Abstract
The purpose of our study was primarily to investigate the relationship between dietary copper intake and abdominal aortic calcification (AAC) in US adults. We used data from the National Health and Nutrition Examination Survey (NHANES) 2013–2014 for our analysis. Multivariate linear regression analysis was used to explore the relationship between copper intake and AAC scores. We also used multivariate logistic regression analysis to explore the association between copper intake and the risk of AAC and severe AAC. We also examined whether there was a nonlinear relationship between copper intake and AAC scores and risk of AAC and severe AAC using restricted cubic splines (RCS) analysis. In addition, we also performed subgroup analysis and interaction tests. A total of 2897 participants were recruited in this study. The mean AAC score of the participants was 1.46 ± 0.11, and the prevalence of AAC and severe AAC among the participants was 28.53% and 7.68%, respectively. In the fully adjusted model, a negative association of copper intake with AAC scores (β = − 0.16, 95%CI: − 0.49 ~ 0.17) and the risk of AAC (OR = 0.85, 95% CI: 0.61–1.19) and severe AAC (OR = 0.82, 95% CI: 0.49–1.38) was observed. Compared to participants in the lowest tertile of copper intake, participants in the highest tertile of copper intake had a 0.37-unit decrease in mean AAC score (β = − 0.37, 95% CI: − 0.90–0.15) and a significant 38% and 22% decrease in risk of AAC (OR = 0.62, 95% CI: 0.41–0.95) and severe AAC (OR = 0.78, 95% CI: 0.34 − 1.77), respectively. The results of subgroup analyses and interaction tests suggested no significant differences in AAC scores and AAC risk between the different strata. In contrast, the risk of severe AAC was significantly dependent on the patients’ diabetes status. Increased copper intake was associated with decreased AAC scores and decreased likelihood of AAC and severe AAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular calcification (VC) is a pathological process of abnormal deposition of calcium, phosphorus, and other mineral components in the walls of blood vessels, commonly found in the aorta, coronary arteries, and aortic valves [1,2,3]. VC is common in patients with diabetes [4] and chronic kidney disease (CKD) [5,6,7]. Coronary artery calcification score could predict all-cause mortality and risk of cardiovascular events in patients with type 2 diabetes [8]. In patients with stage CKD 3–5, moderate to severe coronary artery calcification was associated with an increased risk of cardiovascular events [9]. One study suggested that vascular calcification was also an independent predictor of cardiovascular events in patients receiving peritoneal dialysis [10].
Abdominal aortic calcification (AAC) has received increasing attention in recent years. The prevalence of AAC has been reported to be 28.8% in adults in the USA [11]. Advanced age was associated with an increased incidence of AAC, and the prevalence of AAC has been reported to be as high as about 96% in older women aged 85 years or older [12]. AAC was thought to be associated with poor cardiovascular prognosis in pre-dialysis CKD patients [13]. AAC has also been used to predict the risk of adverse cardiac and cerebrovascular events and the occurrence of left ventricular remodeling in dialysis patients [14, 15]. Even in the general population, AAC remained significantly associated with long-term cardiovascular events and mortality [16]. Kaupplia et al. proposed a method to grade and score calcification of the abdominal aorta by lateral lumbar spine radiographs, which can effectively assess the severity of AAC [17]. Subsequently, Kidney Disease: Improving Global Outcomes (KDIGO) recommended the use of the AAC score to manage arterial calcification in peritoneal dialysis patients [18].
Copper is an important micronutrient for human health and development, since copper cannot be synthesized in the body, it must be obtained daily from foods such as animal offal, nuts, and legumes and from drinking water [19, 20]. Copper is particularly important for the development of the brain, bones, and other organs [21]. In addition, copper can also be used as a cofactor component of enzymes such as cytochrome C oxidase and superoxide dismutase to participate in processes such as energy metabolism and redox mechanisms in the body [22, 23]. One study found that serum copper concentrations were significantly higher in patients with atherosclerosis and showed a positive correlation with the severity of the disease [24]. A study investigating copper and cardiovascular disease risk factors demonstrated a significant positive association between serum copper concentrations and total cholesterol and glycated hemoglobin (HbA1c) levels [25]. In another study, dietary copper intake levels were negatively associated with the risk of myocardial infarction, and this negative association was more pronounced in older women, smokers, and overweight individuals [26]. The researchers also found that inadequate copper intake was not associated with hospitalization and mortality rates in outpatients with heart failure [27]. Plasma copper concentration was also found to be positively associated with the risk of first stroke episode in patients with hypertension [28]. Another study found that an increase in dietary copper intake reduced the risk of stroke [29].
