Abstract
The granulocyte colony-stimulating factor (G-CSF) is a glycoprotein that stimulates cell proliferation and differentiation of precursor cells in the bone marrow. Several cases of G-CSF-producing malignant tumors in various organs have been reported, but there are only nine cases of G-CSF-producing hepatocellular carcinoma (HCC) reported in the English literature. G-CSF-producing tumors grow rapidly and have a high probability of distant metastases; thus, they generally have a poor prognosis. Given that the mechanism of the carcinogenesis of G-CSF-producing HCC remains unclear, an efficient treatment strategy also remains to be elucidated. We report herein a case of G-CSF-producing HCC accompanied by leukocytosis and high serum G-CSF concentrations in the disease progression stage after long-term complete response. We also reviewed previous reports to investigate the clinical behaviors of G-CSF-producing HCC, including our case. Clinicians should consider G-CSF-producing HCC in patients with a hepatic mass and drastic leukocytosis, without any evidence of infection and blood disorders. Early diagnosis and prompt therapy, including radical resection, may provide a more favorable prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leukocytosis is often seen in patients with paraneoplastic syndromes and is related to granulocyte colony-stimulating factor (G-CSF) production. Robinson [1] reported that a colony-stimulating factor was found in the serum and urine in patients with malignant tumors. The granulocyte colony-stimulating factor (G-CSF) is a cytokine produced mainly by macrophages, fibroblasts, and endothelial cells, which functions to induce maturation and proliferation of the precursor of neutrophils in the bone marrow and recruit them into the periphery [2]. Asano et al. [3] first reported a G-CSF-producing lung cancer in 1997; subsequently, various types of G-CSF-producing tumors have been reported, such as the lung [4], bladder [5], gallbladder [6], pancreas [7], thyroid [8], and cervical cancers [9].
However, G-CSF-producing hepatocellular carcinoma (HCC) is extremely rare. G-CSF-producing tumors grow rapidly and have a high probability of distant metastases; thus, they generally have a poor prognosis. Given that the mechanism of the carcinogenesis of G-CSF-producing HCC remains unclear, an efficient treatment strategy also remains to be elucidated.
Here, we report a case of G-CSF-producing HCC accompanied by leukocytosis and high serum G-CSF concentrations in the disease progression stage.
Case report
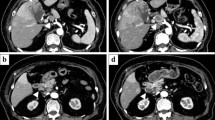
A 78-year-old man with chronic hepatitis C was admitted to our hospital in September 2009 due to general fatigue. Contrast-enhanced computed tomography (CT) showed a 70-mm hepatic mass in the right lobe with peripheral enhancement, and right hepatic vein invasion, with metastasis of both lungs (Fig. 1a, b). The levels of tumor markers on the first admission were elevated as follows: alfa-fetoprotein (AFP), 1528 ng/mL; AFP-L3 fraction, 26.5%; and des-g-carboxy prothrombin (DCP), 110,020 mAU/mL. We, therefore, diagnosed the hepatic mass as HCC; however, the findings of the contrast-enhanced CT showed an unusual enhancement pattern for typical HCC. The patient had initially undergone hepatic arterial infusion chemotherapy (HAIC) with radiotherapy (RT). The HAIC involved cisplatin and a continuous infusion of fluorouracil, referred to as NewFP [10]. RT (50 Gy, in 25 fractions) for the right hepatic vein invasion was initiated with the first cycle of HAIC. After receiving sorafenib, followed by HAIC and RT, he maintained complete response (CR) for several years, and the levels of tumor markers decreased as follows: AFP, 16 ng/mL; AFP-L3 fraction, 0.5%; and DCP, 27 mAU/mL (Fig. 2).
