Abstract
Objectives
Our study objective was to explore whether abnormalities in the subtypes of T cells and B cells were present in peripheral blood of patients with osteoarthritis (OA) and healthy controls (HCs).
Method
Demographic and clinical variables and blood were collected. OA severity was measured using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) questionnaire. Flow cytometry was used to establish the frequencies of lineage subsets. Monoclonal antibodies against 21 surface markers were used to distinguish and evaluate T cells’ and B cells’ subpopulation. The proportion of each subset was compared and correlations between age, immune cells, and clinical data were analyzed.
Results
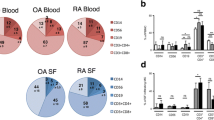
A total of 30 OA patients (male/female = 9/21) and 45 HCs (male/female = 14/31) were included. Median WOMAC pain was 3.0 (2.0). There was no difference in the proportion of T cells, CD8+ T cells, and B cells (p > 0.05). The proportion of CD4+ T cells was higher in OA groups, together with an increased CD4 to CD8 ratio (p = 0.016). CD8+CD45RA+ T cells were reduced after adjustment for age, while CD8+CD45RA− T cells were elevated in OA (p < 0.05). CD4+CD45RA−CCR7+ T cells and CD4+CD45RA−CCR7− T cells were increased (p < 0.004). The proportion of T helper (Th) 17 and T follicular helper (Tfh) 2 cells was higher, but cytotoxic T (Tc) 17 cells were fewer in OA (p < 0.05). CD3−CD19+IgD−IgM−CD27+CD38+ B cells were decreased in OA (p ≤ 0.001). The WOMAC pain score correlated with CD3+CD4+CXCR5−PD-1+ T cells positively (B = 0.404, p = 0.027). CD3−CD19+CD27−IgD+ cells correlated negatively with erythrocyte sedimentation rate (ESR) (B = −0.550, p = 0.005).
Conclusions
The T cell and B cell profiles were proved to have alteration suggesting that acquired immune system may play a substantial role in the pathogenesis of OA.
Key Points • The T cell and B cell profiles were proved to have alteration suggesting that acquired immune system may play a substantial role in the pathogenesis of OA. • The WOMAC pain score correlated with CD3+CD4+CXCR5−PD-1+ T cells and T helper 17 cells positively. • Memory T cells were increased in OA patients, suggesting they could play an important role in OA. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is a degenerative multifactorial joint disease, characterized by progressive joint failure with pain, impaired mobility, joint deformities, and disability worldwide [1] whose exact etiology remains unclear. Except for potential risk factors involving personal factors such as age, sex, obesity, and joint-level factors such as injury, malalignment, and biomechanical stresses [2], the immune system has been shown to be involved in age-related disease, such as OA [3]. Recent findings provide more evidence that OA possesses some characteristics of inflammatory diseases [4]. The interaction between trauma and chronic inflammation has been described as a “triggering factor” that activates the immune responses in the pathogenesis of OA [5]. Emerging evidence suggests the involvement of immunological factors in the development and progression of OA.
Human peripheral blood is readily available for research and offers access to a high number of different T cell subsets [6]. T cells’ contribution to the pathogenesis of OA is still under exploration but a quantity of literature indicates OA synovium has an enriched population of T cells compared with normal synovium [7]. Not only in synovial fluid and synovial membranes of OA patient but also in the T cell profile significant abnormalities have been found with reference to T helper (Th) 1 cells, Th9 cells, Th17 cells, regulatory T (Treg) cells, cytotoxic T (Tc) cells, and memory T (Tm) cells in the peripheral blood [8]. CD4+ T cells, particularly T follicular helper (Tfh) cells, are known to regulate B cell activation and functional differentiation. Tfh cells express various distinguishing genes, such as chemokine (C-X-C motif) receptor 5 (CXCR5); inducible costimulator (ICOS); programmed death (PD)-1; CD40 ligand; the transcription factor, Bcl-6; and IL-21 [9]. CXCR5+CD4+ T cells share the functional properties of Tfh cells which could be considered Tfh cells [10]. A recent study claimed that OA patients showed higher percentages of CXCR5+CD4+ T cells compared with healthy controls [11], but the role of Tfh cells in the pathogenesis of OA has yet to be fully elucidated.
