Abstract
Purpose of the Review
In this review, we will discuss the basic fundamentals of how to perform molecular imaging to better understand the underlying mechanisms contributing to cardiovascular disease.
Report Findings
Molecular imaging combines molecular biology with in vivo imaging. Molecular probes are used to target discrete biological processes such as cell death, inflammation, and angiogenesis. These probes emit signals that are detected by traditional imaging systems. Because the same disease processes can manifest in individuals in different ways, molecular imaging may emerge as an important strategy for delivering precision medicine.
Summary
Molecular imaging is a powerful tool that may help physicians provide more personalized care in the near future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Molecular imaging combines molecular biology with in vivo imaging, enabling clinicians and researchers to visualize cellular processes in living subjects without disturbing them. The ability to image minute changes in molecular processes may lay the foundation for the development of novel strategies to diagnose and treat patients with cardiovascular disease earlier and more effectively. Molecular imaging may address the gaps in patient care left by our reliance on traditional imaging and allow for the development of a more personalized approach to disease management. In this review, we will discuss the basic fundamentals and current strategies for molecular imaging of cardiovascular pathology.
Basic Premise
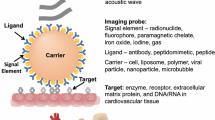
Molecular imaging system requires the following three basic components, as discussed in detail below: (1) a biological target, (2) an imaging probe, and (3) an imaging system. As shown in Fig. 1, a probe designed to target the biological process of interest is administered to the subject. The probe emits a signal, which is detectable by the imaging modality (i.e., single photon emission tomography, positron emission tomography, ultrasound and/or magnetic resonance imaging). The key factors for successful imaging include the probe specificity to discriminate the targeted biological pathway, the probe’s distribution within the tissue, its duration of retention, as well as the sensitivity of the imaging system to detect the signal generated by the probe.
Schematic of the fundamental components of molecular imaging systems. Molecular probes targeting specific cardiovascular disease processes (e.g., cell death, inflammation, fibrosis, and angiogenesis) are conjugated to probes that emit signals that are detected by probe-specific imaging systems (e.g., PET, U/S, and CT)
Molecular Imaging Probes
A molecular imaging probe is an imaging tool that is used to qualitatively evaluate and quantitatively measure biological processes. It is comprised of a signal agent, a targeting moiety, and a linker that couples the target moiety with the signal agent. Signal agents range from bioluminescent or fluorescent molecules for optical imaging, radionuclides for positron emission tomography (PET) and single photon emission computed tomography (SPECT), microbubbles for ultrasound imaging, and magnetic particles for magnetic resonance imaging (MRI). Target moieties can be small molecules, peptides, proteins, antibodies, and nanoparticles, all of which interact with a target (e.g., enzyme, receptor, DNA/RNA, physiological state) in a specific biological process. The linker agent, which joins the target moiety with the signal agent, may also modify the pharmacokinetics of the imaging agent, affecting its biodistribution and tissue retention. The ideal imaging probe has the following properties, as discussed in detail in other comprehensive reviews [1•, 2]: (1) high target affinity, (2) high target specificity, (3) high sensitivity, (4) high contrast ratio, (5) high stability in vivo, (6) low immunogenicity and minimal toxicity, and (7) easy and economical production. Given these prerequisites, it is not surprising that the number of validated probes remains limited. According to the Molecular Imaging and Contrast Agents Database (MICAD), which promotes the development and application of imaging probes to advance the field of molecular imaging [3], there are approximately 1444 agents for both cardiac and noncardiac applications that are currently available (January 2013). Of these, 241 are FDA approved. Based on the modality used for imaging, the largest category of probes described in MICAD are those used for positron emission tomography (41.6%), followed by agents used for single-photon emission computed tomography (30.3%), optical imaging (12.0%), magnetic resonance imaging (9.3%), multimodality imaging (3.4%), ultrasound (2.4%), and X-ray/computed tomography (1.0%). Details of recent advancements in probe development are highlighted below.
Radionuclide Agents
Although most radiolabeled compounds are dedicated to cancer imaging, a few radionuclide probes have been used to image cardiovascular specific markers. In general, PET tracers have advantages over SPECT tracers because some positron emission isotopes (e.g., C, N and O) are principal components of biological molecules and can be used without significantly changing the chemical properties of target moieties. Although the most commonly used radionuclide fluorine 18 (18F) is not a constituent of biomolecules, it is a bioisostere of the biomolecule hydrogen. Fludeoxyglucose, the most frequently used PET probe, has a fluorine-18 substituted for the normal hydroxyl group at the C-2 position in the glucose molecule, and is taken up by metabolically active cells, enabling it to serve as a nonspecific marker for imaging inflammation in the myocardium [4] and atherosclerotic plaques [5•]. More specific agents have also been investigated including 18F-fluoromisonidazole (18F-FMISO), a radiotracer specific for hypoxia, which was been shown to correlate not only with the presence of atherosclerotic plaque, but also its progression [5•]. In addition, fluorinated F18 has been conjugated to annexin and integrin ανβ3 to target cell death and angiogenesis, respectively. Zhu et al. [6] recently demonstrated the feasibility of imaging apoptosis in a rat model of ischemia-reperfusion injury, showing that the area of reperfusion injury had a 7-fold higher standard uptake value than the viable myocardium. Importantly, the imaging signal correlated with histological markers of apoptosis. Finally, Gao et al. [7] evaluated a novel one step labeling ανβ3 integrin probe targeting angiogenesis in a rat model of ischemia reperfusion injury. The tracer 18F-AlF-NOTA-PRGD2 allowed longitudinal noninvasive visualization of angiogenesis induced by ischemia-reperfusion injury, which was consistent with immunohistochemistry analysis of the distribution of CD31 and CD61 (e.g., markers of vascular density and β3 expression, respectively).
