Abstract
Techniques for in vivo assessment of disease-related molecular changes are being developed for all forms of non-invasive cardiovascular imaging. The ability to evaluate tissue molecular or cellular phenotype in patients has the potential to not only improve diagnostic capabilities but to enhance clinical care either by detecting disease at an earlier stage when it is more amenable to therapy, or by guiding most appropriate therapies. These new techniques also can be used in research programs in order to characterize pathophysiology and as a surrogate endpoint for therapeutic efficacy. The most common approach for molecular imaging involves the creation of novel-targeted contrast agents that are designed so that their kinetic properties are different in disease tissues. The main focus of this review is not to describe all the different molecular imaging approaches that have been developed, but rather to describe the status of the field and highlight some of the clinical and research applications that molecular imaging will likely provide meaningful benefit. Specific target areas include assessment of atherosclerotic disease, tissue ischemia, and ventricular and vascular remodeling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Defining Molecular Imaging and Its Potential Role in Medicine
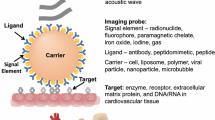
In its broadest sense, the term molecular imaging refers to any technique that can be used to generate an image reflecting a wide array of molecular process such as gene expression, protein synthesis and/or trafficking, metabolic activity, enzyme activity, etc. For medical science, the term molecular imaging is most often applied to describe technologies that can be used in vivo to evaluate phenotype in health or disease in patients or intact animal models of disease. Molecular imaging in cardiology often involves the application of targeted imaging probes paired with conventional clinical and preclinical forms of non-invasive imaging such as radionuclide imaging; magnetic resonance imaging (MRI), ultrasound, computed tomography (CT), and optical imaging.
A fine line does not exist for defining “molecular imaging.” For this discussion, we will limit our comments to techniques that rely on the administration of novel imaging probes that are bioengineered to specifically bind to or are activated by a specific disease-related molecule or class of molecules. Because the field has undergone tremendous expansion, we will not attempt to review all experience with molecular imaging in cardiovascular medicine. Rather, we will focus on how molecular imaging is likely to make an impact in clinical care and science and provide a comparison of the relative utility of different approaches to molecular imaging.
Need Basis for Molecular Imaging
Justification for the development of molecular imaging technologies is based on several considerations. First, molecular imaging could provide some unique biologic insight that will either enhance research capabilities or improve patient care and outcomes, some of which are illustrated in Figure 1. Molecular imaging may also improve efficiency and/or cost-effectiveness in either the research or clinical setting. In preclinical research laboratories, molecular imaging is already being used as a high-throughput approach to evaluate pathophysiology or to screen new therapies. With regards to clinical medicine, there are many precedents where the introduction of a major new technologic advance in cardiovascular imaging that may add cost to initial care can result in eventual cost savings by either preventing adverse events or reducing downstream resource utilization.1,2
High Impact Scenarios for Molecular Imaging
Atherosclerosis
Atherosclerosis is a complex disease process that commonly progresses for decades before becoming clinically evident. The current basis for non-invasive detection of coronary artery disease is to either detect impaired myocardial blood flow or abnormal myocardial contractile function at rest or during stress, or to directly image calcium or plaque in coronary arteries. There are several ways that molecular imaging of atherosclerosis could potentially provide incremental value to the current standard of care.
Imaging vascular phenotype may inform clinicians at a very early stage whether an individual is at exceptionally high risk for developing accelerated and aggressive disease over the ensuing decades. The use of molecular imaging to better discriminate risk is predicated on the limitations of current methods for risk prediction. According to the National Registry of Myocardial Infarction (NRMI) approximately one half of patients experiencing first myocardial infarction (MI) have no or only one risk factor.3 Although biomarkers such as high sensitivity C-reactive protein have been shown to identify a population that may benefit from more aggressive primary prevention,4 it provides only modest benefit in risk prediction and risk reclassification.5 Coronary artery calcium (CAC) on CT has proven to be effective at predicting risk for coronary events, however, incremental value to traditional clinical risk factors has been established mostly in middle age to older individuals.6,7 Molecular imaging may provide an avenue for predicting very high risk in young individuals in whom risk-lowering therapy is likely to be more beneficial. This issue will likely increase in importance with the development of newer more potent anti-atherosclerotic therapies that may have higher cost and rates of adverse effects than statin therapy, and yet work best when given early in the course of disease. Currently, there are off-label or experimental approaches to imaging high risk phenotype in patients including 18F-fluorodeoxyglucose PET, which is thought to reflect increased metabolic activity and/or plaque hypoxic environment; and MRI signal from ultrasmall superparamagnetic iron oxide nanoparticles that are retained by macrophage phagocytosis. These approaches have been shown to represent the degree of plaque inflammatory burden but are not necessarily targeted to any specific disease-related molecular process and it is unknown whether they yield incremental value to current approaches to reduce events.
