Abstract
Amyloidosis is a heterogeneous group of diseases characterized by localized or systemic deposition of insoluble extracellular fibrillary proteins in organs and tissues. Several types of amyloid can infiltrate the heart resulting in a restrictive cardiomyopathy, heart failure, and atrial and ventricular arrhythmias. Scintigraphy is a noninvasive method that may facilitate early diagnosis, distinguish various forms of cardiac amyloid, and may be useful in following disease burden. The amyloid-specific tracers presented in this article have been used with planar imaging and/or single-photon emission computed tomography. To date, there are no approved cardiac amyloid tracers although investigational tracers are currently under examination. This article serves to review the current nuclear imaging modalities available in the detection of cardiac amyloid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiac amyloidosis involves the deposition of insoluble fibrils in the myocardium and is an underdiagnosed cause of heart failure with preserved ejection fraction (HFpEF).1 The most clinically relevant cardiac involvement occurs in primary light-chain (AL) amyloidosis, familial transthyretin amyloidosis (mutant transthyretin, ATTRm), and senile transthyretin amyloidosis (wild-type transthyretin, ATTRwt). Other forms of systemic amyloidosis including secondary AA amyloidosis rarely affect the heart.2
As new subtype-specific therapies for cardiac amyloidosis are developed, the need to identify and distinguish amyloid subtypes reliably and noninvasively has become of increasing importance. Nuclear imaging modalities for cardiac amyloid hold promise for noninvasive identification of myocardial involvement, differentiating amyloid subtypes, and monitoring disease burden, disease progression, and potential response to therapy. This article highlights the current tracers involved in detecting cardiac amyloid.
Epidemiology
ATTRm amyloidosis occurs in an autosomal dominant fashion leading to familial amyloidotic cardiomyopathy (FAC) or familial amyloidotic polyneuropathy (FAP). The exact prevalence of FAC is unknown but pooled data shows up to 3.9% of African Americans are heterozygous carriers of the amyloidogenic allele, V122I, resulting in cardiac amyloid in an age-dependent penetrant manner.1 ATTRwt cardiomyopathy is underdiagnosed and has been shown to have a prevalence similar to autopsy studies with as many as 30% of patients with HFpEF ≥ 75 years.3 Primary AL amyloidosis is the most frequently diagnosed with an annual incidence 6-10 cases per million in the United States and United Kingdom,4 approximately half of whom will have significant cardiac involvement.5 In our experience to date of 210 cases: 53% have AL, 24% have ATTRwt, and 23% have ATTRmt. The numbers of ATTR cases are increasing over the last decade and now account for >50% of the referrals.
Diagnosis
Endomyocardial biopsy (EMB) remains the gold standard for definitive diagnosis of cardiac amyloid. Once amyloid deposits are found, additional testing with immunohistochemistry and/or sequence analysis by mass spectroscopy can identify the precursor protein. EMBs are typically performed in specialized centers and while highly sensitive does not provide sufficient information of extent of disease, progression of disease, prognostic information, nor response to treatment. Complications from EMB are 6% and include arrhythmia, perforation with pericardial tamponade, accidental arterial puncture, and pneumothorax,6 though in our experience in over 150 cases, these complications, especially perforation, are less common. EMB has been given a class IIa recommendation in the most recent American College of Cardiology (ACC) guidelines.7
Treatment
A number of new pharmacotherapies designed to reduce amyloid burden, enhance TTR native state stability or silence TTR production8 and prevent misfolding and aggregation 9,10 show promise. In vivo studies have demonstrated that diflunisal, a nonsteroidal anti-inflammatory drug (NSAID) binds to TTR and enhances stability.11,12 A phase III study in FAP, in which many patients had cardiac involvement, was recently completed and results are expected soon (Diflunisal Study NCT00294671). Tafamidis, a TTR stabilizer modeled after Diflunisal but without any NSAIDs properties, has shown favorable results in phase II and III trial in FAP.13 Other novel therapies aimed at reducing production of TTR through the use of small interfering RNAs8 and antisense oligonucleotides to silence the TTR gene are currently being investigated.14,15
Prognosis
AL amyloidosis is caused by the deposition of monoclonal immunoglobulin light chains and is associated with plasma cell dyscrasias. The prognosis of AL amyloidosis is related to the number and severity of organs involved with cardiac involvement carrying the worst prognosis.16 In addition, the clinical course in AL cardiac amyloidosis is more rapidly progressive than in ATTRm and ATTRwt.17-19 One study comparing AL cardiac amyloid to ATTRwt with heart failure found median survival of 11 and 75 months, respectively.19
Methods
We performed a systematic review of peer-reviewed publications through MEDLINE using the following search terms “myocard*” AND “amyloid*” AND “name of imaging modality*” resulting in a total of 35 articles, of which 8 were excluded and 17 of the remaining 27 articles included. Criteria for selection included English language, clinical relevance, number of patients included in study, and validity based on venue publication. Bibliographies from these references were reviewed, as well as additional articles from content experts. Finally, each imaging modality was searched individually yielding an additional 11 results.