However, the relationship between copper intake and AAC has not been reported previously. Therefore, we used the data from the National Health and Nutrition Examination Survey (NHANES) 2013–2014 to explore the relationship between copper intake and AAC scores and the risk of AAC and severe AAC. The authors hypothesized that a higher copper intake was negatively associated with AAC scores and the risk of AAC and severe AAC.
Materials and Methods
Study Population
The National Health and Nutrition Examination (NHANES) survey is a national cross-sectional survey study based on the US population with the primary purpose of assessing the health and nutritional status of the US population. Participants underwent standardized household interviews and health screenings at mobile examination centers to assess their physical status and laboratory tests to collect their laboratory-related data. The NHANES used a complex stratified, multilevel probability cluster sampling design, resulting in the recruitment of a highly representative sample of the US population. The NHANES study protocol was approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board. All participants provided written informed consent. The detailed NHANES study design and data are publicly available at https://www.cdc.gov/nchs/nhanes/.
We used the 2013–2014 NHANES survey cycle to assess the correlation between dietary copper intake and AAC because this was the only survey cycle that contained complete data on both copper intake and AAC scores. Data on AAC scores were obtained by dual-energy X-ray absorptiometry scanning (DXA), and we excluded participants under the age of 40 years because only those aged 40 years and older underwent DXA.
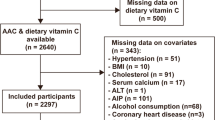
We initially included 10,175 participants in this study, and after excluding data from participants under the age of 40 years (n = 6360), as well as from lack of dietary copper intake (n = 514) and AAC scores (n = 404), a total of 2897 subjects were included in our final analysis (Fig. 1).
Assessment of Copper Intake
All NHANES participants were eligible to participate in two 24-h dietary recall interviews. The first dietary recall interview was collected in person at the mobile testing center. The second interview was collected by telephone 3–10 days later. Dietary intake of copper was obtained from the total nutrient intake file, which contains the total nutrients for all foods and beverages. The average copper intake from two 24-h recalls was used in our analysis.
Definition of Abdominal Aortic Calcification
The AAC score is an assessment system proposed by Kauppila et al. by quantifying the lumbar lateral images obtained by DXA [17]. The total AAC score ranges from 0 to 24. We used the AAC score to assess the severity of abdominal aortic calcification, where a higher AAC score represented a more severe calcification of the abdominal aorta. Based on previous studies, the authors defined participants with an AAC score of 6 or more as having severe AAC [30, 31].
Selection of Covariates
The covariates included in our analysis included age, sex, race, education levels, ratio of family income to poverty (PIR), body mass index (BMI), total cholesterol, total energy intake, hypertension, diabetes (DM), alcohol consumption, and smoking status. BMI was classified as < 25, 25–29.9, and ≥ 30 kg/m2, which corresponded to normal weight, overweight, and obesity for all participants. Hypertension was defined based on a self-reported diagnosis of hypertension, diastolic blood pressure ≥ 90 mmHg or systolic blood pressure ≥ 140 mmHg, or the use of antihypertensive medications [32]. DM was defined base on a self-reported diagnosis of diabetes mellitus, 2-h plasma glucose ≥ 200 mg/dL in an oral glucose tolerance test, HbAlc ≥ 6.5%, use of oral hypoglycemic agents, or fasting glucose ≥ 126 mg/dL [33]. All details regarding these variables are available on the website at www.cdc.gov/nchs/nhanes/.