Molecular imaging of early atherosclerotic disease must rely on the ability to detect processes that are specific to atherosclerosis and are active in the initiation and early rapid progression of disease (Figure 2). Vascular accumulation of oxidized lipids is a critical event in atherogenesis that stimulates the inflammatory response.8 Accordingly, there have been attempts to image this process through the radiolabeling of lipoproteins.9 A much more direct approach has been to create radionuclide probes and MRI nanomicellar contrast agents that are specifically targeted oxidized lipid and lipoprotein epitopes.10-12 This imaging approach has been potentiated by the interest in oxidization-specific ligands for therapy which has, in turn, increased availability of targeting molecules that can be conjugated to contrast agents. Currently, most experience with molecular imaging of oxidation epitopes have been performed in animal models with more advanced atherosclerotic disease where such as with malondialdehyde-targeted 125I-labeled antibodies reflects the underlying inflammatory burden.10 Endothelial cell activation is one of the important inflammatory sequela of oxidative stress and other pro-atherogenic stressors. The expression of endothelial cell adhesion molecules (ECAMs), such as selectins, ICAM-1, and VCAM-1 at plaque-vulnerable regions, results in the capture and adhesion of monocytes and other leukocytes.13,14 Molecular imaging with optical, MRI nanoparticle, and ultrasound contrast agents targeted to ECAMs has been shown to provide an assessment of the degree of underlying intramural inflammatory process.15-17 Recently, contrast-enhanced ultrasound (CEU) molecular imaging of ECAMs has been shown to detect the very earliest stages of disease before fatty streak development when monocytes are just beginning to accumulate in the newly forming neointima (Figure 3).18
Images of the aorta from a murine model of age-dependent atherosclerosis at (A) 10 weeks and (B) 40 weeks of age demonstrating time-dependent increase in plaque by oil red-O (ORO) staining of the thoracic aorta; increased macrophage (Mac-1) accumulation in the neointima, and increased distribution of CEU molecular imaging signal for VCAM-1. (C) Quantitative data for VCAM-1 and P-selectin CEU signal demonstrating the ability to detect very early ECAM expression at disease initiation. Reproduced from Kaufmann et al18
Imaging of CAC with CT can play an important role in detecting established disease that is in progress and may be headed for high risk phenotype (mid-stage disease). By imaging molecular or cellular characteristics plaque, it may be possible to better understand the nature of the plaque with regards to likelihood for future rapid growth or development more high risk features. A more targeted approach has been to use 18F-fluoride PET to detect the active process of plaque calcification.19 Again, imaging oxidatively modified lipids or lipoproteins is a promising approach for defining disease that is progressing in an adverse manner.10,11 Another approach is apply molecular imaging with diffusible probes targeted to monocyte-macrophage markers (e.g. CD68, CD11b) that can provide a readout of inflammatory cell content, or to oxidized LDL receptors or scavenger receptors found on macrophages and endothelial cells such as LOX-1, CD36, and SRA.20-24 Some of these markers, such as LOX-1, have been found in animal models to differentiate between fibrous from inflammatory plaques,21,22 Since monocytes and monocyte-derived cells within the vascular wall can be “polyfunctional,” further differentiating their activity or subtype may prove to be an advantageous approach, although this has not yet been shown.25
In atherosclerosis molecular imaging, there has been a great deal of interest in probes that are able to detect very high risk for acute atherothrombotic complications, so-called “vulnerable plaque” or “vulnerable patient” imaging strategies. Although the exact role of this imaging strategy is yet to be established, recent data showing that the presence of thin cap fibroatheroma can prospectively provide insight regarding likelihood for adverse coronary events suggests that information on plaque phenotype could potentially be used to help guide use of therapies that are being developed to alter biology.26 There are many different strategies that have been investigated for this purpose (Figure 2). Detection of high inflammatory activity with the some of the previously mentioned approaches (ECAM or macrophage imaging) has been shown to detect high risk plaque phenotype.15,16,27 Since proteases derived from activated macrophage and smooth muscle cells are an important contributor to plaque rupture and hemorrhage, there have been significant advances in the non-invasive detection of matrix metalloproteinases (MMPs, particularly gelatinases), cathepsin, myeloperoxidase, and other proteinases.28 These enzymes have been detected with radionuclide and MRI-targeted imaging probes, although optical imaging probes have also been developed which have the added benefit of activation-dependent signal where self-quenching of fluorophores (which causes signal “dimming”) is present for the intact probe but is reversed by proteinase-mediated cleavage.29,30 Because necrosis and apoptosis of various cells are associated with lipid-rich core, radionuclide imaging of high risk plaque with radiolabeled annexins has been shown to be feasible and tends to correlate with markers of plaque proteolytic activity.31 Molecular imaging of plaque thrombosis, or hemorrhage has also been developed for virtually every form of medical imaging. Potential molecular targets have included platelet receptors (GPIIb/IIIa, P-selectin, GP1bα), fibrin, tissue factor, and dysregulated von Willebrand factor.28,32 Results from some of these studies in animal models of disease have led to the notion that some of these targets can provide information on “prothrombotic environment” or microthrombosis rather than overt plaque rupture.33,34 There has been recent success in using fibrin-targeted gadolinium-based MRI contrast agents in humans. There are many other molecular imaging targets that have been proposed that can be found in comprehensive reviews on the molecular basis for atherosclerosis imaging. Although there is hope that these targeted agents will improve patient care, it should be noted that some of these approaches have recently been used to investigate efficacy and mechanisms of new therapies that alter plaque phenotype.35,36
