Abstract
Rheumatoid arthritis is a multisystem disease with underlying immune mechanisms. Osteoarthritis is a debilitating, progressive disease of diarthrodial joints associated with the aging process. Although much is known about the pathogenesis of rheumatoid arthritis and osteoarthritis, our understanding of some immunologic changes remains incomplete. This study tries to examine the numeric changes in the T cell subsets and the alterations in the levels of some cytokines and adhesion molecules in these lesions. To accomplish this goal, peripheral blood and synovial fluid samples were obtained from 24 patients with rheumatoid arthritis, 15 patients with osteoarthritis and six healthy controls. The counts of CD4 + and CD8 + T lymphocytes were examined using flow cytometry. The levels of some cytokines (TNF-α, IL1-β, IL-10, and IL-17) and a soluble intercellular adhesion molecule-1 (sICAM-1) were measured in the sera and synovial fluids using enzyme linked immunosorbant assay. We found some variations in the counts of T cell subsets, the levels of cytokines and sICAM-1 adhesion molecule between the healthy controls and the patients with arthritis. High levels of IL-1β, IL-10, IL-17 and TNF-α (in the serum and synovial fluid) were observed in arthritis compared to the healthy controls. In rheumatoid arthritis, a high serum level of sICAM-1 was found compared to its level in the synovial fluid. A high CD4+/CD8+ T cell ratio was found in the blood of the patients with rheumatoid arthritis. In rheumatoid arthritis, the cytokine levels correlated positively with some clinicopathologic features. To conclude, the development of rheumatoid arthritis and osteoarthritis is associated with alteration of the levels of some cytokines. The assessment of these immunologic changes may have potential prognostic roles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis is a chronic polyarticular disease associated with significant joint destruction and marked physical impairment. In this disorder, tissue damage (synovitis) may result from the activation of the synovial cells and the accumulation of immune cells which release several cytokines including interleukin-1 (IL-1β), tumor necrosis factor-α (TNF- α), IL-8, IL-10 and IL-17 [1–4]. T cells and some cytokines can regulate the inflammatory response and the activity of several connective tissue cells. In rheumatoid arthritis, this contention is supported by the presence of altered T cells [1, 2, 4] and T cells that exhibit the characteristics of macrophages, yet retain their CD3 and T cell receptor expression [5]. T-cell dependent changes were reported in the in vitro and animal models of rheumatoid arthritis [6, 7]. Frye and his colleagues examined the process of cartilage degradation and invasion in rheumatoid arthritis (in vitro model). They found that the normal synoviocytes could be “converted” to a rheumatoid arthritis phenotype by specific cytokines. Invasion of cartilage matrix was enhanced by culturing these synoviocytes in the presence of specific concentrations of IL-1β or TGF-β. Invasion was inhibited by TNF-α, and unaffected by Platelet derived growth factors. Also, synovial fluid from rheumatoid arthritis patients induced invasion of normal synoviocytes while synovial fluid from another inflammatory arthritis (Crohn’s disease) did not augment invasion to the same degree. This “conversion effect” appears to be specific for synoviocytes, since similar effects were not observed with human skin fibroblasts [2].
Osteoarthritis is a common joint disease. It is a degenerative disorder that results from biochemical breakdown of the articular cartilage in the synovial joints. The degenerative changes in osteoarthritis are mediated by the lack of balance between the chondrocyte anabolism and catabolism. Osteoarthritis is thought to be due to excessive wear and tear in the joints. However, secondary nonspecific inflammatory changes were also reported in the synovial membrane of the patients with osteoarthritis [8]. Recent studies indicate that some cytokines (IL-1β, TNF- α and IL-17) are involved in the process of cartilage destruction observed in osteoarthritis [9]. These peptides act by modulating the expression of the matrix metalloproteinases and the cartilage extracellular matrices [8, 10, 11].
Interleukin-1β (IL-1β) is an important cytokine synthesized by the macrophages [7, 12]. It can stimulate B cell growth, activate T cells and induce acute phase protein production. IL-1β has inflammatory effects on the fibroblasts and the endothelial cells [12]. This cytokine can participate in the pathogenesis of arthritis through the direct stimulation of the synovial cells and the augmentation of matrix degradation [13]. In osteoarthritis, the affected cartilage shows increased expression of IL-1β mRNA, which is absent in the normal cartilage. In rheumatoid arthritis the blocking of IL-1β synthesis protects the bones and the cartilages from progressive destruction [14]. IL-1 can modulate the production of nitric oxide and prostaglandin E2. These mediators are undetectable in normal cartilage. The nitric oxide can prevent matrix synthesis and enhances its degradation. It also can react with several oxidants and therefore promotes cellular injury and makes the chondrocytes more susceptible to cytokines-induced apoptosis. The prostaglandin E2 exerts catabolic effects on the chondrocytes [15].
