Abstract
Background
The concept of intraductal papillary neoplasm of the bile duct (IPNB) has been proposed to be the biliary equivalent of intraductal papillary mucinous neoplasm (IPMN) of the pancreas. While the classification of IPMNs is based on their location of duct involvement, such classification has not been fully evaluated for IPNBs. The aim of this study is to investigate the value of IPNB classification based on its location.
Methods
A total of 306 consecutive patients who underwent surgical resection with a diagnosis of bile duct tumor were enrolled. Among these patients, 21 were diagnosed as having IPNB. The IPNBs were classified into two groups as follows: extrahepatic IPNB, which located in the distal or perihilar bile duct, and intrahepatic IPNB, which located more peripherally than the hilar bile duct. The clinicopathological features of the two groups were then compared.
Results
Extrahepatic IPNB tended to show more invasive characteristics than intrahepatic IPNB (presence of invasive component: 40.0 vs. 9.1%, p = 0.084). Moreover, patients with extrahepatic IPNB showed significantly poorer relapse-free survival (RFS) than those with intrahepatic IPNB [5-year RFS rate (%): 81.8 vs. 16.2, p = 0.014].
Conclusion
Patients with intrahepatic IPNB show more favorable pathological characteristics and postoperative survival outcomes than those with extrahepatic IPNB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intraductal papillary neoplasm of the bile duct (IPNB) is a rare tumor that was recently classified as an original pathological entity in the 2010 World Health Organization (WHO) classification [1]. The concept of IPNB was first advocated by Chen and Nakanuma in 2001, who reported that intraductal papillary neoplasm of the liver with goblet cells and colon-like metaplasia was frequently associated with overproduction of mucin and formed a characteristic biliary neoplasm [2]. It is now recognized that mucin production is not a pathologic feature of IPNB and is not present in all cases [3].
IPNB can arise in any part of the biliary tree, from the intrahepatic bile duct to the extrahepatic large bile duct [3, 4], and shows a wide spectrum ranging from low-grade adenoma to noninvasive and invasive carcinoma [5,6,7]. Because of its heterogeneity, the clinicopathological features of IPNB, including the prognosis, are still unclear and an appropriate subclassification system would be desirable.
IPNB is also considered to be the biliary equivalent of intraductal papillary mucinous neoplasm of the pancreas (IPMN-P) [3, 4, 8, 9]. Similarly to IPMN-P, IPNB shows predominantly intraductal growth and causes dilatation of the affected bile ducts; it also seems to show a better prognosis than conventional cholangiocarcinoma, similar to that observed for IPMN-P and conventional pancreatic cancer [4, 10]. IPMN-Ps are classified according to their location of duct involvement, and distinction between the branch-duct type and the main duct type is important because the former arises in younger patients, is more likely to involve the head and uncinated process of the pancreas, and is more frequently associated with an invasive component [11]. However, such classification of IPNB has not been fully evaluated. The aim of this study was to investigate the value of IPNB classification based on its location.
Patients and methods
Definition of IPNB
IPNB was diagnosed in accordance with the WHO 2010 criteria [1], i.e., dilated bile ducts filled with a grossly visible noninvasive papillary biliary neoplasm covering delicate fibrovascular stalks. The covering tumoral cells were well differentiated, although a certain amount of tubular or mucinous carcinoma were occasionally identifiable. IPNB can develop anywhere along the biliary tree, including the intrahepatic bile duct, extrahepatic bile duct and gallbladder. Mucin hypersecretion is occasionally observed, but this is not mandatory for diagnosis of IPNB. So-called papillary adenoma or biliary papilloma or papillomatosis was included in this category [3]. Classic cholangiocarcinomas were defined as grossly solid nodular tumors and microscopically composed of mainly tubular adenocarcinoma. Papillary cholangiocarcinoma was defined as invasive tumors that grossly showed papillary or polypoid lesions and histologically consisted of mainly papillary or papillotubular adenocarcinomas, with an overall architecture that was more complex than that expected in IPNBs (e.g., irregular papillary branching, conspicuous tubular or solid components, and irregularities in the thickness of papillae) [6]. Comparison of the pathological features among IPNB, papillary carcinoma and classical cholangiocarcinoma is summarized in Table 1.
Patients
A total of 306 consecutive patients who underwent surgical resection with a diagnosis of bile duct tumor at our department between April 2000 and March 2015 were retrospectively surveyed in accordance with the above criteria for IPNB by an expert pathologist at our institution. This study had an approval of the institutional reviewer board (approval number 29033). Thirty-six tumors showed grossly visible papillary or polypoid neoplastic lesions in the bile duct lumen. Fifteen tumors were diagnosed as advanced cholangiocarcinoma and excluded from this analysis, and remaining 21 tumors were diagnosed as IPNB and retained in this study.