Nuclear Imaging Modalities
Bone seeking tracers
Radiolabeled phosphate derivatives, initially developed as bone tracers, were first noted to localize to amyloid deposits in 1977 when Kula et al20 visualized calcifications in amyloid deposits with 99mTc-diphosphanate. This association led to the development of several phosphate derivatives tagged with 99mTc including 99mTc-pyrophosphate (PYP), 99mTc-methylene diphosphonate (MDP), and 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) (Table 1).
99mTc-DPD
Of the bone seeking radiotracers, 99mTc-DPD has been the most studied with regards to its use for cardiac amyloid imaging. Currently, this isotope is not approved by the Food and Drug Administration (FDA) and therefore not available for clinical use in the United States. In 2005, Perugini et al performed 99mTc-DPD imaging on 25 patients with cardiac amyloidosis (10 ATTRm, 5 ATTRwt, 10 AL) confirmed by biopsy with immunohistochemistry or by genotyping with typical echocardiographic appearance. All 15 ATTR patients had strong myocardial uptake of 99mTc-DPD while no uptake was observed in AL patients with 99mTc-DPD myocardial uptake being 100% sensitive and 100% specific for diagnosing ATTR cardiac amyloidosis.21
In a larger cohort of 79 patients (28 ATTRm, 17 ATTRwt, and 34 AL) where tracer retention was calculated by a heart-to-whole body ratio (H/WB), the diagnostic accuracy of 99mTc-DPD scintigraphy was found to be lower due to tracer uptake in about one-third of AL patients with sensitivity 100% and specificity 88% using moderate to strong uptake as cutoff. Using visual scoring (VS) (0 = no uptake, 1 = mild uptake, 2 = moderate uptake, 3 = strong uptake), the positive predictive value (PPV) and negative predictive value (NPV) for VS ≥ 1 were 80% and 100%, respectively, compared to 100% and 68% for VS ≥ 3. Using a VS ≥ 2, 99mTc-DPD had a NPV of 100% for excluding AL amyloid while a positive cardiac uptake of 99mTc-DPD had a PPV of 88% for ATTR amyloid.22 The preferential uptake of 99mTc-DPD in ATTR amyloid cardiomyopathy was also supported in a retrospective study of elderly patients with unexplained concentric left ventricular hypertrophy and a non-dilated left ventricle where all 46 patients with positive uptake had biopsy-proven cardiac amyloid (14 ATTRm, 32 ATTRwt).23
In a cohort of 36 patients with ATTRwt amyloid that underwent 99mTc-DPD imaging, heart retention (HR) of the tracer had positive correlation with inter-ventricular septal thickness and severity of cardiac amyloid deposition assessed by impaired longitudinal function (e.g., MAPSE/TAPSE = mitral/tricuspid annular plane systolic excursion). In addition, patients with HR 9.27% had a tendency toward decreased survival.24 Another study of eight biopsy-proven FAP patients, whole body tracer retention and specifically myocardial tracer retention were found to correlate with cardiac disease severity.25 Further studies demonstrated that in ATTR subjects, 99mTc-DPD myocardial uptake is of prognostic value for predicting major adverse cardiac events (MACE), either alone or in combination with LV wall thickness.26
The amyloid tracer 99mTc-DPD is the first radiotracer demonstrating the ability to distinguish ATTR from AL cardiac amyloidosis when tracer retention is either intense or absent. Moderate 99mTc-DPD myocardial uptake was reported to be of indeterminate significance with a prevalence in AL and ATTR amyloid of 18% and 36%, respectively. 99mTc-DPD myocardial uptake also has prognostic significance leading to its widespread use among amyloid centers in Europe.
