Abstract
The aim of our study was to assess the effect of blood cadmium levels (B-Cd) on abdominal aortic calcification (AAC). We used the data from the 2013–2014 NHANES database. A total of 1530 participants were included in our study, with a mean AAC score of 1.40 ± 0.10, and a prevalence of severe AAC of 7.98%. Participants with higher B-Cd quartiles showed a higher prevalence of severe AAC. B-Cd was positively associated with higher AAC scores and increased risk of severe AAC. In the obese population, blood cadmium levels showed a positive association with the risk of severe AAC. There may be a positive correlation between B-Cd levels and AAC scores and risk of severe AAC, and this correlation is more pronounced in the obese population. Therefore, the cadmium load in AAC patients in the obese population should be considered in clinical work.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular calcification (VC) is defined as a pathological process of abnormal deposition of calcium, phosphorus, calcium phosphate complexes, or other minerals in the vessel wall, which eventually leads to vascular sclerosis [1]. In addition to this, VC is also associated with abnormal deposition of lipoproteins in the intima, chronic inflammation, and endoplasmic reticulum stress [2]. VC is a common condition in patients with diabetes and chronic kidney disease (CKD) and the prevalence of VC in patients with CKD is more than 70% [3, 4]. The development of VC may be related to the conversion of vascular smooth muscle cells (VSMCs) to an osteoblast-like phenotype due to mineral deposition [5]. VC is a significant predictor of the occurrence of cardiovascular events and death, and one study found that VC was significantly associated with the risk of cardiovascular disease, myocardial infraction, and heart failure in patients with CKD [6]. The results of a randomized controlled trial indicated that patients treated with intravenous sodium thiosulfate did not develop new heart valve calcifications and their iliac artery calcification was relieved to some extent, although sodium thiosulfate did not improve abdominal aortic calcification in uremia patients [7]. Another phase 2b randomized trial demonstrated that SNF472 (myo-inositol hexaphosphate) significantly reduced the progression of coronary artery calcification and aortic valve calcification in patients with end-stage renal disease treated with hemodialysis [8]. Although the above drugs have shown effective potential in the treatment of VC, large randomized controlled trials are still needed to confirm the therapeutic efficacy of sodium thiosulfate. Therefore, prevention and management of VC in clinical time are crucial.
Abdominal aortic calcification (AAC), which has received increasing attention in recent years, is commonly seen in patients with diabetes and chronic kidney disease [9, 10]. The national prevalence of AAC in the US population is 28.8% [11]. The prevalence of AAC increases with age and can be as high as 96% in people aged 85 years and older [12]. AAC can be used as a prognostic indicator for continuous ambulatory peritoneal dialysis [13]. In addition, AAC is also a good predictor of the risk of cardiovascular events [14]. A meta-analysis showed that patients with CKD with any AAC had a higher risk of cardiovascular events and fatal cardiovascular events [15]. In order to assess the severity of AAC, Kauppila et al. developed a novel scoring system, which was calculated according to lateral X-ray of lumbar region to score the severity of anterior and posterior aortic calcification of each lumbar segment [16]. The AAC score ranges from 0 to 24, with higher AAC scores associated with more severe calcification of the abdominal aorta. This score provides a simple and low-cost assessment for subclinical vascular disease. The AAC score has been widely used in the follow-up clinical research [17].
Cadmium is a common toxic non-essential heavy metal element to human body, cadmium is mainly found in contaminated soil and water sources [18]. Cadmium has a long half-life, the main causes of cadmium exposure include consumption of contaminated food, long-term work in cadmium-contaminated environment, which makes cadmium exposure a global environmental problem that seriouly affects people’s health and brings socio-economic burden [19, 20]. A study evaluating cadmium exposure on blood pressure in the Korean general population showed that blood cadmium levels were higher in hypertensive patients than in non-hypertensive patients, and that systolic and diastolic blood pressure and mean arterial blood pressure were positively correlated with blood cadmium levels in the participants [21]. Another prospective cohort study showed that the adjusted HRs in the highest quantile cadmium exposure level group were 1.73 (95% CI: 1.52–1.97) for all-cause mortality, 1.72 (95% CI: 1.28–2.30) for cardiovascular disease (CVD) mortality compared with the lowest quantile of cadmium exposure level group [22]. Cadmium is also an independent risk factor for atherosclerosis [23, 24].
The increase in overweight and obese populations is a major global public health problem that poses a considerable socioeconomic burden. Globally, the proportion of adults with a body mass index (BMI) of 25 kg/m^2 or greater increased from 28.8 to 36.9% between 1980 and 2013 [25]. As of 2015, there were 603.7 million obese adults worldwide [26]. Obesity is strongly associated with type II diabetes, cardiovascular disease, osteoarthritis, stroke, and other diseases [27]. More than two-thirds of deaths associated with high BMI are due to cardiovascular disease [26].
The relationship between different blood cadmium concentrations and AAC is not well understood, and the association between obesity and AAC is contradictory, with one study finding a negative association between increasing BMI and AAC [11]. We, therefore, use data from the National Health and Nutrition Examination Survey (NHANES) to assess the relationship between cadmium exposure and AAC in people of different weight status. We hypothesized that cadmium exposure is positively associated with AAC scores and the risk of severe AAC, and that this positive correlation is more obvious in overweight and obese people. This may provide new insights into the management and intervention of AAC in clinical practice.