Optical Imaging Agents
Promising optical imaging probes include organic fluorophores, nanoparticle-based contrast agents, and semiconductor crystals. Because of their low molecular weight, high quantum yield, low cost, and well-validated conjugation strategies, organic fluorophores are an ideal optical imaging label. Dyes in the near fluorescent range are of greatest interest owing to their depth of penetration. Using a NIR dye conjugated to an antibody targeting oxidized LDL, for instance, Khamis et al. [8] successfully visualized the atherosclerotic plaques of mice lacking the LDL receptor. In vivo application of these fluorophores, however, is limited by their shallow depth of penetration and the difficulty achieving high target to background ratios [8]. One approach to reduce background noise is to use a smart fluorescent probe, which incorporates a protease that is subsequently cleaved. In the absence of the protease, these probes do not fluoresce due to self-quenching. Cleavage of the peptide linker, in contrast, results in fluorescence. Using this approach, Abd-Ehrahman et al. [9] demonstrated that macrophages in murine atherosclerotic plaques had elevated levels of cathepsin protease activity. The administration of a cathespin inhibitor induced macrophage apoptosis, suggesting that cathespin targeting is a promising approach for the treatment of plaque inflammation. An alternative strategy is to use metallic nanoparticles composed of gold or silver that efficiently scatter light. Gold nanoparticles, for example, have been used to label macrophages injected into an apoliprotein E-deficient mouse to track their recruitment using computed tomography (CT) [10]. Gold nanoparticles have also been conjugated to annexin and Techneitum 99M to target apoptotic macrophages in the vulnerable plaque [11]. A final strategy is to use quantum dots, which are semiconductor nanocrystals with diameters smaller than the Bohr excitation diameter (2–10 nm). Advantages over organic fluorophores include improved brightness due to a high extinction coefficient, enhanced photostability, and increased flexibility to label multiple biomarkers. Recently, Chen et al. [12] developed functionalized quantum dots with VCAM-1 binding peptides to selectively target VCAM-1 expressing endothelial cells to image inflammation as a marker of early atherosclerosis. Although results are preliminary, they demonstrate the feasibility of quantum dots to detect early signs of atherosclerosis.
Ultrasound Imaging Agents
Ultrasound molecular imaging is achieved by using microbubbles containing targeting ligands on the bubble shell. These microbubbles remain intravascular, aggregate in regions expressing the disease biomarker, and appear as bright areas of signal that localize the target. One of the primary advantages of microbubble imaging is that it allows real-time in vivo imaging of evolving disease processes. By conjugating specific anti-GP IIb/IIIa single-chain antibodies to microbubbles, for example, Wang et al. [13] visualized thrombi and its subsequent resolution after the administration of thrombolytics in the diseased carotid arteries of mice. Importantly, microbubbles can also be conjugated to multiple ligands to evaluate different components of the same disease process. To image both platelet adhesion and vascular inflammation in a mouse model of atherosclerosis, for example, Liu et al. [14] used microbubbles that targeted platelet glycoprotein Ib alpha (GPIbα), P selectin, and VCAM-1, respectively. More recently, studies have investigated the feasibility of using ultrasound-targeted microbubble destruction (UTMD) to enhance the transfection efficiency of naked plasmid DNA for delivering cardiovascular gene or drug therapy [15]. Chen et al. [16] used UTMD to deliver the thymosin beta 4 (TB4) gene into the normal rat heart to enhance the differentiation of resident adult cardioprogenitor cells. After the microbubbles reached the target organ, the payload attached to the microbubbles was destroyed by ultrasound. Although results are promising, the duration of exposure, frequency, mode, mechanical index, and payload amounts need further optimization.
Magnetic Resonance Imaging (MRI) Contrast Agents
MRI contrast agents modify the magnetic properties of T1, T2, and susceptibility. The positive contrast agents are typically gadolinium- or magnesium-containing paramagnetic chelates, which predominantly shorten the longitudinal relaxation time (T1) and increase the relaxation rate of water (e.g., relaxivity). Tissues that take up this contrast agent become bright or appear hyperintense on T1-weighted MRI. Negative contrast agents, otherwise known as superparamagnetic or susceptibility contrast agents, are small iron oxide particles that generate local magnetic field gradients that disrupt the primary magnetic field over the tissue, causing a T2* shortening or susceptibility effect and resulting in hypointense signals on T2-, T2*-, and T1-weighted MR images.
The principal challenge of molecular MRI is its relatively low sensitivity (micromolar range) compared to radiotracer and fluorescence techniques (picomolar range). To compensate for this, most approaches using MRI have either selected to image highly expressed molecular targets that are very abundant (e.g., fibrin or thrombi) or delivered contrast agents packaged in liposomes or micelles (e.g., gadolinium chelate) or aggregated as nanoparticles (e.g., iron oxide). Dellinger et al. [17], for example, used liposomes containing gadolinium encased in a carbon shells to increase longitudinal relaxivity 25-fold. Liposomes were coupled with a ligand that binds the surface of macrophage foam cells on atherosclerotic plaques to improve target specificity. Time-dependent accumulation of gadolinium was found in aortas of ApoE−/− mice, which was confirmed by histology and not observed in controls. No toxicity was reported. Similarly, Chen et al. [18] labeled high-density lipoprotein nanoparticles bound to a collagen-specific peptide with gadolinium chelates to target intraplaque macrophages during plaque regression in a mouse model of atherosclerosis. While MRI probes continue to improve, they still lack the sensitivity required to image discrete molecular processes and remain inferior to optical and radionuclide probes.