Mild-to-moderate B lymphocytic infiltration was found in synovial tissues in OA [12], and the degree of B cell infiltration directly correlated with the severity of local inflammation. Some antibodies, such as anti-cyclic citrullinated peptide (anti-CCP) antibodies, have also been shown to be possibly involved in the pathogenesis of early-stage knee OA [13]. Another study found collagen breakdown revealed neo-antigens leading to T/B cell responses [14]. Based on these compelling findings, the autoimmune responses of peripheral immune cells may help to understand the immune-mediated mechanisms in OA.
The aim of our study was to determine lineage composition for CD4+ and CD8+ T cells, B cells, and phenotyped T cells and B cells further for naïve, memory, and regulatory subsets between OA and healthy controls (HCs), and to assess their possible relationship with age and disease parameters in OA.
Methods
Patients
We included 30 symptomatic patients with radiographic evidence of knee OA (Kellgren and Lawrence grade 2) according to American College of Rheumatology criteria from the Third Affiliated Hospital of Sun Yat-sen University and Gaozhou People’s Hospital, assessed by two rheumatologists (one in each center). The patients who had concomitant systemic diseases, such as a history of malignancy, central nervous system diseases, cardiopulmonary diseases, and who ever used glucocorticoids and disease-modifying anti-rheumatic drugs (DMARDs) were excluded. Demographic and clinical variables, including age, sex, height and weight, calculated body mass index (BMI), disease duration, affected joint(s), use of non-steroidal anti-inflammatory drugs (NSAIDs), number of painful and swollen joints, erythrocyte sedimentation rate (ESR), and serum C-reactive protein (CRP), were recorded during the evaluation. The severity of the disease was measured using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) questionnaire containing three sections: (i) pain assessment (five criteria); (ii) stiffness assessment (two criteria); and (iii) functional assessment (seventeen criteria). Patients were rated against each criterion on a 5-point Likert scale (0, none; 1, slight; 2, moderate; 3, severe; 4, extreme) [15].
The HC were recruited from the staff of our center. HCs who reported any previous joint symptoms or a history of OA were excluded from the study. The two rheumatologists confirmed there were no swollen hand joints and knee joints through physical examination in the HCs. Blood samples (heparin sodium tube 3 mL) were collected from OA patients and HCs. The study was approved by the local Research Ethics Committee and all participants gave written an informed consent.
Flow cytometry
Ten microliters of antibodies (anti-CD3, CD25, CD45RA, CD8, CD19 antibodies, Tianjin three arrows; anti-CD4, CD8, CD127, CCR7, CD28, CXCR3, CXCR5, CCR4, PD-1, CD45, CD27, IgD, IgM, CD38, CD24, CD21 antibodies, BD) was added to 50 μl of whole blood sample and incubated for 20 min at room temperature. Then, the lysing agent was added to lyse the red blood cells, and 5μl of fluorescent dye (CD3-PerCP-Cy5.5, CD25-PE, CD45RA-FITC, CD8-PerCP-Cy5.5, CD19-PerCP-Cy5.5, Tianjin Sanjian; CD4-APC-H7, CD8-BV510, CD127-BV421, CCR7-AF647, CD28-PE-Cy7, CD3-APC-H7, CD4-PE-Cy7, CXCR3-Alexa488, CXCR6-BV510, CXCR5-Alexa647, CCR4-BV421, PD-1-PE, CD45-APC-H7, CD27-BV421, IgD-BB515, IgM-BV510, CD38-APC, CD24-PE, CD21-PE-Cy7, BD) was added after washing and stayed for 4 min in the dark at 4 °C. Then, it was washed with