Abstract
Intraductal papillary neoplasm of the bile duct (IPNB) is rare, and its clinicopathological characteristics are still unclear. We retrospectively analyzed the clinicopathological data of IPNB patients who underwent surgical treatment in the Division of Hepatobiliary and Pancreatic Surgery of Zhejiang Provincial People's Hospital from September 2018 to June 2023 and reviewed the IPNB related literature on PubMed. A total of 12 patients with IPNB were enrolled, including 5 males and 7 females, with a median age of 64 years (range, 39–70 years). Abdominal pain (66.7%), jaundice (16.7%) and fever (16.7%) were the most common signs. 66.7% of the patients had a history of biliary stones. 75.0% of the patients had invasive carcinoma. Bile duct dilatation (83.3%), intraductal mass (58.3%) and bile duct wall thickening (25.0%) were the most common imaging findings. Surgical resection is the main treatment. The cumulative survival rates for 1, 3 and 5 years were 100%, 87.5% and 87.5%, respectively. The cumulative recurrence-free survival rates at 1, 3 and 5 years were 90.9%, 68.2% and 51.1%, respectively. More up-to-date research focusing on IPNB is warranted to help us better understand the diagnosis and treatment of this rare disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intraductal papillary neoplasm of bile duct (IPNB) [1, 2] is relatively rare, accounting for approximately 4–15% of biliary tract neoplastic lesions, and is considered as one of the precancerous lesions of cholangiocarcinoma [2,3,4]. Since 2010, IPNB has been officially defined by the World Health Organization (WHO) as an intraductal growing tumor covered by well-differentiated papillary neoplastic epithelium with fine fibrovascular cores in the intra- and extra-hepatic bile ducts [1]. In 2019, WHO included papillary cholangiocarcinoma in the IPNB category [5]. However, this definition is not perfect, and there still is room for continued improvement. The majority of patients were middle-aged and elderly patients, with an average age of 64–67 years [2, 6], but there were also a few young patients. Most recent studies have shown a higher incidence in men [2,3,4, 6]. The incidence of IPNB is higher in areas of the Eastern world compared to the West [2]. Current studies have shown that bile duct stones and Clonorchis sinensis infestation are established risk factors for IPNB [2]. IPNB can occur in various parts of the biliary tree, including the intrahepatic and extrahepatic bile ducts, and even the cystic ducts. A meta-analysis [7] showed that IPNB in Asian patients was more likely to occur within the liver and was less invasive. Herein, we summarized the clinicopathological features, diagnosis and treatment experience of IPNB patients who underwent surgical treatment from September 2018 to June 2023, and reviewed recent IPNB related literature on PubMed.
Material and Method
Patient
Data of patients with hepatobiliary and pancreatic diseases who underwent surgery in the Division of Hepatobiliary and Pancreatic Surgery of Zhejiang Provincial People's Hospital between September 2018 to June 2023 were retrieved from the electronic medical record system. The inclusion criteria were as the following: 1. Patients were pathologically diagnosed as IPNB. 2. Patients underwent curative-intent surgery. Exclusion criteria: 1. Combined with other malignancies. 2. Incomplete clinical data. Surgical indications were decided after discussion by the department team. The patients all gave informed consents of collecting medical information. The current study was approved by the ethics committee of Zhejiang Provincial People's Hospital (Acceptance number, QT2023401).
Data Collection
For enrolled patients, relevant data were obtained by consulting the electronic medical record system. These include age, gender, admission signs, history of related disease, personal history, body mass index (BMI), imaging findings, laboratory tests, preoperative invasive treatment, operation related information, postoperative complications, postoperative hospital stay, pathological information and survival information. According to the electronic medical record system, the postoperative outpatient review of patients was inquired, and the patients who did not have a medical record in our hospital for more than 6 consecutive months were followed up by telephone to inquire about the recent situation.
Data Analysis
The SPSS 26.0 statistical software (SPSS Inc., Chicago, IL, USA).) was used to analyze the data. Categorical data were expressed in absolute numbers and percentages. Continuous data were reported as medians with ranges. The Kaplan⁃Meier method was used to calculate the cumulative survival rates.