Multimodal Imaging Agents
First introduced a decade ago, multimodality imaging agents consist of a target moiety that is conjugated to two signal agents, enabling simultaneous or sequential imaging of biological processes using two complementary imaging systems. Radionuclide and MRI probes joined to optical imaging agents are most frequently used for multimodality imaging. Withana et al. [19], for example, used a dual modality optical and PET/CT probe to noninvasively image activated macrophages in a murine stroke model as well as ex vivo samples of diseased human carotid arteries (Fig. 2). Multimodality imaging has also been used to monitor stem cell fate after transplantation. Transplanted cells transfected with triple fusion reporter genes that incorporate optical (e.g., firefly luciferase for bioluminescence imaging) and radionuclide probes (herpes simplex virus type 1 thymidine kinase, HSV1-tk, for PET imaging) in addition to green fluorescent protein for histological confirmation have been used to evaluate cellular proliferation, migration, and death after injection into small and large animals [20]. By incorporating multiple modality imaging probes, studies can harness the strengths and address the weaknesses of each molecular imaging approach.
Dual modality optical and PET/CT probe, 64Cu-BMV101, for imaging activated macrophages in a murine stroke model. a In situ fluorescence imaging 64Cu-BMV101 in mice with and without ligated carotid arteries. b Coronal and sagittal PET/CT images of the 64Cu-BMV101 in mice with and without ligated carotid arteries. This figure was originally published in JNM. Withana et al. Dual-activity vascular inflammation imaging. J Nucl Med. 2016;57:1583–1590. © by the Society of Nuclear Medicine and Molecular Imaging, Inc.
Imaging Modalities
Like traditional imaging, molecular imaging uses standard equipment to visualize the interaction of the probe and target including nuclear, optical, ultrasound, and magnetic resonance imaging systems. Recent advances in technology have improved the application of these imaging systems for molecular imaging. For additional information on instrumentation, we refer the reader to other comprehensive reviews [21].
The two most common imaging systems to detect nuclear-based probes are SPECT and PET. These imaging systems have superb sensitivity (nanomolar to picomolar range), excellent depth of penetration (unrestricted) and field of view (centimeters to submeter scales), good temporal resolution (seconds to minutes), short examination times (seconds to minute), and a wide selection of probes for cardiovascular disease processes. Importantly, the same probes can be used in humans as well as small animals although modified scanners are required for small animal imaging. The main disadvantage of nuclear imaging, however, is its low spatial resolution, resulting in difficulty identifying the anatomical source of emission. SPECT cameras actually offer the poorest spatial resolution of current imaging techniques. In recent years, the addition of an increasing number of detectors and modified collimator designs have improved sensitivity 4–8-fold, paving the way for clinical use in cardiovascular molecular imaging.
In comparison to nuclear imaging, optical imaging offers similar sensitivity with greater spatial resolution. Despite lower costs and strides in the past 20 years, techniques such as bioluminescence and fluorescence continue to be limited by poor depth of signal penetration (few millimeters to centimeters) due to scatter and attenuation. These factors limit optical imaging to the whole body and intravascular imaging of small animals and humans, respectively. Although the development of 3D optical imaging with diffuse optical tomography and fluorescence molecular tomography offers improved depth sensitivity than planar imaging, imaging depth continues to be limited by photon scatter. Optoacoustic imaging ameliorates this situation by utilizing ultrasound transducers to detect the signal released by the thermal expansion of fluorophores [22]. Because biological tissues are more transparent to sound than light, the depth of imaging is, thus, expanded.
The advantages of ultrasound imaging systems over other imaging modalities include 1) its low cost, portability, and real-time capabilities, 2) improved imaging depth relative to optical imaging, and 3) high temporal resolution. It also possesses the unique ability to remove contrast agents, manipulate contrast distribution with radial force, and combine therapy with imaging. Challenges, however, remain including its limited sensitivity (due to poor contrast retention at the target site and high background signal), relatively small field of view, and inability to accurately quantify signal. One approach to address these challenges is to minimize microbubble destruction by using low amplitudes and higher frequencies. To detect these microbubble echoes that have a low mechanical index, pulse inversion imaging and contrast pulse sequencing can be used. These strategies, however, may add to scan time and reduces frame rates, limiting the clinical translation of ultrasound for molecular imaging.
Unlike ultrasound, MRI provides exquisite high-resolution anatomical information with excellent soft tissue contrast; however, it also suffers from low detection sensitivity, requiring 10 μM to 10 mM of contrast agent for visualization. To compensate for its low sensitivity, MRI can be combined with nuclear and optical imaging to improve target localization and detection of less ubiquitous targets. Recent development of superconducting magnet technology that does not require cryogenic fluid for cooling has also enabled less costly installation and improved accessibility to molecular magnetic resonance molecular imaging.
Biological Processes
Cell Death
The major modes of cell death in the heart include oncosis (ischemic cell death), apoptosis (programmed cell death 1), and autophagic cell death (programmed cell death 2), which have all been detected using electron microscopy, molecular techniques, and immunohistochemical approaches. While readers may be more familiar with the first two processes, autophagy has emerged as an important contributor of myocyte death. Autophagic death is mediated by excessive intracellular lysosome catabolic metabolism that is normally responsible for the degradation and recycling of damaged or dysfunctional cytoplasmic components and intracellular organelles [23, 24]. In essence, autophagy results from the cell “canabilizing” itself from the inside.
