Abstract
Granulocyte colony-stimulating factor (G-CSF) is a naturally occurring glycoprotein that is synthesized by stromal cells in bone marrow. Several cases of G-CSF-producing malignant tumors in various organs have been reported, but it is extremely rare in hepatocellular carcinoma (HCC). Here, we report a rare case of HCC producing G-CSF. The patient presented with a continuous fever and had a huge liver mass in the right lobe with portal vein tumor thrombus (PVTT) in the right first branch. He had marked granulocytosis, and his serum level of G-CSF was elevated. A complete curative liver resection was performed after preoperative radiotherapy to PVTT. The pathological findings of the resected specimen revealed poorly/moderately differentiated HCC, and immunohistochemical staining of G-CSF was negative the first time it was tested, but the second time, it was positive in the cytoplasm of other tumor cells of HCC. Only a few cases of G-CSF-producing HCC have been reported, and they resulted in rapid tumor growth, metastases, and poor prognosis. In our case with PVTT, there was no liver recurrence, although multiple lung metastases occurred at 8 months after curative resection. We should consider G-CSF-producing HCC and diagnose promptly when encountering liver tumor patients with leukocytosis, and we should perform multimodal treatment including radiation, radical surgery, and chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Granulocyte colony-stimulating-factor (G-CSF) is a naturally occurring glycoprotein that stimulates the proliferation and maturation of precursor cells in the bone marrow into fully differentiated neutrophils [1]. Asano et al. first reported a case of G-CSF-producing lung cancer in 1977 [2], and several studies have identified the aberrant expression and production of G-CSF in tumor cells, including thyroid cancer, bladder cancer, gallbladder cancer, gastric cancer, and especially lung cancer [2,3,4,5,6], but only a few cases of G-CSF-producing hepatocellular carcinoma (HCC) have been reported. G-CSF-producing tumors grow rapidly and have a high probability of distant metastases, so they generally have a poor prognosis. Because the mechanism of the carcinogenesis of G-CSF-producing HCC remains unclear, an efficient treatment strategy also remains to be elucidated. This report presents a rare case of G-CSF-producing HCC treated with multimodal therapy including radiation, radical resection and chemotherapy. We describe the clinical, histopathological diagnosis and multimodal treatment strategy of this G-CSF-producing HCC.
Case report
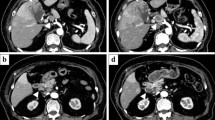
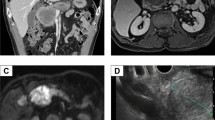
A 62-year-old male with a history of hypertension and diabetes mellitus was admitted due to a prolonged fever over the past 3 months. His temperature remained over 38 °C even though we administered several antibiotics to him. Hematological laboratory data upon admission were as follows: white blood cell count (WBC) 28,900/µl, C-reactive protein (CRP) 4.46 mg/dl, α-fetoprotein (AFP) 7.1 ng/ml, [fucosylated α-fetoprotein (AFP-L3) 76.9%], and protein induced by the absence of vitamin K (PIVKA)-II 27,356 mAU/ml. Hepatitis B surface antigen (HBsAg) and hepatitis C virus antibody (HCV Ab) were negative, but his serum level of G-CSF was elevated to 358 pg/ml (Table 1). Computed tomography (CT) showed a largely enhanced, partially low-density huge mass, 16.0 cm in diameter, located in the right liver lobe. Most of the tumor was enhanced in early phase, contrast-enhanced CT and washed out in the late phase, while the center of the tumor was a low-density area suggestive of necrosis (Fig. 1a). CT revealed the tumor thrombus at the right branch of the portal vein (Fig. 1b), so we diagnosed the hepatic tumor to be HCC with portal vein tumor thrombus (PVTT) in the right first branch. CT did not demonstrate the