Interleukin-10 (IL-10) is a major anti-inflammatory cytokine that exerts several immunomodulatory effects on the immune cells. IL-10 acts primarily by the inhibition of costimulatory properties of the macrophages. It stimulates the proliferation and the differentiation of antibody-forming B-cells [16]. In rheumatoid arthritis, IL-10 is secreted by B lymphocytes and CD4+ CD45RO+ “memory” T cells [17]. IL-10 inhibits the production of both proinflammatory cytokines and chemokine. Interleukin-17 (IL-17) is a T cell-derived proinflammatory cytokine produced by the damaged synovium in rheumatoid arthritis. It activates the production of the inflammatory mediators by the synoviocytes. The tumor necrosis factor-alpha (TNF-α) is released by the macrophages, activated T cells and Natural Killer cells. It can activate both T cells and macrophages. Moreover, it can induce the production of the acute phase proteins [18, 19].
Increased levels of some adhesion molecules were closely linked to the development of endothelial dysfunction in numerous diseases. Soluble intercellular adhesion molecule-1 (sICAM-1) is a member of the immunoglobulin supergene family that exists in the human serum. It is synthesized by the endothelial and mononuclear cells. A fragment of ICAM-1 found in the circulation (sICAM-1) is thought to be cleaved from the surface of ICAM-1 expressing cells. This adhesion molecule plays critical roles in several different inflammatory and immunologic processes [20, 21]. In rheumatoid arthritis, the level of sICAM-1 is elevated in the serum [22].
This study tries to examine the numeric changes in the T cell subsets and the alterations in the levels of some cytokines and adhesion molecules in these lesions. To accomplish this goal, we examined 24 patients with rheumatoid arthritis, 15 patients with osteoarthritis, and six healthy individuals. The immunologic alterations (counts of T cell subsets and cytokine levels) were evaluated in the synovial fluid and in the serum. Also, the associations between the clinicopathologic characteristics and immunologic alterations were determined. Some of these immunologic alterations were checked on an additional set of patients (40 patients with rheumatoid arthritis and 10 healthy individuals).
Patients and Methods
Patients and Healthy Controls
Twenty-four patients with rheumatoid arthritis and 15 patients with osteoarthritis were admitted to the Departments of Rheumatology, Assuit University Hospitals, Faculty of Medicine, Assuit, Egypt. All the patients had joint effusion. Also, six healthy individuals with comparable age and sex were included (healthy control group) in the study. Informed consents were obtained from all the participants. The experimental design was approved by the Ethics Committee (Faculty of Medicine). Full history and clinical examination were performed for all the participants. The diagnosis of rheumatoid arthritis and osteoarthritis was rendered following criteria set by The American College of Rheumatology [23].
Assessment of Disease Activity
The disease activity was assessed using the multivariate analysis according to Mallya and Mace (1981). Each patient was assessed by the following six parameters: two subjective features (the duration of morning stiffness and severity of pain); two semiobjective features (the grip strength and joint tenderness) and two objective features (the ESR and haemoglobin content of blood). The duration of morning stiffness (grade 1: up to 10 min, grade 2: 11 to 30 min, grade 3: 31 to 120 min and grade 4: >120 min). Pain scale was represented by a 10 cm horizontal line marking “worst possible pain” on one end and “no pain” on the other end. The patient was asked to represent the severity of pain by putting a dot which was measured in centimeters, and the severity of pain was graded as follows: grade 1, 0.0–2.4 cm; grade 2: 2.5–4.4 cm; grade 3, 4.5–6.4 cm and grade 4, 6.5–10.0 cm. Grip strength was evaluated using the sphygmomanometer cuff inflated to 20 mmHg. Three trials were done for each hand and the mean of the six readings was graded as follows: grade 1, >200 mmHg; grade 2, 50–200 mmHg; grade 3, 20–49 mmHg and grade 4, <20 mmHg. The joint tenderness was assessed according to Ritchie Articular Index into: score 0 = the patient has no tenderness; score 1 = the patient complains of pain; score 2 = the patient complains of pain and winces; score 3 = the patient complains of pain, winces and withdraws. The joint tenderness score was recorded for each of the following joints: the cervical spine, sternoclavicular, acromioclavicular, shoulder, elbow, wrist, metacarpophalangeal, proximal interphalangeal, hip, ankle, talocalcaneal, midtarsal, and metatarsophalangeal joints. Tenderness of all the above joints were elicited by firm pressure over the joint margin with the exception of the cervical spine, hip joint, talocalcaneal and midtarsal joints, where tenderness was elicited by passive movement of the joint. The articular index was graded into: grade 1, 0; grade 2, 1–7; grade 3, 8–17 and grade 4, ≥18. ESR was determined using the Westergren method. ESR was graded according to Mallya and Mace [50] into: grade 1, 0–20 mm/h, grade 2, 21–45 mm/h, grade 3, 46–80 mm/h and grade 4, >80 mm/h. The haemoglobin (hb) was determined using the coulter counter, Hb content of blood was graded according to Mallya and Mace into: grade 1, >14.0 gm/dl; grade 2, 14.0–13.0 gm/dl; grade 3, 12.9–10.0 gm/dl and grade 4, <10 gm/dl. The scores for these parameters (duration of morning stiffness, severity of pain, grip strength, joint tenderness, ESR and Hb) were summed up together, and the resultant was divided by six to obtain the index of disease activity (IDA) which again was graded into four grades called the mean disease grading activity (MDGA) as follows: grade I (MGDA), 1.0–1.4 (IDA); grade II (MDGA), 1.5–2.4 (IDA); grade III (MDGA), 2.5–3.4 (IDA) and grade IV (MDGA), 3.5–4.0 (IDA) [23].