A number of strategies have been proposed to image cell death. By far, the most experience over the past two decades has been the use of annexin as the target moiety. Annexin binds to phosphatydylserine residues expressed on the outer leaflet of the plasma membranes of dying cells. Annexin V has been coupled with radionuclides for nuclear imaging, magnetic nanoparticles, and gadolinium-containing liposomes for MRI, and fluorescent markers for optical imaging [25]. A major limitation is the large size of annexin derivatives, which may impact its long-term storage, increase its immunogenicity, and limit its access to target tissue. Furthermore, annexin cannot distinguish between oncosis and apoptosis because both processes can exhibit externalization of phosphatidylserine residues while still maintaining cell membrane integrity [26].
Over the last 5 years, studies have evaluated the feasibility of using intracellular rather than extracellular apoptosis markers such as the expression of caspases, which play an important role in the initiation and execution of cell death, and the loss of membrane potential across the inner mitochondrial membrane (Δψm), which accompanies the induction of cell death. The most common strategy is to image caspase activation using optical imaging approaches (e.g., fluorescence or bioluminescence imaging). To detect apoptosis associated with doxurubicin toxicity, for example, Su et al. [27] synthesized an 18F-labeled tetrapeptidic caspase substrate, 18F-CP18, which contains the caspase 3-specific recognition sequence DEVD. DEVD is cleaved intracellularly in the presence of activated caspase 3, resulting in the accumulation of radioactive DEVD in the cytoplasm of apoptotic cells. A 2-fold increase in uptake of CP18 was observed in mice treated with doxorubicin compared to controls, which was verified by ex vivo measures of caspase 3-enzymatic activity and apoptosis. A promising alternative approach is using isatins, which are small molecules that selectively bind and covalently inhibit caspase 3 activation. Thukkani et al. [28], for example, successfully synthesized a radiolabeled istatin 5-sulfonamide ([18F]WC-4116) and demonstrated a 2-fold higher uptake of [18F]WC-4-116 in animals after ischemia reperfusion injury compared to control animals.
Apoptosis can also be quantified by measuring changes in Δψm. Changes in the Δψm can be assessed using fluorescent or radiolabeled lipophilic cations such as triphenylphosphonium (TPP), whose large surface area enables rapid, several hundred fold accumulation in cells [29]. Although TPP has been shown to accurately measure Δψm in vitro [30] and ex vivo [31], an initial study in a swine model of ischemia reperfusion injury using TPP radiolabeled with fluorine (18F-TPP) overestimated Δψm, suggesting that these measures are not yet ready for clinical application [32]. Improved accuracy may be achieved with the use of two TPP compounds simultaneously, which react together to form a stable product that accumulates in the cytosol, thereby, increasing sensitivity to small changes in membrane potential [33••].
While many strategies have been explored to image oncosis and apoptosis, imaging of autophagy has been restricted to reporter imaging models using transgenic mice that express microtubule-associated protein 1A/1B-light chain 3 (e.g., LC3, a marker protein for autophagosomes) and green fluorescent protein. Using an alternative approach of imaging a cathespin-activated fluorochrome, Chen et al. [34] demonstrated the feasibility of in vivo fluorescent tomography of the upregulation of autophagy after rapamycin therapy. Studies of autophagy in humans, however, have not yet been performed.
Inflammation
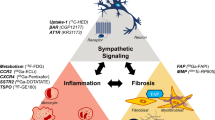
Inflammation is a major component of many types of cardiovascular disease including atherosclerosis, myocarditis, and remodeling. One of the major strategies for imaging inflammation at the molecular level is to target the migration of immune cells as they interact with noxious stimuli. A favorite target in cardiovascular disease is imaging monocyte/macrophage trafficking. Although initial approaches have been nuclear based, magnetic resonance molecular imaging alone or in combination with PET approaches has emerged as promising alternatives. Because of their phagocytic ability, monocytes and macrophages readily take up MRI contrast agents. Macrophage molecular MRI has been used to study atherosclerosis [35, 36, 37•], aortic aneurysm [38], infarction [39,40,41], and myocarditis [42]. In a recent study, Pedersen et al. [37•] used a novel PET ligand [(64)Cu] [1,4,7,10-tetraazacyclododecane-N,N′,N′,N′-tetraacetic acid]-d-Phe1,Tyr3-octreotate ((64)Cu-DOTATATE) to target macrophages, enabling PET-MRI of carotid plaques in ten patients prior to carotid endarterectomy. The study reported higher uptake of the tracer in symptomatic plaques compared to contralateral controls in the same patient, suggesting that the tracer can be used to identify vulnerable plaques.
The other main strategy is to imaging tissue-specific markers of inflammation such as endothelial cell adhesion molecules (e.g., selectins, ICAM-1, and VCAM-1). These proteins signal the presence of endothelial cell activation and attract inflammatory cells into vulnerable atherosclerotic plaques. One limitation of this approach is the low target to background ratios of available agents. In an effort to identify a more sensitive agent, Dimastromatteo et al. [43] tested several derivatives of VCAM-1-specific major histocompatibility complex 1-derived peptide B2702p. They found 2–3-fold increased activity in target tissues in a mouse model of stroke using (99m)Tc-B2702p1, suggesting that this may be a sensitive marker for detecting inflammation in the vulnerable plaque.