Statistical Analysis
Statistical analysis was performed using appropriate sampling weights according to NHANES analysis guidelines and considering complex multi-stage clustering surveys. Continuous variables were presented as mean with standard deviation, and categorical variables were presented as percentages. Weighted Student’s t-test (continuous variables) or a weighted chi-square test (categorical variables) was employed to assess the differences among participants grouped by copper intake and AAC. To examine the association between copper intake and AAC, multivariable linear regression used AAC score as a continuous variable and logistic regression used the risk of AAC (AAC score > 0) and severe AAC (AAC score > 6) as dichotomous variables in three different models. No covariates were adjusted for in Model 1, and in Model 2, age, sex, and race were adjusted. In Model 3, adjustments were made for sex, age, race, education levels, PIR, BMI, total cholesterol, total energy intake, hypertension, DM, alcohol consumption, and smoking status. Subgroup analysis on the associations of copper intake with AAC scores and the risk of AAC and severe AAC was conducted with stratified factors including age, sex, race, diabetes, hypertension, BMI, and smoking status. In addition, an interaction term was added to test the heterogeneity of associations between the subgroups. Restricted cubic spline (RCS) is a popular way to explore the nonlinear relationship in regression models flexibly and is widely used in epidemiology and clinical trials. RCS analysis (with three piecewise points) was performed to evaluate the nonlinear associations between copper intake and AAC scores and the risk of AAC and severe AAC. All analysis was performed using R version 4.2.1 (http://www.R-project.org, The R Foundation). p < 0.05 was considered statistically significant.
Results
Baseline Characteristics of Participants
The weighted baseline characteristics of the included individuals are shown in Table 1. A total of 2897 participants were enrolled in our study, with a mean age of 57.47 ± 0.28 years, of which 52.42% were female and 47.58% were male. The mean AAC score of the participants was 1.46 ± 0.11, and the prevalence of AAC and severe AAC among the participants was 28.53% and 7.68%, respectively. In the lowest copper intake tertile participants, the prevalence of AAC and severe AAC was 32.26% and 10.50%, respectively. Participants in the highest copper intake tertile showed the lowest rate of AAC (23.54%) and severe AAC (5.28%). Among the copper intake tertiles, age, total energy intake, gender, race, education, PIR, BMI, hypertension, diabetes, alcohol consumption, and smoking status were statistically significant (all p < 0.05). Compared to the lowest copper intake group, participants with increased copper intake were more likely to be male, less likely to have hypertension and diabetes, more likely to be never smokers and former smokers, more educated, had lower household poverty, were more likely to be normal weight and overweight, and had higher total energy intake. There were no statistically significant differences in serum creatinine, serum uric acid, and total cholesterol between the tertiles (all p > 0.05).
The Association Between Copper Intake and Increased AAC Scores
Our findings suggested that higher copper intake was associated with decreased AAC scores (Table 2). The correlation between copper intake and AAC score was significant in both our crude model (β = − 0.34, 95% CI: − 0.46 ~ − 0.21) and the minimally adjusted model (β = − 0.25, 95% CI: − 0.46 ~ − 0.04). The negative association between copper intake and AAC score remained stable in the fully adjusted model (β = − 0.16, 95%CI: − 0.49 ~ 0.17), which indicated that each unit increase in copper intake was associated with a 0.16 unit decrease in AAC score. When the authors considered copper intake as tertiles, participants in the highest tertile of copper intake had a mean AAC score decrease of 0.37 units (β = − 0.37, 95% CI: − 0.90–0.15) compared to participants in the lowest tertile. Participants in the middle copper intake tertile also exhibited a decrease in mean AAC scores compared to participants in the lowest tertile (β = − 0.32, 95% CI: − 0.77–0.12).