Result
Clinical Characteristics
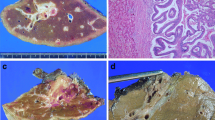
A total of 12 patients were included in this retrospective study, with a median age of 64 years (range 39–70 years). Of these, 58.3% (7/12) were females. 66.7% (8/12) of the patients presented with abdominal pain, 16.7% (2/12) had jaundice, 16.7% (2/12) had fever, 16.7% (2/12) were asymptomatic. 66.7% (8/12) had biliary stones. The details were shown in Table S1. A figure was presented showing imaging and surgical photographs of an IPNB patient with invasive carcinoma undergoing surgical treatment (Fig. 1).
Imaging and surgical photographs of an IPNB patient with invasive carcinoma undergoing surgical treatment. A Preoperative 3D reconstruction showing IPNB lesions (red arrow) and liver tumor lesion (blue triangle); B Magnetic resonance imaging showing IPNB lesion (red arrow); C Photos of laparoscopic exploration of IPNB lesions (red arrow); D Photos of laparotomy; E The IPNB tumor was removed after choledochoscopy; F Photos of IPNB lesions after removal (red arrow); G Liver specimen and liver tumor lesion (blue triangle)
Laboratory Tests
Tumor markers showed a slight increase in carcinoembryonic antigen in 8.3% (1/12) patients, and a slight increase in carbohydrate antigen 199 in 25.0% (3/12) patients. Alpha-fetoprotein was normal in all patients. The details were shown in Table S1.
Imagological Examination
Bile duct dilatation (10/12), intraductal mass (7/12) and bile duct wall thickening (3/12) were the most common imaging findings. However, in only one case was the possibility of IPNB explicitly mentioned in the imaging report. In the remaining 11 cases, 27.3% (3/11) considered benign lesions and 72.7% (8/11) considered malignant lesions. The details were listed in Table S1.
Pathological Results
25.0% (3/12) of the tumors were intrahepatic IPNB (I-IPNB), 58.3% (7/12) were extrahepatic IPNB (E-IPNB), and 16.7% (2/12) involved both. 75.0% (9/12) were pancreaticobiliary type. 75.0% (8/12) had invasive carcinoma. 58.3% (7/12) of the patients underwent lymph node dissection, and no lymph node metastasis was found. The details were shown in Table 1.
Treatment
75.0% (8/12) of the patients underwent laparoscopic surgery, 16.7% (2/12) underwent robot-assisted laparoscopic surgery, and 16.7% (2/12) underwent open surgery. The median operative time was 285 min (range 45–470 min). The median blood loss was 200 ml (range 10–2500 ml). One patient (8.3%) had a severe complication (Clavien-Dindo complication grade ≥ III) of postoperative hematemesis, which was treated and covered by interventional treatment. The median length of postoperative hospital stay was 11 days (range 2–18 days). The details were shown in Table 2.
Prognosis
All 12 patients were followed up, for a median of 34.5 months (range 4–59 months). Among them, 8 patients survived without tumor. 3 patients survived with tumor, and the recurrence time was 6, 18 and 35 months after treatment, respectively. One patient died, the time of tumor recurrence was 13 months after treatment and the time of death was 34 months after treatment. The cumulative survival rates for 1, 3 and 5 years were 100%, 87.5% and 87.5%, respectively. The cumulative recurrence-free survival rates at 1, 3 and 5 years were 90.9%, 68.2% and 51.1%, respectively.
Discussion
Clinical Characteristics
Abdominal pain, jaundice, acute cholangitis and fever are the most common signs [2, 3, 8] in patients with IPNB, which may be related to biliary obstruction caused by bile duct neoplastic lesions and secreted mucus. IPNB may also be prone to forming fistula, such as hepatogastric fistulas [9]. Meanwhile, around 10–15% of patients may have no obvious symptoms [2], indicating the importance of regular physical examination.
