Abstract
Introduction
X-linked hypophosphataemia (XLH) is a rare, genetic renal phosphate-wasting disease, resulting from excess fibroblast growth factor 23 (FGF23) activity, which has a progressive and profound impact on patients throughout life. The monoclonal anti-FGF23 antibody, burosumab, is a subcutaneous injection indicated for the treatment of XLH in children and adults. Originally, burosumab was approved to be administered by a healthcare professional (HCP), but the option of self-administration would enable patient independence and easier access to treatment. Two open-label, single-arm clinical trials, conducted in Japan and Korea, have assessed the safety and efficacy of self-administration of burosumab in both children and adults with XLH.
Methods
In KRN23-003 (n = 15 children aged 1–12 years) and KRN23-004 (n = 5 children aged 3–13 years, n = 4 adults aged 21–65 years), children initially received 0.8 mg/kg of burosumab every 2 weeks and adults initially received 1.0 mg/kg of burosumab every 4 weeks. Self-administration was permitted from Week 4, and patients or carers were provided with training to inject correctly.
Results
In both trials, burosumab had an acceptable safety profile with mainly mild-to-moderate adverse events. Following self-administration, no patients reported serious treatment-emergent adverse events ≥ grade 3, injection-site reactions or hypersensitivity reactions related to burosumab. Serum phosphate and active vitamin D levels increased from baseline in children and adults.
Conclusions
These results indicated that the efficacy and safety of burosumab when administered either by a carer or patient are similar to that when administered by an HCP and show that self-administration is a viable option for patients with XLH.
Trial Registration Numbers
NCT03233126 and NCT04308096.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The option of self-administration of injectable medications for patients is becoming increasingly important as many patients seek the independence and ease of access to treatment which self-administration can offer |
Two open-label, single-arm clinical studies were carried out to assess the safety and efficacy of burosumab, a fully human monoclonal antibody given as a subcutaneous injection every 2 or 4 weeks, in children and adult patients with X-linked hypophosphataemia (XLH). Both studies allowed carer or self-administration of burosumab after appropriate training at the clinical centre |
Carer or self-administration of burosumab in children and adults with XLH had an acceptable safety profile and resulted in an improvement in serum phosphate and active vitamin D levels from baseline |
These studies showed that self-administration of burosumab had a similar efficacy and safety profile to healthcare professional administration and that self-administration of burosumab is a viable alternative option for patients with XLH |
Based on these data, regulatory agencies in Japan, the European Union and the UK have subsequently approved self-administration in their territories |
Introduction
X-linked hypophosphataemia (XLH) is a rare, lifelong, progressive, phosphate-wasting disease affecting approximately 1 in 20,000–60,000 people worldwide [1,2,3]. It is caused by loss-of-function mutations in the phosphate-regulating endopeptidase homologue on the X chromosome (PHEX) gene, leading to excess of the phosphaturic hormone fibroblast growth factor 23 (FGF23) [3, 4]. Excess FGF23 activity leads to reduced renal phosphate reabsorption and suppression of 1,25-dihydroxyvitamin D (1,25[OH]2D), with the resultant hypophosphataemia affecting the musculoskeletal, dental and auditory body systems in patients with XLH [3, 5]. Clinical manifestations arise in childhood and persist or deteriorate over time, with additional complications appearing later in life [6]. The chronic and progressive manifestations experienced by people with XLH contribute to a decrease in their health-related quality of life (QoL) [6,7,8]; therefore, early, optimal and continued treatment of XLH is important to prevent complications and improve patient QoL [9].
Historically, XLH has been treated with conventional therapy comprising oral phosphate and active vitamin D analogues to supplement serum phosphate levels and correct 1,25(OH)2D deficiency, respectively [10]. Despite some improvements in clinical manifestations [11,12,13,14], this treatment regimen is burdensome, associated with numerous side effects, including hyperparathyroidism and nephrocalcinosis, and the symptoms of XLH may persist [9, 14]. A treatment modality, burosumab, is a fully human anti-FGF23 monoclonal antibody that inhibits excess intact FGF23 (iFGF23) activity, thereby increasing renal phosphate reabsorption and enhancing intestinal phosphate absorption through the production of 1,25(OH)2D [15, 16]. This leads to an increase in serum phosphate levels, the amelioration of skeletal and muscular deficits in both children and adults with XLH [17,18,19] and improvements to QoL [20].
