Abstract
Preclinical data indicate a direct anti-tumor effect of zoledronic acid (ZA) outside the skeleton, but its molecular mechanism is still not completely clarified. The aim of this study was to investigate the anti-cancer effects of ZA in human breast cancer cell lines, suggesting that they may in part be mediated via the miR-21/PTEN/Akt signaling pathway. The effect of ZA on cell viability was measured by MTT assay, and cell death induction was analyzed using either a double AO/EtBr staining and M30 ELISA assay. A Proteome Profiler Human Apoptosis Array was executed to evaluate the molecular basis of ZA-induced apoptosis. Cell cycle analysis was executed by flow cytometry. The effect of ZA on miR-21 expression was quantified by qRT-PCR, and the amount of PTEN protein and its targets were analyzed by Western blot. ZA inhibited cell growth in a concentration- and time-dependent manner, through the activation of cell death pathways and arrest of cell cycle progression. ZA downregulated the expression of miR-21, resulting in dephosphorilation of Akt and Bad and in a significant increase of p21 and p27 proteins expression. These results were observed also in MDA-MB-231 cells, commonly used as an experimental model of bone metastasis of breast cancer. This study revealed, for the first time, an involvement of the miR-21/PTEN/Akt signaling pathway in the mechanism of ZA anti-cancer actions in breast cancer cells. We would like to underline that this pathway is present both in the hormone responsive BC cell line (MCF-7) as well as in a triple negative cell line (MDA-MB-231). Taken together these results reinforce the use of ZA in clinical practice, suggesting the role of miR-21 as a possible mediator of its therapeutic efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the most common malignancy in women and the second leading cause of cancer death in women worldwide (Jemal et al. 2010). Although major advances in cancer and adjuvant therapies for BC have been achieved over the past 60 years, a substantial number of patients experience disease relapse. As the disease progresses, approximately 70 % of metastatic BC patients develop bone metastases, which in turn increase the risk of fracture through osteoclast-mediated destruction of the surrounding bone (Roodman 2004). Cancer-induced bone disease is generally treated with a multidisciplinary approach, including chemotherapy, radiotherapy, drugs for pain control, and occasionally orthopedic surgery, to improve the quality of life. The therapeutic approach includes the administration of inhibitors of bone resorption such as bisphosphonates (BPs), resulting in pain relief and reduced incidence of skeletal-related events (Kohono et al. 2005; Rosen et al. 2004). In particular, the most used is zoledronic acid (ZA) that belongs to the group of nitrogen-containing bisphosphonates (N-BPs) and rapidly binds to the bone and it is internalized by osteoclasts. At cytoplasmatic level, ZA inhibits key enzymes of the mevalonate pathways that, in turn, strongly reduces osteoclast activity and reduces bone resorption (Rogers et al. 2011). Recent reports indicate that BPs may also prevent recurrence of BC at peripheral site, suggesting that these drugs may have anti-tumor effects outside the skeleton (Holen and Coleman 2010). Their ability to reduce cell proliferation, to inhibit cell migration, and to induce apoptosis has been demonstrated in numerous studies (Russell 2007); in particular, preclinical data have revealed a direct anti-tumor role of ZA which may act through the inhibition of tumor cell adhesion, invasion, and proliferation as well as acting to induce apoptosis in BC cell lines (Boissier et al. 2000; Verdijk et al. 2007). The mechanism of anti-tumor action of ZA, however, is still not completely understood (Stresing et al. 2007), although the inhibition of the mevalonate pathway has been suggested not only in osteoclasts, but also in tumor cells (Clézardin 2005). According to these in vitro data, emerging clinical data support a wider therapeutic effect of ZA in BC, but there is still an ongoing debate as to what extent these anti-tumor effects are caused by a direct action of ZA on tumors cells, rather than indirectly through its anti-resorptive capacity (Clézardin et al. 2005).
MicroRNAs (miRNAs) are small regulatory non-coding RNA molecules that regulate gene expression by influencing the stability or the translational efficiency of target mRNAs (Ambros 2004), leading to translational repression of molecules involved in the regulation of biological processes, including cell growth, differentiation, and apoptosis, both in physiological conditions and during diseases (Ohtsuka et al. 2015). Dysregulation of miRNA expression has been identified in various cancers, and accumulating data suggest that miRNAs function as classical oncogenes or tumor suppressor genes (Ventura and Jacks 2009). MiRNA-21 (miR-21) is one of the most frequently aberrant miRNAs in human cancer (Bermúdez Brito et al. 2015), and its upregulation has been reported to direct target many tumor suppressors in BC (Yan et al. 2012). Among molecules downregulated by miR-21, our attention was drawn on PTEN (phosphates and tensin homolog, deleted on chromosome ten), a phosphatase protein with a role as tumor suppressor (Yamada and Araki 2001). Inactivation of PTEN stimulates cell cycle progression and proliferation by downregulating p21/CIP1/CDNK1A and p27/Kip1; on the other hand, it also prevents cancer cells from apoptosis by inhibiting apoptotic pathways (Weng et al. 2001). Finally, it inhibits Akt pathway by reversing the phosphorylation of the PI3 kinase (Jiang and Liu 2008). Interestingly, evidence indicate that the miR-21/PTEN/Akt signaling cascade plays a critical role in tumorigenesis (Darido et al. 2011).
