Abstract
Sudden cardiac death (SCD) represents a significant portion of all cardiac deaths. Current guidelines focus mainly on left ventricular ejection fraction (LVEF) as the main criterion for SCD risk stratification and management. However, LVEF alone lacks both sensitivity and specificity in stratifying patients. Recent research has provided interesting data which supports a greater role for advanced cardiac imaging in risk stratification and patient management. In this article, we will focus on nuclear cardiac imaging, including left ventricular function assessment, myocardial perfusion imaging, myocardial blood flow quantification, metabolic imaging, and neurohormonal imaging. We will discuss how these can be used to better understand SCD and better stratify patient with both ischemic and non-ischemic cardiomyopathy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sudden cardiac death (SCD) currently accounts for up to 60% of all cardiac death in the adult population in the United States.1,2 Despite recent advances in our understanding of cardiovascular disease and in cardiac care, effective primary prevention of SCD in the general population is still an aspiration.3,4 The final common pathway in most cases is malignant ventricular arrhythmia.5 Although the fundamental pathophysiology is complex, an underlying anatomical substrate can be identified in the majority of patients,6 with the most frequent being attributed to ischemic heart disease, idiopathic dilated cardiomyopathy (DCM), and hypertrophic cardiomyopathy (HCM).7
Current guidelines focus on the left ventricular ejection fraction (LVEF) as the primary measure for SCD risk stratification and patient management.8,9 One-third of all SCD occur in patients with moderate to severe left ventricular (LV) systolic dysfunction (LVEF ≤ 35%).10 In this group, primary prevention with an implantable cardioverter defibrillator (ICD) significantly prolongs survival.11,12 However, numerous patients with higher LVEF are still at risk of SCD, but would not qualify for ICD placement according to current guidelines. Furthermore, only 35% of patients randomized for ICD placement in the Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) received appropriate shock therapy over a 3-year follow-up.13 Taken together, these facts underscore the lack of sensitivity and specificity of LVEF alone in identifying patients who will suffer from SCD and the need for better markers for SCD.
Emerging data support the potential of cardiac imaging to understand the mechanisms of SCD beyond simple LVEF measurement.14 In this review, we will focus on the possible benefits that nuclear cardiology imaging (including myocardial perfusion imaging (MPI), LV function assessment, accurate flow quantification, metabolic imaging and neurohormonal imaging) offers us to improve risk stratification of SCD in patients with ischemic and non-ischemic cardiomyopathy.
Mechanisms of Sudden Cardiac Death
By definition “sudden,” the acute event is not often witnessed, and therefore SCD is difficult to study. In most cases, it is accepted that the final pathway is malignant ventricular arrhythmia.15,16 The underlying mechanisms of ventricular arrhythmogenesis are complex but can simply be considered as the interaction between a structural or anatomic substrate (myocardial scar from prior myocardial infarction) and a functional trigger, such as ischemia.5,6 In the adult population, most SCD occurs in the setting of an abnormal anatomic substrate from coronary artery disease (CAD),7,17-19 and reentry is the most frequent mechanism of malignant ventricular arrhythmias. Scar and fibrosis create areas of heterogeneous electrophysiological response and aberrant conduction, particularly in the border zones of infarct. What’s more, the presence of ischemia exacerbates regional heterogeneity, further predisposing to malignant arrhythmia,20,21 as does the presence of altered sympathetic innervation.22,23 This implies that these factors (scar, hibernation, ischemia, and innervation) may represent potential imaging targets in the evaluation of SCD in addition to LV function (Table 1).
Left Ventricular Function
LVEF is currently the most studied and most commonly used cardiac imaging marker to assess risk for SCD and is used to guide appropriate therapy in both ischemic and non-ischemic cardiomyopathies. Many studies, dating back to the 1980s, quickly established that LVEF is a strong predictor for overall cardiac mortality.24-26 Not surprisingly, this led to the use of LVEF as a criterion of enrollment in the large clinical trials for the evaluation of ICDs in primary prevention of SCD in the 1990s and 2000s. In the Multicenter Unsustained Tachycardia Trial (MUSTT), Buxton et al demonstrated that in a group of 704 patients with CAD and LVEF ≤ 40%, the risk of sudden cardiac arrest or fatal arrhythmia was significantly reduced in the group treated with ICD when compared with the group without (relative risk 0.24; 95% CI 0.13-0.45; P < .001).27 The Multicenter Automatic Defibrillator Implantation Trial II (MADIT II), another large randomized trial comparing ICD to medical therapy in 1232 patients with LVEF ≤ 30%, had similar results. Over 20 months, the mortality rates were 19.8% in the medical group vs 14.2% in the ICD group, a 31% relative reduction in the risk of death.11 Similar results were again observed in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). 2521 patients with NYHA class II or III heart failure (HF) and LVEF ≤ 35% were randomized to receive either an ICD, amiodarone or a placebo. Median follow-up was 45.4 months. Amiodarone had no effect on survival while ICD therapy decreased risk of death by 23% (0.77; 95% CI 0.62-0.96; P = .007).12 It is thus well established that ICD use in patients with reduced LVEF leads to a decreased risk of death and explains why LVEF plays such an important role in current guidelines regarding the use of ICDs8,9,28-31 (Table 2).
Nuclear cardiac imaging offers many ways to evaluate LV function and LVEF. This includes ECG-gated images as part of MPI using either single-photon emission computer tomography (SPECT) or positron emission tomography (PET) imaging, and also radionuclide angiography (RNA), also called multigated acquisition (MUGA) scan. The use of these modalities to assess LV function and predict cardiac death have all been validated in large studies. Hachamovitch et al, in a study with 5366 patients, demonstrated that LVEF measured with ECG-gated images as part of SPECT MPI was a strong predictor of cardiac death.32 In this study, which aimed at determining the utility of combining perfusion and functional assessment in predicting patient survival with revascularization or medical therapy, LVEF remained the strongest predictor for cardiac death, while ischemia was a better predictor for revascularization benefit (Figure 1). Lertsburapa et al, in a study with 1441 patients undergoing PET MPI with Rubidium-82 chloride, demonstrated that LVEF measured on the ECG-gated images was an independent and incremental prognostic marker. Annualized mortality rates in the group with LVEF above 50%, between 40% and 49%, and below 40% were, respectively, 2.4%, 6.2%, and 9.2% (P < .001).33 Curtis et al, in a study with 7788 patients with known HF, demonstrated similar results using RNA. In their study, over a mean follow-up of 37 months, they demonstrated that mortality increased in a near linear fashion with decreasing LVEF below 45%, while mortality rates were not related to LVEF above 45%.26
Survival free of cardiac death in patients without ischemia (A) and patients with a minimum of 25% ischemic myocardium (B) undergoing either revascularization or optimal medical therapy, stratified by LVEF. Reproduced with permission from J Nucl Cardiol ® 32
RNA is a particularly useful and powerful tool for the evaluation of LVEF and is an integral part of current imaging guidelines and appropriate use criteria documents34,35 (Table 3). Along with cardiac magnetic resonance (CMR), it is currently one of the most accurate and reliable modalities for LVEF measurement and has excellent inter- and intraobserver reproducibility.36-39 Its advantages and disadvantages are summarized in Table 4. Even though LVEF measurement is not enough by itself to accurately predict SCD,10 it remains a strong and important key parameter in current practice and guidelines. Nuclear cardiology offers varied and accurate means to evaluate it.