Myocardial Ischemia
It is widely recognized that there are limitations in the sensitivity and positive predictive value of tests currently used to evaluate patients with suspected acute coronary syndromes (ACS). Rapid point-of-care imaging of molecular events that are upregulated by ischemia represents a possible solution. This approach could be used to detect not only active but recent and resolved ischemia, so-called “ischemic memory” imaging. In certain circumstances, ischemic memory imaging may be useful not only for detection, but also for evaluating the spatial extent of ischemia. Molecular imaging of cell death with anti-myosin antibodies, or targeted peptidomimetics (i.e. glucarate, duramycin) have been used successfully for detecting severe ischemic injury. However, these approaches may not be suitable for detecting ischemia without necrosis. A radionuclide imaging approach has been to use the fatty acid analog 123I-beta-methyl-iodophenylpentadecanoic acid (BMIPP) which detects the switch from fatty acid to glucose metabolism which lasts for hours after resolution of ischemia.37,38 Although in a multicenter study sensitivity and positive predictive value of BMIPP imaging for diagnosing ACS in patients with chest pain were only approximately 70% and 60%, they were still substantially superior to conventional evaluation.38 Ischemic memory imaging with echocardiographic molecular imaging has been proposed and may have distinct advantages in a population with suspected ACS because of speed, convenience, and ability to be performed at the bedside. The most common approach has been to use microbubbles targeted to the selectin class of ECAMs which are involved in the initial rolling of leukocytes along the endothelial surface and require only a mild ischemic stimulus for expression.39
Angiogenesis and Regeneration Therapy
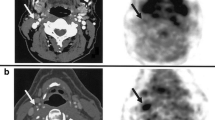
Remodeling of the vascular network is an important adaptive response in many forms of cardiovascular disease. Vascular remodeling can be broadly categorized as angiogenesis which defines the formation of new vessels that generally lack a tunica media, arteriogenesis which generally encompasses growth and network remodeling at the arteriolar or small artery level, and development and/or maturation of large collateral vessels. These processes represent beneficial adaptive responses to tissue ischemia that lower vascular resistance and improve the spatial distribution of perfusion and tissue oxygenation. Hence, imaging of hypoxia, perfusion, and metabolism are effective methods for evaluating the summative impact of all forms of vascular remodeling in ischemic disease. To visualize the underlying processes, molecular imaging probes have been targeted to angiogenesis-related endothelial and leukocyte integrins, growth factor receptors, matrix protease activity which is necessary for large vessel remodeling, receptors for pro-angiogenic cytokines, and molecules involved in the pro-angiogenic inflammatory response.40,41 Although molecular imaging of some of these markers has been applied in humans to detect vascular remodeling after myocardial infarction (Figure 4),42 the main role of molecular imaging thus far has been in the research setting where they can be used to better understand the determinants of successful endogenous and therapeutic angiogenesis.43-46
Images in the horizontal and vertical long-axis planes from a patient with recent anterior MI showing corresponding region of infarct by delayed-enhanced gadolinium MRI (A, D), regional hypoperfusion by 13N-ammonia PET (B, E), and increased PET signal from 18F-labeled RGD peptide targeted to αvβ3 integrin (C, F). Reproduced with permission from Makowski et al42
Not all angiogenesis in cardiovascular disease is thought to be beneficial. Expansion of the vasa vasorum and development of plaque neovessels have been causatively linked with unstable atherosclerotic disease by serving as a source for hemorrhage and a portal of entry for immune cells, lipoproteins, platelets, etc.47 Established non-invasive imaging techniques have been used to detect either enhanced permeability or perfusion through vasa vasorum and plaque neovessels. Molecular imaging techniques have been used as a biologic readout for this process and may provide a method to detect this unwanted process in the aorta or carotid or coronary vessels at an early stage.48
Molecular imaging is playing an increasing role in angiogenic and regenerative stem cell therapy. Figure 5 illustrates just some of the applications for molecular imaging in stem cell therapy. Labeling of stem cells has been possible with non-targeted contrast agents that can be detected by radionuclide, MRI, CT, ultrasound, and optical imaging; and can be used to optimize the route of delivery, and to assess biodistribution and survival.49 Disadvantages of stem cell contrast “loading” are that some probes are not stable over long periods of time or can produce signal even when the stem cell is no longer viable. Most importantly, tracers do not keep pace with cell replication and differentiation. Stable transfection of stem cells with reporter genes that encode for a protein that can be detected by a molecular imaging probe provides a more flexible platform for tracking stem cells over time.49 An example is the transfection of the herpes simplex thymidine kinase gene (HSV-tk) which can be detected by 18F-labeled thymidine analog PET tracers.50 With these types of tools, it becomes possible to fully evaluate cell retention and survival with respect to biologic effect. Increasingly, it is thought that some stem and progenitor cells exert much of their pro-angiogenic or myocellular effects through paracrine mechanisms mediated by growth factors, cytokines, chemokines, proteases, and genetic material within microvesicles. Targeted imaging has recently been used to evaluate these paracrine effects.51
Myocardial Remodeling
Myocardial remodeling is a complex pathophysiological process that occurs in response to pressure overload, volume overload, and ischemic injury and involves a complex cascade of molecular signaling and biological processes including inflammation, angiogenesis, apoptosis, necrosis, repair, and fibrosis. Both acute and chronic remodeling are thought to be critical components of development of heart failure symptoms in many disease processes.