The aim of this study is to investigate the molecular mechanism involved in the anti-tumoral effects of ZA in breast cancer cell lines, suggesting that it may in part be mediated via the miR-21/PTEN/Akt signaling pathway. We firstly examined the effect of ZA in MCF-7 cell line, worldwide used experimental in vitro model of ER+/PgR+ BC cell line (Holliday and Speirs 2011); then, we investigated whether the ZA effect is modified by the receptor loss as it occurred in the triple negative MDA-MB-231 cell line, a commonly used model of BC that can metastasize at the bone level (Peyruchaud et al. 2001).
Material and methods
Cell culture
MCF-7 and MDA-MB-231 cell lines were purchased from the American Type Culture Collection (ATCC) (LGC Promochem, Sesto San Giovanni, MI, Italy) and maintained in culture as suggested by ATCC. Media and supplements were purchased from Euroclone (Pero, MI, Italy). MycoFluor Mycoplasma Detection Kit (Life technologies Italia, Monza, Italy) was routinely used to test cells for mycoplasma contamination.
Drug treatments
Injectable ZA (Zometa®; Novartis Pharmaceuticals Corp, East Hanover, NJ, USA) was used at increasing concentrations (0.1–100 μmol/L) for different times (up to 120 h) in serum-free medium (Fromigue et al. 2000).
Measurement of cell viability
Cell viability was evaluated by 3-(4,5-dimethyl-2-thiazol)-2,5-diphenyl-2H-tetrazolium bromide (MTT) dye reduction assay. Cells (5 ×1 04 cells/well) were treated as above described, and at the end of the treatment period, MTT dye (5 mg/mL) was added to the cell culture medium and then solubilized with DMSO. Absorbance was determined by a spectrophotometer at 540/620 nm (GDV, Roma, Italy).
Double AO/EtBr staining as a marker for apoptosis and necrosis
A double staining with acridine orange (AO) and ethidium bromide (EtBr) was performed to visualize and quantify the number of viable, apoptotic, and necrotic cells (Mironova et al. 2007). Briefly, untreated and ZA-treated cells were centrifuged for 5 min at 700g and resuspended in PBS. The dye mixture (100 μg/mL AO and 100 μg/mL EtBr) was added to cell suspension (1:10 final dilution) and immediately examined by a Zeiss LSM 510 META confocal laser-scanning microscope, with 10× objective (Carl Zeiss AG, Germany). Several fields, randomly chosen, were digitalized, and about 1000 nuclei for each sample were counted and scored by using the NIH Image J software.
M30 antigen quantification
The M30 CytoDeath™ ELISA (Vinci-Biochem, Firenze, Italy) was used to evaluate apoptosis. M30 antibody recognizes a neo-epitope of K18 (K18Asp396) exposed after caspase cleavage (Schutte et al. 2004). Briefly, untreated and ZA-treated cells were processed with NP-40 0.5 % (Sigma Italia, Milano, MI, Italy). To assay M30 antigen, 25 μl of each sample were transferred from each well to a M30 CytoDeath™ ELISA plate and assayed according to the manufacturer’s instructions. Staurosporine (4 μM) was added to MDA-MB-231 cells for 18 h as positive control (Hägg et al. 2002).
Proteome profiler array in cell lysates
The Proteome Profiler Human Apoptosis Array kit (R&D Systems, Space Import-Export, Milano, MI, Italy) simultaneously detects the expression level of 35 apoptosis-related proteins (Li et al. 2011). Briefly, MCF-7 cells were treated as previously described and then solubilized in the kit lysis buffer, with a complete set of protease and phosphatase inhibitors (Roche, Milano, MI, Italy). Membranes, containing immobilized apoptosis-related antibodies, were blocked with BSA for 1 h at room temperature, then incubated with cell lysates (400 μg/membrane) O.N. at 4 °C. The array was conducted as indicated by the manufacturer. Pixel density on developed x-ray film was analyzed using a transmission scanner and GelPro-Analyzer v 6.0 software (MediaCybernetics, Bethesda, MD, USA). To determine the relative change in apoptosis-related protein levels among samples, corresponding signals on different arrays were compared.
