Abstract
Immune checkpoint blockade has demonstrated significant anti-tumor immunity in an array of cancer types, yet the underlying regulatory mechanism of it is still obscure, and many problems remain to be solved. As an inhibitory costimulatory signal of T-cells, the programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) pathway can paralyze T-cells at the tumor site, enabling the immune escape of tumor cells. Although many antibodies targeting PD-1/PD-L1 have been developed to block their interaction for the treatment of cancer, the reduced response rate and resistance to the therapies call for further comprehension of this pathway in the tumor microenvironment. MicroRNAs (miRNAs) and long noncoding RNAs (lncRNAs) are two main types of noncoding RNAs that play critical parts in the regulation of immune response in tumorigenesis, including the PD-1/PD-L1 pathway. Here we summarize the most recent studies on the control of this pathway by noncoding RNAs in cancer and hopefully will offer new insights into immune checkpoint blockade therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The immune system is essential to the maintenance of homeostasis by discriminate “non-self” from “self” through immunological surveillance and elimination of aberrant and carcinogenic cells. However, multiple mechanisms have been elucidated to hinder anti-tumor immunity during tumorigenesis, making the ability to escape from the immune surveillance one of the hallmarks of cancer [1].
The Nobel Prize in Physiology or Medicine 2018 was awarded jointly to James P. Allison and Tasuku Honjo for their discovery and further study of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) [2] and programmed cell death 1 (PD-1) [3], both of which are commonly referred to as immune checkpoints that function as the brakes of the immune system, and the overexpression of them and the PD-1 ligand, programmed cell death ligand 1 (PD-L1), in tumors or immune cells is one of the most studied causes that lead to T cell dysfunction, form immunosuppressive tumor microenvironment, bring about immune tolerance and eventually enable immune escape of tumor cells [4].
PD-1/PD-L1 Pathway and Precision Medicine
The PD-1 cDNA was first isolated by Tasuku Honjo [3] in 1992 while B7 Homolog 1 (B7-H1) was identified independently by Lieping Chen in 1999 [4, 5] and later known as PD-L1 to emphasize their ligand-receptor relationship [6]. PD-1 is a type I transmembrane glycoprotein consisting of an immunoglobulin V (IgV)-type extracellular domain, a transmembrane region, and an intracellular tail and mainly expressed on the surface of T-cells, B-cells and natural killer (NK) cells [7]. PD-L2 (B7-DC) is the other ligand of it besides PD-L1, which is also a member of the B7 family of transmembrane proteins [8].
To be fully activated, T cells must receive two sets of signals from antigen-presenting cells (APCs): the recognition of T cell receptor (TCR) mediated antigen-specific signal of complexes of major histocompatibility complex (MHC) with the antigen on the surface of APCs or tumor cells, and a second costimulatory signal mediated by interaction of CD28 on the T-cells with CD80 (B7–1) or CD86 (B7–2) on APCs [9]. While the interaction of CD28 with B7–1/B7–2 can activate T-cells, the engagement of PD-1 by PD-L1 and PD-L2 can efficiently paralyze T-cells by inhibiting T lymphocytes glucose consumption, proliferation, survival and cytokine production, leading to their malfunction and apoptosis [4, 10], which usually acts as a basic pattern to avoid host autoimmunity [11, 12].
PD-L1 is expressed on the surface of many cell types including T-cells, B-cells, APCs, epithelial cells and monocytes, and rapidly upregulated in response to proinflammatory cytokines like interferon-gamma (IFN-γ) and tumor necrosis factor-α (TNF-α) secreted by tumors [13] while the expression of PD-L2 is mainly restricted to APCs [8]. Also, unlike PD-L2, PD-L1 is a binding partner for B7–1 and competitively binds B7–1 with stronger affinity than CD28, thus impeding the activation of T-cells by B7–1/CD28 pathway [14]. As a result, the anti-tumor effect of PD-L2 is limited, and researchers attach much more importance to PD-L1 study.
Clinically, the PD-1/PD-L1 blockade has demonstrated notable anti-tumor immunity in various tumor types including melanoma [15, 16], non-small cell lung cancer (NSCLC) [17, 18], gastric cancer [19,20,21], colorectal cancer (CRC) [22, 23], renal cell carcinoma (RCC) [24], pancreatic cancer [25], breast cancer [26, 27], ovarian cancer [28, 29], bladder carcinoma [30] and Hodgkin’s lymphoma [25]. In 2015, a miracle in cancer treatment history that the PD-1 inhibitor pembrolizumab (Keytruda®) [31] cured the 91-year-old American former president Jimmy Carter of metastatic melanoma (MM) ignited the enthusiasm of immune checkpoint blockade therapy investigators all over the world, as a result of which the precision medicine has become more and more popular.
The concept of precision medicine is based on the fundamental hypothesis that some specific molecular variations can be regarded as pathogeny of a given malignancy and identifying it will lead to therapeutics that are more discriminating and personized than traditional cancer treatment approaches such as chemotherapeutics and radiotherapeutics. As mentioned above, CTLA-4 and PD-1/PD-L1 are widely studied for their function as brakes of the immune system, especially the anti-tumor immunity. As a result, investigations on checkpoint blockade are in the ascendant and multiple anti-CTLA-4 and anti-PD-1/PD-L1 antibodies have been developed and clinically analyzed for the treatment of all kinds of malignant tumors, showing promising outcomes [25, 32].
The use of immune checkpoint antibodies in treating solid tumors was first established in 2010 when a CTLA-4 inhibitor, ipilimumab, showed to prolong patients’ survival in MM and later approved by the US Food and Drug Administration (FDA) for the treatment of melanoma [33]. On December 22, 2014, the nivolumab of Bristol-Myers Squibb (BMS) became the first FDA approved PD-1 antibody for the treatment of melanoma, and the pembrolizumab mentioned above was approved on September 4, 2014 for treating MM [34].
NSCLC, accounting for 85% of all lung cancer diagnoses [35], has been a significant concern where numerous checkpoint blockade researches take place besides melanoma. As most of NSCLC patients are diagnosed at an advanced stage when the tumor is no longer operable, and chemoradiotherapy is not competent enough, more efficient therapies are incredibly urgent to develop [17, 36, 37]. It was not until March 2015 when FDA approved nivolumab for squamous NSCLC treatment, and eventually for all patients with advanced NSCLC progressing after platinum-based chemotherapy on October 9, 2015. Pembrolizumab was also approved on October 2, 2015 for PD-L1-positive NSCLC treatment. So far, both nivolumab and pembrolizumab have been approved by FDA and used for treatment in melanoma, NSCLC, head and neck cancer, RCC and Hodgkin lymphoma with more and more new drugs under clinical trials both domestically and abroad [38,39,40,41,42,43]. The latest PD-1/PD-L1 related drugs that are launched in the market or currently under clinical trials are listed below (Table 1).