Materials Methods
Study Population
The National Health and Nutrition Examination Survey (NHANES), conducted by the National Center for Health Statistics, is an ongoing program designed to assess the health and nutrition status of the US population. The cross-sectional study employs a complex multi-stage probability sampling design that is continuously updated with survey data in a continuous 2-year replication cycle and has a relatively large representation of participants [28]. The NHANES study protocol was approved by the National Center for Health Statistics Research Ethics Review Committee, and each participant provided written consent prior to pariticipation in the study. All NHANES data are available at https://www.cdc.gov/nchs/nhanes/.
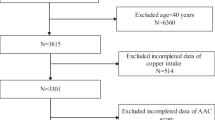
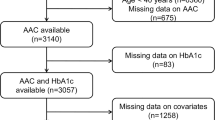
We used data from the NHANES database from 2013 to 2014 because only these 2 years of the survey cycle contained data on AAC scores. We excluded participants younger than 40 years of age because participants younger than 40 years of age did not have DXA scans, so they lacked AAC score data. In addition, we also excluded participants who lacked data on blood cadmium and AAC scores. In our study, we initially inclued 10,175 participants, after excluding 6360 participants aged < 40 years old, and participants lacking blood cadmium and AAC scores, we finally included 1530 participants older than 40 years old with complete blood cadmium and AAC scores in our analysis (Fig. 1).
Exposure and Outcome Definitions
Blood cadmium (B-Cd) was designed as exposure variable. The dilution of the blood in the sample preparation step prior to analysis is a simple dilution of 1 part sample + 1 part water + 48 parts diluent. The effects of the chemicals in the diluent are to release metals bound to red blood cells making them available for ionization, reduce ionization suppression by the biological matrix, prevent clogging of the sample introduction system pathways by undissolved biological solids, and allow introduction of internal standards to be utilized in the analysis step. Tetramethylammonium hydroxide (TMAH, 0.4% v/v) and Triton X-100TM (0.05%) in the sample diluent solubilizes blood components. Whole blood specimens are processed, stored, and shipped to the National Center for Environmental Health, and Centers for Disease Control and Prevention for analysis. Liquid samples are introduced into the mass spectrometer through the inductively coupled plasma (ICP) ionization source. Cadmium ions first passed through a focal region, then the dynamic reaction cell (DRC, ELAN®DRC II, PerkinElmer Norwalk, CT), the quadrupole mass filter, and finally were selectively counted in rapid sequence at the detector where the B-Cd level was accurately measured.When the blood cadmium measurement was below the lower limit of detection (LLOD), they were divided by the square root of 2 [29]. The variable code used for blood cadmium concentration was LBXBCD in NHANES.
We designed the AAC score and severe AAC as outcome variables. The AAC score was used to assess the severity of abdominal aortic calcification, where a higher AAC score was associated with a more severe degree of abdominal aortic calcification. Kauppila et al. invented a scoring system to quantify AAC, which was evaluated based on lumbar spine images obtained using dual-enegry X-ray absorptionmetry, by quantifying the different severity of calcification in each segment of the aortic wall in the anterior region of the lumbar spone L1-4, with a range of 0 to 6 from each segment, with a total AAC score ranging from 0 to 24 [16]. In 2013–2014, lateral DXA scans of the thoraco-lumbar spine were administered in the NHANES mobile examination center (MEC). The variable code used for blood cadmium concentration was DXAAC24 in NHANES. If the AAC score is greater than 6, it is defined as severe AAC, which represents severe abdominal aortic calcification [30].
Covariates
Continuous Variables in this study included age, BMI, fastglucose, hemoglobin A1c (HbA1c), serium creatinine, serium uric acid, serium phosphorus, serium calcium, triglyceride, total chloesterol, systolic bolld pressure, diastolic blood pressure, and the estimated glomerular filtration rate (eGFR). eGFR was calculated according to the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation. Categorical variables in this study included gender (male/female), race (Mexican American/Non-Hispanic Black/Non-Hispanic White/Other races), education level (< 9th grade/9–11th grade/college graduate or above/high school graduate/some college or AA degree), marriage (married/not married), the ratio of family income to poverty (RIP), diabetes, hypertension, alcohol drinker, and smoker. Alcohol use and smoking status was obtained from self-reported information. Hypertension was defined based on a self-reported diagnosis of hypertension, diastolic blood pressure ≥ 90 mmHg or systolic blood pressure ≥ 140 mmHg, or the use of antihypertensive medications [31]. Diabetes mellitus was defined base on a self-reported diagnosis of diabete mellitus, 2-h plasma glucose ≥ 200 mg/dL in an oral glucose tolerance test, HbAlc ≥ 6.5%, use of oral hypoglycemic agents, or fasting glucose ≥ 126 mg/dL [32]. BMI was categorized as < 18.5, 18.5–24.9, 25.0–29.9, and ≥ 30 kg/m2, which corresponded to underweight, normal weight, overweight, and obese groups among the participants. RIP was categorized as < 1, 1–5, and > 5.