Cell cycle analysis by flow cytometry
Untreated and ZA-treated cells were detached by trypsin/EDTA and fixed in 70 % ethanol for 2 h at 4 °C. Cells were then incubated for 30 min in sodium citrate 0.1 % with 50 μg/ml propidium iodide (PI) and 25 μg/ml RNase A (Sigma Italia, Milano, MI, Italy) (Li et al. 2014). Cell cycle analysis was performed using a MACS Quant Analyzer (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), and data were analyzed using FlowJo (TreeStar).
Western blot analysis
Cell pellets were lysed in ice-cold buffer (Fiorentini et al. 2014) with a complete set of protease and phosphatase inhibitors (Roche, Milano, MI, Italy). Protein concentrations were measured using the Bradford Protein Assay and equal amounts were separated by electrophoresis on a 4–12 % NuPAGE Bis-Tris Gel System (Life Technologies, Milano, MI, Italy), then electroblotted to a nitrocellulose membrane, following the manufacturer’s instructions. Membranes were reacted using different primary antibodies: anti-PTEN antibody (Cell Signaling, USA), anti-Akt antibody (BioLegend, San Diego, CA), anti-p-Akt (Ser 473) antibody (EnoGene Biotech, New York, USA), anti-p21 antibody (Santa Cruz Biotechnologies, Heidelberg, Germany), anti-p27 antibody (Abcam, Cambridge, UK), anti-Bad and anti-p-Bad (Ser136) antibodies (Cell Signaling, USA) according to the manufacturer’s instructions. A mouse polyclonal antibody direct against the N-terminal region of human α-Tubulin (Sigma Italia, Milano, MI, Italy) was applied to membranes, to exclude that difference in the band intensity of the corresponding proteins could be due to errors in protein dosage or sample loading. The secondary antibodies were purchase from Santa Cruz Biotechnologies (Heidelberg, Germany) and were applied for 1 h incubation at room temperature. The specific signal was visualized by the ECL-PLUS system (Amersham Italy, Milano, MI, Italy). Densitometric analysis of the immunoblots was performed using the GelPro-Analyzer v 6.0 (MediaCybernetics, Bethesda, MD, USA).
MiR-21 analysis
Total RNA, including miRNAs, was extracted from cells using the miRNeasy kit (Qiagen, Milano, Italia), and 1 μg was transcripted into cDNA using miScript II RT kit (Qiagen, Milano, MI, Italy), following the manufacturer’s protocol. Quantitative real-time PCR (qRT-PCR) was performed with a miScript System (Qiagen, USA), as previously described (Wang et al. 2010), which included specific primers for miR-21 (5′-UAGCUUAUCAGACUGAUGUUGA-3′). Gene expression was evaluated by iCycler iQ real-time PCR detection system (BioRad Laboratories, Milano, MI, Italy), using the SYBRGreen as fluorochrome, as previously described (Johnson et al. 2014). Reactions were performed under the following conditions: 95 °C 15 min; 94 °C 15 s, 55 °C 30 s, 70 °C 30 s, 40 cycles. Variations in expression of miR-21 among different samples were calculated after normalization to U6 and analyzed using the Livak method (2-ΔΔCt) (Livak and Schmitteng 2001).
Transient transfection of miR-21 mimic and inhibitor
MCF-7 cells were transfected with miScript miR-21 mimic and inhibitor (Qiagen, Milano, Italia) using HiPerFect as transfection reagent (Qiagen, Milano, Italia). Sequence of hsa-miR-21 mimic and hsa-miR-21 inhibitor primers were as follows: 5′-UAGCUUAUCAGACUGAUGUUGA-3’and 5′-UAGCUUAUCAGACUGAUGUUGA-3′, respectively. Briefly, MCF-7 cells were seeded in a 6-well plate in appropriate culture medium and under normal growth conditions for 24 h. Transfection complexes were prepared according to the manufacturer’s instructions and delivered drop-wise onto the cells. After 72 h transfection, the expression of PTEN protein was measured by Western blot assay.
Statistical analysis
Data analysis and graphics were obtained using the GraphPad Prism 4 software (GraphPad Software, La Jolla, CA). The statistical analysis was made using the one-way ANOVA, with a post-hoc test for multiple comparisons (Bonferroni’s test), considering p < 0.05 as the threshold for significant difference. Data are expressed as mean ± SEM of at least three experiments run in triplicate, unless otherwise specified.
Results
Effects of ZA on MCF-7 cell viability
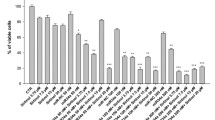
Exposure of cells to increasing concentrations of ZA caused a concentration-dependent reduction of cell viability (Fig. 1). Sigmoidal concentration-response function was used to calculate the IC50 value of ZA in MCF-7 cells, which was 2.67 μmol/L (95 % CI 1.47 to 4.10). Time course experiments up to 120 h demonstrated a significant viability reduction after 72 h of treatment, accordingly to published data (Jagdev et al. 2001) (data not shown). Based on these results, cells were treated with ZA IC50 calculated value for 72 h in all subsequent experiments.