Notwithstanding all the promising results achieved in checkpoint immunotherapies, failure of response to the PD-1/PD-L1 checkpoint blockade therapies makes them inefficacious, and the drug resistance also makes the treatment tougher [44]. The molecular mechanisms making tumors sensitive or not to the PD-1/PD-L1 blockade therapies and under which this pathway is regulated are barely understood. Therefore, further understanding of the underlying regulatory mechanisms of the PD-1/PD-L1 pathway is of noteworthy significance and may provide the basis for the development of more practical and effective targeted anti-cancer therapies.
PD-1/PD-L1 Pathway and Noncoding RNAs
PD-1 is absent on resting T-cells and found initially only in activated mouse T-cells upon TCR engagement [45], yet its expression is commonly upregulated in patients with various cancer types and usually implies a poor prognosis [46,47,48,49]. Likewise, PD-L1 mRNA can be found broadly in various tissues under normal physiological conditions while its protein is only expressed in specific tissue types such as placenta, tonsil and a small proportion of macrophage-like cells in lung and liver [4], suggesting that the expression of PD-1 and PD-L1 is regulated post-transcriptionally, which is typically mediated by noncoding RNAs.
Noncoding RNAs refer to RNAs that have no protein-coding ability, and the most studied types are microRNAs (miRNAs) with a length of about 22 nucleotides and long noncoding RNAs (lncRNAs) which are longer than 200 nucleotides [50, 51].
miRNAs are a class of small single-stranded RNAs that post-transcriptionally modulate gene expression by binding to the mRNA 3′-untranslated region (3′UTR) of target genes, causing mRNA degradation or repression of translation [52]. Currently, it is well-known that miRNAs can be aberrantly expressed in various human cancers [53, 54], as a result of which the majority of studies on miRNAs have focused on their function as oncogenes or tumor suppressors [55,56,57,58,59].
LncRNAs are recognized to play vital roles in the regulation of numerous biological processes such as cell proliferation, RNA splicing, gene expression, and apoptosis, which are altered during cancer development and progression [51]. Due to their highly cell-specific and time-dependent expression patterns, lncRNAs are mainly studied as cellular address code and gene expression modulators, which are often realized by interaction with other noncoding RNAs [60,61,62,63].
Emerging evidence has revealed the pervasive involvement of noncoding RNAs in the regulation of the host immune response, especially in the tumor microenvironment [64,65,66,67,68,69,70,71]. Here we summarize the most recent studies on the regulation of the PD-1/PD-L1 pathway by noncoding RNAs in cancer and aim to contribute to further understanding of the immune checkpoint blockade and precision medicine in cancer immunotherapies.
Regulation of the PD-1/PD-L1 Pathway by Noncoding RNAs in Cancer
Regulation of PD-1/PD-L1 by miRNAs
miRNAs exert their functions mostly by interaction with the 3′UTR of their target genes’ mRNA, which has been a consensus in miRNA study [72]. Elevated PD-L1 expression caused by structural variations disrupting its mRNA 3′UTR uncovered a novel genetic mechanism of immune escape in multiple cancers [73]. Likewise, variant single nucleotide polymorphisms (SNPs) at the binding sites of miRNAs in the PD-L1 3′UTR obstructed the interaction between miRNAs and PD-L1 mRNA, leading to increased risk of cancers [74]. For example, a guanine-to-cytosine mutation at the 3′UTR of PD-L1 mRNA which was further confirmed to locate at the “seed region” of the binding sites of miR-570 was frequently observed in gastrointestinal cancers, as a result of which the interaction between miR-570 and PD-L1 mRNA was hindered, consequently leading to the overexpression of PD-L1 [75].
Meanwhile, PD-L1 expression was induced by IFN-γ and Cryptosporidium parvum in Cholangiocytes through the downregulation of miR-513 [76, 77] while suppressed by miR-155 induction via TNF-α and IFN-γ in primary human cells [78, 79]. A miRNA cluster, the miR-25-93-106b cluster, was also demonstrated to regulate bone marrow metastasis and immune invasion via modulation of PD-L1 in bone marrow (BM) stromal niche [80].
As previously mentioned, most of the studies on noncoding RNAs are focused on the roles they played during the tumorigenesis and development. As NSCLC, melanoma and gastrointestinal cancers are the primary causes of cancer-related deaths worldwide [35], they have undoubtedly drawn much attention. Hereunder, we will mainly discuss the study progress of the PD-1/PD-L1 pathway regulation mechanisms by noncoding RNAs in NSCLC, melanoma, gastrointestinal cancers, and many other cancers.
NSCLC
The role of miRNAs in NSCLC carcinogenesis was indicated as early as in 2004 since the relatively low expression of several miRNAs was demonstrated in lung cancer cell lines [81, 82]. The number of functional studies focusing on the miRNAs’ role in NSCLC has increased and several independent researches revealed the pro- or anti-cancer functions of miRNAs including miR-150 [83], miR-34a [84, 85], miR-486-5p [86,87,88,89], miR-18a [90, 91] and miR-146a [92, 93].
The emergence of numerous predictive bioinformatics tools has offered us unprecedented opportunities and convenience for noncoding RNA function prediction and analysis. miR-140-3p, for example, is predicted to have the potential to bind to the 3′UTR of PD-L1 mRNA in many cancers [94], which was further verified in osteosarcoma [95] and NSCLC [96]. Similarly, the high expression of miR-33a was found to be associated with low PD-1 expression and more prolonged survival of patients in lung adenocarcinoma exploiting The Cancer Genome Atlas (TCGA) database and further confirmed with patients’ tissue samples [97].
miRNAs that share the same seed alignment and consensus secondary structures are considered to belong to a miRNA family [98], and the family members are often found to work synergistically to maximize their function in the biological processes [99]. The miR-34 family has been reported to be a suppressor in various cancer types by targeting the Notch, c-Myc, c-Met, Bcl-2, Src, and p53 [100]. miR-34a especially has shown clinical significance in the treatment of MM [101] and NSCLC [85]. p53 is one of the most commonly mutated genes in cancers and crucial in regulating apoptosis, cell division, DNA damage and repair, senescence, and modulating the immune response [102]. A p53/miR-34/PD-L1 axis was demonstrated in NSCLC, where p53 regulated PD-L1 via the miR-34 family.