99mTc-PYP
A number of a case reports from 1980 and onwards demonstrate myocardial uptake of 99mTc-PYP in amyloid patients. Despite this, 99mTc-PYP scintigraphy has not been validated as a method in identifying cardiac amyloid due to variable sensitivities, lack of identification of amyloid subtype in earlier studies, and failure of a quantitative method for detecting myocardial amyloid.
In 1982, Wizenberg et al reported a group of ten patients with tissue-proven amyloidosis of unidentified subtype (two with histologically proven cardiac amyloid) who underwent 99mTc-PYP myocardial scans and had marked diffuse cardiac uptake on scintigraphy. The authors concluded based on these observations that cardiac amyloid should be strongly suspected in patients with biopsy-proven amyloid combined with echocardiographic features of amyloid and positive myocardial 99mTc-PYP imaging.27 In a larger study of 34 patients with biopsy-proven amyloidosis (undefined subtype), only 3 of 14 retrospective cases and 17 of 20 prospective cases with echocardiographic features of amyloid had myocardial 99mTc-PYP uptake, a finding also found in 15 of 20 controls without amyloid heart disease. These results led to the conclusion that 99mTc-PYP scintigraphy is not sufficiently sensitive to warrant routine screening inpatients with cardiac amyloid.28
In 2012, Yamamoto et al described a quantitative method, the “PYP score,” to assess the utility of 99mTc-PYP to evaluate for cardiac amyloidosis in 13 subjects with heart failure due to amyloid (1 AL, 1 AA, 3 ATTRm, 8 ATTRwt) and 37 subjects with heart failure due to non-amyloid causes. PYP score, defined as the ratio of myocardial mean counts to ventricular cavity mean counts, was found to have a sensitivity of 84.6% and specificity of 94.5% for distinguishing cardiac amyloidosis from non-amyloid causes of heart failure.29
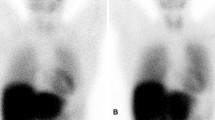
In a recent study at Columbia University by Bokhari et al, 45 subjects (12 AL, 16 ATTRwt, 17 ATTRm) with biopsy-proven amyloidosis amyloid underwent 99mTc-PYP SPECT. Cardiac retention was assessed with both a semi-quantitative visual score (see Figure 1) in relation to bone uptake (0 = no cardiac uptake to 3 = high uptake greater than bone) and by quantitative analysis by drawing a region of interest (ROI) over the heart corrected for contralateral counts and calculating a heart-to-contralateral ratio (H/CL). The degree of cardiac tracer retention in the heart correlated with left ventricular wall thickness and mass (see Figure 2) similar to what has been reported for 99mTc-DPD.22 Subjects with ATTR cardiac amyloid had significantly higher semi-quantitative cardiac VS than the AL cohort as well as a higher quantitative score. Using a H/CL ratio ≥1.5 (see Figure 3) consistent with intensely diffuse myocardial tracer retention had a 97% sensitivity and 100% specificity for identifying ATTR cardiac amyloidosis. The author concluded that 99mTc-PYP cardiac imaging may be a simple, widely available method to identify subjects with ATTR type cardiac amyloidosis.30 This study did not include any normal subjects to know whether uptake in AL subjects is equal to or greater than normal subjects. So, the absence of uptake of bone seeking radiotracers in a patient without myocardial biopsy-proven amyloidosis could mean no disease or AL disease.