The patients were divided into two groups according to the location of their tumors, i.e., extrahepatic and intrahepatic bile duct. In this study, extrahepatic IPNB was defined as a tumor located in the distal bile duct or perihilar bile duct, whereas intrahepatic IPNB was defined as a tumor located more peripherally than the hilar bile duct. The perihilar and intrahepatic bile ducts were defined in accordance with the classification of biliary tract cancers established by the Japanese Society of Hepato-Biliary-Pancreatic Surgery, i.e., the ducts at the left and right borders are defined topologically as being on the right side of the umbilical portion of the left portal vein and the left side of the origin of the right posterior portal vein, respectively [12].
Perioperative factors
The following parameters were compared between the two groups: age, gender, clinical presentation, preoperative levels of AST, ALT, ALP, LDH, γ-GTP, total bilirubin, total protein, albumin, CRP, serum CEA and CA19-9. In terms of pathological characteristics, tumor size, invasion depth, status of lymph node metastasis and phenotype classification were also evaluated and compared between the groups. In addition, we used these clinicopathological parameters to investigate the risk factors for postoperative survival.
Overall, relapse-free and tumor-specific survivals were compared according to the location of IPNB.
Pathological examination
The ductal margin is systematically submitted for intraoperative frozen section analysis to insure R0 resection. Patients with an intraoperative positive margin undergo an additional bile duct resection whenever possible.
The tissue blocks that included the main IPNB tumor were prepared from each paraffin block with 4 μm in thickness. After deparaffinization, thin sections were stained with hematoxylin and eosin, Azan-Mallory, and periodic acid–Schiff after diastase digestion for histological observation. Then the size of the protruding tumor, tumor depth, the presence of mucin production, histological grade, the presence of lymph node metastasis were examined. TNM staging was determined by evaluating these pathological parameters [13]. TNM for intrahepatic bile dutcs was applied for intrahepatic IPNB, TNM for perihilar bile ducts was applied for perihilar IPNB, and TNM for distal extrahepatic bile duct was applied for extrahepatic IPNB. The remaining sections were used for immunohistochemistry as follows. Immunohistochemical staining of CDX2, MUC1, MUC2, MUC5AC, MUC6, CK7, CK20 and CD10 was used to facilitate the phenotyping of IPNB, i.e., gastric, intestinal, pancreatobiliary and oncocytic type [14, 15].
Statistical analysis
All data were expressed as the median and range. Statistical analyses were performed using the SPSS 22.1 software package (SPSS, Chicago, Illinois, USA). Categorical characteristics were analyzed using the Chi-squared test. Continuous characteristics were analyzed using the Mann–Whitney U test. The Cox proportional hazards model was used to identify the independent risk factors associated with postoperative survival. The cumulative survival rates including overall survival, relapse-free survival and cancer-specific survival were analyzed by the Kaplan–Meier method, and log-rank tests were performed to determine the significance of differences between the groups. Cancer-specific survival was the net survival in terms of cancer-related causes of death in the absence of other causes of death.
Results
Clinicopathological features of IPNB in the study patients
The median patient age was 66 year, and the proportion of males was 57.1%. Jaundice was the most frequent presenting feature (40.9%). There were 11 cases of intrahepatic IPNB, 4 affecting the right hemiliver and 7 the left hemiliver. There were 10 cases of extrahepatic IPNB, 5 affecting the hilar bile duct and 5 the distal bile duct. The median tumor size was 27.5 mm, and macroscopic gross mucin production was observed in 10 cases (47.6%). Histologically, 4 cases (19.0%) were revealed to have an invasive component and 17 cases (81.0%) were pre-malignant lesion.
Comparison of characteristics between the groups
The clinical backgrounds of the two groups are summarized in Table 2. There were no intergroup differences in age, gender or the incidence of jaundice. There were also no significant intergroup differences in laboratory parameters, except for the level of serum CA19-9, which was significantly higher in the extrahepatic than in the intrahepatic IPNB group [257 (16–3990) vs. 10 (2–186), p = 0.041]. Operative characteristics are summarized in Table 3. Nine out of eleven patients with intrahepatic IPNB underwent hemihepatectomy. No patient received extrahepatic bile duct resection. On the other hand, among the patients with extrahepatic IPNB, five out of ten patients received pancreaticoduodenectomy, four patients received hemihepatectomy with extrahepatic bile duct resection. There was no significant difference in operation time and the amount of bleeding between the groups. R0 resection rate was 90.9% in intrahepatic IPNB, whereas 90.0% in extrahepatic IPNB (p = 1.000).