phosphate-buffered saline. The instrument settings and cell gating were adopted from previous works to isolate the cells accordingly. And the expression of lymphocyte surface molecules was analyzed. Results are expressed as the percentage of cells expressing a particular marker. All experiments, including sample preparation, were performed under standardized experimental conditions. Without the isotype control, phosphate-buffered saline (PBS) was selected as a blank control. Sample collection and analysis were performed by an operator blinded to clinical features. Samples were analyzed using a FACSCanto flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analyses
Descriptive analysis of the participants was performed. Data with normal distribution were represented using mean± SD, and those with non-normal distribution were presented by median (interquartile range). Following appropriate transformation of variables where necessary to an approximate normal distribution, we used multivariate analysis of covariance (MANCOVA) to determine whether the relative subset frequencies differed between HC and OA, controlling for age. If variance homogeneity cannot be satisfied, comparisons between subgroups were undertaken using 2-tailed independent t tests or the Mann-Whitney U tests. Correlations between age and clinical and serologic data were analyzed using Pearson or Spearman rank correlation test. The level of significance was set at p < 0.05. All the data analyses were performed using SPSS release 20.0 (IBM, Armonk, NY, USA) and GraphPad Prism 7.0 (GraphPad Software, USA).
Results
General characteristics of the participants
There were a total of 30 patients (male/female = 9/21) with confirmed OA diagnosis and 45 HCs (male/female = 14/31) included in the study. Mean age was 57.0 ± 10.3 in OA and 35.9 ± 9.4 in the HC group (p < 0.01). Median disease duration was 0.2 (1.9) years. Median score of WOMAC stiffness, difficulty in daily activity, and pain was all 3.0 (2.0). Mean BMI was 21.1 ± 4.8 in OA patients. Fourteen OA patients had sole complaints of joint pain, while 16 patients also had swollen joints. Median ESR was 22 (17) mm/H, and CRP was 2.1 (1.95) mg/L. Eighteen OA patients were naive to treatment, while 12 patients once used NSAIDs. None of them ever used DMARDs or glucocorticoid for treatment (Table 1).
Lineage blood cell composition between OA and HC
We first compared the frequencies of major circulating immune cell subsets, i.e., CD8+ T cells, CD4+, and B cells (Fig. 1). There was no difference in the proportion of CD3+ T cells between OA and HC (67.5% ± 11.2% vs 71.8% ± 8.7%, p = 0.07), CD3+CD8+ T cells (30.2% ± 7.8% VS 29.8% ± 8.2%, p = 0.82), and CD3−CD19+ B cells (11.9% (5.9%) VS 10.4% (4.9%), p = 0.36). The proportion of CD4+ T cells was higher in the OA group (60.0% ± 9.1% vs 56.5% ± 14.3%, p = 0.001). And meanwhile, patients with OA had an increased CD4 to CD8 ratio (p = 0.016) (see Table 2).
Differences in T cell composition between OA and HC
Altered expression of CD8+ T cell subsets
CD3+CD8+CCR7−CD45RA+ T cells were reduced after adjustment for age (p = 0.026), together with CD3+CD8+CCR7+CD45RA+ T cells (p = 0.032), while both CD3+CD8+CCR7+CD45RA− T cells and CD3+CD8+CCR7−CD45RA− T cells were elevated in the patient group (p = 0.001) (see Fig. 2).
The percentage of CD3+CD8+CD28− T cells, CD3+CD8+CCR7−CD45RA− CD127hi, CD3+CD8+CCR7−CD45RA+ CD127hi T cells, CD3+CD8+CCR7−CD45RA− CD127lo, and CD3+CD8+CCR7−CD45RA+ CD127lo was similar in the two groups (p > 0.05).