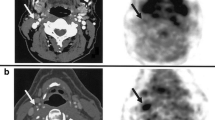
Because calcification is the final process of inflammation, calcium activity has also been imaged using SPECT or PET imaging with 99mTc-phosphonate or 18F-fluoride, respectively. In a promising prospective clinical trial, Joshi et al. [44••] demonstrated the highest uptake of 18F-NaF, which is adsorbed into calcified deposits with high affinity and selectivity [45], at the site of culprit lesion in 37/40 patients presenting with myocardial infarction and 18/40 patients with high-risk coronary arterial plaque (Fig. 3). Whether identifying inflammation within the vulnerable plaque can alter morbidity and mortality needs further exploration.
PET/MRI comparing focal 18F-NaF and 18F-FDG in a patient with an acute ST elevation myocardial infarction. a Invasive angiography showing proximal occlusion of the left anterior descending artery. b Focal uptake of 18F-NaF in the culprit vessel. c No uptake of 18F-FDG is observed in the culprit vessel. Adapted and reprinted with permission from Joshni et al., Lancet. 2014;383:705–13
Fibrosis
Fibrosis is a pathological process characterized by an increased collagen within the extracellular matrix and is a feature of almost all cardiovascular diseases. Following myocyte loss, fibrosis replaces dying myocytes (e.g., replacement fibrosis). Alternatively, stress or inflammation can result in a reactive fibrosis that could lead to ventricular stiffening, functional compromise, and heart failure.
Although traditionally, late gadolinium contrast MRI has been used to identify fibrosis, T1 mapping has emerged as a surrogate measure for fibrosis by evaluating changes in the extracellular matrix. This approach typically uses a modified Look-Locker inversion recovery (MOLLI), which obtains diastolic single-shot images with multiple different T1 sensitivities that are incorporated into a T1 recovery function to create a map. The T1 time has been shown to inversely correlate with histological fibrosis on endomyocardial biopsy in transplant patients [40] and cardiac events in patients with heart failure [46].
For molecular imaging of fibrosis, the most common targets for imaging fibrosis include collagen, myofibroblasts, intracardiac renin-angiotensin axis, matrix metalloproteinases, and matricellular proteins [47]. Collagen can be directly targeted using collagen avid peptide such as CNA35-based probes. Although CNA35 has been can successfully used to image collagen in animal models of abdominal aortic aneurysm [48] and atherosclerosis [49], in vivo cardiac imaging has not yet been demonstrated. Alternatively, the renin angiotensin system (RAS) and/or myofibroblasts can be targeted because cardiomyocyte stretch induces RAS, which promotes fibroblast differentiation into myofibroblasts that secrete collagen. The feasibility of imaging RAS was demonstrated in a study by Dilsizian et al. [50] who showed increased uptake of technetium-99m-labeled lisinopril (an angiotensin receptor blocker) in transgenic rats that overexpress angiotensin-converting enzyme compared to control animals. Because a discussion of all the potential targets for imaging fibrosis is beyond the scope of this review, we refer the reader to other comprehensive reviews for information on other molecular targets for imaging fibrosis [47]. Successful visualization of these targets involved in the development of myocardial fibrosis may help us better understand the mechanism regulating this pathological process.
Angiogenesis
Angiogenesis, the new growth of capillaries from preexisting vessels, is a complex process that is an integral part of atherosclerosis. Although vessels are generally post-mitotic in adults, endothelial cells have retained the ability to become mobile, invade, and sprout into tissue. Perhaps the most well-studied target for imaging angiogenesis is integrin αvβ3, which is a cell adhesion molecule that is low in normal tissue but is highly expressed in activated endothelial cells. Integrin αvβ3 contains a binding site for cyclic Arg-Gly-Asp (RGD) peptide, which has been radiolabeled with SPECT or PET tracers to image angiogenesis in several animal and human studies. In one recent clinical study in 21 patients 2 weeks post ST elevation myocardial infarction, Jenkins et al. [51] demonstrated increased uptake of a novel PET RGD-radiotracer, 18F-fluciclatide, in areas of new myocardial infarction compared to areas of old myocardial infarction and control patients. The tracer preferentially accumulated in areas of subendocardial injury with associated regional wall hypokinesis that subsequently recovered, suggesting that it can serve as a marker of cardiac repair. Integrin αvβ3 expression, however, is not restricted to endothelial cells and is found in macrophages [52]; thus, its specificity for angiogenesis has been questioned. Moreover, these integrins are not required for angiogenesis and can be compensated by the upregulation of VEGF-R2 expression [53]. A promising alternative is to image the expression of integrin α5β1, which is only found on endothelial cells. Comparing the specificity of integrin α5β1 and αvβ3 using 68Ga-based tracers for imaging tumor angiogenesis, Notni et al. [54] confirmed the reliability of targeting α5β1 as a marker of angiogenesis. Although studies in myocardial infarction models are still forthcoming, this study suggests that targeting integrin α5β1 may be a viable approach to image myocardial angiogenesis.