Imaging findings. Abdominal contrast-enhanced computed tomography showed a liver mass in the right lobe, approximately 70 mm in size, with peripheral enhancement and right hepatic vein invasion at the first occurrence in September 2009. a Arterial phase; b equilibrium phase. After the treatment with hepatic arterial infusion chemotherapy with radiation therapy and sorafenib, the tumor obtained complete response for several years; however, abdominal contrast-enhanced computed tomography performed thereafter showed a low-density area in the treated location in February 2016. c Arterial phase; d equilibrium phase
Clinical course and changes in the tumor maker and white blood cell count (WBC) levels. After the patient had undergone hepatic arterial infusion chemotherapy (HAIC) with radiotherapy (RT) and sorafenib, he maintained complete response for several years, and the levels of tumor markers decreased remarkably. In March 2017, a peripheral blood count analysis revealed leukocytosis of up to 26,070/μL at the time of hepatocellular carcinoma recurrence. DCP, des-g-carboxy prothrombin; DEB-TACE; drug-eluting bead transarterial chemoembolization
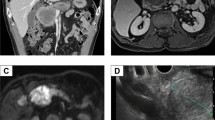
Then, the patient underwent imaging studies regularly, and contrast-enhanced CT revealed a low-density area in the treated location (Fig. 1c, d). This lesion gradually increased over time since February 2016; however, the AFP, AFP-L3, and DCP were not elevated, and we initially suspected biloma and could not determine whether HCC had recurred. We, therefore, followed up the lesion with 4-monthly imaging studies. In December 2016, gadoxetic acid-enhanced magnetic resonance imaging (MRI) showed that the lesion had grown to 50 mm in size, with high signal intensity on T2-weighted image with fat suppression in the anterior segment of the liver. The lesion showed hypo-vascularization in the arterial phase, but peripheral enhancement in the portal phase, and washout appearance in the 20-min delayed phase with restriction on diffusion characterized by high signal on diffusion-weighted imaging (Fig. 3). Since these imaging findings were atypical for HCC and the patient had no symptoms, he strongly desired to be managed with follow-up alone. In March 2017, the patient had loss of appetite and fever, with a body temperature of 37 °C. Physical examination revealed a hard fixed mass palpable at the right hypochondria. As shown in Table 1, a peripheral blood count analysis revealed leukocytosis of up to 26,070/μL with 88.5% neutrophilia, although no leukocytosis or neutrophilia was seen before March 2017. The levels of tumor markers, such as AFP, carcinoembryonic antigen, and carbohydrate antigen 19-9, were slightly elevated, whereas the DCP level was within the normal range. Before the treatment, the serum granulocyte colony-stimulating factor (G-CSF: 93.1 pg/mL; normal range, < 39.0 pg/mL) and interleukin-6 (IL-6 30.5 pg/mL; normal range < 4.0 pg/mL) levels were markedly elevated. In comparison to the grade of leukocytosis, the increase in serum C-reactive protein (CRP) level was lower, and no infection foci were detected by several imaging examinations. Thus, we consider that leukocytosis in this patient was related to the HCC recurrence.
Imaging findings in December 2016. Gadoxetic acid-enhanced magnetic resonance imaging showed that the lesion had grown to 50 mm in size, with high signal intensity on a T2-weighted image with fat suppression (T2FSWI) located in the anterior segment of the liver. The lesion showed hypo-vascularization in the arterial phase, but peripheral enhancement in the portal phase, and washout appearance in the 20 min delayed phase, with restriction on diffusion characterized by high signal on diffusion-weighted imaging (DWI). a T2FSWI; b arterial phase; c portal phase; d 20 min delayed phase; e DWI
Abdominal ultrasonography showed a hypoechoic mass with an irregular demarcation, approximately 100 mm in size and located in the anterior segment of the liver. Sonazoid-enhanced ultrasonography detected a vessel flowing in the marginal region of the tumor in the early vascular phase, which was not enhanced in the late phase. Abdominal contrast-enhanced CT revealed an irregularly demarcated mass, approximately 100 mm in size, located in the anterior segment with peripheral enhancement. The center of the mass was in a low-density area, which was suggestive of degeneration or necrosis (Fig. 4a–d). 18F-fluorodeoxyglucose positron emission tomography/CT imaging showed a liver mass with a maximum standardized uptake value of 13.91 (Fig. 4e).