Altered expression of CD4+ T cell subsets
CD3+CD4+CD45RA+CCR7− T cells went down in OA (p < 0.001), while both CD3+CD4+CD45RA−CCR7+ T cells and CD3+CD4+CD45RA−CCR7− T cells were increased after adjustment for age (p = 0.002, p = 0.003, individually) (see Fig. 3). There was no difference in the proportion of CD3+CD4+CD8+ T cells, CD3+CD4+CD45RA+CCR7+ T cells, CD3+CD4+CD28− T cells, or CD3+CD4+CD28+ T cells (p > 0.05).
Altered expression of Th cell subsets
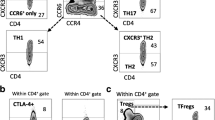
The proportion of CD3+CD4+CXCR5−CXCR3−CCR4−CCR6+ T cells (Th17) was higher in OA than HC after adjustment for age (p = 0.029). But there was no difference in the proportion of CD3+CD4+CXCR5−CXCR3+CCR4−CCR6− T cells (Th1), CD3+CD4+CXCR5−CXCR3−CCR4+CCR6− T cells (Th2), or CD3+CD4+CXCR5−PD-1+ T cells (p > 0.05).
Altered expression of subsets of Tfh cells
No difference was found in the proportion of CD3+CD4+CXCR5+ T cells (Tfh cells) between the two groups (23.3% ± 6.9% VS 21.1% ± 7.5%, p = 0.20), as well as CD3+CD4+CXCR5+CXCR3+CCR4− T cells (Tfh1), CD3+CD4+CXCR5+CXCR3−CCR4−CCR6+ T cells (Tfh17), and CD3+CD4+CXCR5+PD-1+ T cells (p > 0.05). However, CD3+CD4+CXCR5+CXCR3−CCR4+ T cells (Tfh2) went up in OA (p = 0.041) compared with HC.
Altered expression of Tc cell subsets
The percentage of CD3+CD8+CXCR5−CXCR3−CCR4−CCR6+ T cells (Tc17) was lower in the patients than that in HC after adjustment (p = 0.009). No difference was detected in the proportion of CD3+CD8+CXCR5-CXCR3+CCR4− T cells (Tc1) or CD3+CD8+CXCR5−CXCR3−CCR4+ T cells (Tc2) between the two groups (p > 0.05).
Altered expression of Treg cells in OA patients
The percentage of CD3+CD4+CD25+CD127− T cells was decreased in OA patients (3.1% ± 1.5% vs 5.6% ± 5.0%, p = 0.002).
Differences in B cell composition between OA and HC
CD3−CD19+IgD+CD27− CD38lowCD21low B cells went higher in OA after adjustment for age (p < 0.001), while CD3−CD19+IgD−IgM−CD27+CD38+ B cells were decreased compared with those in HC (p = 0.001) (Fig. 4). But there was no difference in the proportion of CD3−CD19+CD27−IgD+ B cells, CD3-CD19+CD27+IgD+ B cells, CD3−CD19+IgM−IgD−CD27−CD38+ B cells, CD3−CD19+IgD−IgM−CD27+CD38− B cells, CD3−CD19+IgD+IgM+CD27+CD24+CD38− B cells, CD3−CD19+IgD+IgM+CD27+CD24+CD38+ B cells (memory B cells), CD3−CD19+IgD+IgM+CD27+CD38+CD24− B cells, CD3−CD19+IgD+IgM+CD27−CD24+CD38+ B cells, or CD3−CD19+IgM+IgD−CD27−CD38+CD24+ B cells between the two groups (p > 0.05).