(A, B) Semi-quantitative method of calculating the distribution of 99mTc-PYP uptake. Raw images of a representative negative (A) and positive subject (B) are shown 1 hour after radiotracer infusion. ROI circles are depicted in green and the contralateral comparison circle is depicted in blue. ROI, region of interest; C/L, contralateral; Cts, counts; StdDev standard deviation
Cardiac tracer retention (H/CL) of 99mTc-PYP vs LV mass index. The degree of cardiac tracer retention (H/CL) positively correlates with LV mass index similar to Tc-DPD study (see Ref.18). H/CL, heart to contralateral ratio; LV left ventricle
Quantitative analysis of mean heart to contralateral ratio according to amyloid subtype. Comparison of 99mTc-PYP mean H/CL ratio between patients with AL, ATTRwt, and ATTRm cardiac amyloidosis. AL and transthyretin-related amyloidoses are differentiated by mean H/CL ratio of 1.5. The outlier with H/CL 1.3 is an ATTRm patient with the unusual Thr59Lys mutation (adapted from Bokhari et al26). AL, amyloid light-chain; ATTRwt, wild-type transthyretin amyloidosis; ATTRm, mutant transthyretin amyloidosis
The discriminatory ability of 99mTc-PYP for ATTR cardiac amyloid with high sensitivity and specificity in the above study may have been related to the selection of patients with more advanced cardiac amyloid and a large percentage of patients with ATTRm had the V122I mutation. Despite these limitations, 99mTc-PYP shows promise in discerning ATTR from AL amyloid and is readily available in the United States. Further multicenter studies with different isotopes, less severe phenotypes, and other mutations are needed to validate its use.
Sympathetic Innervation
123I-MIBG
Metaiodobenzylguanidine (MIBG) is an analog to norepinephrine and shares similar uptake and storage in sympathetic nerve endings. However, unlike norepinephrine, MIBG undergoes little enzymatic degradation. Due to these characteristics, when coupled with 123I, objective evaluation of cardiac sympathetic function is possible.31 123I-MIBG myocardial imaging has been used in Europe and Japan and recently the United States FDA has approved this tracer for the assessment of myocardial sympathetic innervation in patients with New York Heart Association (NYHA) class II or class III heart failure and left ventricular ejection fraction (LVEF) <35%.
In 1995, Nakata et al32 reported the first case of a patient with FAP who had absent myocardial uptake of 123I-MIBG in any cardiac region indicating a lack of sympathetic activity due to amyloid deposits. This was later confirmed by others33-35 indicating that patients with FAP have a high incidence of myocardial adrenergic denervation with viable myocardium and can be identified early in cardiac amyloidosis before clinical heart disease and echocardiographic changes.34 Another clinical trial by Delahaye et al involving 17 patients with rectal or nerve biopsy-proven FAP found that cardiac 123I-MIBG uptake was significantly decreased in FAP patients with no difference in washout rates despite preserved left ventricle systolic function and cardiac perfusion. Furthermore, the clinical severity of polyneuropathy negatively correlated with MIBG uptake at 4 hours.35 These studies suggest that 123I-MIBG imaging may detect early cardiac amyloid specifically in FAP, which is characterized by early autonomic nervous system involvement. Whether MIBG scintigraphy will be as useful in ATTRwt and ATTRm FAC patients who do not have predominant manifestations of autonomic dysfunction requires further study.
In 2002, Hongo et al36 examined the utility of 123I-MIBG in 25 AL amyloid patients and concluded that myocardial uptake and turnover of MIBG in AL amyloid are heterogeneous and dependent on the presence or absence of congestive heart failure and cardiac autonomic dysfunction. A recent study in 2012 that investigated 61 patients (39 AL, 11 AA, 11 ATTRm) found that MIBG late H/M ratio was significantly lower and higher washout rates irrespective of amyloid subtype compared to healthy controls in patients who had echocardiographic features of amyloidosis. In addition, ATTRm patients without echocardiographic signs of amyloidosis had lower H/M ratio compared to other subtypes (AL and AA) which may be related to concomitant neuropathic involvement. The author concluded that 123I-MIBG scintigraphy can detect cardiac denervation in ATTRm patients before signs of amyloidosis on echocardiogram.37 The early detection of cardiac denervation with 123I-MIBG in ATTRm FAP is important as sympathetic denervation occurs early in the disease and all current therapies under investigation, including liver transplantation,38 are aimed at preventing disease progression and not removing pre-existing amyloid.