Pathological characteristics are summarized in Table 4. Mucin production was observed more frequently in intrahepatic IPNB than in extrahepatic IPNB (81.8 vs. 20.0%, p = 0.005). Extrahepatic IPNB tended to show invasive characteristics more frequently than intrahepatic IPNB (40.0 vs. 9.1%, p = 0.084). Lymph node metastasis was observed in two of the 11 cases of extrahepatic IPNB, whereas no metastasis was observed in the cases of intrahepatic IPNB. However, there were no significant intergroup differences in T, N, M factors and final stage. In terms of phenotype classification, the oncocytic type tended to be more frequent in intrahepatic than in extrahepatic IPNB (45.5 vs. 0.0%, p = 0.087).
Postoperative recurrence is summarized in Table 5. One out of eleven cases of intrahepatic IPNB had a relapse as a liver metastasis. Five out of ten cases of extrahepatic IPNB had a relapse. Among them, liver metastasis was the most common type of recurrence. Two cases of carcinoma in situ had a relapse, and one case was local recurrence because the tumor widely spread along the bile duct and curative resection was not achieved in initial surgery. Although the adjacent bile duct was intact macroscopically, microscopic examination showed high-grade dysplasia spread widely to adjacent bile duct from the main tumor in one case. In the remaining cases, the bile duct stump was negative for IPNB.
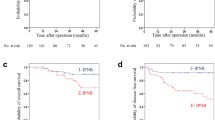
The overall, cancer-specific and recurrence-free survival curves for the two groups are illustrated in Fig. 1a–c. The recurrence-free survival rate was significantly better in patients with intrahepatic IPNB than in those with extrahepatic IPNB (81.8 vs. 16.2%, p = 0.014). Patients with intrahepatic IPNB also tended to show better overall and cancer-specific survival rates than those with extrahepatic IPNB (5-year overall survival rate (%): 81.8 vs. 45.0, p = 0.212; 5-year cancer-specific survival rate (%): 90.9 vs. 45.0, p = 0.070).
Postoperative survival curves stratified by tumor location. a Overall survival curves for the subjects stratified according to tumor location. b Cancer-specific survival curves for the subjects stratified according to tumor location. c Relapse-free survival curves for the subjects stratified according to tumor location
Univariate analyses in relation to overall survival could not determine significant risk factor (Table 6). On the other hand, univariate analyses in relation to relapse-free survival showed that an extrahepatic tumor location and tumor grade were significantly associated with postoperative relapse-free survival (Table 7). Status of the surgical margin was not a significant risk factor for both overall and relapse-free survival.
Details of four invasive cases
The details of the four patients with invasive IPNB are summarized in Table 8. In case 1, primary symptom was fever and jaundice. The lesion arose at left intrahepatic bile duct, producing mucin which occluded the bile duct. The tumor was resected by extended left hemihepatectomy, R0 resection. The patient was dead for peritoneal dissemination 22 months after surgery. In case 2, primary symptom was abdominal pain. The tumor arose at perihilar bile duct, and the tumor was accompanied with mucin hypersecretion. The tumor was resected by extended left hemihepatectomy and extrahepatic bile duct resection. R0 resection was achieved. Regional lymph node metastasis was detected at pathological examination. The patient was still alive 78 months after surgery. In case 3, primary symptom was jaundice. The tumor arose at perihilar bile duct, which was resected by hepatic resection (Couinaud segments 4 and 5) with extrahepatic bile duct resection. The patient was died for recurrence 38 months after surgery. In case 4, primary presentation was abdominal pain. The tumor arose at distal bile duct, and the patient underwent pancreaticoduodenectomy, R0 resection. The patient was alive 29 months after surgery.
Discussion
As both the bile ducts and pancreas develop from the ventral endoderm of the foregut [16], some reports have suggested that these may develop malignancies via similar genetic and molecular oncologic pathways [5, 17]. Furthermore, several reports from Asia have suggested that the progression of papillary biliary neoplasms from benign lesions to invasive cancers may represent a pathway analogous to that of IPMN [3, 18,19,20,21]. Focusing on such similarities, we hypothesized that intrahepatic IPNB might have different clinicopathological features to extrahepatic IPNB and conducted this study.
Our present study demonstrated that patients with extrahepatic IPNB showed significantly poorer relapse-free survival than those with intrahepatic IPNB. Both overall and cancer-specific survivals also tended to be poorer for extrahepatic IPNB than for intrahepatic IPNB. Moreover, an extrahepatic tumor location was an independent factor adversely affecting postoperative survival.
