Abstract
β-Alanine is a versatile amino acid with wide-range industrial applications, but its production from glucose has been limited by a low yield. This study addresses this challenge by developing efficient Escherichia coli strains with modified carbon metabolism as microbial cell factories and implementing a two-stage fermentation strategy. The introduction of aspartate decarboxylase (PanDE56S/I88M) facilitates the conversion of aspartate to β-alanine, while the overexpression of key enzymes such as phosphoenolpyruvate carboxylase and aspartate dehydrogenase increases the carbon flow from phosphoenolpyruvate to aspartate. To mitigate oxidative stress, the glutathione cycle was enhanced by overexpressing BtuE and Gor. In a bioreactor, the optimized strain achieved β-alanine production of 71.7 g/L with a yield of 1.0 mol/mol glucose, reaching a peak of 1.29 mol/mol. Notably, the utilization of acetate as a carbon feedstock enabled the production of 50 g/L of β-alanine with a 0.33 mol/mol acetate yield, showcasing the potential for sustainable production. This research offers valuable insights into improving the carbon yield in β-alanine production, which is of great importance for industrial applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
β-Alanine, a naturally occurring β-type amino acid, plays a crucial role in the biosynthesis of D-pantothenic acid, essential for coenzyme A and acyl-carrier protein production. Its diverse applications in industries such as food, feed, and medicine have generated significant interest [1,2,3,4]. Additionally, β-Alanine combines with another amino acid to form carnosine in the human body, contributing to muscle tissue buffering and enhanced exercise performance [5]. Recent research has even suggested potential cognitive and therapeutic benefits, particularly in neurological disorders [6]. The market demand for β-Alanine is estimated to reach 80,000 tons in 2023 [7].
β-Alanine can be synthesized either chemically or biologically. Chemical methods involve toxic precursors and harsh conditions, making them environmentally unfriendly and unsafe [4, 8]. Bioconversion, although an option, is not economical due to the use of costly substrates, such as L-aspartate or fumaric acid, and the need for purified enzyme such as aspartate decarboxylase (PanD) and/or aspartase (AspA) [4]. Currently, microbial production from glucose, employing recombinant hosts like Escherichia coli or Corynebacterium glutamicum, appears to be the most promising method. Notably, a record β-Alanine titer of 166.6 g/L was achieved with C. glutamicum [9]. However, in all previous studies, the yield from glucose remained low, with the highest reported yield being 0.57 mol/mol, significantly below the theoretical maximum of 2 mol/mol [10].
Improving carbon yield in microbial production presents a formidable challenge. To enhance carbon yield, it is imperative to minimize or discourage competitive pathways and reduce by-product formation. Furthermore, achieving a balance between the synthesis of the carbon skeleton of the target product and energy regeneration from carbon sources is essential. Decoupling growth from production is another critical strategy to maximize product yield, as excessive carbon utilization for cell biomass can hinder yield improvement. Two-stage fermentation, involving a growth stage followed by an extended production period, is commonly employed for decoupling [11,12,13]. A critical challenge in this method, however, lies in maintaining stable cellular activity during the prolonged non-growing stage. E. coli, upon entering the stationary phase, undergoes significant morphological remodeling and develops specific stress resistances. It also substantially downregulates macromolecule biosynthesis [14]. Despite these adaptations, the cellular activity and viability gradually decline over time, ultimately leading to cell death. Additionally, under aerobic conditions, E. coli can generate superoxide in the periplasm through the oxidation of dihydromenaquinone, which accelerates cell death. To counteract oxidative stress, cells implement various protective strategies, including the continuous transmembrane cycling of glutathione [15]. When using fatty acids as substrates, oxidative stress has been observed to severely impair cell viability and β-alanine production due to the high oxygen demand of fatty acid β-oxidation. Metabolite analysis has revealed that elevating glutathione levels and accelerating the glutathione cycle through the overexpression of thioredoxin/glutathione peroxidase (BtuE) and glutathione reductase (Gor) significantly aids in addressing this issue [16].