Statistical Analysis
We followed CDC guidelines when performing all statistical analyses, considering NHANES sampling weights from the complex multistage cluster survey design [33]. Categorical and continuous variables were described as percentages and means ± standard error (S.E.), respectively. We assessed the difference among different B-Cd quartiles via a weighted student’s t test for continuous variables and weighted chi-square test for categorical variables. We used multivariate linear regression to assess the relationship between B-Cd and AAC scores, while β (effect size) was used to present the results. We also tested the independent correlation between B-Cd and severe AAC using multivariate logistic regression, and the results were expressed as OR (odds ratio). In Model 1, no covariates were adjusted. Model 2 was adjusted for age, sex, and races. Model 3 was adjusted for age, sex and races, educational levels, marriage, RIP, BMI, Fastglucose, HbA1c, systolic blood pressure, diastolic blood pressure, diabetes, hypertension, total cholesterol, serum creatinine, serum uric acid, serum phosphorus, serum calcium, eGFR, alcohol use, triglyceride, and total 25-hydroxyvitamin D. Model 4 is a further adjustment of the smoking status based on Model 3. P < 0.05 was considered statistically significant. For the analysis of the correlation between B-Cd and AAC in the population with different weight status, we removed the underweight population because of its small number, only 22 people, accounting for about 1% of the total included population, and we partially adjusted the adjustment variables in the analysis for the population with different weight status in order to ensure the balance between the data. In Model a, no covariates were adjusted. Model b was adjusted for age, sex, and races. Model c was adjusted for age, sex, races, education level, RIP, diabetes, hypertension, serum creatinine, serum uric acid, serum phosphorus, serum calcium, eGFR, alcohol use, and total 25-hydroxyvitamin D. Model d is a further adjustment of the smoking status based on model c. All analysis was performed using R version 4.2.1. (http://www.R-project.org, The R Foundation).
Results
Baseline Characteristics of Participants
The baseline characteristics of the study population stratified by B-Cd quartiles are shown in Table 1. A total of 1530 participants aged 40 years or older were included, with a mean age of 57.24 ± 0.37, of whom 48.71% were men and 51.29% were women, with a mean blood Cd of 0.47 ± 0.02 and a range of B-Cd quartiles of 0.07–0.21, 0.21–0.335, 0.335–0.63, and 0.63–7.23. Among the different quartiles of B-Cd, there were significant differences in age, sex, race, BMI classification, education, systolic blood pressure, diastolic blood pressure, eGFR, serum uric acid, hypertension, diabetes, total 25-hydroxyvitamin D, triglycerides, serum calcium and serum phosphorus, poverty level, smoking, and AAC score. The prevalence of severe AAC increases with increasing BLL quartiles. Compared with the lowest quartile, participants in the highest B-Cd quartile were more likely to be female and Non-Hispanic Black, more likely to have hypertension, a history of alcohol consumption, smoking and poverty, as well as lower diastolic blood pressure, higher systolic blood pressure and triglyceride levels. The highest B-Cd quartile had a blood cadmium concentration of 1.25 ± 0.07ug/L (Fig. 2). In the total population, the mean score for AAC was 1.40 ± 0.1 and the prevalence of severe AAC was 7.98%. Participants in the highest quartile of B-Cd exhibited a higher risk of developing severe AAC compared to the lowest quartile (Q1: 4.05%; Q2: 9.00%; Q3: 10.04%; Q4: 10.37%) (Fig. 3).
The Relationship Between the Blood Cadmium and AAC Score and Severe AAC
The relationship between blood cadmium and AAC scores was observed in Table 2. The association between blood cadmium and AAC scores was not obvious in Model 1 and Model 2 (Model 1: β = 0.28, 95% confidence interval, CI: − 0.18–0.73, P = 0.21; Model2: β = 0.36, 95% confidence interval,CI: − 0.05–0.77, P = 0.08). When age, sex and races, educational levels, marriage, RIP, BMI, Fastglucose, HbA1c, systolic blood pressure, diastolic blood pressure, diabetes, hypertension, total cholesterol, serum creatinine, serum uric acid, serum phosphorus, serum calcium, eGFR, alcohol use, triglyceride, and total 25-hydroxyvitamin D were adjusted in model3, we observed a positive association between blood cadmium levels and AAC socres, a 1-unit increase in log2-transformed blood cadmium levels was associated with a 0.3 unit higher AAC socre (Model 3: β = 0.30, 95% confidence interval,CI: − 0.02–0.61, P = 0.04). When we further adjusted for smoking, blood cadmium and AAC score scores no longer had a positive correlation.
To further assess the correlation between blood cadmium concentrations and AAC scores, we converted blood cadmium concentrations from a continuous variable to a categorical variable (quartiles). In the unadjusted model, we observed an increase of 0.61 and 0.75 units in AAC scores for B-Cd Quartiles 2, 3, respectively, compared to their lowest quartiles. However, when adjusted for the corresponding covariates, the relationship between B-Cd and AAC scores was no longer significant.