Effects of ZA on viability of MCF-7 cells. Results were expressed as percent cell proliferation versus control (untreated cells). The x axis is discontinuous to label the zero concentration control point; other values are expressed in a logarithmic scale, as power of 10. Data are the mean ± SEM of three independent MTT assays performed in triplicate. *p < 0.01; **p < 0.001 vs control
Cell death induced by ZA in MCF-7 cells
To investigate whether ZA exposure induced cell death and if this occurred for apoptosis or necrosis induction, a double staining with AO/EtBr was performed, as described in the “Materials and methods” section. We observed that ZA deeply increased the number of apoptotic cells, 76.18 ± 0.89 % against untreated cells (Fig. 2a), suggesting that apoptosis could be the main mechanism involved in cancer cell growth inhibition induced by ZA. Apoptosis activation by ZA exposure was also confirmed by a significant increase of about 9 times of M30 antigen level in ZA-treated cells against untreated cells (data not shown).
a Double staining of untreated (C) and ZA-treated (T) MCF-7 cells. Cells were stained using a mixture of AO and EtBr, as described in the “Material and methods” section. Viable (green), apoptotic (yellow), and necrotic (red) cells were scored under a confocal laser-scanning microscope. Magnification, 10×. b Representative histograms of DNA content and cell cycle phases (sub G0/G1, G0/G1, S, and G2/M) in untreated (C) and ZA-treated MCF-7 cells (T). Cells were stained with PI for 30 min after fixation by 70 % ethanol and analyzed for DNA content by flow cytometry, as described in the “Materials and methods” section
Protein expression profile of MCF-7
To investigate molecular basis of ZA-induced apoptosis, we performed the Proteome Profiler Human Apoptosis Array, as described in the Materials and methods section. Table 1 shows that by clustering analysis based on protein function, ZA exposure influenced the expression of proteins involved in various aspects of cellular physiology in MCF-7 cells. The apoptotic cell death induced by ZA may find its determinant in the relevant increase of pro-apoptotic proteins, such as Bad, cytochrome C, SMAC/DIABLO, involved in the intrinsic pathway of apoptosis. Furthermore, the extrinsic apoptosis pathway could as well play an important role in mediating the ZA-induced apoptosis, as a strong increase in the expression level of the Death Receptor family proteins has been observed. ZA exposure activated as well some anti-apoptotic proteins, and molecules involved in adaptative response to stress, such as heat shock protein (HPS), paraxonase 2 (PON2), heme oxigenase (HO) etc. This discrepancy may find its rationale in an cells attempt to protect themselves against the apoptotic insult; on the other hand, as cells are treated with the ZA IC50 and we homogenized a whole cell dish, we cannot exclude that surviving cells could activate protective anti-apoptotic mechanisms or cells in different apoptotic o pro-apoptotic phase. ZA exposure induced as well a significant increase of proteins involved in cell cycle regulation.
ZA induced cell cycle arrest in MCF-7 cell line
We subsequently examined the effect of ZA on cell cycle progression. Comparing to the control group, there was a substantial decrease in the percentage of cells at the G2/M phase in ZA-treated cells (from 13.6 ± 0.8 to 7.0 ± 0.5 %) and this phase was shifted towards the sub G0/G1 phase consequently (from 5.5 ± 0.5 to 13.6 ± 0.1 %). The percentage of cells at the G0/G1 phase was not significant different between control and ZA-treated MCF-7 cells (Fig. 2b).
ZA downregulated expression of miR-21 in MCF-7 cells
The level of miR-21 was determined by qRT-PCR. ZA significantly decreased the level of miR-21 expression in MCF-7 cells (−28 ± 1 % versus control). Forced expression of miR-21 by transfection with miR-21 mimic induced a decrease of the PTEN protein levels to −23.4 ± 0.5 % compared to the mock-transfection control values, while the miR-21 inhibitor transfection increased PTEN concentration to +39.6 ± 2.2 % of the mock-transfection control values (Fig.3).
ZA regulated the miR-21/PTEN/Akt pathway to induce apoptosis and cell cycle arrest
MiR-21 has been shown to control the expression and the activities of PTEN and Akt (Meng et al. 2007), which in turn controls its downstream targets: p-Bad, Bad, p21, and p27 to regulate apoptosis and cell cycle arrest. We then investigated whether the miR-21/PTEN/Akt pathway played a role in the effects of ZA in MCF-7 cells. As shown in Fig. 4a, b, PTEN protein was upregulated by ZA treatment (+78.2 ± 2.5 %) compared to control cells, while p-Akt protein was downregulated by ZA (−37.7 ± 1.4 %). Consistently, the expression of p-Bad was decreased by ZA exposure (−45.7 ± 2.8 %), whereas Bad, p21 and p27 were upregulated, respectively, of about 31.3 ± 2.8, 18.2 ± 2, and 28 ± 2.3 % (Fig.4c, d). These results confirmed the data obtained by the protein-array technique.