Moreover, in vivo administration of MRX34 (a liposomal formulation complexed with miR-34a mimic) alone or in combination with radiotherapy could reduce PD-L1 expression in the tumor and liberate T cell from exhaustion, showing a promising anti-tumor potential of miRNAs [103].
miR-200 family is another well studied miRNA family in various malignancies, including acute myeloid leukemia (AML), ovarian cancer, colorectal cancer, and lung cancer [104,105,106]. The miR-200/ZEB1 (zinc-finger E-box-binding homeobox 1) axis has been reported to regulate tumor metastasis through epithelial-to-mesenchymal transition (EMT) progress, and PD-L1 was found to be under the regulation of this miR-200/ZEB1 axis in NSCLC, which subsequently led to debility of CD8+ T-cells in the tumor microenvironment, connecting the CD8+ tumor-infiltrating lymphocytes (TILs) exhaustion with EMT and revealing the significance of immune-suppression to tumor metastasis [107].
Besides directly binding with the 3′UTR of PD-1/PD-L1 mRNA, miRNAs are also found to exert their regulation on PD-1/PD-L1 through their upstream and downstream pathways.
As drug resistance and chemoresistance are the main obstacles affecting the treatment of NSCLC, it seems essential to focus on reversing the resistant state of tumors to sensitive ones. In chemoresistant NSCLC, a miR-197/cyclin-dependent kinases regulatory subunit 1 B (CKS1B)/ signal transducers and activators of transcription 3 (STAT3)-mediated PD-L1 network was revealed in which the downregulation of miR-197 was associated with chemoresistance and worse overall survival [108]. PD-L1 was regulated by STAT3, which was a pivotal target downstream of the miR-197/CKS1B pathway and proved to have the potential of relieving lung cancer drug resistance and tumor progression. On the contrary, miR-3127-5p was found to promote STAT3 phosphorylation and subsequently induced the expression of PD-L1 in NSCLC and eventually resulted in immune escape and chemoresistance [109].
Melanoma
The promising results achieved by anti-PD-1/PD-L1 therapies have inspired more and more studies in checkpoint blockade in melanoma treatment as the low 5-year survival rate and inadequate response to traditional chemotherapies are two main obstacles facing the treatment of melanoma.
PD-L1 expression was upregulated in tissue biopsies after resistance to BRAF or mitogen-activated extracellular signal-regulated kinase (MEK) inhibitors (BRAFi or MEKi) treatment arose in a cohort of 80 MM patients, consequently leading to increased invasiveness and worse prognosis. Plasmatic miR-17-5p levels showed to be inversely correlated with that of PD-L1, which was further verified to be post-transcriptionally regulated by miR-17-5p. Together, these results revealed that miR-17-5p might be used as inverse indicators of PD-L1 levels by the tumor, along with PD-L1 a marker for the aggressiveness of melanoma and a predictor of response to BRAFi or MEKi treatment [110].
miR-28, likewise, through silencing of PD-1, restored the impaired secretion of cytokines interleukin 2 (IL-2) and TNF-α by liberating the exhausted T-cells in melanoma in vivo, thus providing a novel target in melanoma immunotherapies [111].
Tumor-associated macrophages (TAMs) are the dominant and most abundant leukocytic infiltrate in many tumor types that participate in tumor angiogenesis, invasion, metastasis and almost every process during tumor progression. It is commonly acknowledged that TAMs have two dominant distinctive phenotypes: the immunosuppressive, tumor-promoting M2 macrophage and pro-inflammatory and tumoricidal M1 macrophage [112,113,114].
MiRNAs have long been studied for their regulation of host immuno-inflammatory responses [115]. miR-21 and miR-4717 are among the first ones found to modulate host immune response through regulation of PD-1 in virus-induced inflammation and liver diseases [116, 117]. As one of the first identified miRNAs, miR-21 has been reported to be a key regulator of oncogenesis in gastric [118,119,120,121], renal [122, 123], esophageal [124,125,126], colon [127,128,129], lung [130,131,132], prostate [133,134,135], breast cancer [136, 137] and melanoma [138]. By downregulating Janus kinase 2 (JAK2) and signal transducers and activators of transcription 1 (STAT1), miR-21 inhibited the IFN-γ-induced STAT1 signaling pathway, which is required for macrophage M1 polarization. miR-21 depletion was thus confirmed to facilitate tumoricidal inclination through participating in the STAT1-mediated activation of anti-tumor immunity and improved PD-1 immunotherapy [6, 139, 140], which was verified in vivo in melanoma xenografts.
Gastrointestinal Cancers
Gastric cancer and colorectal cancer (CRC) are the two main types of gastrointestinal cancers, which are among the most prevalent cancers in the general population [141]. While Helicobacter pylori (H. pylori) infection is the most common cause of gastric cancer, a novel mechanism was revealed through which H. pylori promotes gastric cancer development by upregulating PD-L1 expression via inhibition of the miR-200b and miR-152 expression, causing immune escape [142]. PD-L1 was further verified to be a target gene of miR-152, and overexpression of the latter could increase immune response via PD-L1 inhibition [143].
In CRC, miR-138-5p expression was downregulated in tissue samples and inversely connected with advanced cancer stage, lymph node metastasis, and overall survival. Moreover, miR-138-5p could bind to PD-L1 mRNA 3′UTR, leading to CRC cell growth suppression in vitro and tumorigenesis inhibition in vivo [144]. Also, in advanced CRC, a PTEN (phosphatase and tensin homolog)-inhibition-induced PD-L1 upregulation was mediated by miR-20b, 21 and -130b, where the PD-L1 overexpression was induced by the inhibition of PTEN, as has been reported before [145], and PTEN silencing was regulated by overexpression of miR-20b, 21 and -130b [146].
Other Cancers
Targeted therapies are showing extraordinary effectiveness in treating all kinds of cancers in this era of precision medicine. As a result, studies on immune checkpoint blockade are also carried out in various malignant tumors.
