Abstract
Purpose
We present a framework to prioritize strategies for monoclonal antibody (mAb) second-generation process development, or post-approval optimization.
Methods
Design of experiments (DoE), in conjunction with principal component analysis (PCA), were employed to identify process parameters that had the most impact on downstream purification cost of goods. Statistically significant parameters were identified through a DoE study, while the PCA characterization was applied as an independent tool to further elucidate the relative importance of these parameters. A stochastic approach incorporating process uncertainties was used to illustrate the distribution of downstream cost of goods under different process conditions.
Results
This framework offered insights on the relative contribution of each parameter to downstream cost of goods, and generated frequency distribution of the downstream cost of goods by incorporating process uncertainty. Such systematic approach to prioritize development strategies under compressed timelines could be useful for biopharmaceutical companies to achieve a competitive advantage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monoclonal antibodies (mAb) have become an increasingly important class of agents that contribute to several major advances in pharmacotherapy. Over the past decade, FDA has approved 15 new therapeutic antibody products spanning four major therapeutic areas [1], with 52% in immunology, 36% in oncology, 4% in infectious disease, and 8% in other areas [2]. For antibody products that are currently in development, more than half (59%) are in the area of oncology [2]. In the past few years, FDA granted breakthrough designation and accelerated the approval of several oncology mAbs such as Keytruda (Merck) and Opdivo (Bristol-Myers). Indeed, the market for monoclonal antibodies is rapidly expanding, and it represents the industry segment with the highest growth rate over the last decade [3].
To respond to the increasing market demand for mAbs, biotech and pharmaceutical industry, in collaboration with academia, have enhanced their strategies to increase bioprocessing productivity. Owing to the improvement in the cell culture process, and the advance in expression technology, mAbs product titer has increased from below 1 g/L to 1–5 g/L, with some companies reporting 10–13 g/L for extended culture duration [4]. The increase in cell culture titer has shifted the manufacturing and economic bottleneck from upstream processing (USP) to downstream processing (DSP). Sommerfeld and Strube have shown that as cell culture titer increases from 0.1 to 1 g/L, the ratio of USP to DSP costs drops from 55:45 to 30:70 [5]. At higher product titer, larger chromatography columns, membrane areas, buffer consumption, and/or additional chromatography or filtration cycles would be required to purify the cell culture harvest from upstream. Experience in the industry shows (which is simulated in our case study later in the paper) that the overall cost per gram of drug substance purified initially decreases as titer increases; however, the cost plateaus as the titer increases further. In a higher titer process, the increase in downstream cost can potentially negate the gains in titer, or overall process throughput.
Given the strong competition in the pharmaceutical industry, the entry of biosimilars into the market, combined with the recent changes in the US healthcare system, the ultimate success for monoclonal antibody process development will be driven by the ability to produce a high throughput and cost-effective process [6, 7]. While there have been a number of efforts trying to increase production capacity by increasing cell culture titer, the industry has been exploring strategies to reduce the cost of goods by optimizing downstream processing. Strategies such as increasing downstream yield, membrane or resin reuse, chromatography or membrane loading capacity, and decreasing cycle time and buffer consumption have been utilized to improve throughput, reduce raw material and consumable usage, shorten processing time, and ultimately achieve a lower downstream cost of goods. Under compressed drug development time scales, it is important for companies to effectively prioritize their strategies to accelerate the process development effort, thus achieving a competitive advantage.
This paper will specifically focus on reducing the downstream cost of goods, which is defined by the downstream cost per unit of drug substance, through optimizing the purification process. Building on our prior modeling work in pharmaceutical manufacturing [8–11], we propose a systematic decision-making framework that (i) utilizes design of experiments (DoE) in conjunction with principal component analysis (PCA) to identify and verify downstream process parameters that impact the downstream cost of goods and (ii) predict the economic outcome of selected strategies through a stochastic analysis (Fig. 1).