Ischemia
Ischemia is a well-recognized trigger for ventricular arrhythmias.40,41 Current ICD therapy guidelines recommend optimal revascularization before ICD therapy.31 Moreover, coronary revascularization has been proven to reduce SCD risk.42-44 Hachamovitch et al, in a large study with 5183 patients who underwent rest/stress dual-isotope (Thallium-201 and Technetium-99m Sestamibi) SPECT MPI, demonstrated that the presence of ischemia yields incremental prognostic data for predicting cardiac death and adverse cardiac events.45 Annual rates of cardiac death increased with increasingly abnormal MPI going from 0.5% in patients with normal scans to 4.2% in patients with severely abnormal MPI (defined as a sum stress score (SSS) of more than 13).45 More recently, Piccini et al, in a study of 6383 patients with angiographically documented CAD, investigated if ischemia on SPECT MPI was a predictor for cardiac death and more specifically for SCD.46 In their final multivariable analysis, the SSS was a significant predictor for SCD, with a hazard ratio of 1.16 per 3-U increase (95% CI 1.08-1.25). The ability of the SSS to predict SCD was comparable to that of LVEF. Interestingly, in the same multivariable analysis, neither the sum rest score (SRS, which reflects scar), nor the sum difference score (SDS, which reflects ischemia), were statistically significant predictors for SCD. Taken together, this implies that it is the combination of scar and ischemia that has the strongest predictive value for SCD, which matches well with our current understanding of SCD mechanisms. Another interesting fact from this study is that the median LVEF of patients who experienced SCD was 47%, again underscoring the lack of sensitivity of simple LVEF measurement in predicting SCD. Furthering this point, in a follow-up study Piccini et al demonstrated that their previous finding held true in a group of 4865 patients, all of whom had LVEF > 35% (median LVEF 56%)47 (Figure 2). In another study, Paganelli et al studied the effect of residual ischemia in patients with prior myocardial infarction. Using programmed ventricular stimulation, they demonstrated that residual ischemia on SPECT MPI carried a 1.6-fold increase in the risk of inducible ventricular arrhythmias.48
Cumulative incidence of sudden cardiac death in patients with summed stress score ≤8 vs >8. Reproduced with permission from JACC 47
Although there is less data regarding PET MPI as a predictor for SCD, recent meta-analyses have shown that PET MPI is superior to SPECT MPI in the detection of CAD.49,50 Its prognostic value is well established.51 Data from a recently completed multicenter registry show an increasing risk of cardiac events and cardiac death in patient with increasingly abnormal stress PET MPI,52 although the authors did not look specifically at SCD. It would appear to be a safe assumption that ischemia as evidenced by PET MPI can play a role similar to SPECT MPI in the prediction and management of SCD, although this will need to be studied further to determine if there is an advantage over SPECT MPI.
In addition, PET MPI permits accurate and reproducible measurement of myocardial blood flow (MBF) at both rest and stress.53 In a recently published paper, Rijnierse et al demonstrated that in patients with ischemic cardiomyopathy, impaired hyperemic MBF and impaired coronary flow reserve (CFR, stress MBF/rest MBF), whether in scar area or remote area, were associated with increased ventricular arrhythmia inducibility during electrophysiological evaluation.54 These results suggest a link between impaired stress MBF and electrical instability, and a potential benefit of PET MPI to help accurately stratify patients at risk for SCD.
Myocardial Scar and Hibernating Myocardium
Myocardial scar is a complex and powerful substrate for arrhythmogenesis. Changes in tissue composition following an infarct create a heterogeneous zone that leads to depolarization abnormalities, autonomic dysfunction, and repolarization disruption; the presence of viable myocardium adjacent to scar tissue often forms the anatomic substrate for reentrant ventricular tachycardia (VT).55,56 Studies have shown that the extent of scar on SPECT MPI is related to the risk of cardiac death. Machecourt et al studied 1926 patients using SPECT MPI and demonstrated that persistent rest and stress imaging defects (scar) were associated with increased cardiovascular death, and that the greater the number of abnormal segments, the worse the prognosis.57 Van der Burg et al studied SCD survivors using SPECT MPI and demonstrated that the presence of more extensive scar was associated with greater risk for recurrent ventricular arrhythmias and cardiac death in both the univariate and multivariate analysis.44 In another study with 106 patients with LVEF ≤ 30%, Morishima et al showed that defect size on rest SPECT MPI (representing scar) was a predictor for lethal arrhythmic events and SCD.58 Using a threshold value of 47.5 mL for defect volume (determined using ROC curve analysis), the risk ratio was 6.34 (95% CI 1.76-22.8, P = .005). This illustrates how, in clinical practice, assessing the extent of scar might help better and more accurately assess SCD risk.
Furthermore, the assessment of hibernating myocardium in current clinical practice is well established. Hibernating myocardium is identified using a combination of metabolic imaging (Fluorine-18 fluorodeoxyglucose (18F-FDG)) and myocardial perfusion (generally Rubidium-82 chloride (82Rb) or Nitrogen-13 ammonia (13NH3)) and will present with a perfusion defect with maintained or even enhanced glucose metabolism (Figure 3). While PET is preferable, in centers which do not have access to PET, it is possible to evaluate hibernating myocardium using SPECT (either with Thallium-201 or Technetium-99m Sestamibi). Viable myocardium is known to represent an unstable electrical region, and it is associated with an increased risk for SCD.59 A study by Di Carli et al looked at 93 patients undergoing PET MPI with 13NH3 and viability assessment with 18F-FDG.60 They showed that an increased perfusion-metabolism mismatch (hibernating myocardium) was associated with an increased cardiac death risk. Patients with at least 5% hibernating myocardium had much lowered annual survival probability than those without (50% vs 92%, P = .007) and were also shown to benefit from revascularization rather than medical therapy.60 Similar results were obtained by Desideri et al, who observed that in 167 patients being treated medically, the extent of hibernating myocardium was strongly related to cardiac mortality (P = .001).61 An 8% increase in mismatch was associated with a 36% increase in cardiac mortality (HR 1.36, 95% CI 1.13 to 1.64). More recently, the PET and Recovery Following Revascularization (PARR-2) trial, a large randomized trial, investigated whether viability imaging with 18F-FDG PET could be used to effectively assist decision making in patients with severe LV dysfunction to reduce cardiac events and cardiac death.62 While the primary study did not demonstrate a clear benefit, a post hoc analysis demonstrated that when PET recommendations were adhered to, a benefit was observed in patients with significant hibernating myocardium, with a hazard ratio of 0.62 for cardiac events (95% CI 0.42-0.93, P = .019).62 Ling et al and Uebleis et al, in separate studies, obtained similar results in patients with ischemic cardiomyopathy and LV dysfunction.63,64 Not all studies have demonstrated similar results. In a substudy of the Surgical Treatment for Ischemic Heart Failure (STICH) trial,65 the presence of viable myocardium was not significantly associated with an improved outcome in the final adjusted analysis. However, it should be noted that in this substudy, viability was assessed using either SPECT or dobutamine echography instead of PET, and that the criteria for classifying myocardium as viable or non-viable did not include wall motion information (in contrast to earlier studies in which viability was assessed only in dysfunctional regions). As well, it is interesting to note the patients were selected from a group already well suited to revascularization and that their population suffered from less comorbidity then what is reported in other studies.66
Viability study demonstrating a large area of hibernating myocardium in the LAD territory. Rest PET perfusion imaging using 13N-NH3 (upper row) shows a moderate reduction in perfusion in the distal anterior, antero-septal, and septal walls as well as the apex. 18F-FDG PET (bottom row) shows a corresponding area of increased glucose metabolism in the same territory
In practice, defining the extent of ischemia, hibernation, and scar is an important step in the work up of patients with SCD and/or ventricular arrhythmia. In light of the possible reversibility of ischemia and hibernating myocardium-induced arrhythmias, accurate evaluation of these conditions appears necessary, a goal which can be achieved using SPECT and PET imaging. Even in cases where revascularization is not possible, the extent of ischemia and hibernation as well as the extent of scar should be taken into account when considering primary prevention for SCD.
Sympathetic Innervation Imaging
Cardiac sympathetic innervation is another novel imaging target in the search for better prediction and prevention of SCD. It plays an important role in cardiac function and may play an important role in future stratification strategies.
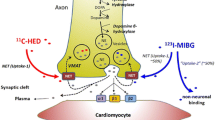
The most widely available and studied non-invasive method to assess cardiac sympathetic innervation is currently Iodine-123-metaiodobenzylguanidine (MIBG). MIBG is a guanethidine analog initially developed in the 1980s to study adrenal medulla tumors and other neuroendocrine tumors.67 It mimics norepinephrine (NE), the main neurotransmitter involved in the sympathetic innervation of the heart. It localizes mainly in presynaptic nerve endings, which it enters by an active energy-dependent transport (uptake 1) before being stored in neurosecretory granules. However, unlike NE, it is not metabolized by monoamine oxidase, which allows it to accumulate in sufficient concentration to permit imaging.68 Cardiac imaging with MIBG is done using a standard gamma camera, with images acquired both in planar views and SPECT 15 minutes after tracer injection, and again 3-5 hours after tracer injection.69 Planar images are then used to calculate the heart-to-mediastinum ratio (H/M ratio) and the cardiac washout of the tracer between the early (15 minutes) and delayed images (3-5 hours), while SPECT images are used mainly to assess for regional uptake and defects.