Acute myeloid leukemia (AML) is a fatal hematological malignancy that features a highly immunosuppressive microenvironment [147]. As the incidence rate of the disease is rapidly growing through the decades, a more profound understanding of its genetic underpinnings is urgent. miR-34a was verified to regulate PD-L1 through interaction with its 3′UTR and could further reduce the PD-L1-induced IL-10 production, sensitizing the PD-L1-overexpressing tumor cells to T cell killing in AML [148]. miR-200c and miR-34a were also identified as crucial regulators of PD-L1 in AML, which is mediated by the heterodimeric oncoprotein MUC1, which could regulate the processing of miRNA. Herein the silencing of MUC1 caused a marked increase in mature miR-200c and miR-34a levels, and a decrease in PD-L1 protein level consequentially [149].
In epithelial ovarian carcinoma (EOC), one leading cause of death in women with gynecological malignancies, miR-424 activated cytotoxic T lymphocytes (CTLs), reduced regulatory cytokine secretions and eventually reversed chemoresistance by downregulating PD-L1 and CD80, as the immune checkpoint was blocked and T cell immune response activated [150]. As a matter of fact, miR-424 belongs to the miR-15/−16/−195/−424/−497/−503 family, whose another two members, miR-16 and miR-195, were also found to be able to enhance radiotherapy in prostate cancer by targeting PD-L1, which subsequently activated T-cells through proliferation of cytotoxic CD8+ T-cells and inhibition restraint of myeloid-derived suppressor cells (MDSCs) and regulatory T-cells (Tregs) [151].
miR-374b [152], miR-375 [153], miR-138 [154], miR-142-5p [155], miR-574-3p [156], miR-195 [157] and miR-873 [158] were also demonstrated to regulate the PD-1/PD-L1 pathway and cancer immune response in liver cancer, head and neck squamous cell carcinoma (HNSCC), glioma, pancreatic cancer, spinal chordoma, diffuse large B cell lymphoma (DLBCL) and breast cancer respectively (Table 2).
Regulation of PD-1/PD-L1 by Other Noncoding RNAs
Besides the miRNAs, other types of noncoding RNAs were also found to be involved in PD-1/PD-L1 pathway regulation in cancers.
Actin filament-associated protein one antisense RNA 1 (AFAP1-AS1), for example, was found to be co-expressed with PD-1 in nasopharyngeal carcinoma (NPC) [159]. Also, the high expression of AFAP1-AS1 and PD-1 was strongly associated with distant metastasis and poor prognosis, revealing a novel marker and candidate target for clinical trials. Another lncRNA, small nucleolar RNA host gene 20 (SNHG20), has been demonstrated recently to promote cell growth and metastasis in esophageal squamous cell carcinoma (ESCC) via modulating ATM (ataxia telangiectasia–mutated kinase)-JAK-PD-L1 pathway [161]. The noncoding RNAs that regulate the PD-1/PD-L1 pathway indirectly are shown in Table 3.
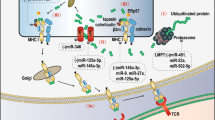
HY4, a member of a newly discovered ncRNA category, Y RNA, is found to be enriched in chronic lymphocytic leukemia (CLL)-derived exosomes. Uptake of hY4 triggered PD-L1 upregulation and cytokine secretion in monocytes, which was further verified to be mediated via Toll-like receptor 7 (TLR7) signaling [160]. Since circulatory noncoding RNAs are mainly transported to the plasma in exosomes to avoid degradation by nuclease, exosomes are widely studied in order to search for novel biomarkers and targets in cancer diagnoses and treatment. This work uncovered a new kind of noncoding RNA participating in cancer immune response regulation and provided new insights into the cancer diagnoses and treatment [162]. The potential role of noncoding RNAs in PD-1/PD-L1 pathway regulation is shown in Fig. 1.
Noncoding RNAs that are involved in regulation of the PD-1/PD-L1 pathway in cancer. The PD-1 is expressed on the surface of T cells, while PD-L1 is usually elevated in tumor cells in response to cytokines secreted by tumors. The PD-1/PD-L1 pathway are under regulation of many noncoding RNAs post-transcriptionally directly or indirectly, most of which are miRNAs. The use of PD-1/PD-L1 antibodies can specifically bind with PD-1 or PD-L1 and block the pathway, after which the T-cells will be activated again to kill the tumor cells
Conclusion and Perspective
As personalized cancer therapies are becoming more and more popular around the world, antibodies targeting different genes in various cancer types are studied and developed, of which the anti-PD-1/PD-L1 antibodies are doubtlessly the most high-profile and successful ones, with several of each kind approved by FDA for treatment of many cancer types [162, 163].
As PD-L1 is often induced by cytokines secreted by cancer cells like TNF-α and IFN-γ to evade the attack by activated T-cells via the interaction between PD-1 and PD-L1, the PD-1/PD-L1 checkpoints blockade therapies regulate the immune response at the tumor sites and fix ongoing immune processes unlike previous immune therapeutic agents that boost systemic immune responses against cancer in the first place. In other words, blockade of the PD-1/PD-L1 interaction can not only ameliorate the host immune response by activating the macrophages or promoting the secretion of anti-tumor cytokines, it can also remove the exhausted T-cells at the tumor sites, thus promoting the proliferation of the T cells and stimulate them to kill the cancer cells, which makes the anti-PD-1/PD-L1 therapies unique and far more effective and powerful than traditional immune therapeutic agents.
Meanwhile, apart from liberating T cells from exhaustion phenotype, PD-1/PD-L1 blockade has also demonstrated a significant impact on reversing the resistance of patients to traditional cancer treatments including chemotherapy and radiotherapy [103, 108,109,110, 150, 151]. Here we can see that once the PD-1/PD-L1 pathway is blocked by miRNA-mediated PD-L1 silencing, the T-cell immune response will be activated and thus reversing the resistance to traditional chemotherapies or radiotherapies or enhancing the efficacy of the therapies. On the contrary, the miRNA-mediated PD-L1 upregulation will often lead to resistance [109] (Table 4). In a sense, miRNAs can function analogously with PD-1/PD-L1 antibodies in cancer treatment to achieve a better outcome, which implies better exploitation of miRNAs in clinical trials.
Despite the success that anti-PD-1/PD-L1 antibodies achieved in clinical, there are still much unknown about the PD-1/PD-L1 pathway and the associated immune responses including the regulation of their expression in different tissues and tumor types, the mechanism of resistance to the blockade therapies and the pathways related in their functions on tumor immunity. At this moment through the work of the regulation of the PD-1/PD-L1 pathway by noncoding RNAs, we can take a glance at the mechanism underlying the expression pattern of the PD-1/PD-L1 pathway in the tumor microenvironment.