Laboratory Tests
IPNB patients are often associated with biliary obstruction, biliary tract infection, and elevated leukocytes and neutrophils. Biliary obstruction in E-IPNB patients often leads to abnormal liver function and jaundice. Current studies have shown that carbohydrate antigen 199 [6], carcinoembryonic antigen [10] and carbohydrate antigen 242 [10] were associated with the malignancy of IPNB. However, our study did not show an association between the two. However, at present, the number of cases included in relevant studies is small, which still needs to be verified by multi-center studies with large sample size.
Imagological Examination
IPNB often presents as a solid mass with bile duct dilatation, bile duct dilatation with intraductal mass, and bile duct dilatation only [8]. Both enhanced ultrasound and enhanced computerized tomography (CT) showed enhancement in arterial phase, and weakened enhancement in venous phase [8]. Magnetic resonance imaging (MRI) showed isointensity or hypointensity on T1, slight hyperintensity on T2, and hyperintensity in arterial phase and portal vein phase after enhancement [11]. Gd-EOB-DTPA enhanced MRI can display mucin components of IPNB and tumor infiltration, and distinguish between inflammation and tumor [12]. Lee S et al. [13] have shown that MRI findings of intraductal mass, tumor diameter ≥ 2.5 cm, multiple tumors, bile duct wall thickening, and adjacent organ invasion are helpful in identifying whether IPNB is invasive cancer. Jin KP et al. [14] have suggested that whole lesion apparent diffusion coefficient (ADC) histogram can distinguish invasive and noninvasive IPNBs.
Positron emission tomography CT (PETCT) may predict the malignancy of IPNB. A higher maximum standardized uptake values (SUVmax) means that patients are more likely to develop invasive cancer. The cut-off value, however, is still controversial. A recent study [15] involving 101 patients identified 3.0 as the optimal cut-off value. However, because PETCT is expensive, it is not a routine examination in clinical practice.
In addition to traditional imaging, transoral choledochoscopy is becoming a very effective way for screening suspected neoplastic bile duct lesions [16, 17]. In 2021, a study [16] involving 207 patients showed that the success rate of transoral choledochoscopy was 96.1%, the success rate of biopsy was 90.3%, and the neoplastic and non-neoplastic lesions could be directly distinguished in 91.6% of patients. Recent studies have shown that the success rate of examination and biopsy has further improved [17]. Meanwhile, transoral choledochoscopy combined with probe-based confocal laser endomicroscopy can be used for real-time diagnosis of IPNB [18].
Pathological Results
Pathological specimens were mainly obtained by surgical resection, choledochoscopic biopsy, and endoscopic ultrasound-guided or percutaneous fine needle aspiration biopsy. However, there is a risk of needle tract seeding of needle aspiration biopsy [19]. In the past, the histological types were divided into pancreaticobiliary, gastric, intestinal, and oncocytic subtypes [20], and there were also mixtures of two or more subtypes. In 2018, pathologists proposed a new subclassification of IPNB [21]. Type I IPNB is histologically similar to intraductal papillary mucinous neoplasms of pancreas (IPMN), whereas type II IPNB is more complex histologically with irregular papillary branches or focal solid tubular components, often including extrahepatic bile ducts [21]. In 2020, pathologists [3] summarized a total of 694 cases of IPNB (520 cases of type I and 174 cases of type II), with type I IPNB being more common in I-IPNB and type II IPNB being more common in E-IPNB. Type I has a higher positive margin rate. Type I IPNB has a better prognosis than type II. This suggests the clinical value of the new classification. However, the boundary between type I and type II is not clear. In 2021, a Japanese study [22] included 181 cases of IPNB showed that a large number of cases were indistinguishable between type I and type II, which means that the pathological classification of IPNB still needs to be further explored. To note, a 2023 Japanese study [23] showed that T staging did not make a significant difference in the prognosis of type II IPNB patients with invasive cancer.