Effects of ZA in MDA-MB-231 cell line
Exposure of MDA-MB-231 cells to ZA caused a concentration-dependent reduction of cell growth; IC50 value was 23.6 μmol/L (95 % CI 18.9 to 29.3), through the activation of cell death and the arrest of cell cycle progression. Indeed, the induction of apoptosis was 68.6 ± 2.9 % analyzed by AO/EtBr double staining (Fig. 5a); a significant increase of M30 antigen level was observed in ZA-treated cells against untreated cells (from 0.57 ± 0.09 to 0.99 ± 0.04). Comparing to the control group, there was a substantial decrease in the percentage of cells at the G2/M phase in ZA-treated cells (from 11.7 ± 0.3 to 2.59 ± 0.2 %) and this phase was shifted towards the sub G0/G1 phase consequently (from 6.86 ± 0.1 to 25.2 ± 0.2 %) (Fig. 5b).
a Double staining of untreated (C) and ZA-treated (T) MDA-MB-231 cells. Cells were stained using a mixture of AO and EtBr, as described in the “Material and methods” section. Viable (green), apoptotic (yellow), and necrotic (red) cells were scored under a confocal laser-scanning microscope. Magnification, 10×. b Representative histograms of DNA content and cell cycle phases (sub G0/G1, G0/G1, S, and G2/M) in untreated (C) and ZA-treated MMDA-MB-231 cells (T). Cells were stained with PI for 30 min after fixation by 70 % ethanol and analyzed for DNA content by flow cytometry, as described in the “Materials and methods” section
ZA downregulated the expression of miR-21 in MDA-MB-231 cells (−35 ± 0.2 %). As shown in Fig. 6a, b, PTEN protein was upregulated by ZA treatment (+46.4 ± 2.6 %) compared to control cells, while p-Akt protein was downregulated (−28.2 ± 3.2 %). Consistently, the expression of p-Bad was decreased by ZA exposure (−35.6 ± 1.8 %) (Fig. 6C), whereas p21 and p27 were upregulated, respectively, of about +39.6 ± 2.0 and +23.2 ± 1.6 % (Fig. 6d).
Discussion
Evidences generated over the past few years suggested a potential direct anti-tumor effect of ZA, in addition to its established role as inhibitor of osteoclast activity and bone resorption (Winter et al. 2008). The mechanism of anti-tumor action of ZA is still not completely understood (Stresing et al. 2007), although the inhibition of the mevalonate pathway has been suggested also in cancer cells (Senaratne and Colston 2002).
Several reports demonstrated that inhibition of cell growth in BC cells was characterized by DNA fragmentation, an altered Bcl-2 to Bax ratio associated with the release of cytochrome C and resulting in caspase activation, all features of apoptotic cell death (Oades et al. 2003). Our study indicated the miR-21/PTEN/Akt pathway as an alternative possible mechanism underlying the effects of ZA in MCF-7 cells. We firstly focused our attention on the MCF-7 cell line, a useful experimental model, as these cells express the same receptor pattern (ER+; PgR+) observed in approximately 70 % of human BC (Lim et al. 2012). The exposure of MCF-7 cells to increasing concentrations of ZA caused a decrease of cell viability, with an IC50 value of about 3 μM. This value is relevant, as not only it is expected to be exceeded locally in bone resorption lacunae (Hughes et al. 1995), but it is also within the peak plasma concentrations of ZA that may be achieved in vivo (Fehm et al. 2012), thus suggesting that a direct inhibition of BC cell viability could be seen at ZA therapeutic doses.
MiR-21 is one of the most anomaly and commonly increased miRNAs in cancer and plays important roles in cancer growth, proliferation, migration, and metastasis by targeting PTEN, PDCD4, Spry1 etc., or by upregulating proteins like Bcl-2 indirectly (Ma et al. 2011). It was demonstrated that anti-miR-21 suppressed the growth and the proliferation of MCF-7 cells in vitro and in vivo (Yan et al. 2011; Zhu et al. 2008). PTEN is an important downstream target of miR-21 and overexpression of PTEN in a tetracycline-controlled inducible system induces apoptosis and blocks cell cycle progression in MCF-7 cells (Weng et al. 1999). In the present study, we demonstrated that ZA inhibited the expression of miR-21 in MCF-7 cells that induced overexpression of PTEN and subsequently inactivate Akt.
