18F-FDG PET/CT
Thirty patients underwent a cardiac dynamic PET acquisition followed by a whole body PET acquisition in 25/30 patients. Blood glucose levels were measured before the injection of the radiopharmaceutical. Patients had a plasma glucose level < 1.8 g/L at the time of injection. The heart was centered inside the scanner field of view using a CT topogram. A 50-min list-mode cardiac 18F-FDG PET/CT scan with a single bed position was immediately performed after an intravenous injection of 4 MBq/kg of 18F-FDG. All patients were kept in a resting state with the arms elevated behind the head during the acquisition. All PET acquisitions were performed on a Biograph TruePoint TrueV (Siemens Medical Solutions, Erlangen, Germany) with a 6-slice spiral CT component. CT acquisitions were achieved with the following parameters: 60mAs, 130 kV, pitch 1, collimation 6 × 2 mm. CT acquisitions were reconstructed with a slice thickness of 3 mm and an increment of 2 mm. The dynamic scan consisted of 33 frames with variable frame length (6 × 5, 6 × 10, 3 × 20, 5 × 30, 5 × 60, 8 × 300 s). PET images were reconstructed using an OSEM algorithm (4 iterations, 8 subsets) and a 5 mm FWHM Gaussian filter. Matrix size was 168 × 168 for all reconstructions. The analysis of dynamic PET data was performed using a dedicated software (Carimas 2.4, Turku PET Centre, Turku, Finland). The net influx constant (Ki) was calculated using a Patlak graphical plot method model without plasma sampling and expressed in min−1 [12].
The characterization of the input function was achieved using blood time-activity curves derived from PET images. ROI were drawn in the cavity of the left ventricle to determine an arterial input function and in the cavity of the right ventricle for spillover correction. The Patlak analysis was performed over the period from 10 to 50 min after injection during steady state. We performed both global and segmental assessment of Ki. The segmental analysis was performed using a 17-segment division of the left ventricle [14]. We estimated the heterogeneity of glucose metabolism using a normalized coefficient of variation (NCOV). The NCOV of Ki was calculated by dividing the standard deviation (SD) of Ki by the global Ki: NCOV = SD Ki (min−1)/global Ki (min−1). All analyses were performed by 2 readers (AM and DA) blinded to any clinical data.
Static 18F-FDG PET/CT
Whole body 18F-FDG PET/CT was acquired after the dynamic cardiac acquisition to assess extra-cardiac sarcoidosis involvement [15]. The whole body images were acquired from the base of skull to mid high. The duration of each bed position was set to 160 s. Static CT acquisitions parameters were the same as those used for dynamic study. Static PET images were reconstructed with a PSF reconstruction algorithm (HD; TrueX, Siemens Medical Solutions; 3 iterations and 21 subsets) without filtering (PSFallpass). Matrix size was 168 × 168 for every reconstruction. Visual analysis was performed by two experimented reader, blinded of any clinical data. Based on previous reports, none or diffuse myocardial 18F-FDG uptake was considered as normal, and a focal or focal in diffuse myocardial 18F-FDG uptake was considered as positive for CS [16].
Data analysis
CS was diagnosed according to the JMHW criteria for clinical diagnosis, revised in 2006 [5]. Data were described as median and interquartile ranges. An unpaired t test or Mann–Whitney test was used to examine comparisons between groups (with and without CS). Statistical significance was set at p value < 0.05. Receiving operating curve (ROC) analysis was performed to determine sensitivity and specificity of cardiac dynamic 18F-FDG PET/CT analysis for CS diagnosis taking the JMHW criteria as the gold standard diagnostic test. The inter- and intra-observer reproducibility of NCOV measurement from dynamic 18F-FDG PET/CT studies were assessed using linear regression and Bland–Altman plots. The intra-observer and inter-observer agreement for visual analysis of static 18F-FDG PET/CT were estimated using kappa coefficients (0.81–1 almost perfect agreement, 0.61–0.8 substantial agreement, 0.41–0.6 moderate agreement and 0.21–0.4 fair agreement). Statistical analyses were done using JMP. Graphs were carried out using Prism (GraphPad software, San Diego, CA).