Association between age and immune cells
CD3+CD4+CD8+ T cells had a positive correlation with age (B = 0.403, p = 0.027). CD3+CD4+CD45RA−CCR7− T cells also correlated with age (B = 0.372, p = 0.043), together with CD3+CD8+CCR7−CD45RA− CD127lo (B = 0.368, p = 0.046). In contrast, CD3+CD4+CD45RA+CCR7+ T cells had a negative correlation with age (B = − 0.366, p = 0.047). Other cells which correlated with age negatively included CD3+CD8+CCR7+CD45RA+ T cells (B = −0.512, p = 0.004), CD3+CD8+CCR7−CD45RA− CD127hi (B = − 0.368, p = 0.046), CD3+CD8+CXCR5−CXCR3−CCR4−CCR6+ T cells (B = − 0.428, p = 0.018), CD3−CD19+CD27−IgD+ B cells (B = − 0.374, p = 0.041), and CD3−CD19+IgD+IgM+CD27−CD24+CD38+ B cells (B = − 0.378, p = 0.04).
Correlation of peripheral immune cells with clinical parameters
We tested whether the immune cells correlated with either the clinical or the serological data of the patients. The WOMAC pain score correlated with CD3+CD4+CXCR5−PD-1+ T cells positively (B = 0.404, p = 0.027), while it correlated with CD3+CD8+CD28− T cells (R = − 0.493, p = 0.006) and Th17/Th2 ratio (R = − 0.409, p = 0.025) negatively.
CD3+CD8+CCR7−CD45RA− T cells (R = 0.420, p = 0.041) and CD3−CD19+IgM−IgD−CD27−CD38+ B cells (B = 0.701, p < 0.001) had a positive correlation with ESR. CD3−CD19+CD27−IgD+ B cells correlated negatively with ESR (B = − 0.550, p = 0.005). However, no significant correlation between Tfh cells or Tc cells and clinical parameters was observed in the current study.
Discussion
Although the pathophysiology of OA remains poorly understood, immune cells, especially T cells and B cells, are widely recognized to exert substantial influence on the pathogenesis of the disease. Our study firstly examined the frequency of different subsets of circulating T cells and B cells in a detailed manner, as well as their association with clinical characteristics of OA, and reported the imbalance of peripheral immune cells in such patients compared with HC, thus providing evidence that the disparity of the immune response could be involved in the development of OA. We first reported Th17 cells correlated with disease severity measured by WOMAC scores, indicating Th17 could be a possible marker of disease severity. Tc17 cells were reduced, while Tfh2 cells went higher in OA patients. Tm cells involving CD3+CD8+ CCR7+ CD45RA− T cells, CD3+CD8+ CCR7− CCD45RA− T cells, CD3+CD4+CD45RA− CCR7+ T cells, and CD3+CD4+CCR7− CD45RA− T cells were all increased in OA patients after the adjustment for age, suggesting Tm cells could play an important role in the pathogenesis of OA.
We found a normal proportion of CD8+ T cells, but a reduced proportion of naive CD8+ T cell (CD3+CD8+CCR7+CD45RA+) and CD3+CD8+CCR7−CD45RA+ T cells. We also confirmed that naive CD8+ T cell frequencies reduced with age in OA. CD8+ T cells also contain minor subsets, such as Tc17 cells that express CCR6+ [16], whose function is incompletely understood. Our study found that the percentage of Tc17 was reduced in OA patients. These results reflected the alteration of peripheral CD8+ T cells in OA patients. In addition, there was no difference in the percentage of CD3+CD8+CCR7−CD45RA− CD127hi T cells, CD3+CD8+CCR7−CD45RA+ CD127hi T cells, CD3+CD8+CCR7−CD45RA− CD127lo T cells, and CD3+CD8+CCR7−CD45RA+ CD127lo T cells between OA and HC, which was indicative of a trivial role of certain virus infection in the pathogenesis of OA.
CD4+ T cells are crucial for directing appropriate immune responses during host defense and for the pathogenesis of inflammatory diseases. We found the proportion of CD4+ T cell and CD4+/CD8+ ratio was significantly higher in OA groups. CD3+CD4+CD45RA+CCR7− T cells went down in OA. Naïve T cells are believed to be a homogeneous population of uncommitted precursor cells that differ only in T cell receptor (TCR) specificities and recognize consequently different antigens [17]. CD4+ T cells contain a minor fraction of CD28− cytotoxic effector cells that express perforin and contribute to the elimination of virus-infected or transformed monocytes and B cells [18]. No difference was found in the proportion of naïve CD4+ T cells, CD3+CD4+CD28− T cells or CD3+CD4+CD28+ T cells in the current study.