Several key molecular signaling pathways in early and chronic post-ischemic remodeling have been evaluated by molecular imaging. One example is the detection of MMPs which are a family of zinc-containing enzymes that play a key role in post-infarct LV dilation and infarct expansion through matrix remodeling (Figure 6).52-54 Hybrid SPECT/CT imaging of MMP activation and perfusion have demonstrated MMP activation within the perfusion defect region, suggesting that MMP activation is taking place primarily within the sites of injury, but also remote areas indicative of global remodeling.54 Factor XIII is another key participant in post-infarct remodeling through its effects on matrix turnover and inflammation that has been targeted with radiolabeled imaging probes.55 In animal models of MI, activity of this probe has been shown to be lower in subjects predisposed to ventricular rupture by treatment with direct thrombin inhibitors.55
(A) Contrast CT (top row), 13N-NH3 PET perfusion (middle row), and molecular imaging of AT1R images using 11C-KR31173 PET in a pig following myocardial infarction. Relative activity of AT1R in the infarct zone is greater than relative perfusion and is also increased in the right ventricle. (B) Fused transaxial 11C-KR31173 PET/CT images in a normal subject through the mid-cardiac region demonstrating homogeneous myocardial uptake of AT1R at baseline (left) at baseline which is reduced after administration of an angiotensin receptor blocker (olmesartan)
Activation of the renin-angiotensin aldosterone system (RAAS) also contributes to adverse left ventricular remodeling and hypertension in ischemic and hypertensive disease, post-myocardial infarction, and heart failure. In particular, activation of angiotensin II type I receptors (AT1) is a final process of RAAS activation that leads to myocyte hypertrophy, collagen deposition, and myocyte apoptosis. Both angiotensin converting enzyme inhibitors and AT1 antagonists have been radiolabeled for targeted molecular imaging of RAAS post-infarction in animal models and in humans.56,57 Importantly, it appears that the different probes used to image RAAS activation tend to reflect different cellular targets of remodeling. Again, these studies suggest that molecular alterations associated with remodeling occur both within the infarct region and to a lesser extent in remote regions.
Dysregulation of collagen formation is prominent aspect of almost all forms of ventricular and atrial remodeling. It has been proposed that radionuclide molecular imaging signal for αvβ3 within an infarct zone reflects not only vascular remodeling (endothelial expression),58 but also myofibroblast activity involved in matrix remodeling.59 It is not unexpected that these processes may be related due to common regulatory pathways. The potential use of these agents in preclinical research is underscored by the finding that molecular imaging of integrins is reduced by agents that interfere with RAAS signaling.59 Molecular imaging probes targeted directly collagen for MRI imaging have also been shown to detect post-infarction remodeling.60
Sympathetic Imaging
The imaging of abnormal pre- or post-synaptic cardiac sympathetic function is another targeted molecular imaging approach that is being studied clinically with PET and SPECT tracers.61,62 In patients with heart failure, reduced 123I-meta-iodobenzylguanidine activity, reflecting reduced pre-synaptic uptake, has been shown to correlate prospectively with risk for adverse heart failure-related and arrhythmic events beyond other conventional markers such as LVEF and BNP.63 Similarly, PET imaging with 11C-meta-hydroxyephedrine, an analog of norepinephrine, is being evaluated for its ability to predict sudden cardiac death in patients with reduced LVEF who are candidates for implantable defibrillators.64 These neuroreceptor imaging approaches have the potential to aid in the selection of patients who would benefit the most from an ICD, leading to more cost-effective utilization.
Other Applications
There are a wide variety of other clinical and research applications for molecular imaging that there is insufficient space to review. Some of the most promising applications include detection of myocarditis or transplant rejection, detection of thrombus, diagnosis of active large vessel vasculitis, and prediction of susceptibility to atrial arrhythmias or unstable course for aortic aneurysms.65
Molecular Imaging Modalities: Matching Technique to Purpose
Contrast Ultrasound
Ultrasound contrast agents that have been developed for clinical use are composed of microbubbles that are several microns in diameter and contain a high molecular weight gas core and stabilized by encapsulation with albumin, lipid/surfactants, or biocompatible polymers. Signal generation from these agents is from either stable cavitation (vibration) or inertial cavitation (disruption with release free gas at high acoustic powers) of these particles within the ultrasound field. Targeting of microbubbles and other acoustically active nanoparticle agents has been achieved by either alteration of shell components or conjugation of ligands to the shell surface using a molecular spacer “arm.” The main disadvantage of this approach is that these agents are confined to the intravascular space. Accordingly, contrast ultrasound has been used primarily for molecular imaging of endothelial phenotype (ECAM expression, angiogenesis), thrombus, platelets, or leukocyte adhesion; but not for imaging myocyte phenotype, matrix changes, or intra-plaque composition. Moreover, as with any particulate contrast agent, the contrast agent and target molecule do not obey a 1:1 relationship but rather signal retention reflects the summed processes that culminate in the adhesion of a multivalent particle within the vascular space. Contrast ultrasound has advantages, however, in terms of practical issues such as cost, availability, portability, and speed (<10 minutes to acquire data). These are important considerations that make the technique attractive for use in screening large populations for a disease process or for rapid bedside detection of ischemia. Contrast ultrasound is characterized by a good balance between sensitivity and spatial resolution which, in general, are inversely related to each other in medical imaging. In the research setting, contrast ultrasound has been used because of the rapid nature of the imaging protocols and the ability to “null” signal from retained agent through inertial cavitation so that repetitive injections of different targeted agents are possible in a short period of time. Moreover, ultrasound imaging algorithms have been developed that allow for elimination of signal from any freely circulating agent. It should also be noted that ultrasound contrast agents have been investigated for various therapeutic purposes where acoustically driven microbubble cavitation can enhance the delivery of gene or drug payload, or accelerate clot lysis (sonothrombolysis). There is convincing evidence that targeting microbubble agents to the disease of interest can enhance these therapeutic applications probably through closer approximation of the microbubble vector to the vessel wall or thrombus.