Future Directions
Although much has been achieved over the last decade in molecular imaging, it still remains a research tool that has not yet made its debut in clinical practice. One potential reason for its lack of routine use is that large-scale studies have yet to demonstrate a clear mortality and morbidity benefit in incorporating molecular imaging into the care of patients with cardiovascular disease. The push toward personalized medicine may present an opportunity to bring these valuable tools to the bedside. With its potential to complement the information obtained from genetic data, molecular imaging may help better characterize individual disease phenotypes and determine who may or may not benefit from therapy. In the next decade, molecular imaging may be incorporated in clinical trials of novel cardiovascular therapeutics to better understand individual variation in response treatment.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Kobayashi H, Choyke PL, Ogawa M. Monoclonal antibody-based optical molecular imaging probes; considerations and caveats in chemistry, biology and pharmacology. Curr Opin Chem Biol. 2016;33:32–8. This is an excellent review of molecular imaging probes.
Charron CL, Hickey JL, Nsiama TK, Cruickshank DR, Turnbull WL, Luyt LG. Molecular imaging probes derived from natural peptides. Nat Prod Rep. 2016;33:761–800.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK5330/
Lee WW, Marinelli B, van der Laan AM, Sena BF, Gorbatov R, Leuschner F, Dutta P, Iwamoto Y, Ueno T, Begieneman MP, Niessen HW, Piek JJ, Vinegoni C, Pittet MJ, Swirski FK, Tawakol A, Di Carli M, Weissleder R, Nahrendorf M. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol. 2012;59:153–63.
• Mateo J, Izquierdo-Garcia D, Badimon JJ, Fayad ZA, Fuster V. Noninvasive assessment of hypoxia in rabbit advanced atherosclerosis using (1)(8)F-fluoromisonidazole positron emission tomographic imaging. Circ Cardiovasc Imaging. 2014;7:312–20. This study introduces 18F-fluoromisonidazole as a novel tool for detecting hypoxia in atherosclerotic lesions.
Zhu J-C, Wang F, Fang W, Hua Z-C, Wang Z-Z. 18F-annexin V apoptosis imaging for detection of myocardium ischemia and reperfusion injury in a rat model. J Radioanal Nucl Chem. 2013;298:1733–8.
Gao H, Lang L, Guo N, Cao F, Quan Q, Hu S, Kiesewetter DO, Niu G, Chen X. PET imaging of angiogenesis after myocardial infarction/reperfusion using a one-step labeled integrin-targeted tracer 18F-AlF-NOTA-PRGD2. Eur J Nucl Med Mol Imaging. 2012;39:683–92.
Khamis RY, Woollard KJ, Hyde GD, Boyle JJ, Bicknell C, Chang SH, Malik TH, Hara T, Mauskapf A, Granger DW, Johnson JL, Ntziachristos V, Matthews PM, Jaffer FA, Haskard DO. Near infrared fluorescence (NIRF) molecular imaging of oxidized LDL with an autoantibody in experimental atherosclerosis. Sci Rep. 2016;6:21785.
Abd-Elrahman I, Kosuge H, Wises Sadan T, Ben-Nun Y, Meir K, Rubinstein C, Bogyo M, McConnell MV, Blum G. Cathepsin activity-based probes and inhibitor for preclinical atherosclerosis imaging and macrophage depletion. PLoS One. 2016;11:e0160522.
Chhour P, Naha PC, O'Neill SM, Litt HI, Reilly MP, Ferrari VA, Cormode DP. Labeling monocytes with gold nanoparticles to track their recruitment in atherosclerosis with computed tomography. Biomaterials. 2016;87:93–103.
Li X, Wang C, Tan H, Cheng L, Liu G, Yang Y, Zhao Y, Zhang Y, Li Y, Zhang C, Xiu Y, Cheng D, Shi H. Gold nanoparticles-based SPECT/CT imaging probe targeting for vulnerable atherosclerosis plaques. Biomaterials. 2016;108:71–80.
Chen Y, Molnar M, Li L, Friberg P, Gan LM, Brismar H, Fu Y. Characterization of VCAM-1-binding peptide-functionalized quantum dots for molecular imaging of inflamed endothelium. PLoS One. 2013;8:e83805.
Wang X, Hagemeyer CE, Hohmann JD, Leitner E, Armstrong PC, Jia F, Olschewski M, Needles A, Peter K, Ahrens I. Novel single-chain antibody-targeted microbubbles for molecular ultrasound imaging of thrombosis: validation of a unique noninvasive method for rapid and sensitive detection of thrombi and monitoring of success or failure of thrombolysis in mice. Circulation. 2012;125:3117–26.
Liu Y, Davidson BP, Yue Q, Belcik T, Xie A, Inaba Y, McCarty OJ, Tormoen GW, Zhao Y, Ruggeri ZM, Kaufmann BA, Lindner JR. Molecular imaging of inflammation and platelet adhesion in advanced atherosclerosis effects of antioxidant therapy with NADPH oxidase inhibition. Circ Cardiovasc Imaging. 2013;6:74–82.
Chen ZY, Lin Y, Yang F, Jiang L, Ge S. Gene therapy for cardiovascular disease mediated by ultrasound and microbubbles. Cardiovasc Ultrasound. 2013;11:11.
Chen S, Shimoda M, Chen J, Grayburn PA. Stimulation of adult resident cardiac progenitor cells by durable myocardial expression of thymosin beta 4 with ultrasound-targeted microbubble delivery. Gene Ther. 2013;20:225–33.
Dellinger A, Olson J, Link K, Vance S, Sandros MG, Yang J, Zhou Z, Kepley CL. Functionalization of gadolinium metallofullerenes for detecting atherosclerotic plaque lesions by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15:7.