Imaging findings at time of recurrence in March 2017. Abdominal contrast-enhanced computed tomography showed an irregularly demarcated liver mass with peripheral enhancement, 100 mm in size, located in the anterior segment of the liver. a Plain; b arterial phase; c portal phase; d equilibrium phase. An 18F-fluorodeoxyglucose positron emission tomography/computed tomography image showed the marginal region of the liver mass with a maximum standardized uptake value of 13.91 (e). Angiography via superior mesenteric artery (f) and celiac artery (g) showed tumor stain in the marginal region of an irregular liver mass
Immunohistochemical examination was performed using samples obtained from a needle biopsy. The histopathological findings revealed atypical poorly differentiated cells with a sheet structure, and the tumor was composed of sarcomatous spindle-shaped cells with necrosis and neutrophilic infiltration (Fig. 5a). The poorly differentiated HCC lesion was found to be positive for cytokeratin (CK) CAM5.2 (Fig. 5b), and the sarcomatous component was found to be negative for CK CAM5.2. Furthermore, the tumor was positive for pankeratin (CK AE1/AE3) (Fig. 5c) and vimentin (Fig. 5d). Immunohistochemical examinations showed that the G-CSF was positive in some cancer cells in the poorly differentiated HCC lesion (Fig. 5e). We found that HCC with sarcomatous changes produced G-CSF, according to the results of the G-CSF immunohistochemical study.
Histopathologic findings. The tumor was composed of sarcomatous spindle-shaped malignant cells, pleomorphic cells with bizarre nuclei, an intense infiltration of neutrophils and histiocytes, focally admixed with poorly differentiated hepatocellular carcinoma with trabecular and compact patterns (a) (hematoxylin eosin staining, × 100). Immunohistochemical findings showed cytokeratin (CK) CAM5.2 positivity in the cancer cells of a poorly differentiated hepatocellular carcinoma (b) (CK CAM5.2, × 100). Immunohistochemical staining of CK AE1/AE3 (c) and vimentin (d) in the sarcomatous area of the tumor (CK AE1/AE3, vimentin, × 100). Immunohistochemical examination showed that granulocyte colony-stimulating factor was positive in a part of cancer cell (e) (granulocyte colony-stimulating factor, × 400)
Given that the angiography via the superior mesenteric (Fig. 4f) and celiac (Fig. 4g) arteries showed a tumor stain in the marginal region of an irregular liver mass, the patient was treated by drug-eluting bead transarterial chemoembolization (DEB-TACE). The serum G-CSF levels decreased at 14 days after DEB-TACE to 41.6 pg/mL. Although the HCC in this patient partially and transiently responded to this treatment, it grew rapidly again, and the patient died from hepatic failure approximately 3 months after the diagnosis (in July 2017).
Discussion
The disease course of this patient suggested two important clinical issues. Clinicians should consider G-CSF-producing HCC in patients with a hepatic mass accompanied by drastic leukocytosis, without any evidence of infection and blood disorders. In the process of dedifferentiation of cancer cells, it might be possible that the cancer cells changed the G-CSF-producing HCC.
G-CSF-producing HCC is extremely rare. To date, only nine cases have been reported in the English literature [11,12,13,14,15,16,17,18,19] (Table 2). The clinical manifestations of G-CSF-producing HCC included general malaise, loss of appetite, continuous fever, and high serum CRP. The characteristics of the CT and MRI findings of G-CSF-producing HCC did not show the same pattern. Some cases showed patterns typical of HCC that enhanced in the early phase of contrast medium infusion and washed out in the late phase; in contrast, some cases showed an unusual pattern that peripherally enhanced, with the center of the tumor in a low-density area, suggestive of necrosis or degeneration, as in our case. The G-CSF-producing tumor has been described as having the following clinical characteristics: (1) drastic white blood cell (WBC) increase; (2) elevation of G-CSF activity; (3) decrease in WBC count after tumor resection; and (4) evidence of G-CSF production in the tumor tissue [3]. In our case, the WBC count and the serum G-CSF level increased remarkably without any evidence of infection and blood disorders. Our case was not due to WBC decrease after tumor resection closely because of an unresectable HCC, and the WBC count and serum G-CSF level considerably decreased after DEB-TACE. In the present case report, the immunohistochemical study showed that G-CSF was positive in the tumor tissues, suggesting the production of G-CSF in HCC. The abovementioned findings supported the diagnosis of G-CSF-producing HCC in our case.