As of 2021, burosumab has been approved for the treatment of XLH in children and adults by several regulatory agencies around the world, including the European Medicines Agency, US Food and Drug Administration and Pharmaceuticals and Medical Devices Agency in Japan [16, 21, 22]. Burosumab is administered as a subcutaneous injection, every 2 weeks for children and every 4 weeks for adults, by a healthcare professional (HCP) [16]. In Japan since December 2020 and in the European Union (EU) since July 2021, burosumab may also be administered by the patient or a carer [23, 24].
Self-administration of subcutaneous therapies is encouraged in hospitals worldwide, particularly in chronic diseases such as diabetes [25], to promote patient independence [26]. The National Prescribing Centre in the UK describes the aims of self-administration as being able to ‘increase the patient’s knowledge and understanding of their medication and promote and maintain patient independence and autonomy’ [26]. Studies have reported a significant improvement in patients’ knowledge of their own medication following self-administration [26]. Self-administration can empower patients to manage their medication, which has been linked to improved adherence, particularly in chronic diseases [27,28,29]. This can lead to improved overall health, reducing future visits to hospital and, therefore, costs [27]. Self-administration can be performed in both a hospital and home setting but, in certain situations, as exemplified and exacerbated during the recent COVID-19 pandemic, hospital care can become inaccessible [30]. Patients administering their own injectable medications can manage their treatment schedule and choose to administer at home, providing continuity of treatment and independence in their personal and professional lives [31]. Self-administration can save time for HCPs and reduce both patient and hospital costs as there is no need for a patient to attend the clinic for treatment [32,33,34]. Psychological benefits of self-administration compared with HCP administration may also include improved patient self-esteem and confidence [26].
For people with XLH, the need for an HCP to administer the burosumab injection in hospital, every 2 or 4 weeks, can be restrictive and a burden in terms of time and travel. The ability of patients or carers of children with XLH to administer burosumab themselves would enable them to fit the medication schedule around their own lives and situations. This is particularly relevant in a chronic and progressive disease like XLH where continued treatment is important, and missed doses, for any unforeseen reason, may lead to a deterioration in health outcomes [35]. Although burosumab is largely administered by HCPs, the option of self-administration would be beneficial and convenient to many patients with XLH. In many other therapy areas, self-administration of subcutaneously injected medicines has been shown to be efficacious and have a good safety profile [36, 37].
Two phase 3 studies, KRN23-003 and KRN23-004, investigated self-administration of burosumab in children and adult patients with XLH in Japan and Korea. This article summarises the safety and efficacy of the self-administration of burosumab from these two studies, while highlighting the benefits and implications of self-administration for patients with XLH, their carers and physicians.
Methods
In the clinical development programme of burosumab, self-administration by either patient or carer was permitted and monitored in patients with XLH in two open-label, single-arm clinical studies conducted in Japan and South Korea: KRN23-003 (NCT03233126) [38] and KRN23-004 (NCT04308096).
For both studies, the study protocol and amendments were reviewed and approved by the institutional review boards (IRB) consulted by the director of each investigative site. The studies were conducted in accordance with the Declaration of Helsinki. All subjects provided informed consent to participate in the studies. The investigative sites are listed in the acknowledgements.
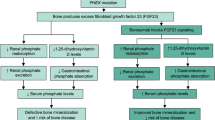
Information on participants who self-administered burosumab in trial KRN23-003 and KRN23-004 can be found in Table 1 and study designs are illustrated in Fig. 1i [38] and Fig. 1ii. In both trials, burosumab was administered as a subcutaneous injection every 2 weeks in children and every 4 weeks in adults. In both trials, the investigator or sub-investigator asked enrolled subjects and their legally acceptable representative whether they wished to self-administer. Patients and their carers who opted for self-administration were given an illustrated ‘Instructions for use’ leaflet to demonstrate how to prepare and give the subcutaneous injection. Investigators or study staff provided further training on the preparation of the drug and how to practice injecting. Self-administration was permitted from Week 4 of treatment, at hospital, where patients or carers were judged to be suitable to administer burosumab appropriately by the investigator or sub-investigator.