Optical Imaging
In vivo optical imaging broadly describes the use of light (ultraviolet, visible, or infrared ranges) for imaging in intact organisms. In cardiovascular disease, intracoronary optical coherence tomography, Raman spectroscopy, and near infrared spectroscopy have been used to evaluate vascular cellular and lipid components. More specific imaging of molecular phenotype has been possible using fluorescent probes that are either retained in a disease tissue through ligand conjugation. A second strategy used to detect macrophage-derived protease activity is to detect augmented photon intensity that occurs when self-quenching of fluorophores is reduced by proteolytic cleavage of a compound fluorophore. Most commonly, near infrared fluorophores with excitation and emission wavelengths generally between 650 and 1,000 nm have been used in optical molecular imaging since there is less in vivo autofluorescence and less absorption from hemoglobin and other naturally occurring compounds in this range. Since these agents are highly diffusible, they can be used to target molecular targets including proteases, ECAMs, macrophages, and markers of angiogenesis. The major advantage of optical imaging is the ease of use and rapid imaging protocols. Although it is possible to perform multi-fluorophore imaging, it is also important to use fluorophores with little overlap in excitation/emission spectra and also to realize that quantitative comparisons are not possible because of different degrees of photon extinction from absorption. Fluorescence tomography is a recent advance that has allowed much better spatial localization of fluorescence signal. However, external imaging with fluorescence tomography is not entirely scalable. Accordingly, high sensitivity molecular imaging of cardiovascular disease in humans is still best achieved with intravascular near infrared detection catheters, some of which have also been hybridized with optical coherence and radionuclide detectors to allow multimodal molecular imaging and high resolution anatomic imaging.66
Radiotracer Imaging
Nuclear approaches including SPECT and PET are well suited for in vivo molecular imaging because of their high sensitivity and the availability of instrumentation and molecular probes. Although spatial resolution is the lowest with these techniques, ultrasensitivity to targeted probes allows detection of abnormal tissues that are much smaller than the stated resolution of SPECT or PET. Both SPECT and PET imaging have become standard approaches for quantitative physiological imaging in patients, and have been readily adapted for quantitative molecular imaging. In recent years dedicated hybrid imaging systems that combine two or more imaging modalities, and provide both anatomical and functional information, have been created. These systems were initially introduced for preclinical cardiovascular imaging, although now are routinely used in clinical practice. Commercial hybrid SPECT-CT and PET-CT systems take advantage of both the high sensitivity of radiotracer imaging (SPECT or PET) and the high spatial resolution of x-ray CT to localize radiotracer within anatomic structures. These hybrid systems have proved to be efficacious in preclinical small animal imaging, drug development studies, and now in clinical practice.40,67 The major limitation is that CT, SPECT, and PET imaging all employ ionizing radiation that can result in late adverse biological effects.
The first direct-targeted radiotracer-based molecular imaging approaches involved use of radiolabeled monoclonal antibodies for imaging of cell-specific surface antigens. The antibody approach evolved to employ engineered recombinant fragments with higher affinity toward target antigens, and more favorable clearance kinetics and biodistribution for in vivo imaging. More recently radiolabeled peptides have been applied since they are small, readily diffusible molecules that possess a high affinity to cell-specific receptors.
As previously mentioned in the cell therapy section, there are also indirect radiotracer-based molecular imaging approaches whereby gene expression is evaluated using cell-specific, drug-controlled expression systems. A reporter gene is transfected into a target tissue by various methods, including viral and non-viral vectors. The reporter gene product can be an enzyme that converts a reporter probe to the metabolite that is selectively trapped within transduced cells. This reporter gene product-probe interaction may involve binding of a radiolabeled ligand to cell surface receptors. The main advantage of this approach is the enzymatic amplification of the probe signal that facilitates imaging the magnitude and location of reporter gene expression. Moreover, this technique can provide information about the regulation of DNA, optimal timing, and dosage of vector delivery, as well as the efficiency of vector transfection into cells. These indirect approaches while extremely valuable for preclinical studies, particularly for tracking cell delivery, they are unlikely to translate into clinical practice because of regulatory issues, and concern for potential alteration of normal cellular function.