The relationship between B-Cd and severe AAC has been revealed in Table 3, We found that increased B-Cd was positively associated with a higher risk of severe AAC in Model 2 and Model 3 (Model 2: OR = 1.79; 95% CI: 1.19–2.69; P = 0.01; Model 3: OR = 2.18; 95% CI: 1.34–3.55; P = 0.004). In addition, we also observed that in the unadjusted model, the OR (reference to Quartile 1) was 2.34 (95% CI: 1.16–4.71; P = 0.02) for Quartile 2, 2.64 for Quartile 3 (95% CI: 1.01–6.88; P = 0.04), and 2.74 for Quartile 4 (95% CI: 1.05–7.14; P = 0.04). In Model 3, the adjusted OR for Quartile 4 was 8.59 (95% CI: 2.35–31.37; P = 0.003) (reference to Quartile 1), suggesting that partipants in Quartile 4 had a significant 759% higher risk of severe AAC than those in the B-Cd Quartile 1. However, we found that a positive relationship between higher B-Cd and increased risk of severe AAC only existed in Quartile 2 (95% CI: 1.03–27.60; P = 0.04) of blood cadmium levels for Model 4.
The Relationship Between the Blood Cadmium and AAC Score and Severe AAC in Population With Different Weight Statuses
For the analysis of the correlation between B-Cd and AAC in the population with different weight status, we removed the underweight population because of its small number, and we partially adjusted the adjustment variables in the analysis for the population with different weight status in order to ensure the balance between the data. In the unadjusted model, we observed a positive correlation between blood cadmium levels and AAC scores in B-Cd Quartile 3 of the overweight population (β = 1.17, 95% confidence interval, CI:0.21–2.12, P = 0.021). But in the fully adjusted model, this correlation no longer existed (Table 4, Fig. 4).
In the model adjusted for age, sex, and race, we observed a positive association between blood cadmium concentrations and the prevalence of severe AAC in overweight and obese populations (Overweight OR 2.49; 95% CI: 1.14–4.71; P = 0.027. Obesity OR 2.08; 95% CI: 1.05–4.12; P = 0.039). In the overweight population, the risk of developing severe AAC increased 149% with each unit of blood cadmium concentration, while in the obese population, the risk of developing severe AAC increased 108% with each unit of cadmium exposure (Table 4). When we further adjusted the education level, RIP, diameter, hypertension, serium creativity, serium uric acid, serium phophorus, serium calculus, eGFR, alcohol use, and total 25-hydroxyvitamin D, We still observed a positive association between increased B-Cd and the risk of severe AAC in overweight and obese people (Overweight OR 2.94; 95% CI: 1.01–8.55; P = 0.048. Obesity OR 3.03; 95% CI: 1.03–8.90; P = 0.044). In Model d, a positive association between increased B-Cd and risk of severe AAC was observed only in the obese population, with a 125% increase in the higher risk of severe AAC with increasing concentrations of cadmium per unit (Obesity OR 2.25; 95% CI: 1.04–4.88; P = 0.041) (Table 4, Fig. 5).
Odds ratios (95% confidence intervals) for the association between blood cadmium concentrations and AAC scores and severe AAC by weight status subgroups in the fully adjusted model. A Odds ratios (95% confidence intervals) for the association between blood cadmium concentrations and severe AAC by weight status subgroups, B Odds ratios (95% confidence intervals) for the association between blood cadmium concentrations and severe AAC by weight status subgroups
In Model c, the adjusted OR (reference to Quartile 1) was 6.64 (OR 6.64; 95% CI: 1.32–33.44; P = 0.025) for Quartile 4, suggesting a positive relationship between higher B-Cd and increased risk of severe AAC with statistical sighificance in overweight population. However, this positive association did not exist in Model d (Table 4, Fig. 6).
Discussion
In our large cross-sectional study that included 1530 participants, we found a possible correlation of B-Cd with AAC scores and severe AAC. In adjusted model 3, we observed that each 1 unit increase in blood cadmium level was associated with a 0.3 unit increase in AAC score, although this correlation disappeared after further adjustment for smoking. In addition to this, we found a positive association between increased B-Cd and increased risk of severe AAC in Model 2 and 3. In the fully adjusted model, we found a positive correlation between increased risk of B-Cd severe AAC only in Quartile 2 with quartiles of blood cadmium levels. We further analyzed the effect of blood cadmium exposure on AAC in people with different weight status, and we found In the unadjusted model, we observed a positive correlation between blood cadmium levels and AAC scores in Quartile 3 of B-Cd in the overweight population. In Model a, Model b, and Model c, we observed a positive association between increased B-Cd and the risk of severe AAC in overweight and obese populations. In Model d, a positive association between increased B-Cd and the risk of severe AAC was observed only in the obese population. Therefore, in our clinical work, we should pay attention to cadmium load in AAC patients, especially in those obese population.
Several studies have shown a positive association between cadmium exposure and the risk of cardiovascular disease [34,35,36]. One study demonstrated that foodborne cadmium exposure accounted for a considerable burden of coronary heart disease and stroke [34]. Ma et al. found that elevated serum cadmium concentrations were positively associated with congestive heart failure, coronary heart disease, heart failure and risk of stroke [35]. Björn Fagerberg et al. reported that cadmium exposure was associated with an increased risk of atherosclerotic plaque formation, and with a 40% increase in the prevalence of large plaques [36]. In a study of young women, cadmium levels were found to be associated with early atherosclerotic vessel wall thickening, and the authors also found significant aortic plaque production in ApoE knockout cadmium-fed mice compared to controls [23]. In addition, cadmium levels at the site of aortic plaque rupture were twice as high as in other parts of the plaque [37]. Aramjoo H et al. found that blood and hair cadmium levels were correlated with hypertension, with hair being the best biological sample to study the relationship between cadmium exposure and hypertension [38]. A study in a Korean population also showed that high blood cadmium levels were significantly associated with high systolic blood pressure, high diastolic blood pressure and increased risk of hypertension in never-smoking women [39].