This study aims to develop a recombinant E. coli strain capable of high-yield β-alanine production from glucose. Strategies to promote carbon utilization for the β-alanine carbon skeleton over energy production were implemented, and decoupling of growth and production was achieved through two-stage fermentation (see Fig. 1 for an overview of metabolic engineering). Additionally, the glutathione cycle was upregulated in an attempt to mitigate oxidative stress during β-alanine production. The performance of the developed strains was assessed at both flask and bioreactor scales, demonstrating an improved yield up to 1.29 mol/mol. Furthermore, the use of acetate as an alternative carbon feedstock was investigated. This study provides valuable insights into the significance of optimizing carbon flux, along with employing a two-stage fermentation, to enhance β-alanine yield.
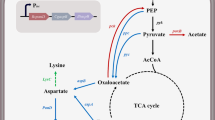
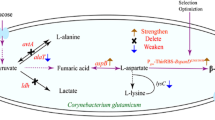
Overall metabolic engineering strategy employed for the development of β-alanine producing Escherichia coli strain. G6P: glucose-6-phosphate, PEP: phosphoenolpyruvate, PYR: pyruvate, OAA: oxaloacetate, CIT: citrate, ICI: isocitrate, KG: α-ketoglutarate, SUCC: succinate, FUM: fumarate, MAL: malate, GOX: glyoxylate, ASP: aspartate, HOM: homoserine, LYS: lysine, MET: methionine, THR: threonine, β-Ala: β-alanine
2 Materials and methods
2.1 Strains
All the strains and plasmids employed in this study are detailed in Table 1. The E. coli DH5α strain served as the host for cloning and plasmid construction purposes. The primers utilized in this study were acquired from Macrogen Co. Ltd. Standard protocols were adhered to for conducting general molecular biological experiments, including PCR, gel electrophoresis, and transformation, to facilitate strain development. The necessary reagents, such as restriction enzymes and pfu DNA polymerase for PCR, were obtained from New England Biolabs and Solgent, respectively.
2.2 Plasmid construction and gene manipulation
To clone aspartate decarboxylase, panDE56S/I88M [17] was amplified by overlapping fragments using Bacillus subtilis strain 168 genome and then ligated to the Ndel/Xhol-digested pM2 plasmid [11] which results in pP plasmid. The AspA aspartase was overexpressed under Pgrac promoter using plasmid pACYC-Duet1 as a backbone. Initially, the aspA gene was PCR-amplified from the genomic DNA of E. coli W3110, followed by its overlap with a Pgrac fragment synthesized using primers. This overlap resulted in the generation of the Pgrac-aspA fragment, which was then ligated with pACYC (previously digested by XbaI and Xhol) to produce the pA plasmid. The ppc gene coding phosphoenolpyruvate carboxylase (PPC) was amplified from E. coli chromosome and inserted into pACYC plasmid at Xhol site to generate pPpc plasmid. The aspDH gene coding aspartate dehydrogenase was amplified from Pseudomonas aeruginosa chromosome and inserted into pPpc plasmid at Xbal site to generate pAPpc plasmid. The btuE and gor genes coding thioredoxin/glutathione peroxidase and glutathione reductase were amplified and overlapped to make butE-gor fragment, and then, it was inserted into pR1 plasmid [11] at Xbal site to generate pBG plasmid.
2.3 Culture conditions
For flask experiments, a single colony was introduced into 10 mL of LB medium (composed of 10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl) and incubated overnight at 37 °C. The LB-cultivated cells were then transferred to 250-mL baffled flasks containing 20 mL of modified M9 medium, adjusted to an optical density of 0.1 at 600 nm (OD600), and cultivated overnight at 37 °C to serve as the inoculum for the main culture. The primary flask culture was carried out in 250-mL baffled flasks with 20 mL of modified M9 medium. The cultivation transpired in a shaking incubator at 37 °C and 220 rpm. The modified M9 medium was prepared according to the previous study [11]. Antibiotics, specifically ampicillin (100 μg/mL), chloramphenicol (25 μg/mL), and kanamycin (50 μg/mL), were incorporated as necessary. For sodium acetate utilization, upon complete consumption of the initial glucose (~ 10 g/L), sodium acetate was introduced at a concentration of 10 g/L.