Results
Population
Six out of 30 patients (20%) with sarcoidosis had cardiac involvement according to the JMHW criteria (Table 1). Histological evidence of non-caseous granuloma was obtained mainly from biopsy specimens of mediastinal lymph nodes and salivary glands (respectively 18 and 8 patients). Most patients had a pulmonary involvement (20 patients, 67%). Nine of the 30 sarcoidosis patients (all without cardiac involvement of sarcoidosis) were receiving a steroid therapy at the time of the 18F-FDG PET/CT examination. There was no significant difference in age, body mass index, blood glucose level, CRP, serum calcium and ACE between sarcoidosis patients with (CS+) and without cardiac involvement (CS−). A male predominance (20/30, 67%) was observed, and CS+ had more often ECG abnormalities than CS− (100 vs. 33%, p < 0.001) and a lower left ventricular ejection fraction (54% [49–60] vs. 65% [60–71], p = 0.04). The disease duration was significantly higher in CS− than in CS+ (88.5 ± 32 vs. 41.7 ± 97.2 months, p = 0.019).
Dynamic 18F-FDG PET/CT findings
Results of cardiac quantitative analysis of 18F-FDG PET/CT are depicted in Fig. 1. Post-processing of dynamic data was not feasible in 2 patients due to the total absence of myocardial uptake, leading to a software failure. In the remaining 28 patients, the global Ki was similar between CS+ and CS− (0.010 ± 0.010 vs. 0.012 ± 0.011, p = ns). The SD of the Ki was not significantly different in CS+ and CS− (0.004 ± 0.004 vs. 0.002 ± 0.002, p = ns). The NCOV values were significantly greater in CS+ compared with CS− (0.513 ± 0.175 vs. 0.205 ± 0.081; p = 0.0007). Using ROC curve analysis, we found a NCOV cut-off value of 0.38 for the diagnosis of CS, with a sensitivity of 100% and a specificity of 91% (Fig. 2). Areas under the curves from ROC analysis of SD Ki and global Ki were 0.674 and 0.602, respectively. Linear regression and Bland–Altman plots demonstrated a high intra- and inter-observer reproducibility, as shown in Fig. 3.
Quantitative analysis of dynamic 18F-FDG PET/CT. a Global Ki b SD Ki, c NCOV. Comparisons between groups were done using a Mann–Whitney test. Data are presented as box plots with means represented by the crosses with the 75th percentiles at the top and the 25th percentiles at the bottom of the boxes. Ranges are depicted as whiskers. CS+ sarcoidosis patients with cardiac involvement according to the JMHW criteria, CS− sarcoidosis patients without cardiac involvement according to the JMHW criteria, Ki net influx constant, NCOV normalized coefficient of variation, SD standard deviation
ROC curves for the differential diagnosis between CS+ and CS−: using the NCOV, p = 1. CS+ sarcoidosis patients with cardiac involvement according to the JMHW criteria, AUC area under the curve, CS− sarcoidosis patients without cardiac involvement according to the JMHW criteria, Ki net influx constant, NCOV normalized coefficient of variation, SE standard error
Static 18F-FDG PET/CT findings
Visual analysis of cardiac 18F-FDG uptake was performed on static whole body 18F-FDG PET/CT in 25 patients. In the remaining 5 patients, the cardiac visual analysis was performed using static data extracting from the dynamic cardiac acquisition (50 min). The inter-observer agreement was fair with a kappa of 0.383 (95% CI 0.022–0.750). The intra-observer agreement was excellent with a 0.833 (95% CI 0.525–1) kappa index. Five CS+ (83%) showed a focal uptake and 1 (17%) showed no cardiac uptake. Among the CS−, 8 (33%) and 4 (17%) showed diffuse or focal uptake patterns respectively, and 12 (50%) showed no cardiac uptake. Both sensitivity and specificity of visual cardiac analysis of the whole body 18F-FDG PET/CT for CS detection were 83%. Four out of the 5 CS+ (80%), and 11/20 CS− (55%) who underwent a whole body 18F-FDG PET/CT showed extra-cardiac 18F-FDG uptake, particularly in mediastinal and hilar lymph nodes.
Other imaging modalities
Using transthoracic echocardiography, mean LVEF was significantly decreased in CS+ compared to CS− (54% [49–60] vs. 65% [60–71], p = 0.04). All CS+ and 3/18 (17%) CS− exhibited abnormal MPI. The sensitivity and the specificity of MPI for diagnosing CS were 100% and 83%, respectively. Table 2 shows the diagnostic performances of each imaging modalities using the 2006 guidelines for CS from the JMHW as a gold standard.