The specific mechanisms linking cadmium exposure to cardiovascular disease are not known. One of the possible mechanisms of vascular calcification due to cadmium exposure may be related to oxidative stress and inflammation. Cadmium exposure may lead to upregulation of the pro-inflammatory cytokines (IL-6,IL-1β, TNF-α) and the inflammatory markers (C-reactive protein) [40, 41]. Cadmium exposure activated the expression of mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-kB) pathways, two major inflammatory signaling pathways that are closely associated with the regulation of apoptosis [42, 43]. Elevated levels of pro-inflammatory cytokines can activate osteogenic proteins by upregulating NF-kB ligand/osteoprotegerin expression, thereby favoring osteoblast differentiation of vascular smooth muscle cells [44]. Cadmium can cause oxidative stress by inducing an antioxidant response that leads to mitophagy, resulting in the accumulation of reactive oxygen species in vivo [45]. In turn, elevated oxidative stress can promote vascular calcification through mechanisms such as DNA damage and extracellular matrix remodeling [46]. Oxidative stress is closely related to the phenotype of vascular smooth muscle cells and can increase osteogenic gene expression and extracellular vesicle secretion in vascular smooth muscle cells, thus contributing to osteoblastic differentiation of vascular smooth muscle cells [47].
The association between blood cadmium and AAC is stronger in obese and overweight populations. It has been suggested that overweight or obesity significantly amplifies the adverse effects of cadmium exposure on the risk of prediabetes [48]. One study found that high urinary cadmium was significantly associated with elevated systolic and diastolic blood pressure in obese people, while such significance was not observed in non-obese people [49]. The opposite result was shown in one study, which found a significant association between urinary cadmium and hypertension in normal weight participants, but not in overweight or obese participants [50]. Although the exact mechanism responsible for this phenomenon has not been fully elucidated, we speculate that it may be related to increased oxidative stress due to obesity. One study suggested that increased production of reactive oxygen species by leukocytes of those obese individuals may be responsible for increased oxidative damage to lipids and proteins, thus leading to atherosclerosis [51].
Cadmium, a toxic heavy metal found in soil, seafood, and water, has caused serious global environmental problems and may lead to a heavy socio-economic burden [52, 53]. Common causes of cadmium exposure mainly include smoking, traffic emissions and ingestion of food contaminated with cadmium [54, 55]. It has been found that deficiency of calcium and zinc, two essential metal elements, leads to increased absorption of cadmium by the body, which further aggravates its toxic effects in the organism [56]. The results of animal experiments also demonstrated that supplementation with zinc ions ameliorated the nephrotoxic effects of cadmium exposure in rats and repaired the expression of tight junction proteins in the kidney [57]. One study found that supplementation with vitamin C reduced cadmium load in liver, kidney, testis, and muscle [58]. Vitamin D supplementation not only ameliorated the inhibition of osteoblast proliferation due to cadmium exposure, but also alleviated cadmium-induced hepatotoxicity through antioxidant and anti-inflammatory pathways [59, 60]. Vitamin E has a protective effect against cadmium-induced apoptosis via the Bax/Bcl2 pathway [61]. The synergistic effect among the micronutrients can also alleviate the toxic effects caused by cadmium exposure. Co-administration of vitamin C and vitamin E ameliorated the elevated markers of renal injury and reduced renal oxidative stress markers in rats exposed to cadmium, resulting in a significant repair of renal function [62]. The combination of vitamin C, vitamin E, and selenium ameliorated the degenerative changes in the stomach induced by cadmium toxicity [63]. Various chelating agents such as ethylenediaminetetraacetic acid (EDTA) have also been used to treat cadmium toxicity [64, 65]. Probiotics have also shown promising potential in reducing cadmium toxicity. Seven probiotics, including Lactobacillus, Bifidobacterium longum, clotting bacillus, Streptococcus valerate, etc., were found to exhibit significant therapeutic effects on cadmium toxicity in preclinical studies [66]. The mechanism of the protective effect of probiotics against cadmium toxicity may be due to their ability to reduce intestinal cadmium absorption, avoid tissue cadmium accumulation, and reduce oxidative stress [67, 68]. In a randomized controlled trial, it was also found that oral administration of L. plantarum not only reduced cadmium levels in the blood of subjects, but also increased the rate of cadmium excretion in the feces, and this modulating effect may be related to the intestinal microbiota, as the authors found that the protective effect of L. plantarum against cadmium toxicity was diminished after administration of antibiotic treatment [69]. These findings above support the development of safe and effective strategies to prevent and control cadmium exposure, such as reducing the bioavailability of cadmium in the body, thus minimizing cadmium exposure, preventing adverse health events and reducing the global economic burden. In addition, the state and government should strengthen environmental monitoring of cadmium, develop relevant policies to help people identify the sources of cadmium contamination and how to avoid cadmium exposure in a timely manner, and conduct a comprehensive health risk assessment to mitigate cadmium exposure in the general population. Cadmium exposure may be a modifiable risk factor for the development and progression of abdominal aortic calcification, and more research on the mechanisms between cadmium toxicity and abdominal aortic calcification is necessary. Identification of molecular targets of cadmium exposure and early biomarkers of cadmium cardiovascular toxicity as well as studies on the interaction between cadmium and other cardiovascular risk factors are also necessary to lay the foundation for the implementation of individualized prevention and control measures.