lung metastasis. Because we reported the efficacy of preoperative radiotherapy (RT) to PVTT in the main trunk or first branch in patients with HCC [7], we chose preoperative RT (30 Gy, in 10 fractions). RT to PVTT was followed by hepatectomy within 2 weeks, and the position of the tip of PVTT did not change though the thrombus itself became slightly bigger. The WBC count did not decrease (WBC: 28,300/µl). G-CSF after RT was not measured. We performed complete resection of the hepatic tumor by right liver lobe hepatectomy and tumor thrombus extraction after preoperative RT. 3 days after the surgery, the WBC count decreased to 7000/µl, and serum G-CSF was 34.2 pg/ml in the normal range. His fever disappeared by postoperative day 3 without any use of antibiotics or nonsteroidal anti-inflammatory drugs. His postoperative course was uneventful, and he was discharged 20 days after surgery. The resected specimen revealed that the tumor (16.0 × 14.0 × 7.0 cm) occupied the right liver lobe invading the right diaphragm. The cut surface of the tumor was off-white and contained massive necrosis (Fig. 2). The pathological findings revealed that the tumor was mostly composed of pleomorphic cells with bizarre nuclei and partially contained spindle-shaped cells making a fascicular arrangement, but there was no intense infiltration of neutrophils or sarcomatous change (Fig. 3a) and the tip of the PVTT became almost necrosis by the therapeutic effect of RT. The tumor showed positive staining for hepatocyte paraffin 1 (Hep-par 1) and glypican 3 (Fig. 3b, c), so the resected specimen was histologically poorly/moderately differentiated hepatocellular carcinoma. Immunohistochemically, the tumor cells were negative for G-CSF staining when tested for the first time, but the other tumor cells from other regions were positive for G-CSF staining the next time (Fig. 3d); thus, we diagnosed this tumor as a G-CSF-producing HCC.
a–c CT findings. A largely enhanced, partially low-density huge mass, 16.0 cm diameter, located in the right liver lobe. Most of the tumor was enhanced in early phase and washed out in the late phase. CT revealed the tumor thrombus at the right branch of the portal vein (white arrowhead). The position of the tip of PVTT did not change, though the thrombus itself become slightly bigger (black arrowhead)
Resected specimen. The tumor (16.0 × 14.0 × 7.0 cm) occupied the right liver lobe. The cut surface of the tumor was off-white and contained massive necrosis. Direct invasion of the right diaphragm was observed (white arrowheads). The tumor thrombus was detected in the right branch of the portal vein (black arrowhead)
Histopathological examination. The tumor was mostly composed of pleomorphic cells with bizarre nuclei, but there was no intense infiltration of neutrophils or sarcomatous change. The tumor was focally admixed with moderately to poorly differentiated HCC (a) [hematoxylin and eosin (H&E) stain, × 100]. Immunohistochemical findings showed Hep-par 1 (b) [Hep-par 1, × 100] and glypican 3 (c) [glypican 3, × 100] positive in the moderately/poorly differentiated HCC region. Immunohistochemical staining of G-CSF was negative for the first time, but the other tumor cells were positive next time in the other site (d) [G-CSF, × 100]. Hep-par 1 hepatocyte paraffin 1, G-CSF granulocyte colony-stimulating factor, HCC hepatocellular carcinoma cell
The patient had intensive follow-ups without any recurrence, but at 8 months after surgery, follow-up CT detected multiple lung metastases but no liver metastasis. We started to treat with systemic chemotherapy using sorafenib tosylate for the multiple lung metastases as soon as possible, so he is alive and his disease progression has been controlled for 2 years after surgery. His serum G-CSF at the time of recurrence was within the normal range, and there has been no recurrence in the liver even now.