Serum and Synovial Fluid Samples
Blood samples were obtained from the patients and healthy controls. The samples were collected in plain test tubes to undergo clotting. After centrifugation, sera were collected and stored at −20°C till analyzed. The synovial fluid samples were collected under complete aseptic conditions from the knee joints (arthrocentesis). The samples were stored in capped tubes containing EDTA, centrifuged and the supernatants were separated and stored at −20°C. Samples were only thawed on the day of analysis for the assessment of the levels of rheumatoid factor (RF), C-reactive protein (CRP), antinuclear antibodies (ANA), TNF-α, IL-1β, IL-17, IL-10 and sICAM-1
Assessment of the Laboratory Changes
All the participants were evaluated for the blood counts, erythrocytic sedimentation rate, rheumatoid factor, C-Reactive Protein and Antinuclear antibodies. The erythrocytic sedimentation rate was determined by Westergreen method using 2-ml citrated blood samples. The first and second hour readings were taken for each sample. The values of C-reactive protein were measured using SAS-CRP-Latex test. It is a rapid latex agglutination test for the qualitative determination of C-reactive Protein in human serum. This test depends on the detection of the immunologic reaction between C-reactive protein as an antigen and the corresponding antibody coated on the surface of biologically inert latex particles. The rheumatoid factor was determined using SAS RF-Latex test. It is a rapid agglutination test for the qualitative determination of RF in human serum. Detection of Antinuclear antibodies was done using indirect immunofluorescence (Dia Sorine). The Quantaflour fluorescent antibody test is an indirect fluorescent antibody procedure that is used for the detection and semiquantitation of human autoantibodies.
Evaluation of CD4+ and CD8+ T cells in the peripheral blood and synovial fluid: Five milliliters of the synovial fluid (patients) and peripheral blood (patients and healthy controls) were obtained in heparinized tubes. The mononuclear cells were isolated on a high density gradient separation medium (Ficoll Hypaque). The cells were washed twice in phosphate buffered saline (PBS) at 400×g for 4 min. They were incubated with FITC-conjugated anti-CD8 mAb (Dako-Cytomation M 0707), and PE conjugated anti-CD4 mAb (Dako-Cytomation M 0716) for 30 min at 4°C. The cells were washed twice in PBS with 2% albumin at 400×g for 4 min, and then suspended into 300 μl PBS. The purity of T lymphocyte was assessed by flow cytometry (PAS – II Dako-Cytomation). Immunophenotyping of T cell subsets was done to determine the counts of CD4+ and CD8+ cells.
Measurement of IL-1β, IL-10, IL-17, TNF-α, and sICAM Levels in the Serum and Synovial Fluid
Ten ml of the synovial fluid (patients) were immediately centrifuged at 3,000 rpm for 20 min at 4°C and the supernatants were stored at −70°C. The serum and synovial fluid levels of IL-1β, IL-10, IL-17, TNF-α were measured. The solid phase high sensitive enzyme linked immunosorbant assay (ELISA) and amplified sensitive EAISA kits (Cat. no. KAC1751, KAC1321, kAC1592 and KSC3011 Biosource Europe, S.A., Nivelles, Belgium) were used following other groups [24]. The Endogen (USA) human IL-10 ELISA (an in vitro ELISA) was used for the quantitative measurement of human IL-10 in the serum and synovial fluid. This ELISA does not cross react with human; IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-6, IL-7, IL-8, IFN-γ, IFN-α, TNF-α and TNF-β. Measurement of sICAM-1 in serum and synovial fluid was done using the Cellfree soluble ICAM-1 (CD54) ELISA (Endogen, USA) which is an enzyme-linked immunosorbent assay for the quantitative measurement of human sICAM-1 (CD54) in serum and SF.