The Negative Association Between Copper Intake and the Risk of AAC and Severe AAC
We also investigated the association of copper intake with the risk of AAC in three different models (Table 3). We observed a negative association between copper intake and the risk of AAC in both the crude and minimally adjusted models, although this correlation was not consistent with statistical significance. In contrast, in the fully adjusted model, we observed that each 1-unit increase in copper intake was associated with a 15% decrease in the risk of AAC (OR = 0.85, 95% CI: 0.61–1.19). When we further adjusted copper intake from a continuous variable to a categorical variable, the risk of AAC decreased by 38% in participants in the highest tertile of copper intake compared to the lowest tertile of copper intake (OR = 0.62, 95% CI: 0.41–0.95).
For severe AAC, we also observed a negative association between copper intake and the increased likelihood of severe AAC with statistical significance (Table 4). In both the crude and minimally adjusted models, the authors found that participants with higher copper intake exhibited a lower risk of severe AAC (Model 1: OR = 0.60, 95% CI: 0.44–0.81; Model 2: OR = 0.68, 95% CI: 0.47–0.98). In the fully adjusted model, the authors observed that participants with higher copper intake were 18% less likely to have severe AAC (Model 3: OR = 0.82, 95% CI: 0.49–1.38). This correlation remained statistically significant when we considered copper intake as tertiles. Participants in the highest tertile had a 22% lower risk of severe AAC compared to those in the lowest tertile of copper intake (Model 3: OR = 0.78, 95% CI: 0.34–1.77).
To further explore the correlation between copper intake and AAC score and risk of AAC and severe AAC, we used RCS analysis (Figs. 2, 3, 4). Our results showed no nonlinear correlation between copper intake and AAC score (p nonlinear = 0.3859) and risk of AAC (p nonlinear = 0.6694) and severe AAC (p nonlinear = 0.4106).
Subgroup Analysis
For the association between copper intake and AAC scores and the risk of AAC, we did not find any statistically significant relationship (Fig. 5, 6).
For the risk of severe AAC, a negative association was found in non-diabetes participants (OR = 0.693) (Fig. 7). In addition, the interaction term reported the influence of diabetes on the association between copper intake and severe AAC (p for interaction = 0.004) (Fig. 7). However, the interaction test showed that this negative association between copper intake and the risk of severe AAC was not significantly influenced by age, sex, BMI, hypertension, and smoking status (pP for interaction > 0.05).
Discussion
In this cross-sectional study of 2897 participants, we observed that participants with higher copper intake had lower AAC scores and lower risk of AAC and severe AAC. The results of subgroup analyses and interaction tests demonstrated that the association between copper intake and AAC scores and risk of AAC was similar across different populations. In contrast, the association between copper intake and risk of severe AAC was significantly dependent on the participants’ diabetes status. Clinicians should be aware of copper intake in patients at risk for AAC.
To the best of our knowledge, this is the first study to assess the relationship between copper intake and AAC, and the results of this study reflect that copper intake is associated with a lower risk of vascular calcification. Previous studies have reported the effects of circulating copper levels and copper intake on cardiovascular disease (CVD). Kunutsor SK et al. conducted a prospective study of 2492 Finnish men and found a positive association between high serum copper levels and increased risk of atherosclerotic CVD in middle-aged men, but the authors did not observe an association between serum copper and venous thromboembolism [34]. Another study by Isiozor NM et al. of 1911 middle-aged Finnish men showed that serum copper levels were positively associated with the risk of death from CVD, and this positive association was more pronounced in obese men [35]. Shi et al. reported a positive association of plasma copper with all-cause mortality and CVD mortality, suggesting that plasma copper levels may serve as a predictor of cardiometabolic risk [36]. Some studies have reported a positive association between dietary copper intake and CVD mortality, regardless of the gender of the participants; in contrast, zinc intake was significantly associated with decreased CVD mortality in men [37]. In a study by Ma et al. exploring copper intake and cardiovascular disease risk levels, a significant negative correlation was found between copper intake and the ratio of total cholesterol to HDL cholesterol, and this correlation was more pronounced in women [38]. Some studies have also shown that copper deficiency could lead to myocardial hypertrophy and an increased risk of valve regurgitation [39]. However, some studies have not concluded that increased copper intake improved long-term cardiovascular health indicators, although increased copper intake has a significant increase in copper enzyme activity [40]. He et al. found a U-shaped association between copper intake and new-onset hypertension, with participants having a significantly lower risk of new-onset hypertension when copper intake did not exceed 1.57 mg/day, yet an increased risk of new-onset hypertension when copper intake exceeded this safe range [41]. In our study, consistent with most studies, we observed that increased copper intake was independently associated with lower AAC scores and a decreased risk of AAC and severe AAC, suggesting that copper intake may have a potentially beneficial effect on cardiovascular health. Although the association between dietary copper intake and calcification is less well studied, previous studies have revealed the influence of dietary factors on calcification. Zaragatski E et al. found that vitamin K supplementation attenuated calcification and endothelial hyperplasia in CKD rats [42]. Peralta-Ramírez A et al. demonstrated that in high phosphorus-induced human vascular smooth muscle cells, vitamin E also showed an effective attenuation of calcification [43]. In addition, the investigators also found that the severity of AAC increased when serum selenium concentrations exceeded 143 µg/L [44].