Study design of i KRN23-003 and ii KRN23-004. Figure i reproduced from Namba et al. [38] by permission of Oxford University Press and the Endocrine Society. aEnrolment took place within 28 days of providing informed consent. If the inclusion criterion for 25-hydroxyvitamin D was not met, enrolment within 49 days was allowed in view of supplement administration and rescreening [38]. bAfter eligibility was confirmed, oral phosphate and active vitamin D therapy were washed out for at least 7 days before the start of burosumab administration. However, washout could be started at the discretion of the investigator or sub-investigator before confirmation of all eligibility criteria [38]. cTreatment was started within 7 days of enrolment [38]. dEnrolled patients included adults with XLH who completed the final observation at Week 96 in Study UX023-CL303 or UX023-CL304 or in paediatric patients with XLH who completed the final observation at Week 64 in Study UX023-CL301. eTreatment was started within 14 days and within 56 days at maximum after the final study visit of the preceding study. EoT end of treatment, XLH X-linked hypophosphataemia
Furthermore, if the patients or their carers had conducted self-administration at least once in hospital, the patient’s serum phosphate levels were deemed stable, and they were considered suitable to self-administer burosumab appropriately at home; then, self-administration at home was allowed at and after Week 10 for paediatric patients and after Week 12 for adult patients. Investigators provided instructions on how to transport, store and manage burosumab for these patients. Self-administration at home was suspended if the dose needed adjustment, and changes to the dose were under the control of the investigator. Self-administration at home could be resumed after the investigator deemed both the dose and serum phosphate levels stable. The safety and efficacy data of patients who self-administered burosumab are reported here. Results from the paediatric patients from KRN23-003 [38] and KRN23-004 are pooled across the two studies. Please note that results for the 15 children in KRN23-003 have already been reported in Namba et al. [38]. Additionally, all data mentioned in this publication can be found in the Supplementary Material.
Patients
In KRN23-003, the key inclusion criteria were children aged ≥ 1 and ≤ 12 years, a confirmed diagnosis of XLH by either a PHEX mutation or a directly related family member with appropriate X-linked inheritance or, alternatively, a serum iFGF23 level ≥ 30 pg/ml. At screening, patients also had open growth plates and evidence of rickets on simple x-ray evaluation as judged by the investigator or clinical symptoms of rickets [38].
In KRN23-004, patients were included if they had completed the last visit of either the phase 3 study UX023-CL301 for children [17] or phase 3 studies UX023-CL303 or UX023-CL304 for adults [18, 19, 39]. Inclusion criteria are provided elsewhere [18, 19, 39].
Study Outcomes
The primary objective for KRN23-003 and KRN23-004 was to assess the safety of burosumab administered subcutaneously every 2 weeks in children and every 4 weeks in adult patients. Safety was assessed by measuring the frequency of adverse events and treatment-emergent adverse events (TEAE). Additionally, laboratory tests, vital signs, 12-lead electrocardiogram, renal ultrasound and echocardiogram were also evaluated [38].
The key secondary objectives of both trials included evaluation of biochemical markers, such as changes in serum phosphate level, 1,25(OH)2D level and ratio of tubular maximum reabsorption of phosphate to glomerular filtration rate (TmP/GFR) from baseline, in children and adult patients.
Demographics, drug exposure, serum phosphate and other pharmacodynamic variables (TmP/GFR and 1,25[OH]2D) and safety results will be reviewed here for those patients who underwent carer/self-administration.
Statistical Analysis
Categorical data were summarised using frequency and percentage, whereas continuous data were summarised using mean and standard deviation (SD) at each time point. Statistical significance was not assessed since no statistical assumptions were provided in either study.
Results
Baseline Characteristics
Paediatric Patients
All 15 of the enrolled children in KRN23-003 and 5 children in KRN23-004 had carer/self-administration both in hospital and at home. The mean age of children was 7.4 years, although it should be noted that patients included in KRN23-004 tended to be older since this was an extension study from previous clinical studies (Table 2). Most paediatric patients (70%) were female. Genetic analysis data were available for 13 patients in KRN23-003 and were positive for a PHEX mutation in all 13 patients. Data were not available for two patients [38].
Adult Patients
In KRN23-004, four adult patients performed self-administration both in hospital and at home. Their baseline characteristics are given in Table 2.
Treatment Duration and Dose
In KRN23-003, all 15 enrolled children self-administered burosumab. In KRN23-004, five children self-administered burosumab. For all 20 children with self/carer administration, the median duration of burosumab exposure at the end of the studies was 114.8 weeks (range 73.9–119.9), with a median dose of 17.36 mg (range 7.52–51.00 mg) every 2 weeks.
In KRN23-004, four adult patients self-administered burosumab. For all adult patients, the median duration of burosumab exposure at the end of study was 100.1 weeks, with a median dose of 52.96 (range 9.35–72.62) mg every 4 weeks.