Statistical analysis: Statistical tests were done using SPSS software. Statistical analysis was done using Analysis of variance (ANOVA). The results were presented as mean ± standard deviation (SD). A p value <0.05 was considered statistically significant. Spearman’s rank test and Mann–Whitney U test were used to determine correlation levels. “r” values (0.0 to 0.2) were regarded as indicating no correlation while “r” values of 0.20 to 0.40 indicate the presence of correlation. The minimum level of significance was set at a p value of <0.05.
Results
Clinicopathologic Features of Rheumatoid Arthritis and Osteoarthritis
The mean age was high in osteoarthritis compared to rheumatoid arthritis. A statistically significant female sex predilection was observed in rheumatoid arthritis and osteoarthritis. The duration of the disease processes was longer in rheumatoid arthritis than in osteoarthritis. In rheumatoid arthritis positive rheumatoid factor and C-reactive protein were observed in 67% and 87% of the patients, respectively. A summary of the clinicopathologic features of rheumatoid arthritis and osteoarthritis are shown in Table 1.
Altered levels of IL-1β, IL-10, TNF-α, IL-17, sICAM-1 in the serum and synovial fluid in the rheumatoid arthritis and osteoarthritis: The serum samples from patients with rheumatoid arthritis and osteoarthritis had a statistically significant high levels of some cytokines (pg/ml) compared to the healthy controls (p < 0.05). The synovial fluid samples from patients with rheumatoid arthritis had statistically significantly high cytokine levels (pg/ml) compared to osteoarthritis. There were statistically significantly high IL-10, and sICAM levels (pg/ml) in the serum of the rheumatoid arthritis patients relative to the healthy controls. Serum IL-17 value was statistically significantly high in the rheumatoid arthritis compared to healthy controls (p < 0.05). Synovial IL-7 value was high in rheumatoid arthritis compared to osteoarthritis (p < 0.05). A summary of these results is shown in Table 2.
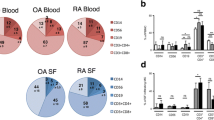
A High CD4+/CD8+ T Cell Ratio in the Blood of the Patients with Rheumatoid Arthritis
The blood from the healthy controls, patients with rheumatoid arthritis and osteoarthritis had a relatively similar cell counts. However, the CD4+/CD8+ ratio was high in rheumatoid arthritis and osteoarthritis compared to the healthy controls. The differences between patients with rheumatoid arthritis and the healthy controls reached the levels of statistical significance (<0.05). The differences between osteoarthritis and healthy controls did not reach the level of statistical significance. A summary of these results is shown in Table 2.
Correlation Between the Immunological Alterations and the Clinical Features in Rheumatoid Arthritis
In rheumatoid arthritis patients, there was a statistically significant positive correlation between the counts of CD4+ cells and the levels of serum TNF-α (p < 0.04). Similarly, a statistically significant positive correlation was observed between the levels of TNF-α in the synovial fluid and some clinicopathologic changes (disease activity: p < 0.05, articular index: p < 0.05 and T cell counts: p < 0.05). There was significant (p < 0.05) positive correlation between sICAM-1 (serum and synovial fluid) and the following parameters: mean disease grading activity, C-reactive protein, and rheumatoid factor levels. Similarly, significant positive correlations were observed between serum IL-10 and the mean disease grading activity (p < 0.05) as well as between the synovial fluid IL-10 and the level of rheumatoid factor (p < 0.05). A positive correlation was observed between sICAM-1 (synovial fluid) and the level of IL-10 (synovial fluid) (p < 0.05). The synovial fluid IL-10 levels negatively correlated with synovial fluid sICAM-1 (p < 0.05). A summary of these results was shown in Table 3. Some of the immunologic alterations (Rheumatoid factor, C-reactive proteins, IL-10, TNF-α and sICAM-1) were checked on an additional set of patients (40 patients with rheumatoid arthritis and 10 healthy individuals) with similar results.
Discussion
Several studies have examined the association between the status of T cell subsets, cytokines and the clinicopathologic features of rheumatoid arthritis and osteoarthritis. However, available reports that bear directly on the correlation between the immunologic alterations in the blood and synovial fluid in rheumatoid arthritis are few. This study tries to address these issues. It demonstrates several observations. There were high levels of serum and synovial fluid IL-1β, IL-10, IL-17 and TNF-α in rheumatoid arthritis and osteoarthritis. A high CD4+/CD8+ T cell ratio was observed in the blood of the patients, especially those with rheumatoid arthritis, compared to healthy controls. The presence of T cell subsets in the local milieu of the synovial fluid suggests that these cells contribute to the development of the tissue damage associated with synovitis. In rheumatoid arthritis there was a positive correlation between the disease activity and some immunologic changes. The rheumatoid arthritis was more common in females than in males. This may be due to an underlying genetic predisposition i.e. association with HLA locus [25]. The high level of C-reactive protein in rheumatoid arthritis may be due to the increased production of TNF-α, which can stimulate the synthesis of this protein by the hepatocytes. The high level of the Rheumatoid factor may reflect the underlying endothelial dysfunction and the ongoing autoimmune response [24].