The underlying mechanism for this negative association between copper intake and AAC is not well understood. The common pathogenesis of VC mainly includes inflammatory cytokine release, extracellular matrix degradation, autophagy inhibition, endoplasmic reticulum stress, and mitochondrial dysfunction [45]. Interleukin (IL)-1β could contribute to the development of vascular calcification by activating the expression of the Wnt/β-catenin signaling pathway, thereby upregulating the expression levels of osteogenic genes in human aortic smooth muscle cells [46]. In a model of phosphate-induced calcification in vascular smooth muscle cells (VSMCs), the authors observed that the autophagic process was inhibited and calcium deposition increased [47]. Decreased mitochondrial membrane potential and reduced ATP production, accompanied by structural disruption of mitochondria and increased reactive oxygen species (ROS) production, were observed in the inorganic phosphate-induced calcification model of VSMCs [48]. Protein carbamylation also occurred during calcification of VSMCs, which led to downregulation of mitochondrial membrane potential and activation of oxidative stress, a process that promoted calcification [49]. Other investigators have also found that oxidative stress induced by mitophagy deficiency can accelerate the calcium deposition process in VSMCs [50]. Insufficient intake of copper may impair extracellular defenses against superoxide [51]. Copper intake not only activates autophagy by inhibiting the mTOR signaling pathway and regulating the expression of autophagy-related factors but also enhances catalase enzyme activity [52, 53]. Dietary copper deficiency may lead to the inhibition of cytochrome c oxidase activity and increased mitochondrial production of hydrogen peroxide [54, 55]. Those may be the possible mechanisms by which copper intake can slow down calcification, and more research is still needed to clarify these insights in the future.
This study has several strengths. First, the study is based on data from NHANES, a national population-based sample, and the sample selection and sample size are sufficiently representative. Second, we adjusted for confounding covariates to reduce confounding bias and to ensure that our findings are more reliable. Nevertheless, our study still has some unavoidable limitations. First, the design of the cross-sectional study did not allow us to obtain a causal relationship between copper intake and AAC. Second, although we have adjusted for some potential covariates, we still cannot guarantee that we have completely excluded other covariates that may cause confounding, such as the use of certain medications and whether patients have comorbidities. Finally, because of the NHANES study design, participants under the age of 40 years did not receive DXA screening, so we were unable to further explore the association between copper intake and AAC in a wide range of age groups. The NHANES database is data for the US population only, and our results may not be widely applicable worldwide.
Conclusion
This study found that increased copper intake was associated with decreased AAC scores and a lower likelihood of AAC and severe AAC. The current findings suggest that clinicians should be concerned about copper intake in patients at risk for AAC. However, further large prospective studies are needed to verify the validity of the authors’ findings.