Discussion
Some cancers have been reported to produce certain humoral factors including cytokines, such as G-CSF, granulocyte macrophage colony-stimulating factor (GM-CSF) or erythropoietin, which cause paraneoplastic syndrome [8, 9]. Pyrexia and a high inflammatory response such as a high WBC or CRP level are not commonly observed in patients with HCC, so we should suspect those signs to be due to humoral factors. G-CSF-producing tumors have been described as having (1) a drastic WBC increase; (2) an elevation of G-CSF activity; (3) WBC decrease after tumor resection; and (4) evidence of G-CSF production in the tumor tissue [2]. Our case fulfilled this definition, but the diagnosis was unclear because the tumor cells did not show G-CSF immunohistochemical staining the first time we tested. The reasons for this result may be that the sensitivity to immunohistochemical staining of G-CSF is low or there may be a possibility of distribution of G-CSF-producing HCC tissue. Some reports discussed that G-CSF production indicated the transformation of the HCC into a more immature and high-grade phenotype [10, 11]. The second immunohistochemical staining was positive for G-CSF staining at poorly differentiated HCC, so the heterogeneity of G-CSF-producing HCC may be related to the discrepancy in G-CSF immunohistochemical staining. When encountering patients with pyrexia, leukocytosis and liver mass, it is crucial to suspect the disease, measure the serum G-CSF level and perform immunohistochemical staining repeatedly to diagnose G-CSF-producing HCC.
G-CSF-producing HCC is extremely rare, and only eight cases have been reported in the English literature (Table 2). G-CSF-producing tumors grow rapidly and have a poor prognosis. Some reports reveal that G-CSF has been linked to tumor cell growth in vitro [12] and that there might be a relationship between the secretion of G-CSF and the degree of cell differentiation [13], but the mechanism is yet to be defined. Because only poorly differentiated HCCs tend to produce G-CSF in vitro using an HCC cell line, it is reasonable that most cases (8/9) were pathologically diagnosed as poorly differentiated HCCs. A sarcomatous change also influences rapid growth and poor prognosis. Patients with sarcomatous changes actually died within approximately 6 months after diagnosis. On the other hand, the prognosis of the patients without sarcomatous changes was longer than 2 years except case 1. It is still controversial whether surgical resection is an effective strategy in this G-CSF-producing HCC because the postoperative outcomes were poor in past reports. Joshita et al. [11] and Ito et al. [14] performed radical surgery, and their cases had more favorable prognosis (4 years and more than 2 years, respectively). In our case, despite the presence of PVTT, there was no liver recurrence though multiple lung metastases occurred 8 months after curative resection. Therefore, radical curative tumor resection is very important to prolong the prognosis of G-CSF-producing HCC. In addition, chemotherapy such as tegafur–uracil [14] or sorafenib tosylate (our case) may be effective for treating recurrence, because the prognosis was prolonged for several years in these patients who received administration of these two agents; therefore, we should initiate systemic chemotherapy as soon as possible if a recurrence occurs. Our case is the first report performing preoperative RT for PVTT in G-CSF-producing HCC patient, so we think preoperative RT is very useful because the pathological findings of the resected PVTT specimen revealed that the tip of the PVTT became almost necrosis by the therapeutic effect of RT and there was no liver recurrence. In our case and the past report [11], the serum G-CSF level had not increased at recurrence. The reason for that may be related to tumor size, heterogeneity or metastasized organs, but the details remain unclear. It is important that we take care of recurrence even if the serum G-CSF level is within the normal range and continue to follow up intensively.
G-CSF-producing HCC is extremely rare, but it generally grows rapidly and has a poor prognosis. G-CSF-producing HCC should be considered and diagnosed promptly when encountering liver tumor patients with leukocytosis, and we suggest performing multimodal treatment including radiation, radical surgery, and systemic chemotherapy. More number of clinical cases and further studies are necessary to understand G-CSF-producing HCC and to establish appropriate treatment strategies.