Chen W, Cormode DP, Vengrenyuk Y, Herranz B, Feig JE, Klink A, Mulder WJ, Fisher EA, Fayad ZA. Collagen-specific peptide conjugated HDL nanoparticles as MRI contrast agent to evaluate compositional changes in atherosclerotic plaque regression. JACC Cardiovasc Imaging. 2013;6:373–84.
Withana NP, Saito T, Ma X, Garland M, Liu C, Kosuge H, Amsallem M, Verdoes M, Ofori LO, Fischbein M, Arakawa M, Cheng Z, McConnell MV, Bogyo M. Dual-modality activity-based probes as molecular imaging agents for vascular inflammation. J Nucl Med. 2016;57:1583–90.
Nguyen PK, Riegler J, Wu JC. Stem cell imaging: from bench to bedside. Cell Stem Cell. 2014;14:431–44.
Wells RG. Instrumentation in molecular imaging. J Nucl Cardiol. 2016;23:1343–7.
Wang LV, Yao J. A practical guide to photoacoustic tomography in the life sciences. Nat Methods. 2016;13:627–38.
Dunn Jr WA. Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol. 1994;4:139–43.
Gatica D, Chiong M, Lavandero S, Klionsky DJ. Molecular mechanisms of autophagy in the cardiovascular system. Circ Res. 2015;116:456–67.
Korngold EC, Jaffer FA, Weissleder R, Sosnovik DE. Noninvasive imaging of apoptosis in cardiovascular disease. Heart Fail Rev. 2008;13:163–73.
Balvan J, Krizova A, Gumulec J, Raudenska M, Sladek Z, Sedlackova M, Babula P, Sztalmachova M, Kizek R, Chmelik R, Masarik M. Multimodal holographic microscopy: distinction between apoptosis and oncosis. PLoS One. 2015;10:e0121674.
Su H, Gorodny N, Gomez LF, Gangadharmath U, Mu F, Chen G, Walsh JC, Szardenings K, Kolb HC, Tamarappoo B. Noninvasive molecular imaging of apoptosis in a mouse model of anthracycline-induced cardiotoxicity. Circ Cardiovasc Imaging. 2015;8:e001952.
Thukkani AK, Shoghi KI, Zhou D, Xu J, Chu W, Novak E, Chen DL, Gropler RJ, Mach RH. PET imaging of in vivo caspase-3/7 activity following myocardial ischemia-reperfusion injury with the radiolabeled isatin sulfonamide analogue [(18)F]WC-4-116. Am J Nucl Med Mol Imaging. 2016;6:110–9.
Smith RA, Hartley RC, Cocheme HM, Murphy MP. Mitochondrial pharmacology. Trends Pharmacol Sci. 2012;33:341–52.
Liberman EA, Topaly VP, Tsofina LM, Jasaitis AA, Skulachev VP. Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria. Nature. 1969;222:1076–8.
Wan B, Doumen C, Duszynski J, Salama G, LaNoue KF. A method of determining electrical potential gradient across mitochondrial membrane in perfused rat hearts. Am J Phys. 1993;265:H445–52.
Gurm GS, Danik SB, Shoup TM, Weise S, Takahashi K, Laferrier S, Elmaleh DR, Gewirtz H. 4-[18F]-tetraphenylphosphonium as a PET tracer for myocardial mitochondrial membrane potential. JACC Cardiovasc Imaging. 2012;5:285–92.
•• Logan A, Pell VR, Shaffer KJ, Evans C, Stanley NJ, Robb EL, Prime TA, Chouchani ET, Cocheme HM, Fearnley IM, Vidoni S, James AM, Porteous CM, Partridge L, Krieg T, Smith RA, Murphy MP. Assessing the mitochondrial membrane potential in cells and in vivo using targeted click chemistry and mass spectrometry. Cell Metab. 2016;23:379–85. This study introduces a novel approach to assess subtle changes in mitochondrial membrane potential.
Chen HH, Mekkaoui C, Cho H, Ngoy S, Marinelli B, Waterman P, Nahrendorf M, Liao R, Josephson L, Sosnovik DE. Fluorescence tomography of rapamycin-induced autophagy and cardioprotection in vivo. Circ Cardiovasc Imaging. 2013;6:441–7.
Lipinski MJ, Frias JC, Amirbekian V, Briley-Saebo KC, Mani V, Samber D, Abbate A, Aguinaldo JG, Massey D, Fuster V, Vetrovec GW, Fayad ZA. Macrophage-specific lipid-based nanoparticles improve cardiac magnetic resonance detection and characterization of human atherosclerosis. JACC Cardiovasc Imaging. 2009;2:637–47.
Majmudar MD, Yoo J, Keliher EJ, Truelove JJ, Iwamoto Y, Sena B, Dutta P, Borodovsky A, Fitzgerald K, Di Carli MF, Libby P, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ Res. 2013;112:755–61.
• Pedersen SF, Sandholt BV, Keller SH, Hansen AE, Clemmensen AE, Sillesen H, Hojgaard L, Ripa RS, Kjaer A. 64Cu-DOTATATE PET/MRI for detection of activated macrophages in carotid atherosclerotic plaques: studies in patients undergoing endarterectomy. Arterioscler Thromb Vasc Biol. 2015;35:1696–703. This study correlates imaging and genetic markers of carotid plaque vulnerabilty in humans.
Briley-Saebo KC, Cho YS, Shaw PX, Ryu SK, Mani V, Dickson S, Izadmehr E, Green S, Fayad ZA, Tsimikas S. Targeted iron oxide particles for in vivo magnetic resonance detection of atherosclerotic lesions with antibodies directed to oxidation-specific epitopes. J Am Coll Cardiol. 2011;57:337–47.