Altered Levels of Serum and Synovial Fluid IL-1β, IL-10, IL-17, TNF-α and sICAM-1 in Rheumatoid Arthritis and Osteoarthritis
The high levels of IL-1β, IL-10, IL-17 and TNF-α in the sera of the patients with arthritis compared to the healthy controls concur with previous studies [7, 8, 26, 27] and may be reasoned to the recruitment of cytokines producing cells (macrophages and T lymphocytes) to the peripheral blood and the synovial membranes of the affected joints [8, 28]. Our findings suggest that IL-1β, IL-10, IL-17 and TNF-α are involved in the pathogenesis of rheumatoid arthritis and osteoarthritis. This contention is supported by several observations. Interleukins IL-1β, IL-17 and TNF-α can modulate the inflammatory process in the synovium in several ways [28]. Interleukin-17 (IL-17) can activate the transcription factor (NF-kappa B) in the synovial fibroblasts and the endothelial cells to secrete IL-6, IL-8, and prostaglandin E2 [29, 30]. Khalkhali-Ellis et al examined the effect of synovial fluid from patients with juvenile rheumatoid arthritis on the proliferation and induction of degradative and invasive phenotype in normal synovial fibroblasts. Their results indicated that the proliferation of normal synovial fibroblasts exposed to synovial fluid (cultured synovial cells) of patients with juvenile rheumatoid arthritis was up to three times greater than untreated controls [31].
Relatively comparable CD4+ and CD8+ T cells counts were observed in the peripheral blood and the synovial fluid of the patients with arthritis. However, there were high levels of TNF-α, IL-10 and IL-17 in the synovial fluid relative to levels in the serum. These findings may be due to the fact that T cells (IL-17 producing cells) and the monocytes (IL-1β and TNF producing cells) infiltrating the damaged synovium are more active than their serum counterparts. However, monocytes/macrophages in the synovial fluid versus peripheral blood were not compared in this study, so it is still possible that an elevated number of these cells could contribute to the observed differences in cytokine levels. In rheumatoid arthritis and osteoarthritis, it is possible that some cells (other than T cells and macrophages) residing in the synovium can effectively share to the production of IL-1β, IL-10, IL-17 and TNF-α. In support, the synovial cell and T lymphocytes producing IL-17 can activate mesenchymal cells (chondrocytes and fibroblasts) leading to an increased proinflammatory pattern sensitive to Th2 cytokines regulation [26]. IL-10 is an immunoregulatory cytokine secreted by the activated T cells, monocytes and B cells. The influence of IL-10 could account for many of the functional changes of T cells, monocytes, macrophages and B cells infiltrating the rheumatoid synovium [32].
High Serum Level of sICAM-1 Compared to its Level in the Synovial Fluid
In rheumatoid arthritis, we found a high serum level of sICAM-1compared to its level in the synovial fluid. This may be reasoned to the fact that ICAM-1 arise from several cells outside the synovium such as the vascular endothelial cells, activated lymphocytes, hepatocytes and smooth muscle cells [33–36].These cells must be acknowledged when considering a significant elevation of sICAM-1 levels. Several studies have indicated that the shedding of sICAM-1 in-vitro is augmented by some cytokines such as TNF-α, IL-1 and IFN-α [37, 38]. Since the rheumatoid arthritis patients in our study had increased serum levels of TNF-α; the elevated sICAM-1 levels may be a consequence of chronic systemic exposure to elevated levels of TNF-α.
A High CD4+/CD8+ T Cell Ratio in the Blood of the Patients with Rheumatoid Arthritis
The high CD4+/CD8+ ratio in the peripheral blood of the patients with rheumatoid arthritis and osteoarthritis relative to the healthy controls are in agreement with other groups [39–44]. However, only these variations between rheumatoid arthritis and healthy controls were statistically significant. The increased CD4+/CD8+ ratio in the blood may be reasoned to the recruitment of increased numbers of CD4+ cells relative to CD8+ T cell. The increased CD4+ cells and decreased CD4+/CD8+ ratio in the synovial fluid of the patients with arthritis compared to the blood values concurs with previous studies [45–49].