Both animal and human models have shown that sympathetic innervation anomalies are associated with ventricular arrhythmias.23,70,71 These anomalies also play an important role in HF and can be associated with worsening LV function and symptoms, as well as an increase in SCD.72 Bax et al demonstrated an association between MIBG defect severity and inducibility of VT in electrophysiology studies.73 The AdreView Myocardial Imaging for Risk Evaluation in Heart Failure (ADMIRE-HF) trial, a prospective study with 961 patients with NYHA class II or III HF and LVEF ≤ 35%, is one of the largest studies that have looked at cardiac MIBG imaging and its prognostic value.74,75 For H/M < 1.60, cardiac death at 2 years was 11.2% vs 1.8% for the group with H/M ≥ 1.6074 (Figure 4). When treated as a continuous variable, over a median follow-up of 17 months, there was a progressive decline in cardiac death from 20% for H/M < 1.10 to 0% for H/M > 1.8074 (Figure 5). Lastly, the risk of arrhythmic event was significantly higher in patient with H/M < 1.60 vs patients with H/M ≥ 1.60 (10.4% vs 3.5%, P < .001).74 These results were further supported by a follow-up analysis in the ADMIRE-HF extension study (ADMIRE-HFX) whose results were recently published.76 In another interesting study, Boogers et al investigated the ability of cardiac MIBG imaging to predict ventricular arrhythmias in patients with ICD. They prospectively recruited 116 HF patient referred for ICD therapy, who all underwent cardiac MIBG SPECT before ICD implantation. Over a follow-up of approximately 2 years, they showed that large defects on late MIBG SPECT imaging were strongly associated with ventricular arrhythmias and appropriate ICD therapy when compared with patients with no or small defects (52% vs 5%, P < .01).77 A similar study by Kawai et al investigated the ability of MIBG to identify patients with HF and reduced LVEF but at low risk for SCD.78 They recruited 81 patients with stable HF and LVEF ≤ 35%, who were then followed-up for a minimum of 5 years. At recruitment, every patient underwent cardiac MIBG and the authors combined the H/M ratio and washout rate to calculate a MIBG score ranging from 1 (normal) to 10 (highly abnormal). The patients were thus stratified into low (1-4), intermediate (5-7), and high (8-10) MIBG scores. This score proved to be a strong predictor for SCD (low = 0%, intermediate = 19%, and high = 36%, P = .001). The positive predictive value of a low MIBG score to identify patients at low risk for SCD was thus 100%. As the authors pointed out, an interesting aspect of their study is that they combined both the H/M ratio and washout rate in one score. We know that these parameters, although they overlap, do not represent the exact same phenomenon, and the authors postulated that combining them might further enhance the predictive value of MIBG.78
Cumulative event curves for arrhythmic events (A) and cardiac death (B) in patients with MIBG heart-to-mediastinum ratio <1.60 vs patient with ratio ≥1.60. Adapted with permission from JACC 74
Cumulative two-year cardiac death rate vs MIBG heart-to-mediastinum ratio. Reproduced with permission from JACC 74
While many studies investigating the role of cardiac MIBG imaging looked at patient with severely reduced LVEF, other studies have also validated its prognostic value in patients with normal or near-normal LVEF.79,80 A high MIBG washout rate is also associated with increased risk for SCD.81 Other authors have also looked at the link between hibernating myocardium and sympathetic innervation. We know that the nerve endings are more sensitive to ischemia than the myocardial cells, and it is thus not surprising that studies in animal models and human subjects have shown that area of mismatched innervation/perfusion (abnormal innervation but preserved perfusion) are arrythmogenic.82-84
PET technology has many intrinsic advantages over SPECT and has become more readily available over the last 10 years, which has led to an increase in research regarding PET imaging of cardiac innervation and the autonomic nervous system using PET norepinephrine analogs including 11C-HED (Carbon-11-meta-hydroxyephedrine), Carbon-11-epinephrine, and beta receptor ligands such as C-11-CGP-12177. These tracers have been used to understand the role of the sympathetic nervous system in cardiomyopathy pathogenesis and the effects of therapy.85 The most investigated of these PET tracers is 11C-HED. Its uptake and storage is similar to that of MIBG, but it has a higher uptake 1 selectivity.86 With the background of (i) persistent innervation damage in patients with previous myocardial infarction and hibernating myocardium,87-90 (ii) the association between MIBG uptake and SCD as per Boogers et al,77 (iii) prior studies suggesting low11 C-HED retention predicts adverse outcomes91 and (iv) that as strong a predictor as LVEF is, we remain unable to better stratify patients for consideration of ICD therapy,13 Fallavollita et al designed the Prediction of ARrythmic Events With Positron Emission Tomography (PAREPET) trial. In a prospective study, they recruited 204 patients with LVEF ≤ 35%, eligible for primary prevention ICD therapy. Their aim was to demonstrate an association between the amount of myocardial sympathetic innervation anomalies and the risk of SCD. All patients had 18F-FDG PET for viability assessment and 11C-HED PET for myocardial innervation assessment and quantification.92 The volume of denervated myocardium as a continuous value was a strong independent predictor of SCD and had the strongest correlation with SCD, with a HR of 1.069 per 1% of LV (95% CI 1.023-1.117, P = .003). The volume of viable, denervated myocardium was also a strong predictor for SCD as a continuous value, with a HR of 1.067 per 1% of LV (95% CI 1.008-1.130, P = .025). When divided by tertiles of sympathetic denervation, the patients in the highest tertile had the highest rate of SCD, while the patients in the lowest tertile had the lowest rate (6.7, 2.2 and 1.2%·year−1, respectively), with a statistically significant difference between all tertiles (Figure 6).
Kaplan-Meier curves showing the relationship between the extent of four different PET-defined myocardial substrates (as continuous variable) and sudden cardiac death: 1 denervated (reduced Carbon-11-meta-hydroxyephedrine (HED) uptake), 2 hibernating (reduced perfusion/maintained Fluorine-18 fluorodeoxyglucose (FDG) mismatch), 3 viable denervated (reduced HED/maintained FDG mismatch), and 4 infarcted (reduced perfusion/FDG match). The total volume of denervated and viable denervated myocardium are both significant predictors for sudden cardiac death. Reproduced with permission from JACC 92
When comparing MIBG and 11C-HED in animals and humans, some studies have reported good correlation between the two,93 while others reported significant differences, with 11C-HED defect being larger than MIBG defects.94 Some data also support the theory that 11C-HED provides a better signal-to-noise ratio,90,95 likely explained by the better imaging characteristics of PET, its higher sensitivity, and the higher specificity of 11C-HED for NE uptake 1. However, the short half-life (20 minutes) of 11C-HED has limited its availability to centers with onsite cyclotron.
In clinical practice, the presence and extent of either global or regional cardiac sympathetic denervation or anomalies should be taken into account when considering ICD therapy if it is available. The current data support a more aggressive approach toward ICD therapy in patient with more extensive or severe sympathetic innervation anomalies, even in patients with normal or near-normal LVEF values, although this has not yet been adopted into practice guidelines.