Data Availability
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
References
Demer LL, Tintut Y (2008) Vascular calcification: pathobiology of a multifaceted disease. Circulation 117(22):2938–2948
Villa-Bellosta R (2021) Vascular calcification: key roles of phosphate and pyrophosphate. Int J Mol Sci 22(24):13536
Yuan C, Ni L, Zhang C, Hu X, Wu X (2021) Vascular calcification: new insights into endothelial cells. Microvasc Res 134:104105
Yahagi K, Kolodgie FD, Lutter C, Mori H, Romero ME, Finn AV et al (2017) Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler Thromb Vasc Biol 37(2):191–204
Górriz JL, Molina P, Cerverón MJ, Vila R, Bover J, Nieto J et al (2015) Vascular calcification in patients with nondialysis CKD over 3 years. Clin J Am Soc Nephrol 10(4):654–666
Chen J, Budoff MJ, Reilly MP, Yang W, Rosas SE, Rahman M et al (2017) Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA Cardiol 2(6):635–643
Zhou Y, Hellberg M, Kouidi E, Deligiannis A, Höglund P, Clyne N (2018) Relationships between abdominal aortic calcification, glomerular filtration rate, and cardiovascular risk factors in patients with non-dialysis dependent chronic kidney disease. Clin Nephrol 90(6):380–389
Kramer CK, Zinman B, Gross JL, Canani LH, Rodrigues TC, Azevedo MJ et al (2013) Coronary artery calcium score prediction of all cause mortality and cardiovascular events in people with type 2 diabetes: systematic review and meta-analysis. BMJ 346:f1654
Wang XR, Yuan L, Shi R, Li H, Wang DG, Wu YG (2021) Predictors of coronary artery calcification and its association with cardiovascular events in patients with chronic kidney disease. Ren Fail 43(1):1172–1179
Martino F, Di Loreto P, Giacomini D, Kaushik M, Rodighiero MP, Crepaldi C et al (2013) Abdominal aortic calcification is an independent predictor of cardiovascular events in peritoneal dialysis patients. Ther Apher Dial 17(4):448–453
Rahman EU, Chobufo MD, Farah F, Elhamdani A, Khan A, Thompson EA et al (2021) Prevalence and risk factors for the development of abdominal aortic calcification among the US population: NHANES study. Arch Med Sci Atheroscler Dis 6:e95–e101
Rodondi N, Taylor BC, Bauer DC, Lui LY, Vogt MT, Fink HA et al (2007) Association between aortic calcification and total and cardiovascular mortality in older women. J Intern Med 261(3):238–244
Suh SH, Oh TR, Choi HS, Kim CS, Bae EH, Oh KH et al (2022) Abdominal aortic calcification and cardiovascular outcomes in chronic kidney disease: findings from KNOW-CKD study. J Clin Med 11(5):1157
Ma D, Yan H, Yang X, Yu Z, Ni Z, Fang W (2020) Abdominal aortic calcification score as a predictor of clinical outcome in peritoneal dialysis patients: a prospective cohort study. BMC Nephrol 21(1):151
Zhang AH, Guo WK, Han DW, Zhu M, Liu WH (2022) Association of abdominal aortic calcification with longitudinal changes in left ventricular mass of patients on hemodialysis and its prognostic value. Clin Nephrol 98(4):171–181
Bastos Gonçalves F, Voûte MT, Hoeks SE, Chonchol MB, Boersma EE, Stolker RJ et al (2012) Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta-analysis. Heart 98(13):988–994
Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW (1997) New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis 132(2):245–250
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group (2017) KDIGO 2017 Clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011) 7(1):1–59
El Sabry MI, Stino FKR, El-Ghany WAA (2021) Copper: benefits and risks for poultry, livestock, and fish production. Trop Anim Health Prod 53(5):487
Bergomi M, Rovesti S, Vinceti M, Vivoli R, Caselgrandi E, Vivoli G (1997) Zinc and copper status and blood pressure. J Trace Elem Med Biol 11(3):166–169