According to previous studies, the dephosphorylation of Akt likely activates Bad protein to trigger intrinsic apoptotic cascades and upregulates p21/CIP1/CDNK1A and p27/Kip1, inducing cell cycle arrest (Lin et al. 2012; She et al. 2005). Indeed, the array results strongly suggested that in MCF-7 cells, the mechanism of action of ZA involved also an inhibitory effect on the cell cycle regulation: proteins involved in cell cycle arrest/inhibition, such as p27/Kip, p21/CIP1/CDNK1A, and claspin, are increased after ZA exposure. In particular, p21/CIP1/CDNK1A is a universal cdk inhibitor, binding to all complexes of cdk 2, 4, and 6, and it likely blocks progression through all stages of G1/S (Dutto et al. 2015). p27/Kip1 has a sequence that is partly related to p21/CIP1/CDNK1A, and also, it binds promiscuously to cdk-cyclin complexes; an overexpression of p27/Kip1 blocks progression through S phase (Noomhorm et al. 2014). The inhibition of these functions was in line with the significant arrest in G1/S phase and decrease of G2/M fraction observed in ZA-treated MCF-7 cells by flow cytometry. Also in a previous study, ZA has been shown to cause tumor cell accumulation in the S phase of the cell cycle (Neville-Webbe et al. 2006).
We then investigated whether these effects of ZA were specific for receptor-positive BC or they were present as well in a metastatic triple-negative BC localization, namely the MDA-MB-231 cell line. Triple negative BC (TNBC), which accounts for approximately 15 % of all breast malignancies, is used to define tumors that lacks ER and PgR expression and HER2 amplification and characterized by aggressiveness (Ibrahim et al. 2013). MDA-MB-231 are highly metastatic cells commonly used as model of BC that when injected into mice metastasizes to bone causing osteolytic lesions by promoting osteoclastic bone resorption and/or suppressing osteoblastic bone formation (Yamaguchi et al. 2015). Results presented here strongly suggest that the anti-tumor effects induced by ZA, through the miR-21/PTEN/Akt pathway were present in both BC cell lines considered.
In conclusion, in this study, we demonstrated that the direct anti-tumor effect of ZA may involve the miR-21/PTEN/Akt pathway, inducing apoptosis and arrest of cell cycle in BC cells and this pathway is graphically proposed in Fig. 7. These findings emphasize the role of ZA not only as therapy in metastatic patients, but as therapeutic opportunity in primary BC. We reported for the first time that miR-21 is downregulated in response to ZA in BC cell line. The identification of miR-21 as a miRNA regulated by ZA may open new avenues for potential therapeutic intervention in BC treatment.
The possible mechanism by which ZA induced anti-cancer effects in BC cells. In the presence of ZA, expression of miR-21 is decreased, which in turn upregulates its target PTEN in BC cells. PTEN upregulation resulted in a decrease in p-Akt which activates P27/Kip1 and P21/CIP1/CDNK1A along with dephosphorylation of p-Bad, leading to cell cycle arrest accompanied by apoptosis
References
Ambros V (2004) The functions of animal microRNAs. Nature 431:350–355
Bermúdez Brito M, Goulielmaki E, Papakonstanti EA (2015) Focus on PTEN regulation. Front Oncol 5:166. doi:10.3389/fonc.2015.00166
Boissier S, Ferreras M, Peyruchaud O, Magnetto S, Ebetino FH, Colombel M, Delmas P, Delaissé JM, Clézardin P (2000) Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res 60:2949–2954
Clézardin P (2005) Anti-tumour activity of zoledronic Acid. cancer Treat Rev 31(Suppl 3):1–8
Clézardin P, Ebetino FH, Fournier PG (2005) Bisphosphonates and cancer-induced bone disease: beyond their antiresorptive activity. Cancer Res 65:4971–4974
Darido C, Georgy SR, Wilanowski T, Dworkin S, Auden A, Zhao Q, Rank G, Srivastava S, Finlay MJ, Papenfuss AT, Pandolfi PP, Pearson RB, Jane SM (2011) Targeting of the tumor suppressor GRHL3 by a miR-21-dependent proto-oncogenic network results in PTEN loss and tumorigenesis. Cancer Cell 20:635–648. doi:10.1016/j.ccr.2011.10.014
Dutto I, Tillhon M, Cazzalini O, Stivala LA, Prosperi E (2015) Biology of the cell cycle inhibitor p21(CDKN1A):molecular mechanisms and relevance in chemical toxicology. Arch Toxicol 89:155–178. doi:10.1007/s00204-014-1430-4