References
Lieschke GJ, Burgess AW (1992) Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. N Engl J Med 327:28–35
Asano S, Urabe A, Okabe T et al (1977) Demonstration of granulopoietic factor(s) in the plasma of nude mice transplanted with a human lung cancer and in the tumor tissue. Blood 49:845–852
Iwasa K, Noguchi M, Mor K et al (1995) Anaplastic thyroid carcinoma producing the granulocyte colony stimulating factor (G-CSF): report of a case. Surg Today 25:158–160
Ito N, Matsuda T, Kakehi Y et al (1990) Bladder cancer producing granulocyte colony-stimulating factor. N Engl J Med 323:1709–1710
Furihata M, Sonobe H, Ohtsuki Y et al (1999) An immunohistochemical study on a case of granulocyte-colony stimulating factor-producing gall-bladder carcinoma. Pathol Int 49:1010–1013
Endo K, Kohnoe S, Okamura T et al (2005) Gastric adenosquamous carcinoma producing granulocyte-colony stimulating factor. Gastric Cancer 8:173–177
Kamiyama T, Nakanishi K, Yokoo H et al (2007) Efficacy of preoperative radiotherapy to portal vein tumor thrombus in the main trunk or first branch in patients with hepatocellular carcinoma. Int J Clin Oncol 12:363–368
Ueno M, Seferynska I, Beckman B et al (1989) Enhanced erythropoietin secretion in hepatoblastoma cells in response to hypoxia. Am J Physiol 257:C743–C749
Tani K, Ozawa K, Ogura H et al (1990) Expression of granulocyte and granulocyte-macrophage colony-stimulating factors by human non-hematopoietic tumor cells. Growth Factors 3:325–331
Aita K, Seki K (2006) Carcinosarcoma of the liver producing granulocyte-colony stimulating factor. Pathol Int 56:413–419
Joshita S, Nakazawa K, Koike S et al (2010) A case of granulocyte-colony stimulating factor-producing hepatocellular carcinoma confirmed by immunohistochemistry. J Korean Med Sci 25:476–480
Berdel WE, Danhauser-Riedl S, Steinhauser G et al (1989) Various human hematopoietic growth factors (interleukin-3, GM-CSF, G-CSF) stimulate clonal growth of nonhematopoietic tumor cells. Blood 73:80–83
Yamamoto S, Takashima S, Ogawa H et al (1999) Granulocyte-colony-stimulating-factor-producing hepatocellular carcinoma. J Gastroenterol 34:640–644
Ito T, Okubo K, Shiomi M et al (2012) [A case of successful treatment of granulocyte colony-stimulating factor producing hepatocellular carcinoma accompanying type B hepatitis with tegafur–uracil.]. Nihon Shokakibyo Gakkai Zasshi 109:2088–2096
Amano H, Itamoto T, Emoto K et al (2005) Granulocyte colony-stimulating factor-producing combined hepatocellular/cholangiocellular carcinoma with sarcomatous change. J Gastroenterol 40:1158–1159
Araki K, Kishihara F, Takahashi K et al (2007) Hepatocellular carcinoma producing a granulocyte colony-stimulating factor: report of a resected case with a literature review. Liver Int 27:716–721
Kohno M, Shirabe K, Mano Y et al (2013) Granulocyte colony-stimulating-factor-producing hepatocellular carcinoma with extensive sarcomatous changes: report of a case. Surg Today 43:439–445
Snyder RA, Liu E, Merchant NB (2012) Granulocyte colony stimulating factor secreting hepatocellular carcinoma. Am Surg 78:821–822
Nagata H, Komatsu S, Takaki W et al (2016) Granulocyte colony-stimulating factor-producing hepatocellular carcinoma with abrupt changes. World J Clin Oncol 7:380–386
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study is approved by the ethics committee of Hokkaido University Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the patient.
About this article
Cite this article
Sakamoto, Y., Kamiyama, T., Yokoo, H. et al. Hepatocellular carcinoma producing granulocyte colony-stimulating factor: diagnosis and treatment. Int Canc Conf J 8, 12–16 (2019). https://doi.org/10.1007/s13691-018-0346-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13691-018-0346-x