Naresh NK, Xu Y, Klibanov AL, Vandsburger MH, Meyer CH, Leor J, Kramer CM, French BA, Epstein FH. Monocyte and/or macrophage infiltration of heart after myocardial infarction: MR imaging by using T1-shortening liposomes. Radiology. 2012;264:428–35.
Alam SR, Shah AS, Richards J, Lang NN, Barnes G, Joshi N, MacGillivray T, McKillop G, Mirsadraee S, Payne J, Fox KA, Henriksen P, Newby DE, Semple SI. Ultrasmall superparamagnetic particles of iron oxide in patients with acute myocardial infarction: early clinical experience. Circ Cardiovasc Imaging. 2012;5:559–65.
Yilmaz A, Dengler MA, van der Kuip H, Yildiz H, Rosch S, Klumpp S, Klingel K, Kandolf R, Helluy X, Hiller KH, Jakob PM, Sechtem U. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: a human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur Heart J. 2013;34:462–75.
Moon H, Park HE, Kang J, Lee H, Cheong C, Lim YT, Ihm SH, Seung KB, Jaffer FA, Narula J, Chang K, Hong KS. Noninvasive assessment of myocardial inflammation by cardiovascular magnetic resonance in a rat model of experimental autoimmune myocarditis. Circulation. 2012;125:2603–12.
Dimastromatteo J, Broisat A, Perret P, Ahmadi M, Boturyn D, Dumy P, Fagret D, Riou LM, Ghezzi C. In vivo molecular imaging of atherosclerotic lesions in ApoE−/− mice using VCAM-1-specific, 99mTc-labeled peptidic sequences. J Nucl Med. 2013;54:1442–9.
•• Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, Yeoh SE, Wallace W, Salter D, Fletcher AM, van Beek EJ, Flapan AD, Uren NG, Behan MW, Cruden NL, Mills NL, Fox KA, Rudd JH, Dweck MR, Newby DE. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705–13. This is the first noninvasive study to identify and localize ruptured and high risk coronary plaque in humans.
Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, Joshi FR, Gallagher FA, Warburton EA, Bennett MR, Brindle KM, Newby DE, Rudd JH, Davenport AP. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun. 2015;6:7495.
Mascherbauer J, Marzluf BA, Tufaro C, Pfaffenberger S, Graf A, Wexberg P, Panzenbock A, Jakowitsch J, Bangert C, Laimer D, Schreiber C, Karakus G, Hulsmann M, Pacher R, Lang IM, Maurer G, Bonderman D. Cardiac magnetic resonance postcontrast T1 time is associated with outcome in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging. 2013;6:1056–65.
de Haas HJ, Arbustini E, Fuster V, Kramer CM, Narula J. Molecular imaging of the cardiac extracellular matrix. Circ Res. 2014;114:903–15.
Klink A, Heynens J, Herranz B, Lobatto ME, Arias T, Sanders HM, Strijkers GJ, Merkx M, Nicolay K, Fuster V, Tedgui A, Mallat Z, Mulder WJ, Fayad ZA. In vivo characterization of a new abdominal aortic aneurysm mouse model with conventional and molecular magnetic resonance imaging. J Am Coll Cardiol. 2011;58:2522–30.
Megens RT, Oude Egbrink MG, Cleutjens JP, Kuijpers MJ, Schiffers PH, Merkx M, Slaaf DW, van Zandvoort MA. Imaging collagen in intact viable healthy and atherosclerotic arteries using fluorescently labeled CNA35 and two-photon laser scanning microscopy. Mol Imaging. 2007;6:247–60.
Dilsizian V, Zynda TK, Petrov A, Ohshima S, Tahara N, Haider N, Donohue A, Aras O, Femia FJ, Hillier SM, Joyal JL, Wong ND, Coleman T, Babich JW, Narula J. Molecular imaging of human ACE-1 expression in transgenic rats. JACC Cardiovasc Imaging. 2012;5:409–18.
Jenkins WS, Vesey AT, Stirrat C, Connell M, Lucatelli C, Neale A, Moles C, Vickers A, Fletcher A, Pawade T, Wilson I, Rudd JH, van Beek EJ, Mirsadraee S, Dweck MR and Newby DE (2016) Cardiac alphaVbeta3 integrin expression following acute myocardial infarction in humans. Heart.
Beer AJ, Pelisek J, Heider P, Saraste A, Reeps C, Metz S, Seidl S, Kessler H, Wester HJ, Eckstein HH, Schwaiger M. PET/CT imaging of integrin alphavbeta3 expression in human carotid atherosclerosis. JACC Cardiovasc Imaging. 2014;7:178–87.
Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–19.
Notni J, Steiger K, Hoffmann F, Reich D, Kapp TG, Rechenmacher F, Neubauer S, Kessler H, Wester HJ. Complementary, selective PET imaging of integrin subtypes alpha5beta1 and alphavbeta3 using 68Ga-Aquibeprin and 68Ga-Avebetrin. J Nucl Med. 2016;57:460–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All of the authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Molecular Imaging
Rights and permissions
About this article
Cite this article
Liang, G., Vo, D. & Nguyen, P.K. Fundamentals of Cardiovascular Molecular Imaging: a Review of Concepts and Strategies. Curr Cardiovasc Imaging Rep 10, 8 (2017). https://doi.org/10.1007/s12410-017-9403-7
Published:
DOI: https://doi.org/10.1007/s12410-017-9403-7