Specific Cardiomyopathy Conditions
Idiopathic Dilated Cardiomyopathy (DCM)
According to some studies, SCD accounts for up to 30% of overall death in patients with DCM,96 which accounts for a significant fraction of overall SCD.6 Current guidelines recommend ICD therapy in patients with non-ischemic cardiomyopathy with LVEF ≤ 35% and NYHA class II-III HF,29 while it may be considered in patients with NYHA class I. Evaluation of LV function is thus indicated in these patients. Additionally, studies have shown that sympathetic innervation abnormalities are present in DCM. Kasama et al, in a study involving 56 patient with DCM, showed that patients with reduced H/M ratio and increased washout rate on MIBG imaging had significantly more late ventricular potentials on signal averaged ECG and were thus at higher risk for SCD.97 Over the average follow-up time of 4.5 years, both the H/M ratio and washout rate were significant predictors for SCD (P = .004 and P = .002 respectively). Other studies have shown similar results, supporting the prognostic role of cardiac sympathetic innervation in DCM.98 Recent studies have also looked at the presence of significant microvascular dysfunction in patients with DCM.99,100 In a study with 510 patients, Majmudar et al investigated the relationship between CFR and major adverse cardiac events (MACE, including cardiac death, SCD, and aborted SCD) in patients with ischemic and non-ischemic cardiomyopathy and LVEF ≤ 45%.100 Reduced CFR was common in both ischemic and non-ischemic cardiomyopathy and was a significant predictor for MACE, with a 32.6%/year rate in the group with CFR ≤ 1.65 and a 15.5%·year−1 rate in the group with CFR > 1.65 (P = .004).100 Although the exact mechanism underlying the reduced CFR in patients with non-ischemic cardiomyopathy is still unclear, another recent study by Rijnierse et al showed that there was a significant association between hyperemic MBF and sympathetic innervation.101 In 70 patients with ischemic cardiomyopathy or DCM, they showed that 11C-HED retention was correlated with resting MBF (r = 0.041, P < .001) and hyperemic MBF (r = 0.055, P < .001) as assessed by Oxygen-15-water PET in non-infarcted myocardium. Whether this is causative or not remains to be determined.
Hypertrophic Cardiomyopathy (HCM)
HCM is a heterogeneous disease with varying presentation and expression, which represents the most common cause of SCD in adults younger than 40 years.29 ICD therapy has been shown to be appropriate in patients with HCM and risk factors for SCD and is part of current guidelines.102,103 Nuclear cardiology can play a role in these patients by evaluating myocardial ischemia, which is known to be an important determinant of the clinical course of HCM.104 In a study of 158 patients with HCM, more than half had abnormal SPECT MPI, and the SSS was significantly associated with cardiovascular death. Patients with a low, intermediate, and high SSS had a 5-year survival of 97%, 94%, and 79%, respectively, (P = .04), and the presence of ischemia was a predictor of cardiovascular death (HR 1.77, 95% CI 1.04-3.02, P = .04) in the final multivariate analysis.104 Microvascular dysfunction is also known to be a feature of HCM.105 Cecchi et al showed using 13NH3 PET that patients with HCM had a severely diminished vasodilator response to dipyridamole when compared with a control group (hyperemic MBF 1.50 ± 0.69 vs 2.71 ± 0.94 mL·g−1·minute−1, P < .001), while resting flow was similar in both groups.105 In addition, in the HCM group, a lower hyperemic MBF was associated with an increased risk of cardiac death.
Some authors have validated the usefulness of sympathetic innervation imaging in patients with HCM.106-108 Terai et al studied the link between cardiac sympathetic innervation and ventricular arrhythmias using MIBG. Patients who experienced ventricular tachy-arrhythmias had a significantly higher MIBG washout rate (26.8 ± 6.4% vs 17.4 ± 5.7%, P < .001),108 supporting the hypothesis that cardiac innervation imaging can help identify HCM patients at higher risk for SCD.
Cardiac Amyloidosis
Amyloidosis is a rare disease marked by the abnormal deposition of amyloid, which is basically inappropriately folded protein. Depending on the type of protein involved, there are four main types of amyloid (AL, AA, ATTR, and AB2M), and the disease can be systemic or restricted to one organ in some cases.
Cardiac involvement is not uncommon and is one of the main determinants of prognosis, and can result in high-degree heart block, severe biventricular dysfunction, and SCD.109-111 Current guidelines support the use of ICD in patients with cardiac involvement and ventricular arrhythmia causing hemodynamic instability.9 Studies have shown that cardiac sympathetic innervation abnormalities are frequently present in patients with cardiac amyloidosis and are often more pronounced in patients with associated HF.112 To our knowledge, no study has specifically investigated the link between sympathetic innervation abnormalities and SCD in this population, but since such a link exists in other populations, we anticipate it translates to this one. The exact role of nuclear cardiology in this disease remains to be determined, but small studies and case reports have shown that both SPECT (using bone agents such as Technetium-99m-pyrophosphate) and PET (using amyloid seeking tracers such as Carbon-11 Pittsburgh compound B and Fluorine-18-florbetapir) can potentially be used to establish the diagnosis of cardiac involvement in amyloidosis.113,114
Cardiac Sarcoidosis (CS) and Other Inflammatory Cardiomyopathies
Sarcoidosis is a systemic inflammatory disease characterized by the formation of noncaseating granulomas. The exact etiology remains unknown, and the disease can involve nearly any organ, although the lungs and lymph nodes are by far the most commonly involved. Cardiac involvement can lead to conduction disturbances, ventricular arrhythmias, HF, and SCD. The exact prevalence of myocardial involvement remains controversial, ranging anywhere from 2% to 40%.115,116 Patients with cardiac involvement are often asymptomatic and initial presentation can range from asymptomatic to SCD or ventricular arrhythmia. In a study by Nery et al, 182 patients presenting with new onset unexplained monomorphic VT underwent comprehensive investigation including 18F-FDG PET. 42% had findings suggestive of CS, underlining the importance of screening for CS.117 Similarly, a significant proportion of patients presenting with unexplained new onset atrioventricular block turn out to have CS.118,119 SCD secondary to arrhythmias is the cause of death in up to 50% of all sarcoidosis deaths, with some studies reporting even higher numbers.120 The diagnosis of cardiac involvement remains challenging, but cardiac magnetic resonance and cardiac PET are emerging as pivotal tools in its assessment.121 Assessment for CS using nuclear cardiology requires two different image acquisitions: one assessing perfusion (which can be done using either SPECT or PET) and 18F-FDG PET to assess for active inflammation. Active CS will classically appear as a focal area of reduced or absent perfusion with increased 18F-FDG uptake.
In the largest study of its kind to date, Blankstein et al studied the prognostic value PET in CS.122 They followed 118 patients with known or suspected CS over a median of 1.5 years. The patients who had scans suggestive of CS at presentation (perfusion defect with associated increased 18F-FDG uptake) were at a significantly increased risk for death or ventricular tachycardia in the final multivariate analysis (HR 2.87, P = .039).
According to the recent HRS consensus statement, ICD is recommended in patients with cardiac sarcoidosis and ventricular arrhythmias or high-degree heart block123—a position supported by the literature124-126 (Figure 7).
This otherwise healthy 57-year-old female presented with new onset complete heart block and no pertinent prior medical history. Echocardiogram was normal, including normal LVEF. Because there was a suspicion for sarcoidosis, she underwent 18F-FDG PET-CT. Wholebody 18F-FDG PET (A) images revealed extensive active systemic sarcoid, with active lesions in the lungs, nodes, spleen, and bones. Cardiac involvement was present. 82Rb MPI PET revealed areas of decreased perfusion mainly in the septum and inferior walls (B) while dedicated ECG-gated 18F-FDG myocardial acquisition (C) revealed areas of severely increased uptake in the septal, anterior, and inferior walls. The decision was taken to go ahead with implantable cardioverter defibrillator (ICD) implantation. The patient received appropriate, life-saving shock 10 months later for ventricular fibrillation arrest
Lastly, a recent paper by Tung et al looked at the incidence of abnormal myocardial 18F-FDG uptake in patients with otherwise unexplained cardiomyopathy referred for ventricular arrhythmias. Out of 103 patients recruited, 50 (49%) were found to have abnormal focal myocardial 18F-FDG uptake, consistent with an active myocardial inflammatory process.127 This impacted therapy in nearly all of these patients, most of which went on to receive immunosuppressive therapy or underwent ablation.
Other Conditions
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a genetically inherited cardiomyopathy which can cause ventricular arrhythmia and SCD. One group has shown that cardiac sympathetic innervation abnormalities are frequently present in these patients, using both MIBG,11C-HED PET, and 11C-CGP-12177 (a marker of post synaptic beta-adrenergic receptors).128,129 They reported the presence of regionally reduced MIBG uptake in 40/48 patients with ARVC, and these areas of abnormal sympathetic innervation were strongly correlated with the site of origin of ventricular tachycardia in patients with right ventricular outflow tract tachycardia.128 In a follow-up study with 42 ARVC patients followed for more than 10 years, they demonstrated that the patients with abnormal MIBG scans were at a significantly increased risk to develop life-threatening VT vs those with normal sympathetic function (88% vs 35% over total follow-up, P < .0005).130
Some evidence also supports a role for cardiac sympathetic innervation imaging in Brugada syndrome, another cause of SCD. Wichter et al demonstrated that regionally decreased MIBG was present in nearly 50% of patients with Brugada syndrome,131 while Kies et al demonstrated that they have abnormal uptake of 11C-HED on PET.132 This, combined with clinical evidence of autonomic nervous system dysfunction,133 supports the hypothesis of autonomic dysfunction in Brugada syndrome’s pathophysiology, but whether or not it has any prognostic value and can help in clinical decision-making remains to be determined.