In KRN23-003, four patients underwent dose adjustment and either self-administered in hospital or had their injections administered by an HCP during dose adjustment. Following the period of dose adjustment, the patients re-initiated self-administration at home. In KRN23-004, there were no instances of dose adjustment.
Safety
Patients in both the KRN23-003 and KRN23-004 studies experienced TEAEs, but most were mild to moderate in severity. Following self-administration, no patients in either study reported serious TEAEs ≥ grade 3, injection-site reactions or hypersensitivity reactions related to burosumab. Furthermore, there were no reports of any patient with TEAEs relating to hyperphosphataemia or ectopic mineralisation. No clinically meaningful changes from baseline were observed for vital signs and no TEAEs led to study discontinuation, treatment discontinuation or death in either trial.
In the 20 paediatric patients from KRN23-003 and KRN23-004 who self-administered burosumab, TEAEs were recorded in 17 patients (85%) after self-administration (Table 3). Five TEAEs that were considered by the investigator as related to the investigational product occurred in two patients (10%) after the start of self-administration, including diarrhoea, dizziness, headache, nausea and decreased levels of serum 25-hydroxycholecalciferol. One patient experienced a serious TEAE, tonsillitis, but this was considered unrelated to burosumab. In addition, all other TEAEs reported in the paediatric subjects who self-administered burosumab were considered by the investigator as unrelated to burosumab and were not mechanistically related to the self-administration procedure. There were no reports of injection-site reactions or hyperphosphataemia in either of the two studies after the start of self-administration. TEAEs of allergic blepharitis, eczema, rash, facial swelling and urticaria occurred in one patient each (5%) who self-administered burosumab (after the initiation of self-administration); however, the investigator considered the events unrelated to burosumab.
In KRN23-004, three out of four adult patients who self-administered burosumab experienced a TEAE following initiation of self-administration (Table 3). None of the reported TEAEs were considered by the investigator as related to burosumab, and none were mechanistically related to the self-administration procedure.
Efficacy
Serum Phosphate
Serum phosphate levels increased with burosumab in both children and adults with XLH. As patients in KRN23-004 had previously participated in other clinical studies with burosumab, their baseline values will remain the effect of those previous studies. In the 20 paediatric patients who self-administered burosumab in KRN23-003 and KRN23-004, serum phosphate levels increased from 2.5 ± 0.4 mg/dl at baseline to 3.5 ± 0.5 mg/dl at Week 8 and were sustained to 3.5 ± 0.4 mg/dl for the 15 children in KRN23-003 at Week 100 (last measurement with all participants) and 3.1 ± 0.3 mg/dl at Week 72 for the 5 children in KRN23-004 (last measurement with all participants) (Fig. 2i). There was little fluctuation in serum phosphate values following the initiation of self-administration at Week 4. The fluctuations that did occur were due to the difference in patient number. Adults in KRN23-004 had an increase in serum phosphate from 2.0 ± 0.4 mg/dl at baseline to 2.1 ± 0.7 mg/dl at Week 96 with burosumab (Fig. 2ii).
Serum phosphate levels in i children in studies KRN23-003 and KRN23-004 (n = 20) and ii adults in KRN23-004 (n = 4). Data are shown as mean ± SD. The number of patients varies because of missing data in each study. Data from paediatric patients in KRN23-003 and KRN23-004 were obtained at different time points and i shows a merged analysis. SD standard deviation
TmP/GFR
TmP/GFR improved for children in both trials following burosumab treatment. In the pooled analysis, TmP/GFR increased from 2.3 ± 0.5 mg/dl at baseline to 3.9 ± 0.8 mg/dl at Week 8. This increase was maintained over time to the last available data for patients in both studies. The decrease shown at Week 48 is due to the difference in patient number (Fig. 3). Adults in KRN23-004 had an increase in TmP/GFR from 1.6 ± 0.3 mg/dl at baseline to 2.0 ± 0.8 mg/dl at Week 96.
TmP/GFR levels in children in studies KRN23-003 and KRN23-004 (n = 20). Data are shown as mean ± SD. The number of patients varies due to missing data in each study. Data from paediatric patients in KRN23-003 and KRN23-004 were obtained at different time points and this figure shows a merged analysis. SD standard deviation
1,25(OH)2D
In both studies, active vitamin D levels increased with burosumab treatment. Children in the KRN23-003 and KRN23-004 studies had levels of 29.3 ± 17.4 pg/ml at baseline, which then increased to 60.4 ± 23.1 pg/ml at Week 24. Increases from baseline were maintained in both studies to the end of treatment. The increase shown at Week 48 is due to the difference in patient number (Fig. 4i). Adult patients in the same study showed an improvement from 37.2 ± 9.6 pg/ml at baseline to 47.8 ± 13.9 pg/ml at Week 96 (Fig. 4ii).