Correlation Between Cytokine Levels and the Clinicopathologic Features in Rheumatoid Arthritis
In rheumatoid arthritis, there was a significant direct correlation between the disease activity and some immunologic changes (cytokine levels and CD8+ T cell counts in the synovial fluid). These findings suggest that the evaluation of these molecules may be a useful adjunct in the clinical assessment of the disease activity and their future outcome. The ability of synovial fluid and serum examination to correctly correlates alteration in cytokines and T cells further highlight its value in the detection of ongoing joint lesions, monitoring its course and choosing the appropriate therapy. Similarly, the correlation between sICAM-1 levels and various clinical and laboratory parameters of disease activity encourage the clinical application of sICAM-1 determinations as a gauge of clinical activity in rheumatoid arthritis.
Here we report alterations of T cell subsets, high levels of key cytokines and S-ICAM-1 in rheumatoid arthritis and osteoarthritis relative to healthy controls. These preliminary findings suggest that the destructive joint events in these pathologies seem to be perpetuated by complex cytokines interactions. The interaction among these cytokines could sustain inflammatory processes within the joint and amplify the involvement of T cells in the pathogenesis of these lesions. Some of these alterations correlate with the clinicopathologic characteristics of these lesions and therefore may be useful in monitoring the disease activity.
References
Maurer D, Felzmann T, Holter W, Petera P, Smolen J, Knapp W (1992) Evidence for the presence of activated CD4 T cells with naive phenotype in the peripheral blood of patients with rheumatoid arthritis. Clin Exp Immunol 87:429–434
Frye CA, Yocum DE, Tuan R, Suyana E, Seftor EA, Seftor RE, Khalkhali-Ellis Z, Moore TL, Hendrix MJ (1996) An in vitro model for studying mechanisms underlying synoviocyte-mediated cartilage invasion in rheumatoid arthritis. Pathol Oncol Res 2:157–166
Khalkhali-Ellis Z, Bulla GA, Schlesinger LS, Kirschmann DA, Moore TL, Hendrix MJ (1999) C1q-containing immune complexes purified from sera of juvenile rheumatoid arthritis patients mediate IL-8 production by human synoviocytes: role of C1q receptors. J Immunol 163:4612–4620
Choy EH, Connolly DJ, Rapson N, Jeal S, Brown JC, Kingsley GH, Panayi GS, Johnston JM (2000) Pharmacokinetic, pharmacodynamic and clinical effects of a humanized IgG1 anti-CD4 monoclonal antibody in the peripheral blood and synovial fluid of rheumatoid arthritis patients. Rheumatology (Oxford) 39:1139–1146
Khalkhali-Ellis Z, Roodman ST, Knutsen AP, Mueller KR, Chauhan B, Moore TL, Hendrix MJ (1998) Expression of macrophage markers by a population of T cells obtained from synovial fluid of a subgroup of patients with juvenile rheumatoid arthritis. J Rheumatol 25:352–360
Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T (1999) IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 103:1345–1352
Zwerina J, Redlich K, Schett G, Smolen JS (2005) Pathogenesis of rheumatoid arthritis: targeting cytokines. Ann N Y Acad Sci 1051:716–729
Malemud CJ (2004) Cytokines as therapeutic targets for osteoarthritis. BioDrugs 18:23–35
Aigner T, Sachse A, Gebhard PM, Roach HI (2006) Osteoarthritis: pathobiology-targets and ways for therapeutic intervention. Adv Drug Deliv Rev 58:128–149
Zangerle PF, De Groote D, Lopez M, Meuleman RJ, Vrindts Y, Fauchet F, Dehart I, Jadoul M, Radoux D, Franchimont P (1992) Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood: II. Application to rheumatoid arthritis and osteoarthritis. Cytokine 4:568–575
Ezawa K, Yamamura M, Matsui H, Ota Z, Makino H (1997) Comparative analysis of CD45RA- and CD45RO-positive CD4 + T cells in peripheral blood, synovial fluid, and synovial tissue in patients with rheumatoid arthritis and osteoarthritis. Acta Med Okayama 51:25–31
Petrovic-Rackov L, Pejnovic N (2005) Clinical significance of IL-18, IL-15, IL-12 and TNF-alpha measurement in rheumatoid arthritis. Clin Rheumatol 25:448–452
Firestein GS (2005) Immunologic mechanisms in the pathogenesis of rheumatoid arthritis. J Clin Rheumatol 11(3 Suppl):S39–44