Future Outlook
Although 11C-HED shows great promise, its use and adoption remain limited because of its short half-life which requires an onsite cyclotron. LMI1195 is a Fluorine-18-based PET tracer with a design similar to MIBG, which could theoretically solve this problem thanks to its longer half-life which allows delivery from a regional cyclotron. Preliminary studies are promising,134,135 and the relationship between myocardial denervation and SCD, along with the potential for an effective Fluorine-18-labeled tracer suggest the potential for LMI1195 to help identify high-risk patients for SCD and guide ICD therapy.
The ability of PET to detect picomolar levels of radiotracers could also eventually lead to imaging of channelopathies, something currently impossible, although appropriate tracers would first have to be developed. Potential applications for PET/MRI hybrid scanners in the field of SCD are numerous. Cardiac magnetic resonance is currently the clinical gold standard in evaluating ventricular function and can accurately assess scar tissue. Combining this data with the functional and physiological data acquired using PET could potentially lead to a better understanding of the underlying mechanism and physiopathology of SCD, and ultimately better risk stratification.
Lastly, it should be noted that further studies evaluating the etiology, outcome, and directing therapy specifically in SCD are required, as some of the data we currently rely on only uses cardiac death, rather than SCD, as an endpoint.
Conclusion
Correctly identifying patients who are at high risk for SCD is of paramount importance since appropriate therapy can be life-saving. While modern medicine has made significant progress in the diagnosis and management of CAD, this is one area where recent progress remains limited. Our current screening process for SCD relies heavily on LVEF assessment, which lacks both sensitivity and specificity. Recent advances in our understanding of the underlying pathophysiology, combined with advances in cardiac imaging, offers hope for a new and better predictive model. Imaging of ischemia, scar tissue, hibernating myocardium, and cardiac innervation all seem to hold the potential to identify additional high-risk features for SCD in patient with or without LV dysfunction. Further validation with large prospective studies will be needed to validate these new risk predictors before they can be widely adopted in the clinical setting and integrated in an improved everyday model of SCD risk prediction that will more accurately guide clinicians in decision-making. Hopefully, this will lead to appropriate ICD therapy in patients in whom it would previously not have been indicated, as well as reduced “inappropriate” ICD therapy in patients with low LVEF who never experience ventricular arrhythmia or SCD.
Abbreviations
- CFR:
-
Coronary flow reserve
- CS:
-
Cardiac sarcoidosis
- DCM:
-
Dilated cardiomyopathy
- HCM:
-
Hypertrophic cardiomyopathy
- H/M:
-
Heart-to-mediastinum ratio
- MBF:
-
Myocardial blood flow
- MPI:
-
Myocardial perfusion imaging
- SDS:
-
Sum difference score
- SRS:
-
Sum rest score
- SSS:
-
Sum stress score
References
Zheng Z-J, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation 2001;104:2158-63.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015;131:e29-322.
Myerburg RJ, Mitrani R, Interian A, Castellanos A. Interpretation of outcomes of antiarrhythmic clinical trials: Design features and population impact. Circulation 1998;97:1514-21.
Deyell MW, Krahn AD, Goldberger JJ. Sudden cardiac death risk stratification. Circ Res 2015;116:1907-18.
Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res 2015;116:1887-906.
Zipes DP, Wellens HJ. Sudden cardiac death. Circulation 1998;98:2334-51.
Myerburg RJ. Sudden cardiac death: Epidemiology, causes, and mechanisms. Cardiology 1987;74:2-9.
Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, Estes NAM, et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012;60:1297-313.
Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793-867.
Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction. Two-year findings from the oregon sudden unexpected death study. J Am Coll Cardiol 2006;47:1161-6.
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877-83.
Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225-37.
Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation 2004;110:3760-5.
Bertini M, Schalij MJ, Bax JJ, Delgado V. Emerging role of multimodality imaging to evaluate patients at risk for sudden cardiac death. Circ Cardiovasc Imaging 2012;5:525-35.
Olshausen KV, Witt T, Pop T, Treese N, Bethge K-P, Meyer J. Sudden cardiac death while wearing a Holter monitor. Am J Cardiol 1991;67:381-6.
Thomsen PEB, Jons C, Raatikainen MJP, Joergensen RM, Hartikainen J, Virtanen V, et al. Long-term recording of cardiac arrhythmias with an implantable cardiac monitor in patients with reduced ejection fraction after acute myocardial infarction the cardiac arrhythmias and risk stratification after acute myocardial infarction (CARISMA) study. Circulation 2010;122:1258-64.
Kuck KH, Cappato R, Siebels J, Rüppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: The Cardiac Arrest Study Hamburg (CASH). Circulation 2000;102:748-54.
Connolly SJ, Gent M, Roberts RS, Dorian P, Roy D, Sheldon RS, et al. Canadian implantable defibrillator study (CIDS): A randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation 2000;101:1297-302.
McAnulty J, Halperin B, Kron J, Larsen G, Rait M, Swenson R, et al. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med 1997;337:1576-84.
Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res 2004;95:754-63.
Luqman N, Sung RJ, Wang C-L, Kuo C-T. Myocardial ischemia and ventricular fibrillation: Pathophysiology and clinical implications. Int J Cardiol 2007;119:283-90.
Zipes DP, Rubart M. Neural modulation of cardiac arrhythmias and sudden cardiac death. Heart Rhythm 2006;3:108-13.
Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 2000;101:1960-9.
Risk stratification and survival after myocardial infarction. N Engl J Med 1983;309:331-6.
Gradman A, Deedwania P, Cody R, Massie B, Packer M, Pitt B, et al. Predictors of total mortality and sudden death in mild to moderate heart failure. Captopril-Digoxin Study Group. J Am Coll Cardiol 1989;14:564-70; discussion 571-572.
Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol 2003;42:736-42.
Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med 1999;341:1882-90.
Dickstein K, Vardas PE, Auricchio A, Daubert J-C, Linde C, McMurray J, et al. 2010 Focused Update of ESC Guidelines on device therapy in heart failure: An update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronization therapy Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Eur Heart J 2010;31:2677-87.
Epstein AE, DiMarco JP, Ellenbogen KA, Estes NAM, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): Developed in Collaboration With the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 2008;117:e350-408.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA Guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128:e240-327.
Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): Developed in Collaboration With the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006;114:e385-484.
Hachamovitch R, Rozanski A, Hayes SW, Thomson LEJ, Germano G, Friedman JD, et al. Predicting therapeutic benefit from myocardial revascularization procedures: Are measurements of both resting left ventricular ejection fraction and stress-induced myocardial ischemia necessary? J Nucl Cardiol 2006;13:768-78.
Lertsburapa K, Ahlberg AW, Bateman TM, Katten D, Volker L, Cullom SJ, et al. Independent and incremental prognostic value of left ventricular ejection fraction determined by stress gated rubidium 82 PET imaging in patients with known or suspected coronary artery disease. J Nucl Cardiol 2008;15:745-53.
Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging. J Am Coll Cardiol 2009;53:2201-29.
Corbett JR, Akinboboye OO, Bacharach SL, Borer JS, Botvinick EH, DePuey EG, et al. ASNC imaging guidelines for nuclear cardiology procedures. J Nucl Cardiol 2009;16:164.
Harel F, Finnerty V, Grégoire J, Thibault B, Marcotte F, Ugolini P, et al. Gated blood-pool SPECT versus cardiac magnetic resonance imaging for the assessment of left ventricular volumes and ejection fraction. J Nucl Cardiol 2010;17:427-34.
Akinboboye O, Nichols K, Wang Y, Dim UR, Reichek N. Accuracy of radionuclide ventriculography assessed by magnetic resonance imaging in patients with abnormal left ventricles. J Nucl Cardiol 2005;12:418-27.