Discussion
Our study showed that myocardial metabolism assessed by cardiac dynamic 18F-FDG PET/CT was significantly heterogeneous in patients with cardiac sarcoidosis. The quantitative indexes of glucose metabolism heterogeneity were significantly increased in CS+ compared to CS−, yielding to high sensitivity and specificity for the diagnosis of cardiac sarcoidosis.
Patchy uptake on cardiac PET or late gadolinium enhancement on CMR in a pattern consistent with CS are some of the criteria used in the recently published consensus statement of the Heart Rhythm Society for the diagnosis of CS [7]. In the present work we decided not to diagnose CS according to HRS criteria because of its lack of objectiveness as no clear definition of patchy pattern is given. As a consequence, CS was diagnosed according to the JMHW criteria, revised in 2006 [5]. In our study, 20% of sarcoidosis patients had a cardiac involvement according to the JMHW criteria. This was in keeping with previous results showing a prevalence of CS of 20% in European populations [17, 18] and of 38–50% in Japanese studies [11, 19].
Tahara et al. [11] previously reported that a heterogeneous myocardial 18F-FDG uptake based on SUV analysis may be a useful diagnostic marker of CS. In this latter study, the coefficient of variation was calculated as the ratio of the standard deviation of segmental uptake divided by the mean left ventricular SUV, and was significantly higher in CS+ (0.25 ± 0.05) compared to CS− (0.14 ± 0.03, p < 0.01) and control patients (0.14 ± 0.03, p < 0.01).
The heterogeneity of myocardial glucose metabolism could be explained by two associated mechanisms: a decreased uptake in some heart regions due to myocardial fibrosis, and an increased uptake in other territories due to active granulomatous lesions. We found low NCOV values in CS−, indicating a homogenous uptake of glucose in normal heart. The high NCOV in CS+ appears as a useful marker of active myocardial inflammation to detect CS.
According to the JMHW criteria as a gold standard, we found a sensitivity of 83% with a specificity of 83% using the static 18F-FDG PET/CT for diagnosing CS. These findings are similar to previous studies reporting a sensitivity of 71–100% and a specificity of 37.5–100% [7, 9, 20]. Youssef et al. reported in a meta-analysis of 7 studies a pooled sensitivity and specificity of static 18F-FDG PET/CT of 89 and 78% respectively, compared to JMWH criteria [21]. According to MPI, the sensitivity and specificity for diagnosing CS were 100 and 83%, respectively. Previous studies reported a sensitivity of 54–100% and a specificity of 76–100% for perfusion imaging [11, 20, 22].
Quantitative analysis using the NCOV of the net influx constant Ki demonstrated an increased inter-observer reproducibility compared with the visual analysis of static 18F-FDG PET/CT imaging. The main finding of our study is that the diagnostic value of cardiac dynamic 18F-FDG PET/CT for CS using a NCOV cut-off value of 0.38 was better than the qualitative analysis of the cardiac 18F-FDG uptake with a sensitivity and a specificity of 100 and 91% respectively. Based on the evaluation of cardiac SUVs, Tahara et al. [11] reported a comparable diagnostic value of 18F-FDG PET/CT using a cut-off COV value of 0.18 for the diagnosis of CS, with a sensitivity and a specificity of 100 and 97%, respectively.
However, the semi-quantification of 18F-FDG uptake using SUV suffers from some limitations. First, SUV measures the total activity in the myocardium, and includes both metabolized 18F-FDG and any unmetabolized 18F-FDG in the plasma, in the intracellular spaces and in the cell, including unphosphorylated 18F-FDG [23]. Dynamic analysis separates these two components because the Patlak slope is determined only by metabolized 18F-FDG. Secondly, SUV also depends on how long after 18F-FDG injection the static acquisition is performed whereas dynamic analysis avoids this time dependence [24]. Moreover, it is well known that the 18F-FDG uptake depends on blood glucose level but this parameter does not appear in the measurement of the SUV while it may be taken into account when using compartmental analysis. Finally, Patlak analysis uses the sum of all the 18F-FDG available to the heart for normalization, whereas SUV approximates this integral by the injected dose divided by the body weight [25]. Quantitative analysis is time-consuming due to the addition of a 50-min dynamic acquisition, reconstruction (33 frames) and post-processing of dynamic datasets Nevertheless, Ki derived from cardiac dynamic 18F-FDG PET/CT appear to better represent myocardial glucose metabolism than does SUV.