As shown in Table 2, among the ten reported G-CSF-producing HCC cases, including our case, six cases [60% (6/10)] were histologically diagnosed as poorly differentiated HCCs, three cases were moderate to poorly differentiated HCCs, and one case was a moderately differentiated HCC. Collectively, our case and previously reported cases might suggest that G-CSF-producing HCCs are poorly and/or moderately differentiated HCCs, not well-differentiated HCCs.
In the previous report by Yamamoto et al. [11], leukocytosis was not found at the initial diagnosis of HCC. The diagnosis of G-CSF-producing HCC was determined after the patient underwent treatments for HCC. Similarly, in our case, the WBC count was within the normal range at the early period of the first treatment for HCC. At 7 years after the first treatment, the HCC of our patient rapidly increased in size, as seen by contrast-enhanced CT, and the WBC count began to increase.
Although the mechanism of the production of G-CSF in HCC remains unclear, an intimate relationship between the production of G-CSF in cancer cells and their dedifferentiation has been reported [11]. Wang et al. [20] compared the production of G-CSF between well-differentiated and poorly differentiated HCCs using cell lines, and concluded that only the poorly differentiated HCCs tend to produce G-CSF. The number of patients with sarcomatoid liver cancers have been increasing since the late 1980s, when clinicians began to use transcatheter arterial embolization (TAE) as a treatment for HCC. In a previous report, sarcomatous changes were found in 27.6% of HCC cases treated with TAE frequently [21]. In our case, HAIC was used to treat HCC, which might induce sarcomatous changes. Thus, patients who maintained long-term CR of HCC should be monitored.
Generally, the G-CSF-producing tumors, including HCC, grow rapidly and have a poor prognosis, and the majority of the patients die within a few months after the initial diagnosis. Baba et al. [22] reported that G-CSF is associated with the rapid growth and metastasis of tumors as an autocrine growth factor in a gastric cancer cell line. Jana et al. [23] reported that G-CSF played an important role in the autonomous proliferation of Caco-2, which is a cell line derived from adenocarcinoma of the colon. The G-CSF-producing tumors induce some inflammatory cytokines, such as IL-1 and IL-6 [24], and IL-6 has emerged as a cytokine involved in cachexia progression with some cancers [25]. Kyo et al. [9] described a case of cervical cancer that exhibited an aggressive clinical course, possibly due to the autocrine stimulation of cell growth by G-CSF and IL-6. In the present case, the G-CSF and IL-6 levels were also high, HCC progression occurred rapidly, and the patient had also a poor prognosis. We speculated that one approach to improve the poor prognosis of patients with tumors producing G-CSF or IL-6 would be the blockade of autocrine and/or paracrine growth-stimulating loops by the inhibition of cytokine production.
In conclusion, clinicians should consider G-CSF-producing HCC in patients with a hepatic mass accompanied by drastic leukocytosis. Early diagnosis and prompt therapy including, radical resection, may provide a more favorable prognosis. Further accumulation and studies of G-CSF-producing HCC are necessary, and other appropriate and effective treatments, such as immunotherapy, should be investigated.
References
Robinson WA. Granulocytosis in neoplasia. Ann N Y Acad Sci. 1974;230:212–8.
Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (1). N Engl J Med. 1992;327:28–35.
Asano S, Urabe A, Okabe T, et al. Demonstration of granulopoietic factor(s) in the plasma of nude mice transplanted with a human lung cancer and in the tumor tissue. Blood. 1977;49:845–52.
Shijubo N, Inoue Y, Hirasawa M, et al. Granulocyte colony-stimulating factor-producing large cell undifferentiated carcinoma of the lung. Intern Med. 1992;31:277–80.
Tachibana M, Miyakawa A, Tazaki H, et al. Autocrine growth of transitional cell carcinoma of the bladder induced by granulocyte-colony stimulating factor. Cancer Res. 1995;55:3438–43.
Ikeda T, Ohgaki K, Miura M, et al. Granulocyte-colony stimulating factor-producing gallbladder cancer without recurrence more than 2 years after resection: report of a case. Surg Today. 2005;35:590–3.
Nakajima A, Takahashi H, Inamori M, et al. Anaplastic carcinoma of the pancreas producing granulocyte-colony stimulating factor: a case report. J Med Case Rep. 2008;2:391.