Schaefer WM, Lipke CSA, Standke D, Kühl HP, Nowak B, Kaiser H-J, et al. Quantification of left ventricular volumes and ejection fraction from gated 99mTc-MIBI SPECT: MRI validation and comparison of the Emory Cardiac Tool Box with QGS and 4D-MSPECT. J Nucl Med 2005;46:1256-63.
Wackers FJ, Berger HJ, Johnstone DE, Goldman L, Reduto LA, Langou RA, et al. Multiple gated cardiac blood pool imaging for left ventricular ejection fraction: Validation of the technique and assessment of variability. Am J Cardiol 1979;43:1159-66.
Virmani R, Burke AP, Farb A. Sudden cardiac death. Cardiovasc Pathol 2001;10:275-82.
Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med 2001;345:1473-82.
Kelly P, Ruskin JN, Vlahakes GJ, Buckley MJ, Freeman CS, Garan H. Surgical coronary revascularization in survivors of prehospital cardiac arrest: Its effect on inducible ventricular arrhythmias and long-term survival. J Am Coll Cardiol 1990;15:267-73.
Holmes DR, Davis KB, Mock MB, Fisher LD, Gersh BJ, Killip T, et al. The effect of medical and surgical treatment on subsequent sudden cardiac death in patients with coronary artery disease: a report from the Coronary Artery Surgery Study. Circulation 1986;73:1254-63.
van der Burg AEB. Impact of viability, ischemia, scar tissue, and revascularization on outcome after aborted sudden death. Circulation 2003;108:1954-9.
Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death differential stratification for risk of cardiac death and myocardial infarction. Circulation 1998;97:535-43.
Piccini JP, Horton JR, Shaw LK, Al-Khatib SM, Lee KL, Iskandrian AE, et al. Single-photon emission computed tomography myocardial perfusion defects are associated with an increased risk of all-cause death, cardiovascular death, and sudden cardiac death. Circ Cardiovasc Imaging 2008;1:180-8.
Piccini JP, Starr AZ, Horton JR, Shaw LK, Lee KL, Al-Khatib SM, et al. Single-photon emission computed tomography myocardial perfusion imaging and the risk of sudden cardiac death in patients with coronary disease and left ventricular ejection fraction > 35%. J Am Coll Cardiol 2010;56:206-14.
Paganelli F, Barnay P, Imbert-Joscht I, Gelisse R, Saadjian A, Mundler O, et al. Influence of residual myocardial ischaemia on induced ventricular arrhythmias following a first acute myocardial infarction. Eur Heart J 2001;22:1931-7.
McArdle BA, Dowsley TF, deKemp RA, Wells GA, Beanlands RS. Does rubidium-82 PET have superior accuracy to SPECT perfusion imaging for the diagnosis of obstructive coronary disease? A systematic review and meta-analysis. J Am Coll Cardiol 2012;60:1828-37.
Parker MW, Iskandar A, Limone B, Perugini A, Kim H, Jones C, et al. Diagnostic accuracy of cardiac positron emission tomography versus single photon emission computed tomography for coronary artery disease: A bivariate meta-analysis. Circ Cardiovasc Imaging 2012;5:700-7.
Dorbala S, Di Carli MF. Cardiac PET perfusion: Prognosis, risk stratification, and clinical management. Semin Nucl Med 2014;44:344-57.
Dorbala S, Di Carli MF, Beanlands RS, Merhige ME, Williams BA, Veledar E, et al. Prognostic value of stress myocardial perfusion positron emission tomography. J Am Coll Cardiol 2013;61:176-84.
Lortie M, Beanlands RSB, Yoshinaga K, Klein R, Dasilva JN, DeKemp RA. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging 2007;34:1765-74.
Rijnierse MT, de Haan S, Harms HJ, Robbers LF, Wu L, Danad I, et al. Impaired hyperemic myocardial blood flow is associated with inducibility of ventricular arrhythmia in ischemic cardiomyopathy. Circ Cardiovasc Imaging 2014;7:20-30.
De Bakker JM, Van Capelle FJ, Janse MJ, Wilde AA, Coronel R, Becker AE, et al. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: Electrophysiologic and anatomic correlation. Circulation 1988;77:589-606.
Bello D, Fieno DS, Kim RJ, Pereles FS, Passman R, Song G, et al. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol 2005;45:1104-8.
Machecourt J, Longère P, Fagret D, Vanzetto G, Wolf JE, Polidori C, et al. Prognostic value of thallium-201 single-photon emission computed tomographic myocardial perfusion imaging according to extent of myocardial defect: Study in 1,926 patients with foilow-up at 33 months. J Am Coll Cardiol 1994;23:1096-106.
Morishima I, Sone T, Tsuboi H, Mukawa H, Uesugi M, Morikawa S, et al. Risk stratification of patients with prior myocardial infarction and advanced left ventricular dysfunction by gated myocardial perfusion SPECT imaging. J Nucl Cardiol 2008;15:631-7.
Di Carli MF, Maddahi J, Rokhsar S, Schelbert HR, Bianco-Batlles D, Brunken RC, et al. Long-term survival of patients with coronary artery disease and left ventricular dysfunction: Implications for the role of myocardial viability assessment in management decisions. J Thorac Cardiovasc Surg 1998;116:997-1004.
Di Carli MF, Davidson M, Little R, Khanna S, Mody FV, Brunken RC, et al. Value of metabolic imaging with positron emission tomography for evaluating prognosis in patients with coronary artery disease and left ventricular dysfunction. Am J Cardiol 1994;73:527-33.
Desideri A, Cortigiani L, Christen AI, Coscarelli S, Gregori D, Zanco P, et al. The extent of perfusion-F18-fluorodeoxyglucose positron emission tomography mismatch determines mortality in medically treated patients with chronic ischemic left ventricular dysfunction. J Am Coll Cardiol 2005;46:1264-9.
Beanlands RSB, Nichol G, Huszti E, Humen D, Racine N, Freeman M, et al. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease. J Am Coll Cardiol 2007;50:2002-12.
Uebleis C, Hellweger S, Laubender RP, Becker A, Sohn H-Y, Lehner S, et al. The amount of dysfunctional but viable myocardium predicts long-term survival in patients with ischemic cardiomyopathy and left ventricular dysfunction. Int J Cardiovasc Imaging 2013;29:1645-53.
Ling LF, Marwick TH, Flores DR, Jaber WA, Brunken RC, Cerqueira MD, et al. Identification of therapeutic benefit from revascularization in patients with left ventricular systolic dysfunction: Inducible ischemia versus hibernating myocardium. Circ Cardiovasc Imaging 2013;6:363-72.
Bonow RO, Maurer G, Lee KL, Holly TA, Binkley PF, Desvigne-Nickens P, et al. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med 2011;364:1617-25.
Mielniczuk LM, Beanlands RS. Does imaging-guided selection of patients with ischemic heart failure for high risk revascularization improve identification of those with the highest clinical benefit? Imaging-guided selection of patients with ischemic heart failure for high-risk revascularization improves identification of those with the highest clinical benefit. Circ Cardiovasc Imaging 2012;5:262-70; discussion 270.
Nakajo M, Shapiro B, Copp J, Kalff V, Gross MD, Sisson JC, et al. The normal and abnormal distribution of the adrenomedullary imaging agent m-[I-131]iodobenzylguanidine (I-131 MIBG) in man: Evaluation by scintigraphy. J Nucl Med 1983;24:672-82.
Hattori N, Schwaiger M. Metaiodobenzylguanidine scintigraphy of the heart: What have we learnt clinically? Eur J Nucl Med 2000;27:1-6.
Bombardieri E, Giammarile F, Aktolun C, Baum RP, Bischof Delaloye A, Maffioli L, et al. 131I/123I-metaiodobenzylguanidine (mIBG) scintigraphy: Procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging 2010;37:2436-46.
Fallavollita JA, Canty JM. Dysinnervated but viable myocardium in ischemic heart disease. J Nucl Cardiol 2010;17:1107-15.
Sasano T, Abraham MR, Chang K-C, Ashikaga H, Mills KJ, Holt DP, et al. Abnormal sympathetic innervation of viable myocardium and the substrate of ventricular tachycardia after myocardial infarction. J Am Coll Cardiol 2008;51:2266-75.