1,25(OH)2D levels in i children in studies KRN23-003 and KRN23-004 (n = 20) and ii adults in KRN23-004 (n = 4). Data are shown as mean ± SD. The number of patients varies because of missing data in each study. Data from paediatric patients in KRN23-003 and KRN23-004 were obtained at different time points and i shows a merged analysis. SD standard deviation
Discussion
The KRN23-003 and KRN23-004 trials demonstrated an acceptable safety profile and efficacy of self-administered burosumab in children and adults with XLH. There were no noteworthy TEAEs, indicating that burosumab can be administrated by both patients and carers without any major safety concerns. Burosumab treatment led to improvements in serum phosphate, TmP/GFR and serum 1,25(OH)2D in children and adults with XLH.
The safety profile from patients with self-administration of burosumab was consistent with those reported in previous phase 3 trials (UX023-CL301, UX023-CL303 and UX023-CL304), where burosumab was administered by HCPs to children and adults [17, 19, 38]. There were no reports of injection-site reactions related to the investigational product or hypersensitivity reactions in the patients who self-administered burosumab in studies KRN23-003 and KRN23-004. There were also no reports of incorrect dosing or overdose for patients who self-administered burosumab.
Results from the KRN23-003 study confirmed the efficacy of self-administered burosumab. Data for each patient who implemented self-administration showed that there were no noticeable fluctuations in serum phosphate following initiation of self-administration. This was also consistent with the individual patient dosing data, which showed that the mg/kg dose of each patient remained consistent over time. Overall, the serum phosphate data and information from the individual patient data do not indicate any change in the pharmacodynamic effect of burosumab following the introduction of self-administration. Supportive data from the nine patients in KRN23-004 who also self-administered burosumab were consistent with data from KRN23-003 with respect to sustained serum phosphate levels.
After routine training, the carers of children with XLH and adults with XLH were able to administer burosumab correctly and safely. The availability of training materials, onsite training and illustrated instructions for use were important for the HCP to ensure patients were comfortable and confident with the procedure. In both studies, no dosing errors, missed doses or safety issues associated with self-administration were reported. All biochemical indicators of efficacy, including serum phosphate levels, were also met, indicating good compliance. Other studies have shown that some patients who appear able to self-inject in the hospital may lack confidence to do so alone [40]. It is imperative for patients and their carers to maintain a link with the treatment centre, which provides the training and support to increase patient confidence and satisfaction with their self-care [41]. In all instances of self-administration, patients and carers must be aware of what to do in case of adverse events or complications and have access to an HCP when needed.
There are limitations associated with the KRN23-003 and KRN23-004 studies, and care must be taken when interpreting the findings. First, the populations studied, Japanese and Korean patients, may not be directly comparable with patient populations in other countries. However, the selection criteria of patients for KRN23-003 were very similar to those of the global paediatric burosumab studies [17], e.g. PHEX mutation positive, or confirmed raised FGF23, hypophosphataemia, open growth plates with evidence of rickets and reduced growth. All patients from KRN23-004 met the selection criteria of the global development studies UX023-CL301 for children [17] or UX023-CL303 or UX023-CL304 for adults [19, 39]. Second, the patient populations were small, but this is not unexpected in studies of a disease as rare as XLH. Third, the self-administration procedure, whether in hospital or at home, was performed in the setting of a clinical trial, which may have influenced compliance. Future studies could investigate the adherence of self-administration of burosumab at home versus HCP administration in hospital. In addition, further elaboration in this publication on the self-administration training provided to patients is unavailable because of the original documents being written in Japanese.
Similar to many other subcutaneously administered treatments, these studies demonstrated that burosumab can be administered by a carer or patient with XLH. While conducted in the clinical trial setting, these data are supported by the real-world experience of patients in Japan, where self-administration has been allowed since December 2020 [23], and in the EU, where self-administration was approved in July 2021 [24]. The option of permitting self-administration during the pandemic has been beneficial in the context of reducing hospital exposure or resource use, and there have been no safety or efficacy issues reported in any country since self-administration has been allowed. It must also be noted that the cost of burosumab may be a factor, and while self-administration of burosumab is an option, it may not be suitable for all patients and carers, particularly where there may be issues with compliance.