Scheiber IF, Mercer JF, Dringen R (2014) Metabolism and functions of copper in brain. Prog Neurobiol 116:33–57
Bost M, Houdart S, Oberli M, Kalonji E, Huneau JF, Margaritis I (2016) Dietary copper and human health: current evidence and unresolved issues. J Trace Elem Med Biol 35:107–115
Cobine PA, Moore SA, Leary SC (2021) Getting out what you put in: copper in mitochondria and its impacts on human disease. Biochim Biophys Acta Mol Cell Res 1868(1):118867
Bagheri B, Akbari N, Tabiban S, Habibi V, Mokhberi V (2015) Serum level of copper in patients with coronary artery disease. Niger Med J 56(1):39–42
Zang X, Huang H, Zhuang Z, Chen R, Xie Z, Xu C et al (2018) The association between serum copper concentrations and cardiovascular disease risk factors in children and adolescents in NHANES. Environ Sci Pollut Res Int 25(17):16951–16958
Wen H, Niu X, Hu L, Sun N, Zhao R, Wang Q et al (2022) Dietary copper intake and risk of myocardial infarction in US adults: a propensity score-matched analysis. Front Cardiovasc Med 9:942000
Torres N, Freire FLA, Dantas-Komatsu RCS, Silva EPD, Queiroz S, Lira NRD et al (2022) Lack of association between inadequate micronutrient intake and prognosis in outpatients with heart failure. Nutrients 14(4):788
Zhang J, Cao J, Zhang H, Jiang C, Lin T, Zhou Z et al (2019) Plasma copper and the risk of first stroke in hypertensive patients: a nested case-control study. Am J Clin Nutr 110(1):212–220
Yang L, Chen X, Cheng H, Zhang L (2022) Dietary copper intake and risk of stroke in adults: a case-control study based on National Health and Nutrition Examination Survey 2013–2018. Nutrients 14(3):409
Chen W, Eisenberg R, Mowrey WB, Wylie-Rosett J, Abramowitz MK, Bushinsky DA et al (2020) Association between dietary zinc intake and abdominal aortic calcification in US adults. Nephrol Dial Transplant 35(7):1171–1178
Qin Z, Jiang L, Sun J, Geng J, Chen S, Yang Q et al (2022) Higher visceral adiposity index is associated with increased likelihood of abdominal aortic calcification. Clinics (Sao Paulo) 77:100114
Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D (2017) Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief 289:1–8
Menke A, Casagrande S, Geiss L, Cowie CC (2015) Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 314(10):1021–1029
Kunutsor SK, Dey RS, Laukkanen JA (2021) Circulating serum copper is associated with atherosclerotic cardiovascular disease, but not venous thromboembolism: a prospective cohort study. Pulse (Basel) 9(3–4):109–115
Isiozor NM, Kunutsor SK, Vogelsang D, Isiozor I, Kauhanen J, Laukkanen JA (2023) Serum copper and the risk of cardiovascular disease death in Finnish men. Nutr Metab Cardiovasc Dis 33(1):151–157
Shi L, Yuan Y, Xiao Y, Long P, Li W, Yu Y et al (2021) Associations of plasma metal concentrations with the risks of all-cause and cardiovascular disease mortality in Chinese adults. Environ Int 157:106808
Eshak ES, Iso H, Yamagishi K, Maruyama K, Umesawa M, Tamakoshi A (2018) Associations between copper and zinc intakes from diet and mortality from cardiovascular disease in a large population-based prospective cohort study. J Nutr Biochem 56:126–132
Ma X, Jiang S, Yan S, Li M, Wang C, Pan Y et al (2020) Association between copper, zinc, iron, and selenium intakes and TC/HDL-C ratio in US adults. Biol Trace Elem Res 197(1):43–51
Malekahmadi M, Firouzi S, Rezayi M, Ghazizadeh H, Ranjbar G, Ferns GA et al (2020) Association of zinc and copper status with cardiovascular diseases and their assessment methods: a review study. Mini Rev Med Chem 20(19):2067–2078
DiSilvestro RA, Joseph EL, Zhang W, Raimo AE, Kim YM (2012) A randomized trial of copper supplementation effects on blood copper enzyme activities and parameters related to cardiovascular health. Metabolism 61(9):1242–1246