Fed-batch experiments in the bioreactor were conducted at 37 °C using a 2-L jar fermentor (Bioflo 3000; New Brunswick Scientific Co.) with an initial working volume of 1 L. The dissolved oxygen concentration was upheld at approximately 20% of saturation through the automatic control of agitation speed, regulated up to 1,000 rpm. pH was maintained at 6.85 ± 0.05 by automatically feeding a 24% NH4OH solution. Glucose concentration was kept within the range of 5 to 10 g/L by intermittently supplying a concentrated glucose solution (700 g/L). During the growth phase, yeast extract and an amino acid mixture (comprising threonine, methionine, and lysine) were added at rates of 0.08 g/L/h and 0.003 g/L/h, respectively. In the stationary phase, only yeast extract was added at a rate of 0.04 g/L/h. When sodium acetate was used as carbon source, cells were first grown on glucose for 9 h and then sodium acetate was added to be 10 g/L. Subsequently, acetic acid (not sodium acetate) was continuously fed in a pH–stat mode to maintain a pH of approximately 6.85 using a 40% solution. The use of acetate (in the culture medium containing sodium ion and acetate ion) leaves sodium ion (Na+), which causes the medium's pH to increase over time. In this pH–stat mode, acetic acid serves a dual purpose: it is both the carbon source and a pH-neutralizing agent, as its addition lowers the culture medium's pH. Acetate concentration was maintained at 5–10 g/L.
2.4 Analytical methods
Cell growth was monitored by assessing OD600 using a UV/VIS spectrophotometer (Lambda 20; PerkinElmer). The conversion factor of 1 OD600 was equal to 0.3 g of dry cell weight per liter. Metabolite quantification in the culture broth, encompassing glucose, amino acids, acetic acid, and pyruvate, was conducted through HPLC (Agilent). Before HPLC analysis, culture supernatants underwent centrifugation at 13,000 g for 10 min and were subsequently purified via a 0.22-µm membrane filter. For substrate analysis, an Aminex HPX-87H ion exclusion column was utilized at a column temperature of 65 °C, employing 0.01 M H2SO4 as the mobile phase at a flow rate of 0.5 mL/min. Similarly, amino acids were analyzed using a Phenomenex column at a column temperature of 30 °C, with a mobile phase consisting of a methanol and 2 mM Cu(OH)2 buffer mixture (15:85) at a flow rate of 1.0 mL/min.
3 Results and discussion
3.1 Introducing PanD mutant enzyme into W-H18 strain for β-alanine production
In our previous study [11], W-H18 strain was developed as an efficient microbial cell factory to produce homoserine from glucose by iterative and rational engineering. The strain had deletions for such by-products as ethanol, acetate, lactate and formate, and upregulation of phosphoenolpyruvate (PEP) carboxylase (PPC) and replacement of PTS (phosphoenolpyruvate: sugar phosphotransferase system) glucose transport system to a glucose permease (glf from C. glutamicum). In a two-stage fed-batch bioreactor experiment, W-H18 strain could produce homoserine at a high titer of 110.8 g/L and yield of 0.96 mol/mol glucose (~ 79% of theoretical maximum). Since aspartate (ASP) is the common intermediate for both homoserine and β-alanine, it is hypothesized that β-alanine is produced at a high level by the W-H18 strain if an efficient aspartate decarboxylase, converting L-aspartate to β-alanine, is introduced. To this end, the mutant aspartate decarboxylase, PanDE56S/I88M from B. subtilis, known to have a high activity for the decarboxylation reaction, was introduced into W-H18 by using the recombinant pP plasmid. In flask experiment, W-H18/pP completely consumed glucose (30 g/L) in 23 h and successfully produce 86 mM β-alanine (Fig. 2A). However, the β-alanine yield was low at 0.54 mol/mol, which is 56% of that observed for homoserine.