Empowering a carer of a child or an adult with XLH to manage their treatment regimen themselves may improve adherence and satisfaction with treatment, as has been shown for other chronic diseases [28, 29]. Further research to assess patient satisfaction and adherence with this alternative means of managing their treatment programme would be useful, as has been done for other injectable medications [41]. These data could inform support programmes to enable patients and carers to be more confident and competent in handling and administering burosumab, thereby reinforcing their connection with their HCPs.
Conclusions
Overall, the results from the KRN23-003 and KRN23-004 studies indicate that the safety and efficacy of burosumab when administered by a patient with XLH or carer are similar to that of HCP administration. This shows that self-administration is a viable alternative option for patients with XLH and their carers.
References
Beck-Nielsen SS, Brock-Jacobsen B, Gram J, Brixen K, Jensen TK. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur J Endocrinol. 2009;160(3):491–7.
Rafaelsen S, Johansson S, Ræder H, Bjerknes R. Hereditary hypophosphatemia in Norway: a retrospective population-based study of genotypes, phenotypes, and treatment complications. Eur J Endocrinol. 2016;174(2):125–36.
Beck-Nielsen SS, Mughal Z, Haffner D, et al. FGF23 and its role in X-linked hypophosphatemia-related morbidity. Orphanet J Rare Dis. 2019;14(1):58.
Dahir K, Roberts MS, Krolczyk S, Simmons JH. X-linked hypophosphatemia: a new era in management. J Endocr Soc. 2020;4(12): bvaa151.
Saraff V, Nadar R, Högler W. New developments in the treatment of X-linked hypophosphataemia: implications for clinical management. Paediatr Drugs. 2020;22(2):113–21.
Skrinar A, Dvorak-Ewell M, Evins A, et al. The lifelong impact of X-linked hypophosphatemia: results from a burden of disease survey. J Endocr Soc. 2019;3(7):1321–34.
Ferizović N, Marshall J, Williams AE, et al. Exploring the burden of X-linked hypophosphataemia: an opportunistic qualitative study of patient statements generated during a technology appraisal. Adv Ther. 2020;37(2):770–84.
Seefried L, Smyth M, Keen R, Harvengt P. Burden of disease associated with X-linked hypophosphataemia in adults: a systematic literature review. Osteoporos Int. 2021;32(1):7–22.
Lambert AS, Zhukouskaya V, Rothenbuhler A, Linglart A. X-linked hypophosphatemia: management and treatment prospects. Joint Bone Spine. 2019;86(6):731–8.
Linglart A, Biosse-Duplan M, Briot K, et al. Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocr Connect. 2014;3(1):R13-30.
Mäkitie O, Doria A, Kooh SW, et al. Early treatment improves growth and biochemical and radiographic outcome in X-linked hypophosphatemic rickets. J Clin Endocrinol Metab. 2003;88(8):3591–7.
BiosseDuplan M, Coyac BR, Bardet C, et al. Phosphate and vitamin D prevent periodontitis in X-linked hypophosphatemia. J Dent Res. 2017;96(4):388–95.
Connor J, Olear EA, Insogna KL, et al. Conventional therapy in adults with X-linked hypophosphatemia: effects on enthesopathy and dental disease. J Clin Endocrinol Metab. 2015;100(10):3625–32.
Haffner D, Emma F, Eastwood DM, et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol. 2019;15(7):435–55.
Yamazaki Y, Tamada T, Kasai N, et al. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res. 2008;23(9):1509–18.
Kyowa Kirin Limited. CRYSVITA (burosumab). Summary of product characteristics.
Imel EA, Glorieux FH, Whyte MP, et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet. 2019;393(10189):2416–27.
Portale AA, Carpenter TO, Brandi ML, et al. Continued beneficial effects of burosumab in adults with X-linked hypophosphatemia: results from a 24-week treatment continuation period after a 24-week double-blind placebo-controlled period. Calcif Tissue Int. 2019;105(3):271–84.
Insogna KL, Rauch F, Kamenický P, et al. Burosumab improved histomorphometric measures of osteomalacia in adults with X-linked hypophosphatemia: a phase 3, single-arm, international trial. J Bone Miner Res. 2019;34(12):2183–91.