Abramson SB, Amin A (2002) Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatology (Oxford) 41:972–980
Attur MG, Patel IR, Patel RN, Abramson SB, Amin AR (1998) Autocrine production of IL-1 beta by human osteoarthritis-affected cartilage and differential regulation of endogenous nitric oxide, IL-6, prostaglandin E2, and IL-8. Proc Assoc Am Physicians 110:65–72
Appel H, Neure L, Kuhne M, Braun J, Rudwaleit M, Sieper J (2004) An elevated level of IL-10- and TGFbeta-secreting T cells, B cells and macrophages in the synovial membrane of patients with reactive arthritis compared to rheumatoid arthritis. Clin Rheumatol 23:435–440
Verhoef CM, van Roon JA, Vianen ME, Bijlsma JW, Lafeber FP (2001) Interleukin 10 (IL-10), not IL-4 or interferon-gamma production, correlates with progression of joint destruction in rheumatoid arthritis. J Rheumatol 28:1960–1966
Miossec P (2004) IL-17 in rheumatoid arthritis: a new target for treatment or just another cytokine? Joint Bone Spine 71:87–90
Ryu S, Lee JH, Kim SI (2006) IL-17 increased the production of vascular endothelial growth factor in rheumatoid arthritis synoviocytes. Clin Rheumatol 25:16–20
Lindsley HB, Smith DD, Davis LS, Koch AE, Lipsky PE (1992) Regulation of the expression of adhesion molecules by human synoviocytes. Semin Arthritis Rheum 21:330–334
Cush JJ, Rothlein R, Lindsley HB, Mainolfi EA, Lipsky PE (1993) Increased levels of circulating intercellular adhesion molecule 1 in the sera of patients with rheumatoid arthritis. Arthritis Rheum 36:1098–1102
Gonzalez-Gay MA, Garcia-Unzueta MT, De Matias JM, Gonzalez-Juanatey C, Garcia-Porrua C, Sanchez-Andrade A, Martin J, Llorca J (2006) Influence of anti-TNF-alpha infliximab therapy on adhesion molecules associated with atherogenesis in patients with rheumatoid arthritis. Clin Exp Rheumatol 24:373–379
Albert DA, Huang G, Dubrow G, Brensinger CM, Berlin JA, Williams HJ (2004) Criteria for improvement in rheumatoid arthritis: alternatives to the American College of Rheumatology 20. J Rheumatol 31(5):856–866
Hussein MR, Hassan HI, Hofny ER, Elkholy M, Fatehy NA, Abd Elmoniem AE, Ezz El-Din AM, Afifi OA, Rashed HG (2005) Alterations of mononuclear inflammatory cells, CD4/CD8 + T cells, interleukin 1beta, and tumour necrosis factor alpha in the bronchoalveolar lavage fluid, peripheral blood, and skin of patients with systemic sclerosis. J Clin Pathol 58:178–184
Barrera P, Faure S, Prud’homme JF, Balsa A, Migliorini P, Chimenti D, Radstake TR, van de Putte LB, Pascual-Salcedo D, Westhovens R, Maenaut K, Alves H, Lopes-Vaz A, Stravopoulos C, Spyropoulou M, Fritz P, Bardin T, Charron D, Lepage V, Alibert, Martinez M, Cornelis F (2001) European genetic study on rheumatoid arthritis: is there a linkage of the interleukin-1 (IL-1), IL-10 or IL-4 genes to RA? Clin Exp Rheumatol 19:709–714
Chabaud M, Page G, Miossec P (2001) Enhancing effect of IL-1, IL-17, and TNF-alpha on macrophage inflammatory protein-3alpha production in rheumatoid arthritis: regulation by soluble receptors and Th2 cytokines. J Immunol 167:6015–6020
Petrovic-Rackov L (2006) Evaluation of the degree of clinical rheumatoid arthritis activity based on the concentrations of cytokines TNF-alpha, IL-12, IL-15, and IL-18 in serum and synovial fluid]. Vojnosanit Pregl 63:21–26
Brennan FM, Hayes AL, Ciesielski CJ, Green P, Foxwell BM, Feldmann M (2002) Evidence that rheumatoid arthritis synovial T cells are similar to cytokine-activated T cells: involvement of phosphatidylinositol 3-kinase and nuclear factor kappaB pathways in tumor necrosis factor alpha production in rheumatoid arthritis. Arthritis Rheum 46:31–41
Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S (1998) T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med 183:2593–2603
Chabaud M, Fossiez F, Taupin JL, Miossec P (1998) Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol 161:409–414
Khalkhali-Ellis Z, Seftor EA, Nieva DR, Seftor RE, Samaha HA, Bultman L, De Larco JE, Ince A, Moore TL, Hendrix MJ (1997) Induction of invasive and degradative phenotype in normal synovial fibroblasts exposed to synovial fluid from patients with juvenile rheumatoid arthritis: role of mononuclear cell population. J Rheumatol 24:2451–2460