Brunner-La Rocca HP, Esler MD, Jennings GL, Kaye DM. Effect of cardiac sympathetic nervous activity on mode of death in congestive heart failure. Eur Heart J 2001;22:1136-43.
Bax JJ, Kraft O, Buxton AE, Fjeld JG, Parízek P, Agostini D, et al. 123 I-mIBG scintigraphy to predict inducibility of ventricular arrhythmias on cardiac electrophysiology testing: A prospective multicenter pilot study. Circ Cardiovasc Imaging 2008;1:131-40.
Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol 2010;55:2212-21.
Verberne HJ, Brewster LM, Somsen GA, van Eck-Smit BLF. Prognostic value of myocardial 123I-metaiodobenzylguanidine (MIBG) parameters in patients with heart failure: A systematic review. Eur Heart J 2008;29:1147-59.
Narula J, Gerson M, Thomas GS, Cerqueira MD, Jacobson AF. 123I-MIBG imaging for prediction of mortality and potentially fatal events in heart failure: The ADMIRE-HFX study. J Nucl Med 2015;56:1011-8.
Boogers MJ, Borleffs CJW, Henneman MM, van Bommel RJ, van Ramshorst J, Boersma E, et al. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J Am Coll Cardiol 2010;55:2769-77.
Kawai T, Yamada T, Tamaki S, Morita T, Furukawa Y, Iwasaki Y, et al. Usefulness of cardiac meta-iodobenzylguanidine imaging to identify patients with chronic heart failure and left ventricular ejection fraction < 35% at low risk for sudden cardiac death. Am J Cardiol 2015;115:1549-54.
Shah AM, Bourgoun M, Narula J, Jacobson AF, Solomon SD. Influence of ejection fraction on the prognostic value of sympathetic innervation imaging with iodine-123 MIBG in heart failure. JACC Cardiovasc Imaging 2012;5:1139-46.
Tamaki S, Yamada T, Okuyama Y, Morita T, Sanada S, Tsukamoto Y, et al. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: Results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J Am Coll Cardiol 2009;53:426-35.
Kasama S, Toyama T, Sumino H, Nakazawa M, Matsumoto N, Sato Y, et al. Prognostic value of serial cardiac 123I-MIBG imaging in patients with stabilized chronic heart failure and reduced left ventricular ejection fraction. J Nucl Med 2008;49:907-14.
Minardo JD, Tuli MM, Mock BH, Weiner RE, Pride HP, Wellman HN, et al. Scintigraphic and electrophysiological evidence of canine myocardial sympathetic denervation and reinnervation produced by myocardial infarction or phenol application. Circulation 1988;78:1008-19.
Simões MV, Barthel P, Matsunari I, Nekolla SG, Schömig A, Schwaiger M, et al. Presence of sympathetically denervated but viable myocardium and its electrophysiologic correlates after early revascularised, acute myocardial infarction. Eur Heart J 2004;25:551-7.
Inoue H, Zipes DP. Results of sympathetic denervation in the canine heart: Supersensitivity that may be arrhythmogenic. Circulation 1987;75:877-87.
Noordzij W, Slart RHJA. PET imaging of the autonomic myocardial function: Methods and interpretation. Clin Transl Imaging 2015;3:365-72.
Rosenspire KC, Haka MS, Van Dort ME, Jewett DM, Gildersleeve DL, Schwaiger M, et al. Synthesis and preliminary evaluation of carbon-11-meta-hydroxyephedrine: A false transmitter agent for heart neuronal imaging. J Nucl Med 1990;31:1328-34.
Allman KC, Wieland DM, Muzik O, Degrado TR, Wolfe ER, Schwaiger M. Carbon-11 hydroxyephedrine with positron emission tomography for serial assessment of cardiac adrenergic neuronal function after acute myocardial infarction in humans. J Am Coll Cardiol 1993;22:368-75.
Matsunari I, Schricke U, Bengel FM, Haase H-U, Barthel P, Schmidt G, et al. Extent of cardiac sympathetic neuronal damage is determined by the area of ischemia in patients with acute coronary syndromes. Circulation 2000;101:2579-85.
Canty JM. Hibernating myocardium: Chronically adapted to ischemia but vulnerable to sudden death. Circ Res 2004;94:1142-9.
Luisi AJ Jr, Suzuki G, DeKemp R, Haka MS, Toorongian SA, Canty Jr JM, et al. Regional 11C-hydroxyephedrine retention in hibernating myocardium: Chronic inhomogeneity of sympathetic innervation in the absence of infarction. J Nucl Med 2005;46:1368-74.
Pietilä M, Malminiemi K, Ukkonen H, Saraste M, Någren K, Lehikoinen P, et al. Reduced myocardial carbon-11 hydroxyephedrine retention is associated with poor prognosis in chronic heart failure. Eur J Nucl Med 2001;28:373-6.
Fallavollita JA, Heavey BM, Luisi AJ, Michalek SM, Baldwa S, Mashtare TL, et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol 2014;63:141-9.
Matsunari I, Aoki H, Nomura Y, Takeda N, Chen W-P, Taki J, et al. Iodine-123 metaiodobenzylguanidine imaging and carbon-11 hydroxyephedrine positron emission tomography compared in patients with left ventricular dysfunction. Circ Cardiovasc Imaging 2010;3:595-603.
Rischpler C, Fukushima K, Isoda T, Holt D, Dannals R, Bengel F, et al. Comparison of the sympathetic nerve imaging tracers hydroxyephedrine (HED) and metaiodobenzylguanidine (MIBG) in rat hearts. J Nucl Med 2011;52:332.
Luisi AJ, Fallavollita JA, Suzuki G, Canty JM. Spatial inhomogeneity of sympathetic nerve function in hibernating myocardium. Circulation 2002;106:779-81.
Tamburro P, Wilber D. Sudden death in idiopathic dilated cardiomyopathy. Am Heart J 1992;124:1035-45.
Kasama S, Toyama T, Kaneko Y, Iwasaki T, Sumino H, Kumakura H, et al. Relationship between late ventricular potentials and myocardial 123I-metaiodobenzylguanidine scintigraphy in patients with dilated cardiomyopathy with mild to moderate heart failure: Results of a prospective study of sudden death events. Eur J Nucl Med Mol Imaging 2012;39:1056-64.
Manrique A, Bernard M, Hitzel A, Bauer F, Ménard J-F, Sabatier R, et al. Prognostic value of sympathetic innervation and cardiac asynchrony in dilated cardiomyopathy. Eur J Nucl Med Mol Imaging 2008;35:2074-81.
Neglia D, Michelassi C, Trivieri MG, Sambuceti G, Giorgetti A, Pratali L, et al. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation 2002;105:186-93.
Majmudar MD, Murthy VL, Shah RV, Kolli S, Mousavi N, Foster CR, et al. Quantification of coronary flow reserve in patients with ischaemic and non-ischaemic cardiomyopathy and its association with clinical outcomes. Eur Heart J Cardiovasc Imaging 2015;16:900-9.
Rijnierse MT, Allaart CP, de Haan S, Harms HJ, Huisman MC, Wu L, et al. Sympathetic denervation is associated with microvascular dysfunction in non-infarcted myocardium in patients with cardiomyopathy. Eur Heart J Cardiovasc Imaging 2015;16:788-98.
Maron BJ, Spirito P, Shen W-K, Haas TS, Formisano F, Link MS, et al. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA 2007;298:405-12.
Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: The task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733-79.
Sorajja P, Chareonthaitawee P, Ommen SR, Miller TD, Hodge DO, Gibbons RJ. Prognostic utility of single-photon emission computed tomography in adult patients with hypertrophic cardiomyopathy. Am Heart J 2006;151:426-35.
Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med 2003;349:1027-35.
Hiasa G, Hamada M, Saeki H, Ogimoto A, Ohtsuka T, Hara Y, et al. Cardiac sympathetic nerve activity can detect congestive heart failure sensitively in patients with hypertrophic cardiomyopathy. Chest 2004;126:679-86.
Matsuo S, Nakamura Y, Tsutamoto T, Kinoshita M. Impairments of myocardial sympathetic activity may reflect the progression of myocardial damage or dysfunction in hypertrophic cardiomyopathy. J Nucl Cardiol 2002;9:407-12.