A minority of T cells persist as Tm cell; however, most T cells undergo apoptosis once activated. There is some evidence for the possible involvement of Tm cells in the pathogenesis of OA including the accumulation of CD45RO+ memory CD4+ T cells, secreted chemokine in the peripheral blood, and synovial fluid of OA patients [19, 20]. Consistent with the current knowledge [21], our study showed that naïve CD4+ T cell (CD3+CD4+CD45RA+CCR7+) frequencies were reduced with age, while memory CD4+ T cell (CD3+CD4+CD45RA−CCR7−) frequencies were increased with age. Our study also found that Tm cells involving CD3+CD8+CCR7+CD45RA− T cells, CD3+CD8+CCR7−CD45RA− T cells, CD3+CD4+CD45RA−CCR7+ T cells, and CD3+CD4+CD45RA−CCR7− T cells were all increased in OA patients after the adjustment for age. The lymph node homing receptors CCR7, typical for naive T cells, are also expressed on a major fraction of human CD4+CD45RA− and on a minor fraction of CD8+CD45RA− memory T cells [22]. These central memory T cells are characterized by low effector function and high secondary expansion potential and appear thus arrested at an intermediate stage of T cell differentiation [23]. These memory T cells seem to be critical to the pathogenesis of OA which needs further investigation.
Th17 cells provide protection against bacterial infection and are associated with the development of autoimmune diseases [24]. There is controversy on alternations in OA synovium with some studies showing similar peripheral Th17 cells’ percentage as healthy controls [25], but others found Th17 cells were increased in OA synovium [26]. Variation in the markers can result in discrepancy. Our study revealed that the proportion of peripheral Th17 cells (CD3+CD4+CXCR5−CXCR3−CCR4-CCR6+) became elevated in OA patients after adjusting for age, indicating circulating Th17 cells may have certain contribution to the pathogenesis of OA but needs further investigation. The measurement of IL-17 could help explain the role of circulating Th17 cells in the pathogenesis of OA. In RA, the number of Th17 cells in peripheral blood correlates with disease activity [27]. We found Th17 cells correlated with disease severity measured by the WOMAC score, indicating Th17 could be a possible marker of disease severity. Besides, our study was in consistent with previous studies that both Th1 cells and Th2 cells underwent limited alteration in the peripheral blood [8, 25]. As Th1 cells have been shown to accumulate in the synovial fluid and synovial membranes of OA patients, a further study of the synovial fluid and synovial membranes of OA could help to explain the function of Th1 on the pathogenesis of OA. No association between peripheral Th cells and clinical parameters was discovered in the current study. More data are needed to detect a relationship between Th cells and disease variables in OA patients.
Tfh cells contribute to both host protection and immune-mediated inflammatory diseases by providing help to B cell and antibody production and producing lineage-specific cytokines such as IFN-γ, IL-4, and IL-17. These cells are identified on the basis of their location in the germinal centers in vivo and by the surface expression of CXCR5 and programmed cell death 1 molecules [9]. We observed no difference in the proportion of Tfh cells between the two groups. However, Tfh2 was higher in OA patients. Our result regarding circulating Tfh cells were different from a recent study, and no association between Tfh and clinical variables was found [11], possibly due to different markers of the Tfh cells. What’s more, the study did not further classify Tfh into several types. Further studies with larger sample sizes could help to illuminate the pathogenic mechanism of Tfh cells.
Treg cells are essential for the maintenance of immunological self-tolerance and the prevention of various autoimmune diseases [28]. We found that OA patients had fewer Treg cells, which was in consistence with previous findings [21]. But no association was found between Treg and disease severity in our study.