Although the function of lncRNAs in different biological activities have been studied comprehensively and understanding of them have been thriving, apparently there are still much unknown about their roles in immune checkpoint regulation. The studies focusing on the regulation of the PD-1/PD-L1 pathway by noncoding RNAs mostly revealed microRNA-mediated mechanisms, leaving much work for investigation of other unrevealed noncoding RNAs, especially lncRNAs. It must be admitted that there is still a long way ahead of us to achieve a full understanding of this pathway and the role it plays in the tumor immunology. Much more work is needed to reveal the complete picture of these critical immune checkpoints, which will facilitate the discovery and design of novel clinically applicable approaches in cancer immunotherapies.
Data Availability
The datasets analyzed in this article are available from the corresponding author on reasonable request.
References
Teng MW et al (2008) Immune-mediated dormancy: an equilibrium with cancer. J Leukoc Biol 84(4):988–993
Schneider H et al (2006) Reversal of the TCR stop signal by CTLA-4. Science 313(5795):1972–1975
Ishida Y et al (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11(11):3887–3895
Dong H et al (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8(8):793–800
Dong H et al (1999) B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 5(12):1365–1369
Freeman GJ et al (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192(7):1027–1034
Zhang X et al (2004) Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity 20(3):337–347
Lesterhuis WJ, Steer H, Lake RA (2011) PD-L2 is predominantly expressed by Th2 cells. Mol Immunol 49(1–2):1–3
Chen L (2004) Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol 4(5):336–347
Sheppard KA et al (2004) PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett 574(1–3):37–41
Francisco LM, Sage PT, Sharpe AH (2010) The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 236:219–242
Zamani MR et al (2016) PD-1/PD-L and autoimmunity: a growing relationship. Cell Immunol 310:27–41
Sharpe AH et al (2007) The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 8(3):239–245
Butte MJ et al (2007) Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 27(1):111–122
Schadendorf D et al (2015) Pooled analysis of Long-term survival data from phase II and phase III trials of Ipilimumab in Unresectable or metastatic melanoma. J Clin Oncol 33(17):1889–1894
Wolchok JD et al (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369(2):122–133
Younes RN et al (2011) Chemotherapy beyond first-line in stage IV metastatic non-small cell lung cancer. Rev Assoc Med Bras (1992) 57(6):686–691
Reck M et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung Cancer. N Engl J Med 375(19):1823–1833
D'Alterio C et al (2016) CXCR4-CXCL12-CXCR7, TLR2-TLR4, and PD-1/PD-L1 in colorectal cancer liver metastases from neoadjuvant-treated patients. Oncoimmunology 5(12):e1254313
Sasaki S et al (2018) EBV-associated gastric cancer evades T-cell immunity by PD-1/PD-L1 interactions. Gastric Cancer
Saito H et al (2018) Highly activated PD-1/PD-L1 pathway in gastric Cancer with PD-L1 expression. Anticancer Res 38(1):107–112
Liu S et al (2017) PD-1/PD-L1 interaction up-regulates MDR1/P-gp expression in breast cancer cells via PI3K/AKT and MAPK/ERK pathways. Oncotarget 8(59):99901–99912
Shi W et al (2018) Follicular helper T cells promote the effector functions of CD8(+) T cells via the provision of IL-21, which is downregulated due to PD-1/PD-L1-mediated suppression in colorectal cancer. Exp Cell Res 372(1):35–42
Motzer RJ et al (2015) Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol 33(13):1430–1437
Sharma P, Allison JP (2015) The future of immune checkpoint therapy. Science 348(6230):56–61
Schutz F et al (2017) PD-1/PD-L1 pathway in breast Cancer. Oncol Res Treat 40(5):294–297
Ge Y et al (2013) Blockade of PD-1/PD-L1 immune checkpoint during DC vaccination induces potent protective immunity against breast cancer in hu-SCID mice. Cancer Lett 336(2):253–259
Strickland KC et al (2016) Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget 7(12):13587–13598
Tan D, Sheng L, Yi QH (2018) Correlation of PD-1/PD-L1 polymorphisms and expressions with clinicopathologic features and prognosis of ovarian cancer. Cancer Biomark 21(2):287–297
Powles T et al (2014) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515(7528):558–562
Kwok G et al (2016) Pembrolizumab (Keytruda). Hum Vaccin Immunother 12(11):2777–2789
Topalian SL et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26):2443–2454
Hodi FS et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723
Topalian SL et al (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32(10):1020–1030
Bray F et al (2018) Global Cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin
Barlesi F et al (2014) Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann Oncol 25(5):1044–1052
Ettinger DS et al (2012) Non-small cell lung cancer. J Natl Compr Cancer Netw 10(10):1236–1271
Shamai S, Merimsky O (2018) Efficacy and safety of Nivolumab in non-small cell lung cancer patients in Tel-Aviv tertiary medical center: facing the reality. Mol Clin Oncol 9(4):419–422
Osa A et al (2018) Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight:3(19)
Nizam A, Aragon-Ching JB (2018) Frontline immunotherapy treatment with nivolumab and ipilimumab in metastatic renal cell cancer: a new standard of care. Cancer Biol Ther:1–2
Long GV et al (2018) Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat-dosing schedule in patients with cancer. Ann Oncol
Hida T (2018) Nivolumab for the treatment of Japanese patients with advanced metastatic non-small cell lung cancer: a review of clinical trial evidence for efficacy and safety. Ther Adv Respir Dis 12:1753466618801167
Grimm SE et al (2018) Nivolumab for treating metastatic or Unresectable urothelial Cancer: An evidence review Group perspective of a NICE single technology appraisal. Pharmacoeconomics
Keir ME et al (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704