Performance of recombinant Escherichia coli W-H18/pP overexpressing PanD by pP plasmid. A Time course profiles. B Sectional and overall metrics of fermentation results. The error bars represent standard deviation from duplicate experiments. The vertical line in the graphs delineates the transition from rapid cell growth (stage 1) to product formation (stage 2). OD: optical density
In flask experiment, there was a discernible increase in the production rate at 9 h when the cell growth rate was significantly reduced (Fig. 2A). This observation suggests a redirection of carbon flux in the glucose metabolism of the W-H18 strain, shifting from cell growth to β-alanine production at the later period. Consequently, the fermentation could be divided into two distinct stages: the active growth phase identified as "stage 1," and the subsequent production phase marked as "stage 2″. Despite a similar glucose consumption rate, the β-alanine production rate in stage 2 was significantly higher, resulting in a ~ threefold increase in yield, compared to stage 1 (Fig. 2B). This enhancement in stage 2 is attributed to a shift in carbon flux toward β-alanine synthesis without the deliberate induction of genes. The depletion of yeast extract in the modified M9 medium (2 g/L) appears to play a crucial role in this transition by reducing the cell growth rate, similar to our previous findings in homoserine production [11]. Furthermore, the upregulation of PPC in W-H18 likely contributed to the decreased carbon flow through the TCA cycle following yeast extract depletion, thereby diminishing cell growth. Further optimization of carbon flux, especially proper carbon distribution between the carbon oxidation through the oxidative TCA cycle and the β-alanine synthesis pathway, is anticipated to increase the yield and production of β-alanine.
3.2 Examining the role of TCA cycle-glyoxylate shunt on β-Alanine production
The yield of β-alanine, when produced from ASP, greatly depends on the carbon flux distribution at the PEP node. There can be three distinct pathways (models) for producing β-alanine (as shown in Fig. S1): (1) the ppc-linked anaplerotic reaction, (2) the glyoxylate (GOX) shunt, and (3) the oxidative TCA cycle [10]. The material and energy balance for each pathway can be summarized as follows:
In Eq. (1), it is assumed that ASP is synthesized by AspC (glutamate-dependent transaminase) solely from oxaloacetate (OAA) which is formed from PEP by PPC. In contrast, in Eqs. (2) and (3), ASP is assumed to be synthesized from fumarate (FUM) by AspA (aspartate-ammonia-lyase). The highest β-alanine yield (close to 2 mol/mol) is achievable in the scenario of Eq. (1). However, when OAA is converted to ASP by AspC, NADPH (not NADH) is required to regenerate glutamate, which is used as an amino donor for the transaminase reaction. NADPH can be synthesized via the pentose phosphate pathway, the TCA cycle (by α-ketoglutarate dehydrogenase), and/or transhydrogenase (encoded by PntAB) from NADH and ATP. Thus, to ensure the supply of NADPH, a certain amount of glucose carbon needs to be oxidized. Therefore, even in the case of Eq. (1), the maximum yield is less than 2 mol/mol (as indicated ‘-ʼ sign for ATP in the right hand side), the exact value of which depends on the energy efficiency in producing NADPH. It is noteworthy that in the pathways of the GOX shunt (Eq. 2) or the oxidative TCA cycle (Eq. 3), where AspA plays a role, NADPH is not required. In practice, it is expected that all three pathways collectively contribute to β-alanine production, each to varying extents.
Previously, when homoserine was produced from glucose, upregulating AspA was beneficial, leading to an increase in both titer and yield [11]. Since a low β-alanine production yield in W-H18 can be attributed to insufficient NADPH supply, the effect of AspA upregulation was studied by overexpressing it episomally using the recombinant pA plasmid (Fig. 3). The glucose consumption, particularly during the production phase, increased by 20%, and fermentation was terminated at 19 h, about 4 h earlier than the one without the pA plasmid. However, this enhanced glucose consumption did not translate into an increased β-alanine production yield. In fact, the overall production yield decreased by 19%. It is assumed that the overexpression of AspA increased carbon flux toward the oxidative TCA cycle and/or GOX shunt, leading to a higher carbon loss through CO2 release.