Method
Monoclonal Antibody Production Process
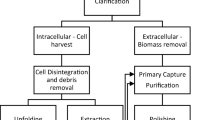
An example of a typical monoclonal antibody production process is depicted in Fig. 2. The process consists of an upstream process section and a downstream process section. While the USP includes inoculation, cell culture, and primary recovery, the DSP consists of protein A chromatography, followed by two ion exchange steps (flowthrough and bind-and-elute), virus filtration and ultrafiltration and diafiltration. The downstream purification train is designed to capture the antibody, reduce impurities, such as aggregates, host cell protein, DNA, leached protein A and adventitious virus, and finally concentrate and formulate the product.
Process Simulation Software—SuperPro
A process simulation tool such as SuperPro Designer Version 9.0, Build 9, Special Build 2200 (Intelligen, Scotch Plains, NJ http://www.intelligen.com/) has the capability of integrating both process and business modeling functions to facilitate strategic decision making during process development and large-scale manufacturing. Some might attempt to model the cost of goods using excel. However, since changing in one parameter will cascade the impact on multiple parameters downstream, SuperPro is more efficient to capture this impact compared to excel. Input to this tool includes a detailed description of the process steps, and their related costs, such as capital investment, total spending in infrastructure, labor, and raw material costs. Figure 3 shows the SuperPro model (more detailed on parameter values are provided in Appendix Tables 4 and 5) that we used in this study. The model consists of the major process steps shown in Fig. 2, and the associated steps, such as sterile filtration, buffer exchange, and media prep. The parameter values, raw material, and facility costs used in building this model derived from literature and process experience. The resulting throughput and cost models generated can then be utilized to estimate the final unit cost of drug substance or drug product generated at manufacturing scale [3].
Design of Experiments
A design of experiments approach was taken to explore the impact of selected parameters on the downstream cost of goods. Compared to a One Factor at A Time (OFAT) approach, which can become very cumbersome when there are more parameters (typically >2), DoE is more efficient in understanding the effect of parameters, their interactions, and generating a predictive model with a minimum number of experiments. Table 1 shows the six parameters studied in our DoE. These parameters can potentially reduce the downstream cost of goods by increasing the throughputs (yield) or decrease the consumable cost (loading). The current process ranges are ranges typically experienced in early phase process development, while the optimized range represents the reasonable target ranges after further optimization. This DoE study encompasses both the current and optimized ranges and is designed to explore whether the changes in parameters within these ranges have a statistical significant impact on downstream COGs. A central composite design was utilized in this DoE study and a total of 45 simulations of downstream COGs were performed for titer set point at 0.5, 2, 4, and 7 g/L.
An R 2 value of greater than 95% is required to establish the validity of the DoE model. A p value of <0.05 is required to indicate statistical significance.
Principal Component Analysis
To further verify the conclusions derived from the DoE analysis, the same set of data were processed using principal component analysis (PCA) [12]. PCA is a statistical tool that converts a set of possibly correlated variables into a set of values of linearly uncorrelated variables called principal components (PC). The first PC captures the largest part of variance in the data, the second PC the next largest, and so on. The result of PCA is displayed as loading plots, showing contributions from original variables [12].
Monte Carlo Simulation
Since there are inherent uncertainties in large-scale bioprocess production, it is important to account for these uncertainties during process modeling for representative results. The key sources of technical uncertainties affecting the biomanufacturing often arise from cell culture titer, chromatography loading, yields, and process cycle time [13]. Stochastic modeling with Monte Carlo simulation was utilized in this case study to understand the output distribution of downstream COGs, by incorporating the effect of uncertainties in process parameters. The details of our Monte Carlo approach are described below.
Case Study
A simulation case study was set up to compare the different DSP optimization strategies for high titer (1–7 g/L) process. Typically, during second-generation process development, or post-approval process optimization, efforts are devoted to improve cell culture titer and ultimately to increase production capacity and improve process economic.