Cush JJ, Splawski JB, Thomas R, McFarlin JE, Schulze-Koops H, Davis LS, Fujita K, Lipsky PE (1995) Elevated interleukin-10 levels in patients with rheumatoid arthritis. Arthritis Rheum 38:96–104
Macchioni P, Boiardi L, Casali B, Nicoli D, Farnetti E, Salvarani C (2000) Intercellular adhesion molecule 1 (ICAM-1) gene polymorphisms in Italian patients with rheumatoid arthritis. Clin Exp Rheumatol 18:553–558
Blann AD, Herrick A, Jayson MI (1995) Altered levels of soluble adhesion molecules in rheumatoid arthritis, vasculitis and systemic sclerosis. Br J Rheumatol 34:814–819
Dolezalova P, Telekesova P, Nemcova D, Hoza J (2002) Soluble adhesion molecules ICAM-1 and E-selectin in juvenile arthritis: clinical and laboratory correlations. Clin Exp Rheumatol 20:249–254
Hanyuda M, Kasama T, Isozaki T, Matsunawa MM, Yajima N, Miyaoka H, Uchida H, Kameoka Y, Ide H, Adachi M (2003) Activated leucocytes express and secrete macrophage inflammatory protein-1alpha upon interaction with synovial fibroblasts of rheumatoid arthritis via a beta2-integrin/ICAM-1 mechanism. Rheumatology (Oxford) 42:1390–1397
Pigott R, Dillon LP, Hemingway IH, Gearing AJ (1992) Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine activated cultured endothelial cells. Biochem Biophys Res Commun 187:584–589
Lindsley HB, Smith DD, Cohick CB, Koch AE, Davis LS (1993) Proinflammatory cytokines enhance human synoviocyte expression of functional intercellular adhesion molecule-1 (ICAM-1). Clin Immunol Immunopathol 68:311–320
Fitzgerald JE, Ricalton NS, Meyer AC, West SG, Kaplan H, Behrendt C, Kotzin BL (1995) Analysis of clonal CD8 + T cell expansions in normal individuals and patients with rheumatoid arthritis. J Immunol 154:3538–3547
Burmester GR, Stuhlmuller B, Keyszer G, Kinne RW (1997) Mononuclear phagocytes and rheumatoid synovitis. Mastermind or workhorse in arthritis? Arthritis Rheum 40:5–18
Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B (2005) Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis 64:1263–1267
Miltenburg AM, van Laar JM, de Kuiper R, Daha MR, Breedveld FC (1992) T cells cloned from human rheumatoid synovial membrane functionally represent the Th1 subset. Scand J Immunol 35:603–610
Rittner HL, Zettl A, Jendro MC, Bartz-Bazzanella P, Goronzy JJ, Weyand CM (1997) Multiple mechanisms support oligoclonal T cell expansion in rheumatoid synovitis. Mol Med 3:452–465
Steiner G, Tohidast-Akrad M, Witzmann G, Vesely M, Studnicka-Benke A, Gal A, Kunaver M, Zenz P, Smolen JS (1999) Cytokine production by synovial T cells in rheumatoid arthritis. Rheumatology (Oxford) 38:202–213
Franz JK, Kolb SA, Hummel KM, Lahrtz F, Neidhart M, Aicher WK, Pap T, Gay RE, Fontana A, Gay S (1998) Interleukin-16, produced by synovial fibroblasts, mediates chemoattraction for CD4 + T lymphocytes in rheumatoid arthritis. Eur J Immunol 28:2661–2671
Mamoune A, Durand V, Le Goff P, Pennec YL, Youinou P, Le Corre R (2000) Abnormal distribution of CD45 isoforms expressed by CD4 + and CD8 + T cells in rheumatoid arthritis. Histol Histopathol 15:587–591
Hatachi S, Iwai Y, Kawano S, Morinobu S, Kobayashi M, Koshiba M, Saura R, Kurosaka M, Honjo T, Kumagai S (2003) CD4 + PD-1 + T cells accumulate as unique anergic cells in rheumatoid arthritis synovial fluid. J Rheumatol 30:1410–1419
van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS (2004) CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum 50:2775–2785
Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O (2005) CD4 + CD25 + T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol 140:360–367
Mallya RK, Mace BE (1981) The assessment of disease activity in rheumatoid arthritis using a multivariate analysis. Rheumatol Rehabil 20:14–17
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hussein, M.R., Fathi, N.A., El-Din, A.M.E. et al. Alterations of the CD4+, CD8+ T Cell Subsets, Interleukins-1β, IL-10, IL-17, Tumor Necrosis Factor-α and Soluble Intercellular Adhesion Molecule-1 in Rheumatoid Arthritis and Osteoarthritis: Preliminary Observations. Pathol. Oncol. Res. 14, 321–328 (2008). https://doi.org/10.1007/s12253-008-9016-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-008-9016-1