Our study is based on data from the NHANES database, a national population-based sample, and obtained through standard protocols. Appropriate NHANES sampling weights were considered for all analyses. We also adjusted covariates based on previous studies to reduce confounding bias. However, our study still has some limitations. First, because our study was a cross-sectional study, a causal relationship between blood cadmium levels and AAC could not be obtained, and a longitudinal study with a larger sample size may be needed to further demonstrate this. Second, because the participants in our study were all from the same country, the results of this study may not be generalizable to many other countries around the world. Finally, our study may not be able to exclude the effect of drug use on vascular calcification, and we were unable to analyze this association in a broad age group because patients younger than 40 years of age did not have DXA scans and therefore had missing AAC scores.
Conclusions
Blood cadmium concentration was positively correlated with AAC score and the risk of severe AAC. This correlation was more significant in overweight and obese populations. Consider the adverse effects of cadmium exposure on the cardiovascular system. We should consider the cadmium load in patients with AAC in our clinical work, especially in overweight and obese populations. Larger prospective studies are needed to assess the exact causality of this relationship.
Data Availability
Data described in the manuscript, codebook, and analytic code will be made publicly and freely available without restriction at www.cdc.gov/nchs/nhanes/
References
Villa-Bellosta R. Vascular calcification: key roles of phosphate and pyrophosphate. Int J Mol Sci. 2021;22(24):13536.
Yuan C, Ni L, Zhang C, Hu X, Wu X. Vascular calcification: new insights into endothelial cells. Microvasc Res. 2021;134:104105.
Liu ZH, Yu XQ, Yang JW, Jiang AL, Liu BC, Xing CY, et al. Prevalence and risk factors for vascular calcification in Chinese patients receiving dialysis: baseline results from a prospective cohort study. Curr Med Res Opin. 2018;34(8):1491–500.
Ghosh S, Luo D, He W, Chen J, Su X, Huang H. Diabetes and calcification: the potential role of anti-diabetic drugs on vascular calcification regression. Pharmacol Res. 2020;158:104861.
Leopold JA. Vascular calcification: mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc Med. 2015;25(4):267–74.
Chen J, Budoff MJ, Reilly MP, Yang W, Rosas SE, Rahman M, et al. Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA Cardiol. 2017;2(6):635–43.
Djuric P, Dimkovic N, Schlieper G, Djuric Z, Pantelic M, Mitrovic M, et al. Sodium thiosulphate and progression of vascular calcification in end-stage renal disease patients: a double-blind, randomized, placebo-controlled study. Nephrol Dial Transplant. 2020;35(1):162–9.
Raggi P, Bellasi A, Bushinsky D, Bover J, Rodriguez M, Ketteler M, et al. Slowing progression of cardiovascular calcification with SNF472 in patients on hemodialysis: results of a randomized phase 2b Study. Circulation. 2020;141(9):728–39.
Bendix EF, Johansen E, Ringgaard T, Wolder M, Starup-Linde J. Diabetes and abdominal aortic calcification-a systematic review. Curr Osteoporos Rep. 2018;16(1):42–57.
Zhou Y, Hellberg M, Kouidi E, Deligiannis A, Höglund P, Clyne N. Relationships between abdominal aortic calcification, glomerular filtration rate, and cardiovascular risk factors in patients with non-dialysis dependent chronic kidney disease. Clin Nephrol. 2018;90(6):380–9.
Rahman EU, Chobufo MD, Farah F, Elhamdani A, Khan A, Thompson EA, et al. Prevalence and risk factors for the development of abdominal aortic calcification among the US population: NHANES study. Arch Med Sci Atheroscler Dis. 2021;6:e95–101.
Rodondi N, Taylor BC, Bauer DC, Lui LY, Vogt MT, Fink HA, et al. Association between aortic calcification and total and cardiovascular mortality in older women. J Intern Med. 2007;261(3):238–44.
Selvan K, Sampathkumar K, Sampath D, Rajiv A. Abdominal aortic calcification as prognostic marker in continuous ambulatory peritoneal dialysis. Clin Nephrol. 2022;98(6):267–73.
Golestani R, Tio R, Zeebregts CJ, Zeilstra A, Dierckx RA, Boersma HH, et al. Abdominal aortic calcification detected by dual X-ray absorptiometry: a strong predictor for cardiovascular events. Ann Med. 2010;42(7):539–45.
Leow K, Szulc P, Schousboe JT, Kiel DP, Teixeira-Pinto A, Shaikh H, et al. Prognostic value of abdominal aortic calcification: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. 2021;10(2):e017205.
Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132(2):245–50.
Mäkelä S, Asola M, Hadimeri H, Heaf J, Heiro M, Kauppila L, et al. Abdominal aortic calcifications predict survival in peritoneal dialysis patients. Perit Dial Int. 2018;38(5):366–73.
Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure, and health outcomes. Environ Health Perspect. 2010;118(2):182–90.