It is well known that most main-duct-type IPMNs have high malignant potential at the time of diagnosis [22, 23], in sharp contrast to branch-type IPMNs. From the viewpoint of phenotypic classification, it was documented that intestinal-type IPMNs are typically main duct type [7]. Moreover, invasive carcinoma was identified in a third of intestinal IPMNs resected and such tumor reported to show surprisingly protracted clinical course [7]. In contrast, oncocytic IPMNs were reported to have surprisingly rare incidence of invasive component, although they were typically present as large, multilocular tumor [7, 22]. Previous studies have suggested that IPNB shares many clinicopathological features with main-duct-type IPMN, but not with branch-type IPMN, as clinically detectable IPNBs frequently have malignant potential already [3, 24].
In the present study, intestinal type tended to be more frequent in extraheptic IPNB than intrahepatic IPNB, and oncocytic type was the most predominant in intrahepatic IPNB. Although it was not statistically significant, extrahepatic IPNB tended to accompany invasive lesion and lymph node metastasis more frequently than intrahepatic IPNB in our series and it might influence postoperative survivals. However, it was also assumed that there was considerable biological difference between intrahepatic and extraheptic disease on the ground of the phenotypic results and such biological difference could be a reason of marked discrepancy of postoperative outcome between the groups.
In this study, the incidence of mucin hypersecretion was significantly frequent in intrahepatic IPNB than in extrahepatic IPNB. As not only tumor itself but also hypersecreted mucin can obstruct the bile flow and emphasize clinical and imaging finding [24], the discrepancy of the tumor stage between the groups might be one of the results for such biological differences. These results agree well with the study by Nakanuma et al., who classified IPNB on the basis of histopathologic similarities to IPMN and revealed that tumors resembling IPMN were located more frequently at the intrahepatic or perihilar bile duct, accompanied by mucin secretion and demonstrating less aggressive pathological characteristics than those of IPMN. On the other hand, tumors not resembling IPMN arose more frequently from the distal or perihilar bile duct, showing characteristics that were more aggressive than those of tumors resembling IPMN [5]. Moreover, of the aforementioned study, in terms of phenotype classification, the distribution of oncocytic type was more frequent in the tumors resembling IPMN than in those not resembling IPMN, which also supports our idea that intrahepatic IPNB shows pathological similarity to branched type IPMN [5]. Although it might be a novel approach for evaluation of IPNB tumorigenesis to use the pathological resemblance, classification based on tumor location is more easily applicable to clinical use because it can classify IPNB on the basis of preoperative imaging studies alone.
As the present entity of IPNB includes tumors with varying status, there is ongoing confusion in defining such tumors [4, 25]. The results of the present study suggest that it might be reasonable to classify IPNB into intrahepatic and extrahepatic types, which would be helpful for better clinical decision-making.
In our series, one case had a relapse probably due to metachronous multicentric tumor, and another case developed local recurrence after R1 surgery because of extensive superficial spread. Vibert et al. has reported the good results of resecting whole biliary tract by pancreaticoduodenectomy and liver transplantation in very selected patients [26]. Of course it is highly debatable whether such an aggressive strategy is justified, the optimal management for postoperative recurrence is a critical problem for managing IPNB, and further investigation should be warranted.
The limitation of this study was a relatively small, single-center, retrospective fashion. Nevertheless, we were able to show a clear clinicopathological contrast between intrahepatic and extrahepatic IPNB. Naturally, however, further investigation of a multicenter larger cohort will be needed in order to ascertain whether this type of classification is truly workable.
In summary, as both the clinical and pathological features of intrahepatic IPNB are significantly better than those of extrahepatic IPNB, classification based on the level of bile duct involvement appears to be useful for accurate prognostic stratification of IPNB.