Padidela R, Whyte MP, Glorieux FH, et al. Patient-reported outcomes from a randomized, active-controlled, open-label, phase 3 trial of burosumab versus conventional therapy in children with X-linked hypophosphatemia. Calcif Tissue Int. 2021;108:622–33.
Lamb YN. Burosumab: first global approval. Drugs. 2018;78(6):707–14.
PMDA. New drugs approved in FY 2019. https://www.pmda.go.jp/files/000235289.pdf. Accessed December 2022.
Kyowa Kirin Announces Crysvita® now reimbursed for self-injection in Japan. December 2020. https://www.kyowakirin.com/media_center/news_releases/2020/e20201204_01.html. Accessed December 2022.
PharmaTimes. EU approval for self-administered Crysvita. http://www.pharmatimes.com/news/eu_approval_for_self-administered_crysvita_1373330. Accessed December 2022.
American Diabetes Association. Insulin administration. Diabetes Care. 2004;27(Suppl 1):S106–9.
Richardson SJ, Brooks HL, Bramley G, Coleman JJ. Evaluating the effectiveness of self-administration of medication (SAM) schemes in the hospital setting: a systematic review of the literature. PLoS One. 2014;9(12): e113912.
van den Bemt BJF, Gettings L, Domańska B, Bruggraber R, Mountian I, Kristensen LE. A portfolio of biologic self-injection devices in rheumatology: how patient involvement in device design can improve treatment experience. Drug Deliv. 2019;26(1):384–92.
Marengo MF, Suarez-Almazor ME. Improving treatment adherence in patients with rheumatoid arthritis: what are the options? Int J Clin Rheumtol. 2015;10(5):345–56.
Kaday R, Ratanajamit C. Inpatient self-administered medication under the supervision of a multidisciplinary team: a randomized, controlled, blinded parallel trial. Pharm Pract (Granada). 2020;18(2):1766.
Brizola E, Adami G, Baroncelli GI, et al. Providing high-quality care remotely to patients with rare bone diseases during COVID-19 pandemic. Orphanet J Rare Dis. 2020;15(1):228.
Chilton F, Collett RA. Treatment choices, preferences and decision-making by patients with rheumatoid arthritis. Musculoskelet Care. 2008;6(1):1–14.
Sørensen CA, Olesen C, Lisby M, Enemark U, de Thurah A. Self-administration of medication during hospitalization-a randomized pilot study. Pilot Feasibility Stud. 2020;6:116.
Touati M, Lamarsalle L, Moreau S, et al. Cost savings of home bortezomib injection in patients with multiple myeloma treated by a combination care in Outpatient Hospital and Hospital care at Home. Support Care Cancer. 2016;24(12):5007–14.
Elliott RA, Thornton J, Webb AK, Dodd M, Tully MP. Comparing costs of home- versus hospital-based treatment of infections in adults in a specialist cystic fibrosis center. Int J Technol Assess Health Care. 2005;21(4):506–10.
Kamenicky P, Briot K, Brandi ML, et al. Maintenance of effect of burosumab treatment and the impact of treatment interruption across a 96-week phase 3 study and 48 weeks of a phase 3b study in adults with X-linked hypophosphatemia (XLH). Presented at the World Congress on Osteoporosis, Osteoarthritic and Musculoskeletal Disease. Abstract OC9; 2021.
Ochs HD, Gupta S, Kiessling P, Nicolay U, Berger M, Subcutaneous IgG Study Group. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26(3):265–73.
Kuter DJ, Arnold DM, Rodeghiero F, et al. Safety and efficacy of self-administered romiplostim in patients with immune thrombocytopenia: results of an integrated database of five clinical trials. Am J Hematol. 2020;95(6):643–51.
Namba N, Kubota T, Muroya K, et al. Safety and efficacy of burosumab in pediatric patients with X-linked hypophosphatemia: a phase 3/4 open-label trial. J Endocr Soc. 2022;6(5):1–10.
Insogna KL, Briot K, Imel EA, et al. A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: week 24 primary analysis. J Bone Miner Res. 2018;33(8):1383–93.
Bailey K, Mountian I, Bruggraber R, Sunderland K, Tilt N, Szegvari B. Patient satisfaction with CIMZIA® (certolizumab pegol) AutoClicks® in the UK. Adv Ther. 2020;37(4):1522–35.
Schiff M, Saunderson S, Mountian I, Hartley P. Chronic disease and self-injection: ethnographic investigations into the patient experience during treatment. Rheumatol Ther. 2017;4(2):445–63.