Treatment
Early surgical treatment is the main treatment after detection. Since IPNB may occur anywhere within the biliary tree, the primary purpose is to remove the tumor and ensure a negative margin. The surgery included hepatectomy, pancreaticoduodenectomy, and radical resection of hilar cholangiocarcinoma. For elderly patients who cannot tolerate surgery, transoral choledochoscopy provides options for endoscopic interventional therapy, such as endoscopic stent placement and argon plasma coagulation [24, 25]. Regular follow-up is also an appropriate option for patients with abnormal imaging findings but no tumor tissue found on biopsy. Hasebe T et al. [26] reported an IPNB patient who underwent liver resection after 13 years of long-term observation, and pathology confirmed IPNB with high-grade atypia. The recurrence rate of IPNB patients with invasive cancer is higher than that of IPNB patients without invasive cancer, and the prognosis is worse. Most IPNB patients without invasive cancer do not receive follow-up after surgery, which is what we need to improve in the future.
Prognosis
The overall prognosis of IPNB patients is better than that of biliary malignancies. Current published studies have shown that the 5-year OS of IPNB is around 65%-93.9% [6, 7, 27], which is consistent with the 5-year survival rate of 87.5% observed in the current study. However, there is a large difference in prognosis between patients with type I IPNB and type II IPNB. A study [3] of 694 patients showed that the 5-year OS of type I IPNB and type II IPNB was 75.2% and 50.9%, respectively, and the 5-year disease-free survival was 64.1% and 35.3%, respectively. You Y et al. [28] found that cytokeratin 20, pancreaticobiliary type, tumor invasion outside the duct wall, tumor invasion to adjacent organs, and invasive carcinoma occurred more common in E-IPNB than in I-IPNB.
Another aspect that cannot be ignored may be related to the surgical procedure. In particular, pancreaticoduodenectomy is more likely to be planned for E-IPNB. A recent European multicenter retrospective study [29] showed that failure to achieve textbook outcomes (no prolonged hospital stay, no Clavien-Dindo complication grade ≥ III, readmission, or death within 90 days) was an independent risk factor for OS. IPNB patients who underwent hepatectomy were more likely to have a textbook outcome (64.8% vs 32.0%) than those who underwent pancreatectomy, which correlated with more complications after pancreatic surgery. Khodr J et al. [30] reported a new surgical procedure to perform common bile duct resection and avoid pancreatic resection in patients with IPNB of the distal bile duct, which may help to improve the prognosis, but the preoperative diagnosis and intraoperative guarantee of negative surgical margins should be confirmed. Positive surgical margin has significant influence on the prognosis. One study [31] showed that the 5-year survival rates for positive and negative surgical margins were 38% and 87%, respectively. The expression of MUC5AC and MUC6 may be associated with good prognosis, while expression of MUC1and cytokeratin 20 may be associated with poor prognosis [2, 5, 7].
Elevated carbohydrate antigen 199 [6], positive surgical margin [6, 31], lymph node metastasis [31] were associated with tumor recurrence. Local recurrence was the most common site of first recurrence. Distal metastasis may involve the liver, peritoneum and retroperitoneal lymph nodes [28]. You Y et al. [28] found that I-IPNB had a higher recurrence rate within 1 year (83.3% vs 33.3%) than that of E-IPNB, and E-IPNB had a higher recurrence rate within 1–3 years (50% vs 0%) than that of I-IPNB. This suggests that different types of IPNB require different follow-up plans.
Summary
More up-to-date research focusing on IPNB is warranted to help us better understand the diagnosis and treatment of this rare disease.
Data Availability
The data gathered in this study are available from the authors on reasonable request.