Amyloid Deposits
99mTc-aprotinin
The observation of the presence of anti-proteases in amyloid deposits led to the development of radiolabeled 99mTc-aprotinin as a potential amyloid tracer. Aprotinin is a low molecular protease inhibitor and was first used in 1995 in 25 patients (24 AL and 1 ATTRm) with myocardial uptake seen in 10 of 24 AL patients and 1 ATTRm patient. Although endomyocardial biopsies were not done to confirm this finding, the author concluded that 99mTc-aprotinin can be a potential tracer for detection of cardiac amyloid.39 Similar findings were reported 7 years later with myocardial uptake seen in 6 of 18 AL patients and 2 of 2 ATTRm patients.40 Another prospective study of 18 biopsy-proven amyloid patients (14 AL, 3 AA, 1 ATTRm), noted positive cardiac uptake in five patients (4 of 14 AL, 1 ATTRm), all who had echocardiographic and magnetic resonance imaging (MRI) features of cardiac involvement, compared to absent uptake in the remaining patients who had no clinical or echocardiographical features of amyloid.41 These studies may suggest a role of 99mTc-aprotinin scintigraphy but limitations include poor specificity for amyloid subtype and inadequate number of ATTR patients to draw conclusions.
Other tracers
Serum amyloid P component (SAP) is a constituent of all amyloid subtypes and was first reported by Hawkins et al.42 When combined with radiolabeled iodine, 123I-SAP can detect amyloid deposition in the liver, spleen, kidneys, bones, and adrenals. However, due to blood pool content and decreased permeability of tracer in the myocardium, 123I-SAP is not useful for detection of cardiac amyloidosis.
The use of gallium, 67Ga, in cardiac amyloid is limited to few case reports and currently does not play a role in cardiac amyloid imaging. 111Indium-antimyosin imaging is limited to one study of seven patients with biopsy-proven cardiac amyloid noted to have abnormal cardiac antimyosin uptake.
PET Scanning
Pittsburgh compound B (11C-PIB) is a positron emission tomography (PET) tracer developed for β-amyloid in Alzheimer disease and believed to bind to amyloid fibrils of any type. In a case report of a patient with suspected systemic amyloidosis in whom the subtype was not defined, the increased 11C-PIB concentration in the left ventricle at 2 minutes post-injection with subsequent tracer clearance by 5 minutes was consistent with normal bio-distribution, suggesting unsuitability for cardiac amyloid.43 However, in a study of ten patients with systemic amyloidosis (7 AL, 2 ATTRm, 1 ATTRwt) and cardiac involvement (5 biopsy proven), 11C-PIB uptake was seen in all patients 15-25 minutes after injection of tracer compared to absent uptake in five controls suggesting a possible role in cardiac amyloid imaging.44
A French multicenter study of 10 AL amyloid patients who underwent FDG-PET/CT imaging during follow-up identified positive extra-cardiac uptake in 70% of patients namely broncho-pulmonary and nasopharynx and was concordant with known organ impairment in 6 of 7 cases. However, FDG-PET uptake was negative in the patient with known cardiac amyloidosis.45 Other PET tracers include 11C-BF-227 which has shown significant cardiac retention compared to a control in a patient with ATTRm amyloidosis 46 and 124I-m11-1F4, a murine amyloid-reactive monoclonal antibody, currently in phase I clinical trial developed for passive immunotherapy in AL amyloid.47
Recently, the success of visualizing β-amyloid plaques in the brain with PET has resulted in the approval of 18F-florbetapir by the US FDA for imaging β-amyloid plaques in Alzheimer patients. In an open-label, multicenter brain imaging study of 18F-florbetapir PET imaging on 32 patients (16 Alzheimer patients, 16 controls), mean cortical standardized uptake value ratios (SUVRs) were significantly higher in Alzheimer patients compared to healthy controls.48 The role of 18F-florbetapir in cardiac amyloidosis holds promise in the future and is currently in clinical trial (NCT01683825).