Performance of recombinant Escherichia coli W-H18/pP/pA overexpressing PanD (pP plasmid) and AspA (pA plasmid). A Time course profiles. B Sectional and overall metrics of fermentation results. The error bars represent standard deviation from duplicate experiments. The vertical line in the graphs delineates the transition from rapid cell growth (stage 1) to product formation (stage 2). OD: optical density
3.3 Overexpression of PPC and AspDH
The upregulation of AspA, which is dependent on the pathways outlined in Eqs. (2) and (3), proved to be ineffective. Consequently, an opposite approach was employed: enhancing the role of AspC through upregulation of PPC as outlined in Eq. (1), which could increase the availability of OAA. This approach involved the use of the recombinant plasmid pPpc, leading to significant improvements in glucose consumption rate, titer, and yield during the production phase for the W-H18/pP/pPpc strain, with increase of over 20% in each metric (Fig. 4A). Notably, the β-alanine production rate in stage 2 rose by over 50%. These results suggest that, PPC overexpression was beneficial in the current strain, likely by enhancing the contribution of the ppc-linked anaplerotic reaction among the three β-alanine production pathways.
Performance of recombinant Escherichia coli W-H18/pP/pPpc and W-H18/pP/pAPpc. A Time course profiles of W-H18/pP/pPpc. B Sectional and overall metrics from W-H18/pP/pPpc fermentation. C Time course profiles of W-H18/pP/pAPpc. D Sectional and overall metrics from W-H18/pP/pAPpc fermentation. The plasmid pP, pPpc, and pAPpc carries PanD, PPC, and both AspDH and PPC, respectively. The error bars represent standard deviation from duplicate experiments. The vertical line in the graphs delineates the transition from rapid cell growth (stage 1) to product formation (stage 2). OD: optical density
To reduce the NADPH requirement in the ppc-linked pathway, the NADH-dependent aspartate dehydrogenase (AspDH) from P. aeruginosa was introduced. This enzyme facilitates the reversible conversion of OAA to aspartate, using ammonium as the amino group donor. If this enzyme functions exclusively in the forward direction and is solely responsible for the conversion of OAA to ASP, the theoretical maximum yield can reach 2 mol/mol of glucose, as per Eq. (1). The W-H18/pP strain was then transformed with the recombinant pAPpc plasmid, which overexpresses both PPC and AspDH. This alteration resulted in an approximate 15% increase in β-alanine production yield compared to the W-H18/pP/pPpc strain, as depicted in Fig. 4C. However, the β-alanine production rate remained at the same level, and a minor reduction in glucose consumption rate was noted, mainly due to decreased cell growth. While the reduction in NADPH demand via AspDH overexpression improves β-alanine yield, its influence on glucose consumption and cell growth requires further investigation.
3.4 Improvement of glutathione cycle
The implementation of two-stage fermentation has consistently proven advantageous for increasing product yield across all our developed strains. However, a critical challenge in this method is the loss of cellular activity during the non-growing stage, often linked to oxidative stress. Using fatty acid as carbon source, β-alanine production was significantly improved in the later period by overexpressing BtuE and Gor, reducing oxidative stress [16].
Even with glucose as carbon substrate, it was hypothesized that enhancing the tolerance to oxidative stress would improve β-alanine production in stage 2. To test this, two enzymes, BtuE and Gor, were overexpressed episomally in the recombinant W-H18/pP/pAPpc/pBG strain. Flask experiments with this new strain demonstrated notable improvements in β-alanine production titer (approximately 9%), rate (around 9%), and yield (about 8%) during the production phase (Fig. 5). Furthermore, it was observed that the inhibition of cell growth, previously noted with the overexpression of AspDH, was alleviated. This indicates that mitigating oxidative stress not only enhances cell growth but also boosts product formation during the non-growing stage.
Performance of recombinant Escherichia coli W-H18/pP/pAPpc/pBG with overexpression of PanD (pP plasmid), AspDH and PPC (pAPpc plasmid), and BtuE and Gor (pBG plasmid). A Time course profiles. B Sectional and overall metrics of fermentation results. The error bars represent standard deviation from duplicate experiments. The vertical line in the graphs delineates the transition from rapid cell growth (stage 1) to product formation (stage 2). OD: optical density
3.5 Production of β-alanine from acetate
Microbial production is heavily dependent on the choice of carbon source. Traditional agricultural products, often used as carbon sources, have limitations due to cost, and competition with food supply chains. Acetate, a two-carbon molecule that is both low-cost and readily available, offers a promising alternative for microbial production [18]. However, using acetate in microbial fermentation poses challenges due to its toxicity at high concentrations, slow assimilation rate, and low energy content [19, 20].