Iwasa K, Noguchi M, Mori K, et al. Anaplastic thyroid carcinoma producing the granulocyte colony stimulating factor (G-CSF): report of a case. Surg Today. 1995;25:158–60.
Kyo S, Kanaya T, Takakura M, et al. A case of cervical cancer with aggressive tumor growth: possible autocrine growth stimulation by G-CSF and Il-6. Gynecol Oncol. 2000;78:383–7.
Nagamatsu H, Hiraki M, Mizukami N, et al. Intra-arterial therapy with cisplatin suspension in lipiodol and 5-fluorouracil for hepatocellular carcinoma with portal vein tumour thrombosis. Aliment Pharmacol Ther. 2010;32:543–50.
Yamamoto S, Takashima S, Ogawa H, et al. Granulocyte-colony-stimulating-factor-producing hepatocellular carcinoma. J Gastroenterol. 1999;34:640–4.
Amano H, Itamoto T, Emoto K, et al. Granulocyte colony-stimulating factor-producing combined hepatocellular/cholangiocellular carcinoma with sarcomatous change. J Gastroenterol. 2005;40:1158–9.
Araki K, Kishihara F, Takahashi K, et al. Hepatocellular carcinoma producing a granulocyte colony-stimulating factor: report of a resected case with a literature review. Liver Int. 2007;27:716–21.
Joshita S, Nakazawa K, Koike S, et al. A case of granulocyte-colony stimulating factor-producing hepatocellular carcinoma confirmed by immunohistochemistry. J Korean Med Sci. 2010;25:476–80.
Kohno M, Shirabe K, Mano Y, et al. Granulocyte colony-stimulating-factor-producing hepatocellular carcinoma with extensive sarcomatous changes: report of a case. Surg Today. 2013;43:439–45.
Snyder RA, Liu E, Merchant NB. Granulocyte colony stimulating factor secreting hepatocellular carcinoma. Am Surg. 2012;78:821–2.
Ito T, Okubo K, Shiomi M, et al. A case of successful treatment of granulocyte colony-stimulating factor producing hepatocellular carcinoma accompanying type B hepatitis with tegafur-uracil. Nihon Shokakibyo Gakkai Zasshi. 2012;109:2088–96.
Nagata H, Komatsu S, Takaki W, et al. Granulocyte colony-stimulating factor-producing hepatocellular carcinoma with abrupt changes. World J Clin Oncol. 2016;7:380–6.
Sakamoto Y, Kamiyama T, Yokoo H, et al. Hepatocellular carcinoma producing granulocyte colony-stimulating factor: diagnosis and treatment. Int Cancer Conf J. 2019;8:12–6.
Wang SY, Chen LY, Tsai TF, et al. Constitutive production of colony-stimulating factors by human hepatoma cell lines: possible correlation with cell differentiation. Exp Hematol. 1996;24:437–44.
Kojiro M, Sugihara S, Kakizoe S, et al. Hepatocellular carcinoma with sarcomatous change: a special reference to the relationship with anticancer therapy. Cancer Chemother Pharmacol. 1989;23:S4-8.
Baba M, Hasegawa H, Nakayabu M, et al. Establishment and characteristics of a gastric cancer cell line (HuGC-OOHIRA) producing high levels of G-CSF, GM-CSF, and IL-6: the presence of autocrine growth control by G-CSF. Am J Hematol. 1995;49:207–15.
Jana S, Patel H. Expression of human granulocyte colony stimulating factor (hG-CSF) in colon adenocarcinoma cell line (Caco-2). Biotechnol Lett. 2012;34:1791–6.
Suzuki A, Takahashi T, Okuno Y, et al. Liver damage in patients with colony-stimulating factor-producing tumors. Am J Med. 1993;94:125–32.
Narsale AA, Carson JA. Role of interleukin-6 in cachexia: therapeutic implications. Curr Opin Support Palliat Care. 2014;8:321–7.
Acknowledgements
We would like to thank Editage (https://www.editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Human/animal rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from the patient for being included in this case report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nomura, T., Morishita, A., Tani, J. et al. A case report of granulocyte colony-stimulating factor-producing hepatocellular carcinoma that recurred after long-term complete response. Clin J Gastroenterol 14, 204–211 (2021). https://doi.org/10.1007/s12328-020-01239-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-020-01239-9