Conclusion
EMB remains the gold standard for detection of cardiac amyloidosis and a class IIa recommendation by the ACC. However, EMBs are limited to specialized centers, are not a minimal risk procedure and do not provide information on extent of disease, progression of disease or response to treatment. With the development of novel drug agents aimed at prevention of cardiac amyloid, noninvasive methods to diagnose early amyloid and follow disease progression will become critical. The radioactive isotopes predominately involved in cardiac amyloid include 99mTc-DPD, 99mTc-PYP, and 123I-MIBG, the latter which may detect cardiac denervation and autonomic dysfunction in cardiac amyloid.
Multimodal nuclear imaging with MIBG can be employed for early detection of cardiac amyloid before echocardiographic features emerge and can be combined with Tc-PYP or Tc-DPD for differentiation of amyloid subtype in patients with echocardiographic amyloid. Differentiating immunoglobulin AL amyloidosis from ATTR-related cardiac amyloidosis is imperative given implications for prognosis, therapy, and genetic counseling. Currently, the bone seeking tracers 99mTc-DPD and more recently 99mTc-PYP have been shown to differentiate AL from ATTR cardiac amyloid with high sensitivity and specificity in patients with advanced amyloid. Studies to identify the role of bone seeking tracers for early cardiac amyloid detection and quantifying amount of amyloid burden with serial scans in order to track progression of disease and monitor treatment response have yet to be investigated. In the near future, the use of standardized nuclear imaging protocols for cardiac amyloidosis may lead to earlier detection, expedited treatment, delayed disease progression, and improvement of quality of life for patients.
References
Dharmarajan K, Maurer MS. Transthyretin cardiac amyloidoses in older North Americans. J Am Geriatr Soc 2012;60:765-74.
Desai HV, Aronow WS, Peterson SJ, Frishman WH. Cardiac amyloidosis: Approaches to diagnosis and management. Cardiol Rev 2010;18:1-11.
Sultan AM, Edwards WD, Mohammed SF, Hammill SC, Bailey KR, Ballard DJ, et al. Cardiac amyloid deposition is common in elderly patients with heart failure and preserved ejection fraction. Circulation 2010;122:A17926.
Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD. Updates in cardiac amyloidosis: A review. J Am Heart Assoc 2012;1:e000364.
Falk RH. Cardiac amyloidosis: A treatable disease, often overlooked. Circulation 2011;124:1079-85.
Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation 2007;116:2216-33.
Yancy CW, Jessup M, Bozkurt B, Masoudi FA, Butler J, McBride PE, et al. ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128:e240–327.
Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med 2013;369:819-29.
Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis 2010;52:347-61.
Macario AJ, Conway de Macario E. Sick chaperones and ageing: A perspective. Ageing Res Rev 2002;1:295-311.
Sekijima Y, Dendle MA, Kelly JW. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid 2006;13:236-49.
Tojo K, Sekijima Y, Kelly JW, Ikeda S. Diflunisal stabilizes familial amyloid polyneuropathy-associated transthyretin variant tetramers in serum against dissociation required for amyloidogenesis. Neurosci Res 2006;56:441-9.
Coelho T, Maia LF, Martins da Silva A, Waddington Cruz M, Planté-Bordeneuve V, Lozeron P, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: A randomized, controlled trial. Neurology 2012;79:785-92.
Sah D. Phase I safety, pharmacokinetic and pharmacodynamics results for ALN-TTR01, a novel RNAi therapeutic for the treatment of transthyretin amyloidosis. VIIIth International Symposium on Familial Amyloid Polyneuropathy; 2011.
Benson MD, Pandey S, Witchell D, Jazayeri A, Siwkowski A, Monia B, et al. Antisense oligonucleotide therapy for TTR amyloidosis. Amyloid 2011;18:60.