Agata Y et al (1996) Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 8(5):765–772
Zaric B et al (2018) PD-1 and PD-L1 protein expression predict survival in completely resected lung adenocarcinoma. Clin Lung Cancer 19:e957–e963
Karim R et al (2009) Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res 15(20):6341–6347
PD-1 inhibitors raise survival in NSCLC. Cancer Discov, 2014. 4(1):6
Yang ZZ et al (2015) PD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survival. Blood Cancer J 5:e281
Prasanth KV, Spector DL (2007) Eukaryotic regulatory RNAs: an answer to the 'genome complexity' conundrum. Genes Dev 21(1):11–42
Enfield KS et al (2012) Mechanistic roles of noncoding RNAs in lung Cancer biology and their clinical implications. Genet Res Int 2012:737416
Mohr AM, Mott JL (2015) Overview of microRNA biology. Semin Liver Dis 35(1):3–11
Bentwich I et al (2005) Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet 37(7):766–770
Berezikov E et al (2005) Phylogenetic shadowing and computational identification of human microRNA genes. Cell 120(1):21–24
Voorhoeve PM (2010) MicroRNAs: oncogenes, tumor suppressors or master regulators of cancer heterogeneity? Biochim Biophys Acta 1805(1):72–86
Lee YS, Dutta A (2006) MicroRNAs: small but potent oncogenes or tumor suppressors. Curr Opin Investig Drugs 7(6):560–564
Chen CZ (2005) MicroRNAs as oncogenes and tumor suppressors. N Engl J Med 353(17):1768–1771
Ortholan C et al (2009) MicroRNAs and lung cancer: new oncogenes and tumor suppressors, new prognostic factors and potential therapeutic targets. Curr Med Chem 16(9):1047–1061
Wozniak M, Mielczarek A, Czyz M (2016) miRNAs in melanoma: tumor suppressors and oncogenes with prognostic potential. Curr Med Chem 23(28):3136–3153
Bergmann JH, Spector DL (2014) Long non-coding RNAs: modulators of nuclear structure and function. Curr Opin Cell Biol 26:10–18
Cabili MN et al (2011) Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev 25(18):1915–1927
Dinger ME et al (2008) Long noncoding RNAs in mouse embryonic stem cell pluripotency and differentiation. Genome Res 18(9):1433–1445
Batista PJ, Chang HY (2013) Long noncoding RNAs: cellular address codes in development and disease. Cell 152(6):1298–1307
Pei X, Wang X, Li H (2018) LncRNA SNHG1 regulates the differentiation of Treg cells and affects the immune escape of breast cancer via regulating miR-448/IDO. Int J Biol Macromol 118(Pt A):24–30
Guo Q et al (2015) Comprehensive analysis of lncRNA-mRNA co-expression patterns identifies immune-associated lncRNA biomarkers in ovarian cancer malignant progression. Sci Rep 5:17683
Rusek AM et al (2015) MicroRNA modulators of epigenetic regulation, the tumor microenvironment and the immune system in lung cancer. Mol Cancer 14:34
Ray M, Ruffalo MM, Bar-Joseph Z (2018) Construction of integrated microRNA and mRNA immune cell signatures to predict survival of patients with breast and ovarian cancer. Genes Chromosom Cancer
Miao BP et al (2015) Nasopharyngeal cancer-derived microRNA-21 promotes immune suppressive B cells. Cell Mol Immunol 12(6):750–756
Li ZH et al. (2018) MicroRNA-92a promotes tumor growth and suppresses immune function through activation of MAPK/ERK signaling pathway by inhibiting PTEN in mice bearing U14 cervical cancer. Cancer Med
Korsunsky I et al (2017) Two microRNA signatures for malignancy and immune infiltration predict overall survival in advanced epithelial ovarian cancer. J Investig Med 65(7):1068–1076
Khorrami S et al (2017) MicroRNA-146a induces immune suppression and drug-resistant colorectal cancer cells. Tumour Biol 39(5):1010428317698365
Afonso-Grunz F, Muller S (2015) Principles of miRNA-mRNA interactions: beyond sequence complementarity. Cell Mol Life Sci 72(16):3127–3141
Kataoka K et al (2016) Aberrant PD-L1 expression through 3'-UTR disruption in multiple cancers. Nature 534(7607):402–406
Du W et al (2017) Variant SNPs at the microRNA complementary site in the B7-H1 3′-untranslated region increase the risk of non-small cell lung cancer. Mol Med Rep 16(3):2682–2690
Wang W et al (2012) A frequent somatic mutation in CD274 3'-UTR leads to protein over-expression in gastric cancer by disrupting miR-570 binding. Hum Mutat 33(3):480–484
Gong AY et al (2009) MicroRNA-513 regulates B7-H1 translation and is involved in IFN-gamma-induced B7-H1 expression in cholangiocytes. J Immunol 182(3):1325–1333
Gong AY et al (2010) Cryptosporidium parvum induces B7-H1 expression in cholangiocytes by down-regulating microRNA-513. J Infect Dis 201(1):160–169
Yee D et al (2017) MicroRNA-155 induction via TNF-alpha and IFN-gamma suppresses expression of programmed death ligand-1 (PD-L1) in human primary cells. J Biol Chem 292(50):20683–20693
Zhang J, Braun MY (2014) PD-1 deletion restores susceptibility to experimental autoimmune encephalomyelitis in miR-155-deficient mice. Int Immunol 26(7):407–415
Cioffi M et al (2017) The miR-25-93-106b cluster regulates tumor metastasis and immune evasion via modulation of CXCL12 and PD-L1. Oncotarget 8(13):21609–21625
Takamizawa J et al (2004) Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res 64(11):3753–3756
Yu SL et al (2008) MicroRNA signature predicts survival and relapse in lung cancer. Cancer Cell 13(1):48–57
Wang DT et al (2013) miR-150, p53 protein and relevant miRNAs consist of a regulatory network in NSCLC tumorigenesis. Oncol Rep 30(1):492–498
Fu Y et al (2018) Silencing of Long non-coding RNA MIAT sensitizes lung Cancer cells to Gefitinib by epigenetically regulating miR-34a. Front Pharmacol 9:82
Shi Y et al (2014) The microRNA miR-34a inhibits non-small cell lung cancer (NSCLC) growth and the CD44hi stem-like NSCLC cells. PLoS One 9(3):e90022
Wang J et al (2014) Downregulation of miR-486-5p contributes to tumor progression and metastasis by targeting protumorigenic ARHGAP5 in lung cancer. Oncogene 33(9):1181–1189
Shao Y et al (2016) Direct repression of the oncogene CDK4 by the tumor suppressor miR-486-5p in non-small cell lung cancer. Oncotarget 7(23):34011–34021