MR Imaging
Molecular imaging with MRI most commonly relies on the detection of targeted paramagnetic contrast agents composed of either gadolinium chelates that shorten T1, or superparamagnetic iron oxide particles that produce negative signal on T2*-weighted images.67,68 Although micron-range particles have been used, most constructs are several tens of nanometers in size so that they can be used to target both intravascular events as well as events that occur in tissues where there is enhanced permeability (e.g. atherosclerotic plaque, angiogenesis).68,69 An advantage of MR contrast agents is the superb spatial resolution and the ability to readily co-register anatomic and contrast data. A disadvantage is the relatively low sensitivity for detecting probe, generally requiring micromolar concentration for detection. It is this low sensitivity that has justified the nanoparticle construct which provides a large surface area for the incorporation of multiple binding ligands to improve targeting efficacy, and multiple paramagnetic chelates to amplify the signal enhancement.69,70 The major advantage of these complex nanoparticle MR imaging constructs is that the MR imaging probes can be integrated with other imaging beacons for multimodality imaging capability, or linked to therapeutic delivery. It should be noted, however, that rapid imaging protocols are somewhat hindered by the long circulation times of most nanoparticle MRI contrast agents.
Summary
Presently, molecular imaging for cardiovascular disease is beginning to emerge from being an exploratory technique and is now being applied in animal models to gain insight into pathophysiology and to assess response to new therapies. There is still hope that for certain clinical applications, molecular imaging will evolve and improve either diagnostic performance or patient outcomes. Although there are technical challenges for the development of agents for human use, the most important hurdle for clinical translation is the availability of data that demonstrate that molecular imaging provides useful information that is incremental in value to established diagnostic methods.
References
Kurt M, Shaikh KA, Peterson L, Kurrelmeyer KM, Shah G, Nagueh SF, et al. Impact of contrast echocardiography on evaluation of ventricular function and clinical management in a large prospective cohort. J Am Coll Cardiol 2009;53:802-10.
Halpern EJ, Savage MP, Fischman DL, Levin DC. Cost-effectiveness of coronary CT angiography in evaluation of patients without symptoms who have positive stress test results. AJR Am J Roentgenol 2010;194:1257-62.
Canto JG, Kiefe CI, Rogers WJ, Peterson ED, Frederick PD, French WJ, et al. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA 2011;306:2120-7.
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195-207.
Yousuf O, Mohanty BD, Martin SS, Joshi PH, Blaha MJ, Nasir K, et al. High-sensitivity C-reactive protein and cardiovascular disease: A resolute belief or an elusive link? J Am Coll Cardiol 2013;62:397-408.
Erbel R, Mohlenkamp S, Moebus S, Schmermund A, Lehmann N, Stang A, et al. Coronary risk stratification, discrimination, and reclassification Improvement based on quantification of subclinical coronary atherosclerosis: The Heinz Nixdorf recall study. J Am Coll Cardiol 2010;56:1397-406.
Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012;308:788-95.
Lee S, Birukov KG, Romanoski CE, Springstead JR, Lusis AJ, Berliner JA. Role of phospholipid oxidation products in atherosclerosis. Circ Res 2012;111:778-99.
Lees AM, Lees RS, Schoen FJ, Isaacsohn JL, Fischman AJ, McKusick KA, et al. Imaging human atherosclerosis with 99mTc-labeled low density lipoproteins. Arteriosclerosis 1988;8:461-70.
Torzewski M, Shaw PX, Han KR, Shortal B, Lackner KJ, Witztum JL, et al. Reduced in vivo aortic uptake of radiolabeled oxidation-specific antibodies reflects changes in plaque composition consistent with plaque stabilization. Arterioscler Thromb Vasc Biol 2004;24:2307-12.
Briley-Saebo KC, Shaw PX, Mulder WJ, Choi SH, Vucic E, Aguinaldo JG, et al. Targeted molecular probes for imaging atherosclerotic lesions with magnetic resonance using antibodies that recognize oxidation-specific epitopes. Circulation 2008;117:3206-15.
Tsimikas S, Palinski W, Halpern SE, Yeung DW, Curtiss LK, Witztum JL. Radiolabeled MDA2, an oxidation-specific, monoclonal antibody, identifies native atherosclerotic lesions in vivo. J Nucl Cardiol 1999;6:41-53.
Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol 1998;18:842-51.
Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, et al. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res 1999;85:199-207.
Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, et al. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation 2006;114:1504-11.
Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation 2007;116:276-84.
McAteer MA, Schneider JE, Ali ZA, Warrick N, Bursill CA, von zur Muhlen C, et al. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler Thromb Vasc Biol 2008;28:77-83.
Kaufmann BA, Carr CL, Belcik JT, Xie A, Yue Q, Chadderdon S, et al. Molecular imaging of the initial inflammatory response in atherosclerosis: Implications for early detection of disease. Arterioscler Thromb Vasc Biol 2010;30:54-9.
Dweck MR, Chow MW, Joshi NV, Williams MC, Jones C, Fletcher AM, et al. Coronary arterial 18F-sodium fluoride uptake: A novel marker of plaque biology. J Am Coll Cardiol 2012;59:1539-48.
Mulder WJ, Strijkers GJ, Briley-Saboe KC, Frias JC, Aguinaldo JG, Vucic E, et al. Molecular imaging of macrophages in atherosclerotic plaques using bimodal PEG-micelles. Magn Reson Med 2007;58:1164-70.
Ishino S, Mukai T, Kuge Y, Kume N, Ogawa M, Takai N, et al. Targeting of lectinlike oxidized low-density lipoprotein receptor 1 (LOX-1) with 99mTc-labeled anti-LOX-1 antibody: Potential agent for imaging of vulnerable plaque. J Nucl Med 2008;49:1677-85.