In a proof-of-concept investigation, acetate was tested as an alternative carbon source for β-alanine production using two recombinant strains, W-H18/pP and W-H18/pP/pA. The cells were initially grown on glucose (~ 60 mM) for 13 h, after which ~ 120 mM of acetate was added to facilitate β-alanine production. As shown in Fig. 6, cell growth stopped completely at 13 h coinciding with the depletion of glucose. During the production phase, both recombinant strains showed a linear increase in β-alanine levels without any notable cell growth. The β-alanine production from acetate reached 25 mM in W-H18/pP and 40 mM in W-H18/pP/pA. Notably, W-H18/pP/pA, which has upregulated AspA, exhibited better performance, with a 50% higher specific production rate of β-alanine from acetate compared to W-H18/pP, and a significantly higher yield (52%; 0.21 vs. 0.32 mol/mol). The linear increase in β-alanine concentration in both strains suggests the possibility of growth-independent, or bioconversion-like, β-alanine production using acetate. These results mark the first report of β-alanine production from acetate, highlighting its potential as a viable carbon source.
β-Alanine production from acetate by Escherichia coli W-H18/pP and W-H18/pP/pA. A Time course profiles for W-H18/pP, overexpressing PanD via the pP plasmid. B Metrics of fermentation results for W-H18/pP during the acetate utilization period (13–21 h). C Time course profiles for W-H18/pP/pA, overexpressing PanD via the pP plasmid and AspA via the pA plasmid. D Metrics of fermentation results for W-H18/pP/pA during the acetate utilization period (13–21 h). For both strains, cells were initially grown on 60 mM glucose for 13 h, followed by the addition of 120 mM acetate. Error bars represent the standard deviation from duplicate experiments. The vertical line in the graphs indicates the transition from rapid cell growth (stage 1) to product formation (stage 2). 'qs' denotes the specific acetate consumption rate, and 'qp' denotes the specific β-alanine production rate. OD: optical density
When acetate serves as the carbon source, it must supply both the energy and the carbon skeleton for β-alanine synthesis. The performance differences between W-H18/pP and W-H18/pP/pA can be explained by the variance in the three β-alanine synthesis pathways (Fig. S1). Specifically, the ppc-linked pathway (Eq. 1) is less relevant for β-alanine synthesis from acetate due to limited PEP availability. The contribution of the GOX shunt (Eq. 2) and the oxidative TCA cycle (Eq. 3) appears to vary based on AspA activity, with higher AspA levels likely enhancing the role of the GOX shunt relative to the oxidative TCA throughput. When carbon flux is directed more toward the GOX shunt, a higher product yield is achieved, as the oxidative TCA cycle fully oxidizes acetyl-CoA and does not contribute to β-alanine's carbon skeleton. This outcome contrasts with glucose as the carbon source, where the ppc-linked pathway plays a significant role in β-alanine synthesis.
3.6 Two-stage fed-batch fermentation
Fed-batch bioreactor experiments were conducted with two recombinant strains and two carbon sources: W-H18/pP/pAPpc/pBG using glucose, and W-H18/pP/pA using acetate. In the two-stage bioreactor, the W-H18/pP/pAPpc/pBG strain exhibited consistent β-alanine production for almost 60 h, including a 36-h non-growing period. Notably, a high β-alanine production of 805.6 mM (71.7 g/L) was achieved after 61 h of fermentation (Fig. 7A), with an overall yield of 1 mol/mol glucose. The sectional yields were 0.77 mol/mol in stage 1 and 1.29 mol/mol in stage 2. To our knowledge, this yield of 1 mol/mol from the W-H18/pP/pAPpc/pBG strain is the highest recorded, surpassing the previous best by 75% (Table 2) [7, 9, 14, 21, 22]. This finding demonstrates that high β-alanine yields can be attained through two-stage fermentation with an appropriately modified recombinant E. coli strain.