References
Nakanuma Y, Curado MP, Franceschi S et al (2010) World Health Organization Classification of Tumours of the Digestive System[M], 4th edn. IARC, Lyon, pp 217–224
Kim JR, Jang KT, Jang JY (2023) Intraductal papillary neoplasm of the bile duct: review of updated clinicopathological and imaging characteristics. Br J Surg 110(9):1229–1240
Kubota K, Jang JY, Nakanuma Y et al (2020) Clinicopathological characteristics of intraductal papillary neoplasm of the bile duct: a Japan-Korea collaborative study. J Hepatobiliary Pancreat Sci 27(9):581–597
Harada F, Matsuyama R, Mori R et al (2019) Outcomes of surgery for 2010 WHO classification-based intraductal papillary neoplasm of the bile duct: Case-control study of a single Japanese institution’s experience with special attention to mucin expression patterns. Eur J Surg Oncol 45(5):761–768
Nakanuma Y, Basturk O, Esposito I et al (2019) Intraductal papillary neoplasm of the bile ducts. In: Board E (ed) WHO Classification of Tumours of the Digestive System, 5th edn. IARC, Lyon, pp 279–282
Youn JM, Hwang S, Ahn CS et al (2022) Clinicopathological features and long-term outcomes of intraductal papillary neoplasms of the bile duct of the liver: single-institution experience with 146 patients. J Gastrointest Surg 26(7):1394–1405
Gordon-Weeks AN, Jones K, Harriss E et al (2016) Systematic review and meta-analysis of current experience in treating IPNB: clinical and pathological correlates. Ann Surg 263(4):656–663
Zheng Q, Ruan SM, Shan QY et al (2019) Clinicopathological findings and imaging features of intraductal papillary neoplasm of the bile duct: comparison between contrast-enhanced ultrasound and contrast-enhanced computed tomography. Abdom Radiol (NY) 44(7):2409–2417
Chan WH, Chen CM, Wang SY et al (2023) Intraductal papillary neoplasm of the bile duct presenting with hepatogastric fistula: a case report and literature review. Front Oncol 19(13):1193918
Zhang H, Zhong Z, Kong G et al (2020) Clinicopathological findings and imaging features of intraductal papillary neoplasms in bile ducts. PeerJ 30(8):e10040
Aslam A, Wasnik AP, Shi J et al (2020) Intraductal papillary neoplasm of the bile duct (IPNB): CT and MRI appearance with radiology-pathology correlation. Clin Imaging 66:10–17
Ying SH, Teng XD, Wang ZM et al (2015) Gd-EOB-DTPA-enhanced magnetic resonance imaging for bile duct intraductal papillary mucinous neoplasms. World J Gastroenterol 21(25):7824–7833
Lee S, Kim MJ, Kim S et al (2019) Intraductal papillary neoplasm of the bile duct: assessment of invasive carcinoma and long-term outcomes using MRI. J Hepatol 70(4):692–699
Jin KP, Rao SX, Sheng RF et al (2019) Skewness of apparent diffusion coefficient (ADC) histogram helps predict the invasive potential of intraductal papillary neoplasms of the bile ducts (IPNBs). Abdom Radiol (NY) 44(1):95–103
Choi JU, Hwang S, Ahn CS et al (2022) Diagnostic and prognostic impact of fluorodeoxyglucose-positron emission tomography in diagnosing intraductal papillary neoplasms of the bile duct of the liver. Ann Surg Treat Res 102(6):335–341
Shin IS, Moon JH, Lee YN et al (2021) Use of peroral cholangioscopy to screen for neoplastic bile duct lesions in patients with bile duct stones (with videos). Gastrointest Endosc 94(4):776–785
Shin IS, Moon JH, Lee YN et al (2023) Detection and endoscopic classification of intraductal neoplasms of the bile duct by peroral cholangioscopy with narrow-band imaging (with videos). Gastrointest Endosc 97(5):898–910
Wen J, Ji JM, Gong B (2019) Cholangioscopy and probe-based confocal laser endomicroscopy for real-time diagnosis of intraductal papillary mucinous neoplasm of the bile duct. Dig Endosc 31(5):595
Takahashi N, Taniguchi T, Adachi M (2014) A case of needle tract seeding of an intraductal papillary neoplasm of the bile duct (IPNB) after percutaneous biopsy. Eur J Dermatol 24(1):128–30