Fehm T, Zwirner M, Wallwiener D, Seeger H, Neubauer H (2012) Antitumor activity of zoledronic acid in primary breast cancer cells determined by the ATP tumor chemosensitivity assay. BMC Cancer 23:12–308. doi:10.1186/1471-2407-12-308
Fiorentini C, Bodei S, Bedussi F, Fragni M, Bonini SA, Simeone C, Zani D, Berruti A, Missale C, Memo M, Spano P, Sigala S (2014) GPNMB/OA protein increases the invasiveness of human metastatic prostate cancer cell lines DU145 and PC3 through MMP-2 and MMP-9 activity. Exp Cell Res 323:100–111. doi:10.1016/j.yexcr.2014.02.025
Fromigue O, Lagneaux L, Body JJ (2000) Bisphosphonates induce breast cancer cell death in vitro. J Bone Miner Res 15:2211–2221
Hägg M, Bivén K, Ueno T, Rydlander L, Björklund P, Wiman KG, Shoshan M, Linder S (2002) A novel high-through-put assay for screening of pro-apoptotic drugs. Investig New Drugs 20:253–259
Holen I, Coleman RE (2010) Bisphosphonates as treatment of bone metastases. Curr Pharm Des 16:1262–1271
Holliday DL, Speirs V (2011) Choosing the right cell line for breast cancer research. Breast Cancer Res 13:215. doi:10.1186/bcr2889
Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF (1995) Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res 10:1478–1487
Ibrahim T, Liverani C, Mercatelli I, Sacanna E, ZanoniM FF, Zoli W, Amadori D (2013) Cisplatin in combination with zoledronic acid: a synergistic effect in triple-negative breast cancer cell lines. Int J Oncol 42:1263–1270. doi:10.3892/ijo.2013.1809
Jagdev SP, Coleman RE, Shipman CM, Rostami-H A, Croucher PI (2001) The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: evidence for synergy with paclitaxel. Br J Cancer 84:1126–1134
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics. CA Cancer J Clin 60:277–300. doi:10.3322/caac.20073
Jiang BH, Liu LZ (2008) PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim Biophys Acta 1784:150–158
Johnson DJ, Andersen C, Scriven KA, Klein AN, Choi MR, Carroll C, de Leon RD (2014) A molecular method to correlate bloodstains with wound site for crime scene reconstruction. J Forensic Sci 259:735–742. doi:10.1111/1556-4029.12377
Kohno N, Aogi K, Minami H, Nakamura S, Asaga T, Iino Y (2005) Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol 23:3314–3321
Li J, Cho YY, Langfald A, Carper A, Lubet RA, Grubbs CJ, Ericson ME, Bode AM (2011) Lapatinib, a preventive/therapeutic agent against mammary cancer, suppresses RTK-mediated signaling through multiple signaling pathways. Cancer Prev Res (Phila) 4:1190–1197. doi:10.1158/1940-6207.CAPR-10-0330
Li X, Wang K, Ren Y, Zhang L, Tang XJ, Zhang HM, Zhao CQ, Liu PJ, Zhang JM, He JJ (2014) MAPK signaling mediates sinomenine hydrochloride-induced human breast cancer cell death via both reactive oxygen species-dependent and -independent pathways: an in vitro and in vivo study. Cell Death Dis 5:e1356. doi:10.1038/cddis.2014.321
Lim E, Metzger-Filho O, Winer EP (2012) The natural history of hormone receptor-positive breast cancer. Oncology 26(688–694):696
Lin LQ, Li XL, Wang L, Du WJ, Guo R, Liang HH, Liu X, Liang DS, Lu YJ, Shan HL, Jiang HC (2012) Matrine inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells. Cell Physiol Biochem 30:631–641. doi:10.1159/000341444
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T) method. Methods 25:402–408
Ma X, Kumar M, Choudhury SN, Becker Buscaglia LE, Barker JR, Kanakamedala K, Liu MF, Li Y (2011) Loss of the miR-21 allele elevates the expression of its target genes and reduces tumorigenesis. Proc Natl Acad Sci U S A 304:1073–1081. doi:10.1073/pnas.1103735108
Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T (2007) MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 133:647–658
Mironova EV, Evstratova AA, Antonov SM (2007) A fluorescence vital assay for the recognition and quantification of excitotoxic cell death by necrosis and apoptosis using confocal microscopy on neurons in culture. J Neurosci Methods 163:1–8
Neville-Webbe HL, Evans CA, Coleman RE, Holen I (2006) Mechanisms of the synergistic interaction between the bisphosphonate zoledronic acid and the chemotherapy agent paclitaxel in breast cancer cells in vitro. Tumour Biol 27:92–103
Noomhorm N, Chang CJ, Wen CS, Wang JY, Chen JL, Tseng LM, Chen WS, Chiu JH, Shyr YM (2014) In vitro and in vivo effects of xanthorrhizol on human breast cancer MCF-7 cells treated with tamoxifen. J Pharmacol Sci 125:375–385