Gao ZJ et al (2018) miR-486-5p functions as an oncogene by targeting PTEN in non-small cell lung cancer. Pathol Res Pract 214(5):700–705
Yu S, Geng S, Hu Y (2018) miR-486-5p inhibits cell proliferation and invasion through repressing GAB2 in non-small cell lung cancer. Oncol Lett 16(3):3525–3530
Liang C et al (2017) MicroRNA-18a-5p functions as an oncogene by directly targeting IRF2 in lung cancer. Cell Death Dis 8(5):e2764
Shen Z et al (2015) Effect of miR-18a overexpression on the radiosensitivity of non-small cell lung cancer. Int J Clin Exp Pathol 8(1):643–648
Chen G et al (2013) miR-146a inhibits cell growth, cell migration and induces apoptosis in non-small cell lung cancer cells. PLoS One 8(3):e60317
Park DH et al (2015) MicroRNA-146a inhibits epithelial mesenchymal transition in non-small cell lung cancer by targeting insulin receptor substrate 2. Int J Oncol 47(4):1545–1553
Kapodistrias N, Bobori C, Theocharopoulou G (2017) MiR-140-3p Downregulation in Association with PDL-1 Overexpression in Many Cancers: A Review from the Literature Using Predictive Bioinformatics Tools. Adv Exp Med Biol 988:225–233
Ji X, Wang E, Tian F (2018) MicroRNA-140 suppresses osteosarcoma tumor growth by enhancing anti-tumor immune response and blocking mTOR signaling. Biochem Biophys Res Commun 495(1):1342–1348
Xie WB et al (2018) MiR-140 expression regulates cell proliferation and targets PD-L1 in NSCLC. Cell Physiol Biochem 46(2):654–663
Boldrini L et al (2017) Role of microRNA-33a in regulating the expression of PD-1 in lung adenocarcinoma. Cancer Cell Int 17:105
Jiang D et al (2006) Duplication and expression analysis of multicopy miRNA gene family members in Arabidopsis and rice. Cell Res 16(5):507–518
Senfter D et al (2016) The microRNA-200 family: still much to discover. Biomol Concepts 7(5–6):311–319
Wang R et al (2013) Functional role of miR-34 family in human cancer. Curr Drug Targets 14(10):1185–1191
Vergani E et al (2016) Overcoming melanoma resistance to vemurafenib by targeting CCL2-induced miR-34a, miR-100 and miR-125b. Oncotarget 7(4):4428–4441
Huarte M et al (2010) A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142(3):409–419
Cortez MA et al (2016) PDL1 Regulation by p53 via miR-34. J Natl Cancer Inst:108(1)
Feng X et al (2014) MiR-200, a new star miRNA in human cancer. Cancer Lett 344(2):166–173
Humphries B, Yang C (2015) The microRNA-200 family: small molecules with novel roles in cancer development, progression and therapy. Oncotarget 6(9):6472–6498
Diaz T et al (2014) Role of miR-200 family members in survival of colorectal cancer patients treated with fluoropyrimidines. J Surg Oncol 109(7):676–683
Chen L et al (2014) Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 5:5241
Fujita Y et al (2015) The clinical relevance of the miR-197/CKS1B/STAT3-mediated PD-L1 network in chemoresistant non-small-cell lung cancer. Mol Ther 23(4):717–727
Tang D et al (2018) The miR-3127-5p/p-STAT3 axis up-regulates PD-L1 inducing chemoresistance in non-small-cell lung cancer. J Cell Mol Med 22:3847–3856
Audrito V et al (2017) PD-L1 up-regulation in melanoma increases disease aggressiveness and is mediated through miR-17-5p. Oncotarget 8(9):15894–15911
Li Q et al (2016) miR-28 modulates exhaustive differentiation of T cells through silencing programmed cell death-1 and regulating cytokine secretion. Oncotarget 7(33):53735–53750
Rivera LB, Bergers G (2013) Location, location, location: macrophage positioning within tumors determines pro- or antitumor activity. Cancer Cell 24(6):687–689
Jahangiri A et al (2013) Gene expression profile identifies tyrosine kinase c-met as a targetable mediator of antiangiogenic therapy resistance. Clin Cancer Res 19(7):1773–1783
Biswas SK, Mantovani A (2010) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11(10):889–896
Liu G, Abraham E (2013) MicroRNAs in immune response and macrophage polarization. Arterioscler Thromb Vasc Biol 33(2):170–177
Choi B et al (2015) The relevance of miRNA-21 in HSV-induced inflammation in a mouse model. Int J Mol Sci 16(4):7413–7427
Zhang G et al (2015) microRNA-4717 differentially interacts with its polymorphic target in the PD1 3′ untranslated region: A mechanism for regulating PD-1 expression and function in HBV-associated liver diseases. Oncotarget 6(22):18933–18944
Zhang Z et al (2008) miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab Investig 88(12):1358–1366
Yang SM et al (2013) miR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicology 306:162–168
Xu Y et al (2012) miR-21 Is a Promising Novel Biomarker for Lymph Node Metastasis in Patients with Gastric Cancer. Gastroenterol Res Pract 2012:640168
P LA et al (2018) Up-Regulation of miR-21, miR-25, miR-93, and miR-106b in Gastric Cancer. Iran Biomed J 22(6):367–373
Zhang H et al (2012) miR-21 downregulated TCF21 to inhibit KISS1 in renal cancer. Urology 80(6):1298–1302 e1
An F, Liu Y, Hu Y (2017) miR-21 inhibition of LATS1 promotes proliferation and metastasis of renal cancer cells and tumor stem cell phenotype. Oncol Lett 14(4):4684–4688
Winther M et al (2015) Evaluation of miR-21 and miR-375 as prognostic biomarkers in esophageal cancer. Acta Oncol 54(9):1582–1591
Wen SW et al (2015) Association of miR-21 with esophageal cancer prognosis: a meta-analysis. Genet Mol Res 14(2):6578–6582
Fu C et al (2014) The expression of miR-21 and miR-375 predict prognosis of esophageal cancer. Biochem Biophys Res Commun 446(4):1197–1203
Zhang J et al (2012) miR-21, miR-17 and miR-19a induced by phosphatase of regenerating liver-3 promote the proliferation and metastasis of colon cancer. Br J Cancer 107(2):352–359
Yu Y et al (2015) miR-21 and miR-145 cooperation in regulation of colon cancer stem cells. Mol Cancer 14:98
Deng J et al (2014) Targeting miR-21 enhances the sensitivity of human colon cancer HT-29 cells to chemoradiotherapy in vitro. Biochem Biophys Res Commun 443(3):789–795
Xue X et al (2016) MiR-21 and MiR-155 promote non-small cell lung cancer progression by downregulating SOCS1, SOCS6, and PTEN. Oncotarget 7(51):84508–84519