Fukumura Y, Nakanuma Y, Kakuda Y et al (2017) Clinicopathological features of intraductal papillary neoplasms of the bile duct: a comparison with intraductal papillary mucinous neoplasm of the pancreas with reference to subtypes. Virchows Arch 471(1):65–76
Nakanuma Y, Jang KT, Fukushima N et al (2018) A statement by the Japan-Korea expert pathologists for future clinicopathological and molecular analyses toward consensus building of intraductal papillary neoplasm of the bile duct through several opinions at the present stage. J Hepatobiliary Pancreat Sci 25(3):181–187
Onoe S, Ebata T, Yokoyama Y et al (2021) A clinicopathological reappraisal of intraductal papillary neoplasm of the bile duct (IPNB): a continuous spectrum with papillary cholangiocarcinoma in 181 curatively resected cases. HPB (Oxford) 23(10):1525–1532
Mitake Y, Onoe S, Igami T et al (2023) Is a specific T classification needed for extrahepatic intraductal papillary neoplasm of the bile duct (IPNB) type 2 associated with invasive carcinoma? J Hepatobiliary Pancreat Sci 30(6):745–754
Cha B, Park JS, Jeong S et al (2019) Direct cholangioscopy with argon plasma coagulation of an intraductal papillary mucinous neoplasm of the bile duct. Korean J Intern Med 34(4):940–941
Hinokuchi M, Iwaya H, Ido A (2020) Endoscopic ultrasound-guided hepaticogastrostomy for intraductal papillary neoplasm of bile duct with obstructive jaundice and cholangitis due to mucus overproduction. Dig Endosc 32(4):e80–e81
Hasebe T, Sawada K, Hayashi H et al (2019) Long-term growth of intrahepatic papillary neoplasms: a case report. World J Gastroenterol 25(36):5569–5577
Kim JR, Jang KT, Jang JY et al (2020) Clinicopathologic analysis of intraductal papillary neoplasm of bile duct: Korean multicenter cohort study. HPB (Oxford) 22(8):1139–1148
You Y, Choi SH, Choi DW et al (2020) Recurrence after resection for intraductal papillary neoplasm of bile duct (IPNB) according to tumor location. J Gastrointest Surg 24(4):804–812
Lluís N, Serradilla-Martín M, Achalandabaso M et al (2023) Intraductal papillary neoplasms of the bile duct: a European retrospective multicenter observational study (EUR-IPNB study). Int J Surg 109(4):760–771
Khodr J, Truant S, El Amrani M (2021) Surgical ampullectomy with resection of the common bile duct for biliary papillomatosis. J Gastrointest Surg 25(4):1087–1088
Uemura S, Higuchi R, Yazawa T et al (2021) Prognostic factors for surgically resected intraductal papillary neoplasm of the bile duct: a retrospective cohort study. Ann Surg Oncol 28(2):826–834
Acknowledgements
The current study was supported by the fund of Public Welfare Technology Research Program/Social Development of Zhejiang Provincial Natural Science Foundation of China No. LGF21H030011 to Wei FQ; The Outstanding Young Personnel Research Fund of Zhejiang Provincial People’s Hospital (No. ZRY2020B012) to Wei FQ. The fund of the Joint Project of Department of Science and Technology of State Administration of Traditional Chinese Medicine and Zhejiang Administration of Traditional Chinese Medicine (No.GZY-ZJ-KJ-23059) to Wei FQ.
Author information
Authors and Affiliations
Contributions
Protocol/project development: Wei Fangqiang, Sun Xiaodong, Gu Jing, Jin Lei, Wang Zhimin
Data collection or management: Wei Fangqiang, Sun Xiaodong, Gu Jing, Jin Lei, Wang Zhimin
Data analysis: Wei Fangqiang, Sun Xiaodong, Gu Jing, Jin Lei, Wang Zhimin
Manuscript writing: Wei Fangqiang, Sun Xiaodong, Gu Jing, Jin Lei, Wang Zhimin
Manuscript editing: Wei Fangqiang, Sun Xiaodong, Gu Jing
Corresponding authors
Ethics declarations
Conflict of Interest
The authors have no financial disclosures or other conflicts of interest in relation to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gu, J., Jin, L., Wang, Z. et al. Clinicopathological Features of 12 Cases of Intraductal Papillary Neoplasm of the Bile Duct: A Case Series. Indian J Surg (2024). https://doi.org/10.1007/s12262-024-04029-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12262-024-04029-6