Terai H, Shimizu M, Ino H, Yamaguchi M, Hayashi K, Sakata K, et al. Cardiac sympathetic nerve activity in patients with hypertrophic cardiomyopathy with malignant ventricular tachyarrhythmias. J Nucl Cardiol 2003;10:304-10.
Glaudemans AWJM, Slart RHJA, Zeebregts CJ, Veltman NC, Tio RA, Hazenberg BPC, et al. Nuclear imaging in cardiac amyloidosis. Eur J Nucl Med Mol Imaging 2009;36:702-14.
Grogan M, Dispenzieri A. Natural history and therapy of AL cardiac amyloidosis. Heart Fail Rev 2015;20:155-62.
Mohty D, Damy T, Cosnay P, Echahidi N, Casset-Senon D, Virot P, et al. Cardiac amyloidosis: Updates in diagnosis and management. Arch Cardiovasc Dis 2013;106:528-40.
Hongo M, Urushibata K, Kai R, Takahashi W, Koizumi T, Uchikawa S, et al. Iodine-123 metaiodobenzylguanidine scintigraphic analysis of myocardial sympathetic innervation in patients with AL (primary) amyloidosis. Am Heart J 2002;144:122-9.
Bokhari S, Shahzad R, Castaño A, Maurer MS. Nuclear imaging modalities for cardiac amyloidosis. J Nucl Cardiol 2014;21:175-84.
Antoni G, Lubberink M, Estrada S, Axelsson J, Carlson K, Lindsjö L, et al. In vivo visualization of amyloid deposits in the heart with 11C-PIB and PET. J Nucl Med 2013;54:213-20.
Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Bresnitz EA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001;164:1885-9.
Mehta D, Lubitz SA, Frankel Z, Wisnivesky JP, Einstein AJ, Goldman M, et al. Cardiac involvement in patients with sarcoidosis: Diagnostic and prognostic value of outpatient testing. Chest 2008;133:1426-35.
Nery PB, Mc Ardle BA, Redpath CJ, Leung E, Lemery R, Dekemp R, et al. Prevalence of cardiac sarcoidosis in patients presenting with monomorphic ventricular tachycardia. Pacing Clin Electrophysiol 2014;37:364-74.
Kandolin R, Lehtonen J, Kupari M. Cardiac sarcoidosis and giant cell myocarditis as causes of atrioventricular block in young and middle-aged adults. Circ Arrhythm Electrophysiol 2011;4:303-9.
Nery PB, Beanlands RS, Nair GM, Green M, Yang J, McArdle BA, et al. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. J Cardiovasc Electrophysiol 2014;25:875-81.
Lynch JP, Hwang J, Bradfield J, Fishbein M, Shivkumar K, Tung R. Cardiac involvement in sarcoidosis: Evolving concepts in diagnosis and treatment. Semin Respir Crit Care Med 2014;35:372-90.
Aggarwal NR, Snipelisky D, Young PM, Gersh BJ, Cooper LT, Chareonthaitawee P. Advances in imaging for diagnosis and management of cardiac sarcoidosis. Eur Heart J Cardiovasc Imaging 2015;16:949-58.
Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014;63:329-36.
Kron J, Sauer W, Mueller G, Schuller J, Bogun F, Sarsam S, et al. Outcomes of patients with definite and suspected isolated cardiac sarcoidosis treated with an implantable cardiac defibrillator. J Interv Card Electrophysiol 2015;43:55-64.
Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014;11:1305-23.
Betensky BP, Tschabrunn CM, Zado ES, Goldberg LR, Marchlinski FE, Garcia FC, et al. Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart Rhythm 2012;9:884-91.
Kron J, Sauer W, Schuller J, Bogun F, Crawford T, Sarsam S, et al. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace 2013;15:347-54.
Tung R, Bauer B, Schelbert H, Lynch JP, Auerbach M, Gupta P, et al. Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: The potential role of occult inflammation in arrhythmogenesis. Heart Rhythm 2015;12:2488-98.
Wichter T, Hindricks G, Lerch H, Bartenstein P, Borggrefe M, Schober O, et al. Regional myocardial sympathetic dysinnervation in arrhythmogenic right ventricular cardiomyopathy. An analysis using 123I-meta-iodobenzylguanidine scintigraphy. Circulation 1994;89:667-83.
Wichter T, Schäfers M, Rhodes CG, Borggrefe M, Lerch H, Lammertsma AA, et al. Abnormalities of cardiac sympathetic innervation in arrhythmogenic right ventricular cardiomyopathy: Quantitative assessment of presynaptic norepinephrine reuptake and postsynaptic beta-adrenergic receptor density with positron emission tomography. Circulation 2000;101:1552-8.
Paul M, Wichter T, Kies P, Gerss J, Wollmann C, Rahbar K, et al. Cardiac sympathetic dysfunction in genotyped patients with arrhythmogenic right ventricular cardiomyopathy and risk of recurrent ventricular tachyarrhythmias. J Nucl Med 2011;52:1559-65.
Wichter T, Matheja P, Eckardt L, Kies P, Schäfers K, Schulze-Bahr E, et al. Cardiac autonomic dysfunction in Brugada syndrome. Circulation 2002;105:702-6.
Kies P, Wichter T, Schäfers M, Paul M, Schäfers KP, Eckardt L, et al. Abnormal myocardial presynaptic norepinephrine recycling in patients with Brugada syndrome. Circulation 2004;110:3017-22.
Kostopoulou A, Koutelou M, Theodorakis G, Theodorakos A, Livanis E, Maounis T, et al. Disorders of the autonomic nervous system in patients with Brugada syndrome: A pilot study. J Cardiovasc Electrophysiol 2010;21:773-80.
Werner RA, Rischpler C, Onthank D, Lapa C, Robinson S, Samnick S, et al. Retention kinetics of the 18F-labeled sympathetic nerve PET tracer LMI1195: Comparison with 11C-hydroxyephedrine and 123I-MIBG. J Nucl Med 2015;56:1429-33.
Higuchi T, Yousefi BH, Kaiser F, Gartner F, Rischpler C, Reder S, et al. Assessment of the 18F-labeled PET tracer LMI1195 for imaging norepinephrine handling in rat hearts. J Nucl Med 2013;54:1142-6.
Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: Executive summary a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012;126:3097-137.
Members Task Force, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, et al. ESC guidelines on the management of stable coronary artery disease: The task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;2013:2949-3003.
McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787-847.
Mancini GBJ, Gosselin G, Chow B, Kostuk W, Stone J, Yvorchuk KJ, et al. Canadian Cardiovascular Society guidelines for the diagnosis and management of stable ischemic heart disease. Can J Cardiol 2014;30:837-49.
O’Meara E, Mielniczuk LM, Wells GA, deKemp RA, Klein R, Coyle D, et al. Alternative imaging modalities in ischemic heart failure (AIMI-HF) IMAGE HF Project I-A: Study protocol for a randomized controlled trial. Trials 2013;14:218.
Acknowledgments
RSB is a career investigator supported by the Heart and Stroke Foundation of Ontario, a Tier 1 Research Chair supported by the University of Ottawa, and the University of Ottawa Heart Institute Vered Chair in Cardiology. DJ is a Cardiac Imaging Fellow at the University of Ottawa Heart Institute supported by a grant from the CHUM and CHUM Foundation. JK is supported by the Centre of Excellence of Cardiovascular and Metabolic Disease, Academy of Finland.
Disclosures
RSB is or has been a consultant for and receives grant funding from GE Healthcare, Lantheus Medical Imaging, and Jubilant DraxImage. TDR receives research grant funding from GE Healthcare, Advanced Accelerator Applications, and AstraZeneca. BJC holds the Saul and Edna Goldfarb Chair in Cardiac Imaging Research. He receives research support from GE Healthcare and educational support from TeraRecon Inc. JK has been a consultant of Lantheus Medical Imaging and received a grant from CardiRad Inc.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Juneau, D., Erthal, F., Chow, B.J.W. et al. The role of nuclear cardiac imaging in risk stratification of sudden cardiac death. J. Nucl. Cardiol. 23, 1380–1398 (2016). https://doi.org/10.1007/s12350-016-0599-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-016-0599-8