B cells can contribute to disease development by producing autoantibodies, presenting autoantigens to autoreactive T cells and secreting pro-inflammatory cytokines and chemokines which leads to the amplification of the inflammatory response [29]. Inadequate work showed evidence of a limited disturbance in B cells. Our result showed that the proportion of B cells, including naïve B cells, the CD24+CD38+ transitional B cells, and memory B cells, did not differ between OA and HC. Looking at individual phenotypes, CD3-CD19+IgD+CD27− CD38lowCD21low B cell frequency went higher in OA patients, while CD3−CD19+IgD−IgM−CD27+CD38+ B cells were decreased compared with those in HC. Previous study only examined the presence of B lymphocytes in inflamed knee OA synovium [12]. Oligoclonal B cell expansion seen in OA synovial membranes suggests that different B cell reactivities are also present in OA joints, and some of them may induce, sustain, and modify the disease course of OA [30]. Future studies involving cytokine and autoantibodies produced by B cells are needed to understand how B cells affect OA pathology in general.
There were certain limitations of the present study which should be carefully considered for the clinical translation of the findings. First, OA is a heterogeneous disease with different subtypes according to disease localization, stage, and severity and personal-level factors. It is unknown whether the described T cell and B cell alterations are widely applicable to different forms of OA. That was one of the reasons why we chose the patients on early stage of the disease. Second, some significant cytokines could add the value to the analysis of immune cell composition and its possible mechanism during the interpretation of the results. Third, the failure to involve the study of synovium fluid and synovium membrane in OA may lead to less convincing and integrated findings.
The findings of the present study confirmed the significant immune cell alteration, especially in T cells in OA patients, leading to the conclusion that T cells and B cells may be involved in the pathogenesis of OA. Th17 cells could be a possible marker of disease severity. Understanding the significance of the altered T cell and B cell profiles to the pathogenesis of OA can open up novel directions for preventing and treating OA by modulating immune responses.
References
Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, March L (2014) The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 73:1323–1330
Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, Sowers M, McAlindon T, Spector TD, Poole AR, Yanovski SZ, Ateshian G, Sharma L, Buckwalter JA, Brandt KD, Fries JF (2000) Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med 133:635–646
Pawelec G (2012) Hallmarks of human “immunosenescence”: adaptation or dysregulation? Immun Ageing 9:15
Berenbaum F (2013) Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr Cartil 21:16–21
Huber-Lang M, Kovtun A, Ignatius A (2013) The role of complement in trauma and fracture healing. Semin Immunol 25:73–78
Geginat J, Paroni M, Facciotti F, Gruarin P, Kastirr I, Caprioli F, Pagani M, Abrignani S (2013) The CD4-centered universe of human T cell subsets. Semin Immunol 25:252–262
de Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ et al (2012) Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthr Cartil 20:1484–1499
Li YS, Luo W, Zhu SA, Lei GH (2017) T cells in osteoarthritis: alterations and beyond. Front Immunol 8:356
Crotty S (2011) Follicular helper CD4 T cells (TFH). Annu Rev Immunol 29:621–663
Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh SK, Sabzghabaei N, Lavecchio EM, Punaro M, Pascual V, Banchereau J, Ueno H (2011) Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34:108–121
Shan Y, Qi C, Liu Y, Gao H, Zhao D, Jiang Y (2017) Increased frequency of peripheral blood follicular helper T cells and elevated serum IL21 levels in patients with knee osteoarthritis. Mol Med Rep 15:1095–1102