Fed-batch bioreactor experiments for β-alanine production using glucose and acetate as carbon sources. A Escherichia coli W-H18/pP/pAAP/pBG with glucose as carbon source. B E. coli W-H18/pP/pA with acetate as carbon source. For the experiment in B, cells were initially grown on 18 g/L glucose for 9 h, followed by feeding with acetate. OD: optical density
In addition, when using acetate as the carbon source, the W-H18/pP/pA strain produced 555.7 mM (approximately 50.0 g/L) of β-alanine with a yield of 0.33 mol/mol (Fig. 7B). Similar to the glucose experiments, β-alanine production progressed linearly throughout the entire production period on acetate, including the 41-h non-growing phase. This recombinant strain, in conjunction with two-stage fermentation, allows for flexible substrate switching between the growth and production phases. Currently, this is the highest level of amino acid production achieved from acetate. The work presented in this paper demonstrates the feasibility of scalable and sustainable β-alanine production using advanced methodologies. It also contributes to a growing knowledge base in industrial microbiology and biochemical engineering, offering a framework for future studies and technological developments in microbial production.
4 Conclusion
This study enhances β-alanine production through metabolic engineering of E. coli, complemented by a strategic fermentation approach. The method involved altering carbon flux and employing a two-stage fermentation process, leading to increased β-alanine yields. Specifically, the engineered W-H18/pP/pAPpc/pBG strain successfully produced 71.7 g/L β-alanine from glucose, achieving a peak yield of 1.29 mol/mol. Furthermore, this study demonstrated the feasibility of β-alanine production from acetate, achieving 50 g/L with a yield of 0.33 mol/mol. These results underscore the effectiveness of pathway engineering, particularly focusing on the synthesis of the key metabolite aspartate (ASP), and the use of two-stage fermentation to improve amino acid production. The techniques developed in this research could offer a more economical and environmentally sustainable method for β-alanine production, and potentially for manufacturing other valuable chemicals derived from ASP as well.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
References
Leonardi R, Jackowski S (2007) Biosynthesis of pantothenic acid and coenzyme A. EcoSal Plus. https://doi.org/10.1128/ecosalplus.3.6.3.4
Liu J, Zhao C, Liu B et al (2019) Analgesia and curative effect of pamidronate disodium combined with chemotherapy on elderly patients with advanced metastatic bone cancer. Oncol Lett 18:771–775. https://doi.org/10.3892/ol.2019.10340
de SallesPainelli V, Nemezio KM, Pinto AJ et al (2018) High-intensity interval training augments muscle carnosine in the absence of dietary beta-alanine intake. Med Sci Sports Exerc 50:2242–2252. https://doi.org/10.1249/MSS.0000000000001697
Wang L, Mao Y, Wang Z et al (2021) Advances in biotechnological production of β-alanine. World J Microbiol Biotechnol 37:79. https://doi.org/10.1007/s11274-021-03042-1
Saunders B, Elliott-Sale K, Artioli GG et al (2017) β-alanine supplementation to improve exercise capacity and performance: a systematic review and meta-analysis. Br J Sports Med 51:658–669. https://doi.org/10.1136/bjsports-2016-096396
Ostfeld I, Hoffman JR (2023) The effect of β-alanine supplementation on performance, cognitive function and resiliency in soldiers. Nutrients 15:1039. https://doi.org/10.3390/nu15041039
Zhou HY, Tang YQ, Peng JB et al (2022) Re-designing Escherichia coli for high-yield production of β-alanine by metabolic engineering. Biochem Eng J 189:108714. https://doi.org/10.1016/j.bej.2022.108714
Ohara T, Sato T, Shimizu N et al (2020) Acrylic acid and derivatives. Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH, Weinheim
Ghiffary MR, Prabowo CPS, Adidjaja JJ et al (2022) Systems metabolic engineering of corynebacterium glutamicum for the efficient production of β-alanine. Metab Eng 74:121–129. https://doi.org/10.1016/j.ymben.2022.10.009