Kyle RA, Gertz MA. Primary systemic amyloidosis: Clinical and laboratory features in 474 cases. Semin Hematol 1995;32:45-59.
Dubrey SW, Cha K, Skinner M, LaValley M, Falk RH. Familial and primary (AL) cardiac amyloidosis: Echocardiographically similar diseases with distinctly different clinical outcomes. Heart 1997;78:74-82.
Gertz MA, Kyle RA, Thibodeau SN. Familial amyloidosis: A study of 52 North American-born patients examined during a 30-year period. Mayo Clin Proc 1992;67:428-40.
Ng B, Connors LH, Davidoff R, Skinner M, Falk RH. Senile systemic amyloidosis presenting with heart failure: A comparison with light chain-associated amyloidosis. Arch Intern Med 2005;165:1425-9.
Kula RW, Engel WK, Line BR. Scanning for soft-tissue amyloid. Lancet 1977;1:92-3.
Perugini E, Guidalotti PL, Salvi F, Cooke RM, Pettinato C, Riva L, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol 2005;46:1076-84.
Rapezzi C, Quarta CC, Guidalotti PL, Longhi S, Pettinato C, Leone O, et al. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur J Nucl Med Mol Imaging 2011;38:470-8.
Quarta CC, Guidalotti PL, Longhi S, Pettinato C, Leone O, Ferlini A, et al. Defining the diagnosis in echocardiographically suspected senile systemic amyloidosis. JACC Cardiovasc Imaging 2012;5:755-8.
Kristen AV, Haufe S, Schonland SO, Hegenbart U, Schnabel PA, Röcken C, et al. Skeletal scintigraphy indicates disease severity of cardiac involvement in patients with senile systemic amyloidosis. Int J Cardiol 2011;43:1862-7.
Puille M, Altland K, Linke RP, Steen-Müller MK, Kiett R, Steiner D, et al. 99mTc-DPD scintigraphy in transthyretin-related familial amyloidotic polyneuropathy. Eur J Nucl Med Mol Imaging 2002;29:376-9.
Rapezzi C, Quarta CC, Guidalotti PL, Pettinato C, Fanti S, Leone O, et al. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging 2011;4:659-70.
Wizenberg TA, Muz J, Sohn YH, Samlowski W, Weissler AM. Value of positive myocardial technetium-99m-pyrophosphate scintigraphy in the non-invasive diagnosis of cardiac amyloidosis. Am Heart J 1982;103:468-73.
Gertz MA, Brown ML, Hauser MF, Kyle RA. Utility of technetium Tc 99m pyrophosphate bone scanning in cardiac amyloidosis. Arch Intern Med 1987;147:1039-44.
Yamamoto Y, Onoguchi M, Haramoto M, Kodani N, Komatsu A, Kitagaki H, et al. Novel method for quantitative evaluation of cardiac amyloidosis using (201)TlCl and (99m)Tc-PYP SPECT. Ann Nucl Med 2012;26:634-43.
Bokhari S, Castaño A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. 99mTc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging 2013;6:195-201.
Camacho V, Carrio I. Targeting neuronal dysfunction and receptor imaging. Curr Opin Biotechnol 2007;18:60-4.
Nakata T, Shimamoto K, Yonekura S, Kobayashi N, Sugiyama T, Imai K, et al. Cardiac sympathetic denervation in transthyretin-related familial amyloidotic polyneuropathy: Detection with iodine-12-MIBG. J Nucl Med 1995;36:1040-2.
Arbab AS, Koizumi K, Toyama K, Arai T, Yoshitomi T, Araki T. Scan findings of various myocardial SPECT agents in a case of amyloid polyneuropathy with suspected myocardial involvement. Ann Nucl Med 1997;11:139-41.
Tanaka M, Hongo M, Kinoshita O, Takabayashi Y, Fujii T, Yazaki Y, et al. Iodine-123 metaiodobenzylguanidine scintigraphic assessment of myocardial sympathetic innervation in patients with familial amyloid polyneuropathy. J Am Coll Cardiol 1997;29:168-74.