Acknowledgements
Funding
The studies were both funded entirely by Kyowa Kirin Co., Ltd., Otemachi Financial City Grand Cube, 1-9-2 Otemachi, Chiyoda-ku, Tokyo, Japan. Kyowa Kirin Co., Ltd., have also funded the Rapid Service and Open Access fees for this journal.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing and Editorial Assistance
Medical writing support was provided by Bhavini Chauhan of Sciterion and funded by Kyowa Kirin Co., Ltd.
Author Contributions
All investigative authors: Substantial contributions to the design of the work; and the acquisition and interpretation of data for the work; and revising the work critically for important intellectual content; and final approval of the version to be published. Masanori Kanematsu: Substantial contributions to the conception and design of the work; and revising the manuscript critically for important intellectual content; and final approval of the version to be published. Wei Sun: Substantial contributions to the analysis and interpretation of data for the work; and revising the manuscript critically for important intellectual content; and final approval of the version to be published.
Study Investigators The study investigators for KRN23-003 were Koji Muroya, Noriyuki Namba (up to 21 August 2019), Hiroyuki Yamada (from 21 August 2019), Takuo Kubota and Hiroyuki Tanaka. The study investigators for KRN23-004 were Akie Nakamura, Nobuaki Ito, Yasuhiro Takeuchi, Koji Muroya, Noriyuki Namba (up to 21 August 2019), Hiroyuki Yamada (from 21 August 2019), Takuo Kubota, Yasuo Imanishi, Hiroyuki Tanaka, Hae-Il Cheong (up to 31 December 2020), Hee Gyang Kang (from 1 January 2021) and Han-Wook Yoo. Please note, the investigators listed may not all have had patients who self-administered burosumab.
Participants
We would like to thank all participants enrolled in both the KRN23-003 and KRN23-004 trials.
Disclosures
Takuo Kubota, Noriyuki Namba, Hiroyuki Tanaka, Koji Muroya, Yasuhiro Takeuchi and Yoshiki Seino have received consulting fees from Kyowa Kirin Co., Ltd. Yasuo Imanishi has received research grants from Kyowa Kirin Co., Ltd. Keiichi Ozono has received honorarium from Kyowa Kirin Co., Ltd, Alexion and Novo Nordisk. Masanori Kanematsu and Wei Sun are employees of Kyowa Kirin.
Compliance with Ethics Guidelines
For both studies, the study protocol and amendments were reviewed and approved by the IRBs consulted by the director of each investigative site. For KRN23-003, IRBs included Kanagawa Children’s Medical Centre IRB, Japan Community Healthcare Organisation Osaka Hospital IRB, Osaka University Hospital IRB and Okayama Saiseikai General Hospital IRB. For KRN23 004, IRBs included Hokkaido University Hospital IRB, University of Tokyo Hospital IRB, Toranomon Hospital IRB, Kanagawa Children’s Medical Centre IRB, Japan Community Healthcare Organisation Osaka Hospital IRB, Osaka University Hospital IRB, Osaka City University Hospital IRB, Okayama Saiseikai General Hospital IRB, Seoul National University Hospital IRB and Asan Medical Centre Children’s Hospital IRB. The studies were conducted in accordance with the Declaration of Helsinki. All subjects provided informed consent to participate in the studies. For KRN23-003, approval was obtained after modifications for Osaka University Hospital. Additional investigative sites in KRN23-003 were Kanagawa Children’s Medical Centre, Japan Community Healthcare Organisation Osaka Hospital and Okayama Saiseikai Outpatient Centre Hospital. For KRN23-004, approval was obtained after modifications for Seoul National University Hospital, Osaka University Hospital, Toranomon Hospital and Kanagawa Children’s Medical Centre. Additional investigative sites in KRN23-004 were Hokkaido University Hospital, the University of Tokyo Hospital, Japan Community Healthcare Organisation Osaka Hospital, Osaka City University Hospital, Okayama Saiseikai Outpatient Centre Hospital and Asan Medical Centre Children’s Hospital.
Data Availability
The data generated or analysed during this study are included as a supplementary information file.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kubota, T., Namba, N., Tanaka, H. et al. Self-Administration of Burosumab in Children and Adults with X-Linked Hypophosphataemia in Two Open-Label, Single-Arm Clinical Studies. Adv Ther 40, 1530–1545 (2023). https://doi.org/10.1007/s12325-022-02412-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02412-x