Oades GM, Senaratne SG, Clarke IA, Kirby RS, Colston KW (2003) Nitrogen containing bisphosphonates induce apoptosis and inhibit the mevalonate pathway, impairing Ras membrane localization in prostate cancer cells. J Urol 170:246–252
Ohtsuka M, Ling H, Doki Y, Mori M, Calin GA (2015) MicroRNA Processing and Human Cancer. J Clin Med 4:1651–1667. doi:10.3390/jcm4081651
Peyruchaud O, Winding B, Pecheur I, Serre CM, Delmas P, Clezardin P (2001) Early detection of bone metastases in a murine model using fluorescent human breast cancer cells: application to the use of the bisphosphonate zoledronic acid in the treatment of osteolytic lesions. J Bone Miner Res 16:2027–2034
Rogers MJ, Crockett JC, Coxon FP, Mönkkönen J (2011) Biochemical and molecular mechanisms of action of bisphosphonates. Bone 49:34–41. doi:10.1016/j.bone.2010.11.008
Roodman GD (2004) Mechanisms of bone metastasis. N Engl J Med 350:1655–1664
Rosen LS, Gordon DH, Dugan W Jr, Major P, Eisenberg PD, Provencher L, Kaminski M, Simeone J, Seaman J, Chen BL, Coleman RE (2004) Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer 100:36–43
Russell G (2007) Bisphosphonates: mode of action and pharmacology. Pediatrics 119:S150–S162
Schutte B, Henfling M, Kölgen W, Bouman M, Meex S, Leers MP, Nap M, Björklund V, Björklund P, Björklund B, Lane EB, Omary MB, Jörnvall H, Ramaekers FC (2004) Keratin 8/18 breakdown and reorganization during apoptosis. Exp Cell Res 297:11–26
Senaratne SG, Colston KW (2002) Direct effects of bisphosphonates on breast cancer cells. Breast Cancer Res 4:18–23
She QB, Solit DB, Ye Q, O’Reilly KE, Lobo J, Rosen N (2005) The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell 8:287–297
Stresing V, Daubiné F, Benzaid I, Mönkkönen H, Clézardin P (2007) Bisphosphonates in cancer therapy. Cancer Lett 257:16–35
Ventura A, Jacks T (2009) MicroRNAs and cancer: short RNAs go a long way. Cell 136:586–591. doi:10.1016/j.cell.2009.02.005
Verdijk R, Franke HR, Wolbers F, Vermes I (2007) Differential effects of bisphosphonates on breast cancer cell lines. Cancer Lett 246:308–312
Wang JF, Yu ML, Yu G, Bian JJ, Deng XM, Wan XJ, Zhu KM (2010) Serum miR-146a and miR-223 as potential new biomarkers for sepsis. Biochem Biophys Res Commun 394:184–188. doi:10.1016/j.bbrc.2010.02.145
Weng LP, Brown JL, Eng C (2001) PTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum Mol Genet 10:599–604
Weng LP, Smith WM, Dahia PL, Ziebold U, Gil E, Lees JA, Eng C (1999) PTEN suppresses breast cancer cell growth by phosphatase activity-dependent G1 arrest followed by cell death. Cancer Res 59:5808–5814
Winter MC, Holen I, Coleman RE (2008) Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev 34:453–475. doi:10.1016/j.ctrv.2008.02.004
Yamada KM, Araki M (2001) Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J Cell Sci 114:2375–2382
Yan LX, Wu QN, Zhang Y, Li YY, Liao DZ, Hou JH, Fu J, Zeng MS, Yun JP, Wu QL, Zeng YX, Shao JY (2011) Knockdown of miR-21 in human breast cancer cell lines inhibits proliferation, in vitro migration and in vivo tumor growth. Breast Cancer Res 13:R2. doi:10.1186/bcr2803
Yamaguchi M, Vikulina T, Weitzmann MN (2015) Gentian violet inhibits MDA-MB-231 human breast cancer cell proliferation and reverses the stimulation of osteoclastogenesis and suppression of osteoblast activity induced by cancer cells. Oncol Rep 34:2156–2162. doi:10.3892/or.2015.4190
Yan T, Yin W, Zhou Q, Zhou L, Jiang Y, Du Y, Shao Z, Lu J (2012) The efficacy of zoledronic acid in breast cancer adjuvant therapy: a meta-analysis of randomised controlled trials. Eur J Cancer 48:187–195. doi:10.1016/j.ejca.2011.10.021
Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY (2008) MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res 18:350–359. doi:10.1038/cr.2008.24
Acknowledgments
This work was supported by A.O. Istituti Ospitalieri di Cremona, Cremona (Italy) and by ARCO, Associazione Ricerca in Campo Oncologico, Cremona (Italy). Zoledronic Acid (Zometa®) was kindly given by Novartis Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fragni, M., Bonini, S.A., Bettinsoli, P. et al. The miR-21/PTEN/Akt signaling pathway is involved in the anti-tumoral effects of zoledronic acid in human breast cancer cell lines. Naunyn-Schmiedeberg's Arch Pharmacol 389, 529–538 (2016). https://doi.org/10.1007/s00210-016-1224-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-016-1224-8