Li D, Patel AR, Klibanov AL, Kramer CM, Ruiz M, Kang BY, et al. Molecular imaging of atherosclerotic plaques targeted to oxidized LDL receptor LOX-1 by SPECT/CT and magnetic resonance. Circ Cardiovasc Imaging 2010;3:464-72.
Lipinski MJ, Amirbekian V, Frias JC, Aguinaldo JG, Mani V, Briley-Saebo KC, et al. MRI to detect atherosclerosis with gadolinium-containing immunomicelles targeting the macrophage scavenger receptor. Magn Reson Med 2006;56:601-10.
Ayala-Lopez W, Xia W, Varghese B, Low PS. Imaging of atherosclerosis in apoliprotein e knockout mice: Targeting of a folate-conjugated radiopharmaceutical to activated macrophages. J Nucl Med 2010;51:768-74.
Swirski FK, Nahrendorf M. Imaging macrophage development and fate in atherosclerosis and myocardial infarction. Immunol Cell Biol 2013;91:297-303.
Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226-35.
Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ Res 2005;96:327-36.
Quillard T, Libby P. Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development. Circ Res 2012;111:231-44.
Chen J, Tung CH, Mahmood U, Ntziachristos V, Gyurko R, Fishman MC, et al. In vivo imaging of proteolytic activity in atherosclerosis. Circulation 2002;105:2766-71.
Deguchi JO, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, et al. Inflammation in atherosclerosis: Visualizing matrix metalloproteinase action in macrophages in vivo. Circulation 2006;114:55-62.
Haider N, Hartung D, Fujimoto S, Petrov A, Kolodgie FD, Virmani R, et al. Dual molecular imaging for targeting metalloproteinase activity and apoptosis in atherosclerosis: Molecular imaging facilitates understanding of pathogenesis. J Nucl Cardiol 2009;16:753-62.
Lindner JR. Molecular imaging of thrombus: Technology in evolution. Circulation 2012;125:3057-9.
Hamilton AJ, Huang SL, Warnick D, Rabbat M, Kane B, Nagaraj A, et al. Intravascular ultrasound molecular imaging of atheroma components in vivo. J Am Coll Cardiol 2004;43:453-60.
McCarty OJ, Conley RB, Shentu W, Tormoen GW, Zha D, Xie A, et al. Molecular imaging of activated von Willebrand factor to detect high-risk atherosclerotic phenotype. JACC Cardiovasc Imaging 2010;3:947-55.
Majmudar MD, Keliher EJ, Heidt T, Leuschner F, Truelove J, Sena BF, et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation 2013;127:2038-46.
Liu Y, Davidson BP, Yue Q, Belcik T, Xie A, Inaba Y, et al. Molecular imaging of inflammation and platelet adhesion in advanced atherosclerosis effects of antioxidant therapy with NADPH oxidase inhibition. Circ Cardiovasc Imaging 2013;6:74-82.
Dilsizian V. Metabolic imaging for identifying antecedent myocardial ischemia and acute coronary syndrome in the emergency department. Curr Cardiol Rep 2011;13:96-9.
Kontos MC, Dilsizian V, Weiland F, DePuey G, Mahmarian JJ, Iskandrian AE, et al. Iodofiltic acid I 123 (BMIPP) fatty acid imaging improves initial diagnosis in emergency department patients with suspected acute coronary syndromes: A multicenter trial. J Am Coll Cardiol 2010;56:290-9.
Davidson BP, Kaufmann BA, Belcik JT, Xie A, Qi Y, Lindner JR. Detection of antecedent myocardial ischemia with multiselectin molecular imaging. J Am Coll Cardiol 2012;60:1690-7.
Nahrendorf M, Sosnovik DE, French BA, Swirski FK, Bengel F, Sadeghi MM, et al. Multimodality cardiovascular molecular imaging, part II. Circ Cardiovasc Imaging 2009;2:56-70.
Sadeghi MM, Krassilnikova S, Zhang J, Gharaei AA, Fassaei HR, Esmailzadeh L, et al. Detection of injury-induced vascular remodeling by targeting activated αvβ3 integrin in vivo. Circulation 2004;110:84-90.
Makowski MR, Ebersberger U, Nekolla S, Schwaiger M. In vivo molecular imaging of angiogenesis, targeting αvβ3 integrin expression, in a patient after acute myocardial infarction. Eur Heart J 2008;29:2201.
Winter PM, Caruthers SD, Allen JS, Cai K, Williams TA, Lanza GM, et al. Molecular imaging of angiogenic therapy in peripheral vascular disease with αvβ3-integrin-targeted nanoparticles. Magn Reson Med 2010;64:369-76.
Carr CL, Qi Y, Davidson B, Chadderdon S, Jayaweera AR, Belcik JT, et al. Dysregulated selectin expression and monocyte recruitment during ischemia-related vascular remodeling in diabetes mellitus. Arterioscler Thromb Vasc Biol 2011;31:2526-33.
Willmann JK, Chen K, Wang H, Paulmurugan R, Rollins M, Cai W, et al. Monitoring of the biological response to murine Hindlimb ischemia with 64Cu-labeled vascular endothelial growth factor-121 positron emission tomography. Circulation 2008;117:915-22.
Dobrucki LW, Tsutsumi Y, Kalinowski L, Dean J, Gavin M, Sen S, et al. Analysis of angiogenesis induced by local IGF-1 expression after myocardial infarction using microSPECT-CT imaging. J Mol Cell Cardiol 2010;48:1071-9.
Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, et al. Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 2005;25:2054-61.
Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with αvβ3-integrin-targeted nanoparticles. Circulation 2003;108:2270-4.
Chen IY, Wu JC. Molecular imaging: The key to advancing cardiac stem cell therapy. Trends Cardiovasc Med 2013;23:201-10.
Cao F, Lin S, Xie X, Ray P, Patel M, Zhang X, et al. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation 2006;113:1005-14.
Ryu JC, Davidson BP, Xie A, Qi Y, Zha D, Belcik JT, et al. Molecular imaging of the paracrine proangiogenic effects of progenitor cell therapy in limb ischemia. Circulation 2013;127:710-9.
Spinale FG. Matrix metalloproteinases: Regulation and dysregulation in the failing heart. Circ Res 2002;90:520-30.
Su H, Spinale FG, Dobrucki LW, Song J, Hua J, Sweterlitsch S, et al. Noninvasive targeted imaging of matrix metalloproteinase activation in a murine model of postinfarction remodeling. Circulation 2005;112:3157-67.
Sahul ZH, Mukherjee R, Song J, McAteer J, Stroud RE, Dione DP, et al. Targeted imaging of the spatial and temporal variation of matrix metalloproteinase activity in a porcine model of postinfarct remodeling: Relationship to myocardial dysfunction. Circ Cardiovasc Imaging 2011;4:381-91.
Nahrendorf M, Aikawa E, Figueiredo JL, Stangenberg L, van den Borne SW, Blankesteijn WM, et al. Transglutaminase activity in acute infarcts predicts healing outcome and left ventricular remodelling: Implications for FXIII therapy and antithrombin use in myocardial infarction. Eur Heart J 2008;29:445-54.
Shirani J, Dilsizian V. Imaging left ventricular remodeling: Targeting the neurohumoral axis. Nat Clin Pract Cardiovasc Med 2008;5(Suppl 2):S57-62.
Fukushima K, Bravo PE, Higuchi T, Schuleri KH, Lin X, Abraham MR, et al. Molecular hybrid positron emission tomography/computed tomography imaging of cardiac angiotensin II type 1 receptors. J Am Coll Cardiol 2012;60:2527-34.
Meoli DF, Sadeghi MM, Krassilnikova S, Bourke BN, Giordano FJ, Dione DP, et al. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J Clin Invest 2004;113:1684-91.
van den Borne SW, Isobe S, Verjans JW, Petrov A, Lovhaug D, Li P, et al. Molecular imaging of interstitial alterations in remodeling myocardium after myocardial infarction. J Am Coll Cardiol 2008;52:2017-28.
Helm PA, Caravan P, French BA, Jacques V, Shen L, Xu Y, et al. Postinfarction myocardial scarring in mice: Molecular mr imaging with use of a collagen-targeting contrast agent. Radiology 2008;247:788-96.
Carrio I, Cowie MR, Yamazaki J, Udelson J, Camici PG. Cardiac sympathetic imaging with mIBG in heart failure. JACC Cardiovasc Imaging 2010;3:92-100.
Link JM, Caldwell JH. Diagnostic and prognostic imaging of the cardiac sympathetic nervous system. Nat Clin Pract Cardiovasc Med 2008;5(Suppl 2):S79-86.
Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol 2010;55:2212-21.
Fallavollita JA, Luisi AJ Jr, Michalek SM, Valverde VM, deKemp RA, Haka MS, et al. Prediction of arrhythmic events with positron emission tomography: PAREPET study design and methods. Contemp Clin Trials 2006;27:374-88.
Pugliese F, Gaemperli O, Kinderlerer AR, Lamare F, Shalhoub J, Davies AH, et al. Imaging of vascular inflammation with [11C]-PK11195 and positron emission tomography/computed tomography angiography. J Am Coll Cardiol 2010;56:653-61.
Yoo H, Kim JW, Shishkov M, Namati E, Morse T, Shubochkin R, et al. Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo. Nat Med 2011;17:1680-4.
Sinusas AJ, Bengel F, Nahrendorf M, Epstein FH, Wu JC, Villanueva FS, et al. Multimodality cardiovascular molecular imaging, part I. Circ Cardiovasc Imaging 2008;1:244-56.
Sosnovik DE. Molecular imaging in cardiovascular magnetic resonance imaging: Current perspective and future potential. Top Magn Reson Imaging 2008;19:59-68.
Choudhury RP, Fisher EA. Molecular imaging in atherosclerosis, thrombosis, and vascular inflammation. Arterioscler Thromb Vasc Biol 2009;29:983-91.
Winter PM, Caruthers SD, Lanza GM, Wickline SA. Quantitative cardiovascular magnetic resonance for molecular imaging. J Cardiovasc Magn Reson 2010;12:62.
Acknowledgments
Dr Lindner is supported by Grants R01-HL078610 and RC01-100659 from the National Institutes of Health. Dr Sinusas is supported by Grants R01-HL113352 and P01-HL107205 from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lindner, J.R., Sinusas, A. Molecular imaging in cardiovascular disease: Which methods, which diseases?. J. Nucl. Cardiol. 20, 990–1001 (2013). https://doi.org/10.1007/s12350-013-9785-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-013-9785-0