Da RR, Qin Y, Baeten D, Zhang Y (2007) B cell clonal expansion and somatic hypermutation of Ig variable heavy chain genes in the synovial membrane of patients with osteoarthritis. J Immunol 178:557–565
Du H, Masuko-Hongo K, Nakamura H et al (2005) The prevalence of autoantibodies against cartilage intermediate layer protein, YKL-39, osteopontin, and cyclic citrullinated peptide in patients with early-stage knee osteoarthritis: evidence of a variety of autoimmune processes. Rheumatol Int 26:35–41
Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, Lee YJ, Song YW, Herzog C, Theilmeier G, Pap T (2009) Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med 15:1072–1076
Bellamy N, Buchanan WW, Goldsmith CH et al (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15:1833–1840
Kondo T, Takata H, Takiguchi M (2007) Functional expression of chemokine receptor CCR6 on human effector memory CD8+ T cells. Eur J Immunol 37:54–65
Hermiston ML, Xu Z, Weiss A (2003) CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol 21:107–137
Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, Easterbrook P, Grey P, Smith D, McMichael AJ, Cooper DA, Rowland-Jones SL, Kelleher AD (2002) Characterization of CD4(+) CTLs ex vivo. J Immunol 168:5954–5958
Haynes MK, Hume EL, Smith JB (2002) Phenotypic characterization of inflammatory cells from osteoarthritic synovium and synovial fluids. Clin Immunol 105:315–325
Ezawa K, Yamamura M, Matsui H, Ota Z, Makino H (1997) Comparative analysis of CD45RA- and CD45RO-positive CD4+T cells in peripheral blood, synovial fluid, and synovial tissue in patients with rheumatoid arthritis and osteoarthritis. Acta Med Okayama 51:25–31
Ponchel F, Burska AN, Hensor EM et al (2015) Changes in peripheral blood immune cell composition in osteoarthritis. Osteoarthr Cartil 23:1870–1878
Sallusto F, Lenig D, Forster R et al (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712
Sallusto F, Geginat J, Lanzavecchia A (2004) Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 22:745–763
Miossec P, Kolls JK (2012) Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov 11:763–776
Zhang L, Li JM, Liu XG, Ma DX, Hu NW, Li YG, Li W, Hu Y, Yu S, Qu X, Yang MX, Feng AL, Wang GH (2011) Elevated Th22 cells correlated with Th17 cells in patients with rheumatoid arthritis. J Clin Immunol 31:606–614
Qi C, Shan Y, Wang J, Ding F, Zhao D, Yang T, Jiang Y (2016) Circulating T helper 9 cells and increased serum interleukin-9 levels in patients with knee osteoarthritis. Clin Exp Pharmacol Physiol 43:528–534
Kim J, Kang S, Kim J, Kwon G, Koo S (2013) Elevated levels of T helper 17 cells are associated with disease activity in patients with rheumatoid arthritis. Ann Lab Med 33:52–59
Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, Rudensky A, Sakaguchi S, Shevach EM, Vignali DAA, Ziegler SF (2013) Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol 14:307–308
Wilkinson M, Rosser EC (2019) B cells as a therapeutic target in paediatric rheumatic disease. Front Immunol 10:214
Shiokawa S, Matsumoto N, Nishimura J (2001) Clonal analysis of B cells in the osteoarthritis synovium. Ann Rheum Dis 60:802–805
Acknowledgments
We owed special thanks to Professor Zhinan Yin from Jinan University for his assistance in the study.
Funding
The study was financially supported by the Municipal Healthcare Joint-Innovation Major Project of Guangzhou (No. 201604020013) and Prevention and Treatment of Multiple Immune Diseases and Allergic Diseases (No. 82000-41070001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Ethics Commitee of the Third Affiliated Hospital of Sun Yat-sen University ([2018]-02-349-01) and all participants gave a written informed consent
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wanshou Zhu, Xi Zhang, and Yutong Jiang contribute equally as first authors.
Rights and permissions
About this article
Cite this article
Zhu, W., Zhang, X., Jiang, Y. et al. Alterations in peripheral T cell and B cell subsets in patients with osteoarthritis. Clin Rheumatol 39, 523–532 (2020). https://doi.org/10.1007/s10067-019-04768-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04768-y