He P, Li H, Liu C, Liu M, Zhang Z, Zhang Y et al (2022) U-shaped association between dietary copper intake and new-onset hypertension. Clin Nutr 41(2):536–542
Zaragatski E, Grommes J, Schurgers LJ, Langer S, Kennes L, Tamm M et al (2016) Vitamin K antagonism aggravates chronic kidney disease-induced neointimal hyperplasia and calcification in arterialized veins: role of vitamin K treatment? Kidney Int 89(3):601–611
Peralta-Ramírez A, Montes de Oca A, Raya AI, Pineda C, López I, Guerrero F et al (2014) Vitamin E protection of obesity-enhanced vascular calcification in uremic rats. Am J Physiol Renal Physiol 306(4):F422-429
Lu YY, Chen WL (2021) Clinical relevance of serum selenium levels and abdominal aortic calcification. Biol Trace Elem Res 199(8):2803–2810
Lee SJ, Lee IK, Jeon JH (2020) Vascular calcification-new insights into its mechanism. Int J Mol Sci 21(8):2685
Saito Y, Nakamura K, Miura D, Yunoki K, Miyoshi T, Yoshida M et al (2017) Suppression of Wnt signaling and osteogenic changes in vascular smooth muscle cells by eicosapentaenoic acid. Nutrients 9(8):858
Liberman M, Johnson RC, Handy DE, Loscalzo J, Leopold JA (2011) Bone morphogenetic protein-2 activates NADPH oxidase to increase endoplasmic reticulum stress and human coronary artery smooth muscle cell calcification. Biochem Biophys Res Commun 413(3):436–441
Kim H, Kim HJ, Lee K, Kim JM, Kim HS, Kim JR et al (2012) α-Lipoic acid attenuates vascular calcification via reversal of mitochondrial function and restoration of Gas6/Axl/Akt survival pathway. J Cell Mol Med 16(2):273–286
Mori D, Matsui I, Shimomura A, Hashimoto N, Matsumoto A, Shimada K et al (2018) Protein carbamylation exacerbates vascular calcification. Kidney Int 94(1):72–90
Zhu Y, Ji JJ, Yang R, Han XQ, Sun XJ, Ma WQ et al (2019) Lactate accelerates calcification in VSMCs through suppression of BNIP3-mediated mitophagy. Cell Signal 58:53–64
DiSilvestro RA (1988) Influence of copper intake and inflammation on rat serum superoxide dismutase activity levels. J Nutr 118(4):474–479
Liu H, Deng H, Cui H, Jian Z, Guo H, Fang J et al (2021) Copper induces hepatocyte autophagy via the mammalian targets of the rapamycin signaling pathway in mice. Ecotoxicol Environ Saf 208:111656
Arablou T, Aryaeian N, Djalali M, Shahram F, Rasouli L (2019) Association between dietary intake of some antioxidant micronutrients with some inflammatory and antioxidant markers in active rheumatoid arthritis patients. Int J Vitam Nutr Res 89(5–6):238–245
Johnson WT, Newman SM Jr (2003) Copper deficiency: a potential model for determining the role of mitochondria in cardiac aging. J Am Aging Assoc 26(1–2):19–28
Johnson WT, Newman SM Jr (2007) Hearts in adult offspring of copper-deficient dams exhibit decreased cytochrome c oxidase activity, increased mitochondrial hydrogen peroxide generation and enhanced formation of intracellular residual bodies. J Nutr Biochem 18(2):97–104
Acknowledgements
The authors thank the staff and the participants of the NHANES study for their valuable contributions.
Author information
Authors and Affiliations
Contributions
CL analyzed the data and wrote the primary manuscript. DL reviewed and revised the manuscript. All the authors have approved the manuscript for publication.
Corresponding authors
Ethics declarations
Ethics Approval
The studies involving human participants were reviewed and approved by NCHS Research Ethics Review Board (ERB).
Consent to Participate
Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, C., Liang, D. High Copper Intake Is Associated with Decreased Likelihood of Abdominal Aortic Calcification in Middle-Aged and Older US Adults. Biol Trace Elem Res 202, 1390–1400 (2024). https://doi.org/10.1007/s12011-023-03765-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03765-2