Delahaye N, Dinanian S, Slama MS, Mzabi H, Samuel D, Adams D, et al. Cardiac sympathetic denervation in familial amyloid polyneuropathy assessed by iodine-123 metaiodobenzylguanidine scintigraphy and heart rate variability. Eur J Nucl Med 1999;26:416-24.
Hongo M, Urushibata K, Kai R, Takahashi W, Koizumi T, Uchikawa S, et al. Iodine-123 metaiodobenzylguanidine scintigraphic analysis of myocardial sympathetic innervation in patients with AL (primary) amyloidosis. Am Heart J 2002;144:122-9.
Noordzij W, Glaudemans AW, Rheenen RW, Hazenberg BP, Tio RA, Dierckx RA, et al. (123)I-Labelledmetaiodobenzylguanidine for the evaluation of cardiac sympathetic denervation in early stage amyloidosis. Eur J Nucl Med Mol Imaging 2012;39:1609-17.
Delahaye N, Rouzet F, Sarda L, Tamas C, Dinanian S, Plante-Bordeneuve V, et al. Impact of liver transplantation on cardiac autonomic denervation in familial amyloid polyneuropathy. Medicine (Baltimore) 2006;85:229-38.
Aprile C, Marinone G, Saponaro R, Bonino C, Merlini G. Cardiac and pleuropulmonary AL amyloid imaging with technetium-99m labeled aprotinin. Eur J Nucl Med 1995;22:1393-401.
Schaadt BK, Hendel HW, Gimsing P, Jønsson V, Pedersen H, Hesse B, et al. 99mTc-aprotinin scintigraphy in amyloidosis. J Nucl Med 2003;44:177-83.
Han S, Chong V, Murray T, McDonagh T, Hunter J, Poon FW, et al. Preliminary experience of 99mTc-aprotinin scintigraphy in amyloidosis. Eur J Haematol 2007;79:494-500.
Hawkins PN, Myers MJ, Lavender JP, Pepys MB. Diagnostic radionuclide imaging of amyloid: Biological targeting by circulating human serum amyloid P component. Lancet 1988;1:1413-8.
Minamimoto R, Ishii K, Kubota K, Morooka M, Okasaki M, Ito K, et al. Amyloid imaging mismatch. Clin Nucl Med 2012;37:807-9.
Antoni G, Lubberink M, Estrada S, Axelsson J, Carlson K, Lindsjö L, et al. In vivo visualization of amyloid deposits in the heart with 11C-PIB and PET. J Nucl Med 2012;54:213-20.
Mekinian A, Jaccard A, Soussan M, Launay D, Berthier S, Federici L, et al. 18F-FDG PET/CT in patients with amyloid light-chain amyloidosis: Case-series and literature review. Amyloid 2012;19:94-8.
Furukawa K, Ikeda S, Okamura N, Tashiro M, Tomita N, Furumoto S, et al. Cardiac positron-emission tomography images with an amyloid-specific tracer in familial transthyretin-related systemic amyloidosis. Circulation 2012;125:556-7.
Wall JS, Kennel SJ, Stuckey AC, et al. Radioimmunodetection of amyloid deposits in patients with AL amyloidosis. Blood 2010;116:2241-4.
Wong DF, Rosenberg PB, Zhou Y, et al. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F18). J Nucl Med 2010;51:913-20.
Disclosures
Dr Maurer is supported by a K24 Award from the National Institute on Aging (AG036778-02), receives research support from Alnylam and Pfizer and serves on the executive board of Transthyretin Amyloid Outcomes Survey (THAOS) an international registry of patients with ATTR amyloidosis, and funded by FoldRx Pharmaceuticals, Inc, a wholly owned subsidiary of Pfizer, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bokhari, S., Shahzad, R., Castaño, A. et al. Nuclear imaging modalities for cardiac amyloidosis. J. Nucl. Cardiol. 21, 175–184 (2014). https://doi.org/10.1007/s12350-013-9803-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-013-9803-2