Xia H et al (2017) miR-21 modulates the effect of EZH2 on the biological behavior of human lung cancer stem cells in vitro. Oncotarget 8(49):85442–85451
Su C et al (2018) MiR-21 improves invasion and migration of drug-resistant lung adenocarcinoma cancer cell and transformation of EMT through targeting HBP1. Cancer Med 7(6):2485–2503
Yang Y, Guo JX, Shao ZQ (2017) miR-21 targets and inhibits tumor suppressor gene PTEN to promote prostate cancer cell proliferation and invasion: An experimental study. Asian Pac J Trop Med 10(1):87–91
Reis ST et al (2012) miR-21 may acts as an oncomir by targeting RECK, a matrix metalloproteinase regulator, in prostate cancer. BMC Urol 12:14
Amankwah EK et al (2013) miR-21, miR-221 and miR-222 expression and prostate cancer recurrence among obese and non-obese cases. Asian J Androl 15(2):226–230
Zadeh MM, Ranji N, Motamed N (2015) Deregulation of miR-21 and miR-155 and their putative targets after silibinin treatment in T47D breast cancer cells. Iran J Basic Med Sci 18(12):1209–1214
Fragni M et al (2016) The miR-21/PTEN/Akt signaling pathway is involved in the anti-tumoral effects of zoledronic acid in human breast cancer cell lines. Naunyn Schmiedeberg's Arch Pharmacol 389(5):529–538
Yang CH et al (2015) The oncogenic microRNA-21 inhibits the tumor suppressive activity of FBXO11 to promote tumorigenesis. J Biol Chem 290(10):6037–6046
Iliopoulos D et al (2011) The negative costimulatory molecule PD-1 modulates the balance between immunity and tolerance via miR-21. Eur J Immunol 41(6):1754–1763
Xi J et al (2018) miR-21 depletion in macrophages promotes tumoricidal polarization and enhances PD-1 immunotherapy. Oncogene 37(23):3151–3165
Tran E et al (2015) Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 350(6266):1387–1390
Xie G et al (2017) Helicobacter pylori promote B7-H1 expression by suppressing miR-152 and miR-200b in gastric Cancer cells. PLoS One 12(1):e0168822
Wang Y et al (2017) MicroRNA-152 regulates immune response via targeting B7-H1 in gastric carcinoma. Oncotarget 8(17):28125–28134
Zhao L et al (2016) The tumor suppressor miR-138-5p targets PD-L1 in colorectal cancer. Oncotarget 7(29):45370–45384
Xu C et al (2014) Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell 25(5):590–604
Zhu J et al (2014) MiR-20b, −21, and -130b inhibit PTEN expression resulting in B7-H1 over-expression in advanced colorectal cancer. Hum Immunol 75(4):348–353
Shlush LI, Mitchell A (2015) AML evolution from preleukemia to leukemia and relapse. Best Pract Res Clin Haematol 28(2–3):81–89
Wang X et al (2015) Tumor suppressor miR-34a targets PD-L1 and functions as a potential immunotherapeutic target in acute myeloid leukemia. Cell Signal 27(3):443–452
Pyzer AR et al (2017) MUC1 inhibition leads to decrease in PD-L1 levels via upregulation of miRNAs. Leukemia 31(12):2780–2790
Xu S et al (2016) miR-424(322) reverses chemoresistance via T-cell immune response activation by blocking the PD-L1 immune checkpoint. Nat Commun 7:11406
Tao Z et al (2018) MiR-195/−16 family enhances radiotherapy via T cell activation in the tumor microenvironment by blocking the PD-L1 immune checkpoint. Cell Physiol Biochem 48(2):801–814
Huang F et al (2018) MicroRNA-374b inhibits liver cancer progression via down regulating programmed cell death-1 expression on cytokine-induced killer cells. Oncol Lett 15(4):4797–4804
Wu Q et al (2018) miR-375 inhibits IFN-gamma-induced programmed death 1 ligand 1 surface expression in head and neck squamous cell carcinoma cells by blocking JAK2/STAT1 signaling. Oncol Rep 39(3):1461–1468
Wei J et al (2016) MiR-138 exerts anti-glioma efficacy by targeting immune checkpoints. Neuro-Oncology 18(5):639–648
Jia L et al (2017) miR-142-5p regulates tumor cell PD-L1 expression and enhances anti-tumor immunity. Biochem Biophys Res Commun 488(2):425–431
Zou MX et al (2018) Clinicopathologic implications of CD8(+)/Foxp3(+) ratio and miR-574-3p/PD-L1 axis in spinal chordoma patients. Cancer Immunol Immunother 67(2):209–224
He B, Yan F, Wu C (2018) Overexpressed miR-195 attenuated immune escape of diffuse large B-cell lymphoma by targeting PD-L1. Biomed Pharmacother 98:95–101
Gao L et al (2019) MiR-873/PD-L1 axis regulates the stemness of breast cancer cells. EBioMedicine 41:395–407
Tang Y et al (2017) Co-expression of AFAP1-AS1 and PD-1 predicts poor prognosis in nasopharyngeal carcinoma. Oncotarget 8(24):39001–39011
Haderk F et al (2017) Tumor-derived exosomes modulate PD-L1 expression in monocytes. Science Immunology 2(13)
Zhang C et al (2019) Upregulation of long noncoding RNA SNHG20 promotes cell growth and metastasis in esophageal squamous cell carcinoma via modulating ATM-JAK-PD-L1 pathway. J Cell Biochem
Gilligan KE, Dwyer RM (2017) Engineering Exosomes for Cancer Therapy. Int J Mol Sci:18(6)
Kazandjian D et al (2016) FDA approval summary: Nivolumab for the treatment of metastatic non-small cell lung Cancer with progression on or after platinum-based chemotherapy. Oncologist 21(5):634–642
Tanaka K et al. (2013) Tumor-suppressive function of protein-tyrosine phosphatase non-receptor type 23 in testicular germ cell tumors is lost upon overexpression of miR142-3p microRNA. J Biol Chem 288(33): p. 23990-9
Acknowledgments
Our work is supported by The National Natural Science Foundation of China grants 81572122 to Shanghai Sixth People’s Hospital, affiliated to Shanghai Jiao Tong University.
Funding
The National Natural Science Foundation of China (81572122).
Author information
Authors and Affiliations
Contributions
Data collection and analysis, manuscript writing: L-D. Funding Supporter: SD-L. Manuscript revision and supervision: SD-L, YL-L.
Corresponding authors
Ethics declarations
Ethical Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
There is no conflict of interest regarding this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ding, L., Lu, S. & Li, Y. Regulation of PD-1/PD-L1 Pathway in Cancer by Noncoding RNAs. Pathol. Oncol. Res. 26, 651–663 (2020). https://doi.org/10.1007/s12253-019-00735-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-019-00735-9