Rani A, Kumar A, Lal A, Pant M. Cellular mechanisms of cadmium-induced toxicity: a review. Int J Environ Health Res. 2014;24(4):378–99.
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A. The effects of cadmium toxicity. Int J Environ Res Public Health. 2020;17(11):3782.
Eum KD, Lee MS, Paek D. Cadmium in blood and hypertension. Sci Total Environ. 2008;407(1):147–53.
Li Z, Fan Y, Tao C, Yan W, Huang Y, Qian H, et al. Association between exposure to cadmium and risk of all-cause and cause-specific mortality in the general US adults: a prospective cohort study. Chemosphere. 2022;307(Pt 4):136060.
Messner B, Knoflach M, Seubert A, Ritsch A, Pfaller K, Henderson B, et al. Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arterioscler Thromb Vasc Biol. 2009;29(9):1392–8.
Barregard L, Sallsten G, Harari F, Andersson EM, Forsgard N, Hjelmgren O, et al. Cadmium exposure and coronary artery atherosclerosis: a cross-sectional population-based study of Swedish middle-aged adults. Environ Health Perspect. 2021;129(6):67007.
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81.
Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27.
Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88.
Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat 1. 201310(56):1–37.
Westgard JO, Barry PL, Hunt MR, Groth T. A multi-rule Shewhart chart for quality control in clinical chemistry. Clin Chem. 1981;27(3):493–501.
Górriz JL, Molina P, Cerverón MJ, Vila R, Bover J, Nieto J, et al. Vascular calcification in patients with nondialysis CKD over 3 years. Clin J Am Soc Nephrol. 2015;10(4):654–66.
Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017;289:1–8.
Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–9.
Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat 2. 2013;10(161):1–24.
Liu J, Li Y, Li D, Wang Y, Wei S. The burden of coronary heart disease and stroke attributable to dietary cadmium exposure in Chinese adults, 2017. Sci Total Environ. 2022;825:153997.
Ma S, Zhang J, Xu C, Da M, Xu Y, Chen Y, et al. Increased serum levels of cadmium are associated with an elevated risk of cardiovascular disease in adults. Environ Sci Pollut Res Int. 2022;29(2):1836–44.
Fagerberg B, Barregard L, Sallsten G, Forsgard N, Ostling G, Persson M, et al. Cadmium exposure and atherosclerotic carotid plaques–results from the Malmö diet and Cancer study. Environ Res. 2015;136:67–74.
Bergström G, Fagerberg B, Sallsten G, Lundh T, Barregard L. Is cadmium exposure associated with the burden, vulnerability and rupture of human atherosclerotic plaques? Plos One. 2015;10(3):e0121240.
Aramjoo H, Arab-Zozani M, Feyzi A, Naghizadeh A, Aschner M, Naimabadi A, et al. The association between environmental cadmium exposure, blood pressure, and hypertension: a systematic review and meta-analysis. Environ Sci Pollut Res Int. 2022;29(24):35682–706.
Kwon JA, Park E, Kim S, Kim B. Influence of serum ferritin combined with blood cadmium concentrations on blood pressure and hypertension: from the Korean National Health and Nutrition Examination Survey. Chemosphere. 2022;288(Pt 1):132469.
Arikan TA, Kelles M. Plasma selenium and cadmium levels in patients with chronic otitis media in a Turkish population and their relation to inflammation markers. Biol Trace Elem Res. 2019;189(1):55–63.
Hong H, Xu Y, Xu J, Zhang J, Xi Y, Pi H, et al. Cadmium exposure impairs pancreatic β-cell function and exaggerates diabetes by disrupting lipid metabolism. Environ Int. 2021;149:106406.
Li T, Quan H, Zhang H, Lin L, Ou Q, Chen K. Silencing cyclophilin A improves insulin secretion, reduces cell apoptosis, and alleviates inflammation as well as oxidant stress in high glucose-induced pancreatic β-cells via MAPK/NF-kb signaling pathway. Bioengineered. 2020;11(1):1047–57.
Liu L, Zhao L, Liu Y, Yu X, Qiao X. Rutin ameliorates cadmium-induced necroptosis in the chicken liver via inhibiting oxidative stress and MAPK/NF-κB pathway. Biol Trace Elem Res. 2022;200(4):1799–810.
Ono T, Hayashi M, Sasaki F, Nakashima T. RANKL biology: bone metabolism, the immune system, and beyond. Inflamm Regen. 2020;40:2.
Park JH, Lee BM, Kim HS. Potential protective roles of curcumin against cadmium-induced toxicity and oxidative stress. J Toxicol Environ Health B Crit Rev. 2021;24(3):95–118.
Hu CT, Shao YD, Liu YZ, Xiao X, Cheng ZB, Qu SL, et al. Oxidative stress in vascular calcification. Clin Chim Acta. 2021;519:101–10.
Petsophonsakul P, Burgmaier M, Willems B, Heeneman S, Stadler N, Gremse F, et al. Nicotine promotes vascular calcification via intracellular Ca2+-mediated, Nox5-induced oxidative stress, and extracellular vesicle release in vascular smooth muscle cells. Cardiovasc Res. 2022;118(9):2196–210.