Abbreviations
- Alb:
-
Albumin
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- CA19-9:
-
Carbohydrate antigen 19-9
- CEA:
-
Carcinoembryonic antigen
- CRP:
-
C-reactive protein
- γ-GTP:
-
Gamma-glutamyltransferase
- IPMN-P:
-
Intraductal papillary mucinous neoplasm of the pancreas
- IPNB:
-
Intraductal papillary neoplasm of the bile duct
- PLT:
-
Platelet
- TP:
-
Total protein
- WHO:
-
World Health Organization
References
Bosman FT (2010) World Health Organization., international agency for research on cancer. WHO classification of tumours of the digestive system. Lyon, International agency for research on cancer
Chen TC, Nakanuma Y, Zen Y et al (2001) Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology 34:651–658
Ohtsuka M, Shimizu H, Kato A et al (2014) Intraductal papillary neoplasms of the bile duct. Int J Hepatol 2014:10
Nakanuma Y, Sato Y, Ojima H et al (2014) Clinicopathological characterization of so-called “cholangiocarcinoma with intraductal papillary growth” with respect to “intraductal papillary neoplasm of bile duct (IPNB)”. Int J Clin Exp Pathol 7:3112–3122
Nakanuma Y, Kakuda Y, Uesaka K et al (2016) Characterization of intraductal papillary neoplasm of bile duct with respect to histopathologic similarities to pancreatic intraductal papillary mucinous neoplasm. Hum Pathol 51:103–113
Fujikura K, Fukumoto T, Ajiki T et al (2016) Comparative clinicopathological study of biliary intraductal papillary neoplasms and papillary cholangiocarcinomas. Histopathology 69(6):950–961
Adsay V, Mino-Kenudson M, Furukawa T et al (2016) Pathologic evaluation and reporting of intraductal papillary mucinous neoplasms of the pancreas and other tumoral intraepithelial neoplasms of pancreatobiliary tract: recommendations of verona consensus meeting. Ann Surg 263:162–177
Ji Y, Fan J, Zhou J et al (2008) Intraductal papillary neoplasms of bile duct. A distinct entity like its counterpart in pancreas. Histol Histopathol 23:41–50
Rocha FG, Lee H, Katabi N et al (2012) Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology 56:1352–1360
Koh Y-X, Chok A-Y, Zheng H-L et al (2014) Systematic review and meta-analysis comparing the surgical outcomes of invasive intraductal papillary mucinous neoplasms and conventional pancreatic ductal adenocarcinoma. Ann Surg Oncol 21:2782–2800
Salvia R, Castillo CF, Bassi C et al (2004) Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg 239:678–687
Miyazaki M, Ohtsuka M, Miyakawa S et al (2015) Classification of biliary tract cancers established by the Japanese Society of Hepato-Biliary-Pancreatic Surgery: 3rd English edition. J Hepatobiliary Pancreat Sci 22:181–196
Union for International Cancer Control (UICC) TNM Classification of Malignant Tumors, Wiley Blackwell, New Jersey
Kim KM, Lee JK, Shin JU et al (2012) Clinicopathologic features of intraductal papillary neoplasm of the bile duct according to histologic subtype. Am J Gastroenterol 107:118–125
Wan X-S, Xu Y-Y, Qian J-Y et al (2013) Intraductal papillary neoplasm of the bile duct. World J Gastroenterol 19:8595–8604
Deutsch G, Jung J, Zheng M et al (2001) A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development 128:871–881
Bennett S, Marginean EC, Paquin-Gobeil M et al (2015) Clinical and pathological features of intraductal papillary neoplasm of the biliary tract and gallbladder. HPB 17(9):811–818
Nakanuma Y (2010) A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: is the biliary tract an incomplete pancreas? Pathol Int 60:419–429
Minagawa N, Sato N, Mori Y et al (2013) A comparison between intraductal papillary neoplasms of the biliary tract (BT-IPMNs) and intraductal papillary mucinous neoplasms of the pancreas (P-IPMNs) reveals distinct clinical manifestations and outcomes. Eur J Surg Oncol 39:554–558
Zen Y, Fujii T, Itatsu K et al (2006) Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology 44:1333–1343
Suh KS, Roh HR, Koh YT et al (2000) Clinicopathologic features of the intraductal growth type of peripheral cholangiocarcinoma. Hepatology 31:12–17
Fong Y, Blumgart LH (2000) Surgery of the liver and biliary tract. WB Saunders, Philadelphia
Salvia R, Fernandez-del Castillo C, Bassi C et al (2004) Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg 239:678–685 discussion 685–677
Kubota K, Nakanuma Y, Kondo F et al (2014) Clinicopathological features and prognosis of mucin-producing bile duct tumor and mucinous cystic tumor of the liver: a multi-institutional study by the Japan biliary association. J Hepatobiliary Pancreat Sci 21:176–185
Gordon-Weeks AN, Jones K, Harriss E et al (2016) Systematic review and meta-analysis of current experience in treating IPNB: clinical and pathological correlates. Ann Surg 263:656–663
Vibert E, Dokmak S (2010) Belghiti J Surgical strategy of biliary papillomatosis in Western countries. J Hepatobiliary Pancreat Sci 17:241–245
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matsumoto, T., Kubota, K., Hachiya, H. et al. Impact of Tumor Location on Postoperative Outcome of Intraductal Papillary Neoplasm of the Bile Duct. World J Surg 43, 1313–1322 (2019). https://doi.org/10.1007/s00268-019-04913-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-04913-3