Piao X, Wang L, Lin B et al (2019) Metabolic engineering of Escherichia coli for production of L-aspartate and its derivative β-alanine with high stoichiometric yield. Metab Eng 54:244–254. https://doi.org/10.1016/j.ymben.2019.04.012
Vo TM, Park S (2022) Metabolic engineering of Escherichia coli W3110 for efficient production of homoserine from glucose. Metab Eng 73:104–113. https://doi.org/10.1016/j.ymben.2022.07.001
Lama S, Seol E, Park S (2020) Development of Klebsiella pneumoniae J2B as microbial cell factory for the production of 1,3-propanediol from glucose. Metab Eng 62:116–125. https://doi.org/10.1016/j.ymben.2020.09.001
Zhu YX, Hu WW, Yao LY et al (2015) Improvement of fumigaclavine C production in a two-stage culture of Aspergillus fumigatus with molasses as a cost-effective ingredient. Biotechnol Bioprocess Eng 20:1106–1113. https://doi.org/10.1007/s12257-015-0193-y
Schellhorn HE (2020) Function, evolution, and composition of the RpoS regulon in Escherichia coli. Front Microbiol 11:560099. https://doi.org/10.3389/fmicb.2020.560099
Smirnova GV, Tyulenev AV, Muzyka NG et al (2020) Study of the relationship between extracellular superoxide and glutathione production in batch cultures of Escherichia coli. Res Microbiol 171:301–310. https://doi.org/10.1016/j.resmic.2020.07.004
Miao Y, Liu J, Wang X et al (2022) Fatty acid feedstocks enable a highly efficient glyoxylate-TCA cycle for high-yield production of β-alanine. mLife 1:171–182. https://doi.org/10.1002/mlf2.12006
Wang JY, Rao ZM, Xu JZ et al (2021) Enhancing β-alanine production from glucose in genetically modified corynebacterium glutamicum by metabolic pathway engineering. Appl Microbiol Biotechnol 105:9153–9166. https://doi.org/10.1007/s00253-021-11696-y
Kim Y, Lama S, Agrawal D et al (2021) Acetate as a potential feedstock for the production of value-added chemicals: metabolism and applications. Biotechnol Adv 49:107736. https://doi.org/10.1016/j.biotechadv.2021.107736
Elkasaby T, Hanh DD, Kawaguchi H et al (2023) Co-utilization of maltose and sodium acetate via engineered Corynebacterium glutamicum for improved itaconic acid production. Biotechnol Bioprocess Eng 28:790–803. https://doi.org/10.1007/s12257-023-0091-7
Lama S, Kim Y, Nguyen DT et al (2021) Production of 3-hydroxypropionic acid from acetate using metabolically-engineered and glucose-grown Escherichia coli. Bioresour Technol 320:124362. https://doi.org/10.1016/j.biortech.2020.124362
Wang P, Zhou HY, Li B et al (2021) Multiplex modification of Escherichia coli for enhanced β-alanine biosynthesis through metabolic engineering. Bioresour Technol 342:126050. https://doi.org/10.1016/j.biortech.2021.126050
Li B, Zhang B, Wang P et al (2022) Rerouting fluxes of the central carbon metabolism and relieving mechanism-based inactivation of l-aspartate-α-decarboxylase for fermentative production of β-alanine in Escherichia coli. ACS Synth Biol 11:1908–1918. https://doi.org/10.1021/acssynbio.2c00055
Acknowledgements
This work was financially supported by the National Research Foundation of Korea through the Engineering Research Centre (ERC) at UNIST (NRF-2020R1A5A1019631).
Funding
This study was funded by National Research Foundation of Korea, NRF-2020R1A5A1019631, Sunghoon Park.
Author information
Authors and Affiliations
Contributions
Toan Minh Vo was involved in the conceptualization, investigation, methodology, formal analysis, data curation, writing—original draft, and review and editing. Sunghoon Park contributed to the conceptualization, formal analysis, funding acquisition, project administration, supervision, validation, visualization, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Neither ethical approval nor informed consent was required for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vo, T.M., Park, S. High-yield β-alanine production from glucose and acetate in Escherichia coli. Biotechnol Bioproc E 29, 650–660 (2024). https://doi.org/10.1007/s12257-024-00107-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-024-00107-4