Jiang F, Zhi X, Xu M, Li B, Zhang Z. Gender-specific differences of interaction between cadmium exposure and obesity on prediabetes in the NHANES 2007–2012 population. Endocrine. 2018;61(2):258–66.
Vallée A, Gabet A, Grave C, Blacher J, Olié V. Associations between urinary cadmium levels, blood pressure, and hypertension: the ESTEBAN survey. Environ Sci Pollut Res Int. 2020;27(10):10748–56.
Wang Q, Wei S. Cadmium affects blood pressure and negatively interacts with obesity: findings from NHANES 1999–2014. Sci Total Environ. 2018;643:270–6.
Dandona P, Mohanty P, Ghanim H, Aljada A, Browne R, Hamouda W, et al. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab. 2001;86(1):355–62.
Clemens S, Ma JF. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu Rev Plant Biol. 2016;67:489–512.
Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich A, et al. The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol. 2006;1:22.
Böhlandt A, Schierl R, Diemer J, Koch C, Bolte G, Kiranoglu M, et al. High concentrations of cadmium, cerium and lanthanum in indoor air due to environmental tobacco smoke. Sci Total Environ. 2012;414:738–41.
Yang Q, Li Z, Lu X, Duan Q, Huang L, Bi J. A review of soil heavy metal pollution from industrial and agricultural regions in China: pollution and risk assessment. Sci Total Environ. 2018;642:690–700.
Reeves PG, Chaney RL. Marginal nutritional status of zinc, iron, and calcium increases cadmium retention in the duodenum and other organs of rats fed rice-based diets. Environ Res. 2004;96(3):311–22.
Jacquillet G, Barbier O, Cougnon M, Tauc M, Namorado MC, Martin D, et al. Zinc protects renal function during cadmium intoxication in the rat. Am J Physiol Renal Physiol. 2006;290(1):F127-137.
Grosicki A. Influence of vitamin C on cadmium absorption and distribution in rats. J Trace Elem Med Biol. 2004;18(2):183–7.
Dal Ulutas A, Turgut Cosan D, Mutlu F. Protective and curative role of vitamin D and hormones on the cadmium-induced inhibition of proliferation of human osteoblast cells. J Basic Clin Physiol Pharmacol. 2020;32(5):995–1000.
El-Boshy M, Refaat B, Almaimani RA, Abdelghany AH, Ahmad J, Idris S, et al. Vitamin D(3) and calcium cosupplementation alleviates cadmium hepatotoxicity in the rat: enhanced antioxidative and anti-inflammatory actions by remodeling cellular calcium pathways. J Biochem Mol Toxicol. 2020;34(3):e22440.
Amanpour P, Khodarahmi P, Salehipour M. Protective effects of vitamin E on cadmium-induced apoptosis in rat testes. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(3):349–58.
Milton Prabu S, Shagirtha K, Renugadevi J. Quercetin in combination with vitamins (C and E) improves oxidative stress and renal injury in cadmium intoxicated rats. Eur Rev Med Pharmacol Sci. 2010;14(11):903–14.
Bolkent S, Sacan O, Yanardag R, Bolkent S. Effects of vitamin E, vitamin C, and selenium on gastric fundus in cadmium toxicity in male rats. Int J Toxicol. 2008;27(2):217–22.
Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013;309(12):1241–50.
Mousavi A, Pourakbar L, Moghaddam SS. Effects of malic acid and EDTA on oxidative stress and antioxidant enzymes of okra (Abelmoschus esculentus L.) exposed to cadmium stress. Ecotoxicol Environ Saf. 2022;248:114320.
Bhattacharya S. The role of probiotics in the amelioration of cadmium toxicity. Biol Trace Elem Res. 2020;197(2):440–4.
Daisley BA, Monachese M, Trinder M, Bisanz JE, Chmiel JA, Burton JP, et al. Immobilization of cadmium and lead by Lactobacillus rhamnosus GR-1 mitigates apical-to-basolateral heavy metal translocation in a Caco-2 model of the intestinal epithelium. Gut Microbes. 2019;10(3):321–33.
Zhai Q, Wang G, Zhao J, Liu X, Tian F, Zhang H, et al. Protective effects of Lactobacillus plantarum CCFM8610 against acute cadmium toxicity in mice. Appl Environ Microbiol. 2013;79(5):1508–15.
Zhai Q, Liu Y, Wang C, Zhao J, Zhang H, Tian F, et al. Increased cadmium excretion due to oral administration of lactobacillus plantarum strains by regulating enterohepatic circulation in mice. J Agric Food Chem. 2019;67(14):3956–65.
Funding
The study was supported by The First batch of key Disciplines on Public Health in Chongqing.
Author information
Authors and Affiliations
Contributions
DL and CL wrote the paper. MY reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The studies involving human participants were reviewed and approved by the NCHS Ethics Review Board. Written informed consent was obtained from all participants in this study.
Consent for Publication
All authors contributed to the article and approved the submitted version.
Competing Interests
The authors declare no competing interests.
Additional information
Associate Editor Laura Iop oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, D., Liu, C. & Yang, M. Blood Cadmium and Abdominal Aortic Calcification in Population with Different Weight Statuses: a Population-Based Study. J. of Cardiovasc. Trans. Res. 16, 1425–1438 (2023). https://doi.org/10.1007/s12265-023-10414-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-023-10414-5