The myocardium is able to metabolize either free fatty acids (FFA) or glucose as a substrate for metabolic energy [26]. Under fasting state, the majority of myocardial energy is obtained from oxidation of the FFA, and therefore the 18F-FDG uptake is expected to be low. The performance of 18F-FDG PET/CT sarcoidosis studies is closely linked to the adherence to diet which includes many prohibited foods and could lead to an improper adherence. According to our department procedures, patient’s observance was insured by written and oral (phone call) instructions given to patients prior to the examination.
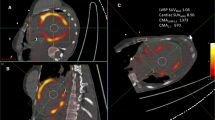
Additionally to the cardiac dynamic acquisition, 25 sarcoidosis patients underwent a whole body 18F-FDG PET/CT which may provide evidence of sarcoidosis and guide sites for biopsy in detecting 18F-FDG uptake within mediastinal or hilar lymph nodes [15]. Figure 4 shows an example of a CS+ patient referred for a second degree atrioventricular block. Both cardiac dynamic and whole body 18F-FDG PET/CT acquisitions were helpful to diagnose CS and extra-cardiac sarcoidosis. Additionally, 18F-FDG PET/CT has the advantage of being able to image patients with implanted pacemakers or defibrillators and those with impaired renal function and might also be useful in determining the requirement for device treatment.
Representative findings of a patient with a cardiac sarcoidosis referred for an advanced atrioventricular block. The diagnosis of sarcoidosis was biopsy-proven and an implantable cardioverter defibrillator was placed to prevent sudden death. a ECG showed a second degree atrioventricular block. b 18F-FDG PET maximum intensity projection image: increased accumulation of 18F-FDG is shown in the heart (blue arrow) and in several mediastinal lymph nodes (black arrow). c Fusion 18F-FDG PET/CT axial view at cardiac level depicted focal accumulation of 18F-FDG in inter-ventricular septal and lateral walls at basal level (white arrows). d Polar map display of the 17 LV segments assessed by cardiac dynamic 18F-FDG PET/CT showed heterogeneity of the Ki with a NCOV = 0.542
Limitations
We did not use the prolonged fasting [10] nor the unfractionned heparin [20] to suppress the physiological myocardial 18F-FDG uptake. Few patients received a steroid therapy at the time of examination, a situation that may affect the sensitivity of 18F-FDG PET/CT to detect CS. However, no CS+ was treated by steroid at the time of the examination.
In this study, we used the 2006 JMWH criteria for the diagnosis of CS. The use of the Japanese criteria is justified because the diagnostic yield of endomyocardial biopsy is low and has a relatively high invasiveness and complication rates [3]. Some authors have speculated that these criteria might lead to an underestimation of cardiac involvement in patients with sarcoidosis [27]. 18F-FDG PET may depict early-stage sarcoidosis lesions in the heart even in patients who do not meet the diagnostic criteria. Additionally, ventricular tachycardia, which is the second clinical manifestation (23%) of CS [28], is not considered as a major criteria in the 2006 JMHW guidelines and therefore lead to a case considered as a false positive in the NCOV ROC analysis in our study.
Conclusion
In this study, heterogeneous myocardial glucose metabolism assessed using a normalized coefficient of variation (NCOV) of the net influx constant Ki derived from cardiac dynamic 18F-FDG PET/CT was a highly suggestive feature of CS. Our results suggest that quantitative analysis of cardiac dynamic 18F-FDG PET/CT after a HFLC diet could be a useful tool for the diagnosis of cardiac involvement in patients with sarcoidosis.
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- CRP:
-
C-reactive protein
- CS:
-
Cardiac sarcoidosis
- CT:
-
Computed tomography
- FFA:
-
Free fatty acids
- 18F-FDG:
-
8F-fluoro-2-deoxyglucose
- 18F-FDG PET/CT:
-
Cardiac dynamic 18F-fluoro-2-deoxyglucose positron emission tomography
- FWHM:
-
Full width at half maximum
- HFLC:
-
High-fat and low-carbohydrate
- Ki:
-
Net influx constant
- MPI:
-
Myocardial perfusion imaging
- NCOV:
-
Normalized coefficient of variation
- PET:
-
Positron emission tomography
- ROC:
-
Receiving operating curve
- ROI:
-
Region of interest
- SUV:
-
Standard uptake value
References
Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet P-Y. Müller-Quernheim J Sarcoidosis Lancet. 2014;383:1155–67.
Sekiguchi M, Yazaki Y, Isobe M, Hiroe M. Cardiac sarcoidosis: diagnostic, prognostic, and therapeutic considerations. Cardiovasc Drugs Ther Spons Int Soc Cardiovasc Pharmacother. 1996;10:495–510.
Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–11.
Hamzeh NY, Wamboldt FS, Weinberger HD. Management of cardiac sarcoidosis in the United States: a Delphi study. Chest. 2012;141:154 – 62.
Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis Granulomatous Disord. 2007;27:89–102.
Soejima K, Yada H. The work-up and management of patients with apparent or subclinical cardiac sarcoidosis: with emphasis on the associated heart rhythm abnormalities. J Cardiovasc Electrophysiol. 2009;20:578 – 83.
Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–23.
Brudin LH, Valind SO, Rhodes CG, Pantin CF, Sweatman M, Jones T, et al. Fluorine-18 deoxyglucose uptake in sarcoidosis measured with positron emission tomography. Eur J Nucl Med. 1994;21:297–305.
Williams G, Kolodny GM. Suppression of myocardial 18F-FDG uptake by preparing patients with a high-fat, low-carbohydrate diet. AJR Am J Roentgenol. 2008;190:151–6.
Langah R, Spicer K, Gebregziabher M, Gordon L. Effectiveness of prolonged fasting 18f-FDG PET-CT in the detection of cardiac sarcoidosis. J Nucl Cardiol. 2009;16:801 – 10.
Tahara N, Tahara A, Nitta Y, Kodama N, Mizoguchi M, Kaida H, et al. Heterogeneous myocardial FDG uptake and the disease activity in cardiac sarcoidosis. JACC Cardiovasc Imaging. 2010;3:1219–28.
Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7.
Fahy GJ, Marwick T, McCreery CJ, Quigley PJ, Maurer BJ. Doppler echocardiographic detection of left ventricular diastolic dysfunction in patients with pulmonary sarcoidosis. Chest. 1996;109:62–6.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539–542.
Sobic-Saranovic D, Artiko V, Obradovic V. FDG PET imaging in sarcoidosis. Semin Nucl Med. 2013;43:404 – 11.
Ishimaru S, Tsujino I, Takei T, Tsukamoto E, Sakaue S, Kamigaki M, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J. 2005;26:1538–43.
Smedema J-P, Snoep G, van Kroonenburgh MPG, van Geuns R-J, Dassen WRM, Gorgels APM, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol. 2005;45:1683–90.
Soussan M, Brillet P-Y, Nunes H, Pop G, Ouvrier M-J, Naggara N, et al. Clinical value of a high-fat and low-carbohydrate diet before FDG-PET/CT for evaluation of patients with suspected cardiac sarcoidosis. J Nucl Cardiol. 2013;20:120–7.
Ohira H, Tsujino I, Ishimaru S, Oyama N, Takei T, Tsukamoto E, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008;35:933 – 41.
Okumura W, Iwasaki T, Toyama T, Iso T, Arai M, Oriuchi N, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med. 2004;45:1989–98.
Youssef G, Leung E, Mylonas I, Nery P, Williams K, Wisenberg G, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012;53:241–8.
Yamagishi H, Shirai N, Takagi M, Yoshiyama M, Akioka K, Takeuchi K, et al. Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET. J Nucl Med. 2003;44:1030–6.
Freedman NMT, Sundaram SK, Kurdziel K, Carrasquillo JA, Whatley M, Carson JM, et al. Comparison of SUV and Patlak slope for monitoring of cancer therapy using serial PET scans. Eur J Nucl Med Mol Imaging. 2003;30:46–53.
Hamberg LM, Hunter GJ, Alpert NM, Choi NC, Babich JW, Fischman AJ. The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification? J Nucl Med. 1994;35:1308–12.
Zasadny KR, Wahl RL. Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-d-glucose: variations with body weight and a method for correction. Radiology. 1993;189:847–50.
Bengel FM, Higuchi T, Javadi MS, Lautamäki R. Cardiac positron emission tomography. J Am Coll Cardiol. 2009;54:1–15.
Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–77.
Banba K, Kusano KF, Nakamura K, Morita H, Ogawa A, Ohtsuka F, et al. Relationship between arrhythmogenesis and disease activity in cardiac sarcoidosis. Heart Rhythm. 2007;4:1292–9.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lebasnier, A., Legallois, D., Bienvenu, B. et al. Diagnostic value of quantitative assessment of cardiac 18F-fluoro-2-deoxyglucose uptake in suspected cardiac sarcoidosis. Ann Nucl Med 32, 319–327 (2018). https://doi.org/10.1007/s12149-018-1250-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-018-1250-3