In this case study, we first examined the impact of titer on the overall cost of drug substance through Monte Carlo simulation. Previous research has suggested that the high purification cost can sometimes negate the benefits gained from the increase in titer and throughput [4]. Hence, to reduce the downstream purification cost for higher titer process, we first identified a list of parameters that could have potential impact on downstream cost of goods and then ranked them based on previous process knowledge. With this process, we have identified six parameters to carry forward in our study (Table 1). These parameters, such as chromatographic yield and loading, could reduce the downstream COGs by increasing throughput and reducing the raw material and consumable consumption. When prioritizing development efforts under compressed timelines, it is advantageous to select strategies that can maximize the reduction of downstream COGs. A design of experiments (DoE) approach was utilized to explore the impact of selected parameters on downstream COGs and identify statistically significant parameters on COGs. To further confirm and refine our conclusions from the DoE study, principal component analysis (PCA) was used as an alternative method to understand the contribution of each parameter to the change in downstream COGs. Finally, for the top parameters selected to pursue forward, Monte Carlo simulation was performed to evaluate the frequency distribution of downstream COGs by incorporating process uncertainties and variability. This systematic approach help prioritize strategies to reduce downstream COGs.
There were several key assumptions made in the DoE and Monte Carlo simulations:
-
1.
The cost of upstream process is assumed to be independent of titer, although the upstream cost can sometimes vary depend on the feeding strategy and culture duration at different titers. In this paper, we focus on identifying strategies to reduce the downstream cost, which is unlikely to be impacted by the upstream cost.
-
2.
The number of chromatography cycle is fixed in this case study, and the facility is assumed to have the flexibility to adjust the column size depending on the incoming streams.
-
3.
While some companies will continue to manufacture the second-generation process at the original commercial site, other might move to a different site due to facility fit, cost, and other business and strategic reasons. Here, we are assuming that second-generation process will be manufactured at a new commercial site, and hence, the capital cost is relevant in our case.
Monte Carlo Simulation Results at Current Downstream Operating Conditions
In order to account for the uncertainties inherent in large-scale biomanufacturing, representative triangle distributions of the current operating range indicated in Table 1 were assigned to the six parameters: protein A loading, IEX I loading, IEX II loading, protein A yield, IEX I yield, and IEX II yield. The base value represents the most likely value, bounded by the maximum and minimum values, which were formed by taking into account process fluctuations. The triangular distribution was derived based on data from manufacturing, pilot, and laboratory scale. Triangular distribution was also what we typically experienced across various monoclonal antibody programs and was used in an antibody cost analysis by Farid et al. [6]. The range for cell culture titer in this study ranged from 0.5 to 7 g/L, and a uniform distribution was used to ensure that all titer set points within the range were simulated with equal probabilities. A total of 300 simulations were performed and the simulated cost per gram of drug substance at different titers is shown in Fig. 4.
Initially as titer increases from 0.5 to 2 g/L, the cost per gram of drug substance drops from $700 to $200/g/L, or a 3.5-fold reduction. However, as the titer increases further beyond 2 g/L, the cost per gram of drug substance plateaus—minimal to no change is observed with the increase in titer. At lower titers of 0.5–2 g/L, the production cost of antibody is more dominant and therefore maximizing the throughput by increasing the titer is generally an efficient strategy to decrease cost of goods [3]. However, at titers greater than 2 g/L, larger chromatography columns, which translate into larger resin and buffer consumption, larger membrane areas, or possibly longer processing time would be required to accommodate for the increased amount of antibody coming from upstream. Although the throughput continues to increase with titer, the purification cost increases at a much faster rate that it negates the gains from the increase in throughput. As a result, improvement in downstream efficiency, either by increase throughput, decrease operation cost or a combination of both could further reduce the downstream COGs.
Design of Experiments to Explore the Impact of Selected DSP Parameters on Downstream COGs
A central composite response surface was used to explore the effect of selected chromatographic parameters on downstream COGs at a titer range of 0.5–7 g/L. For all DoE models generated, the R-sq. values were greater than 95%, indicating the validity of the model. Table 2 displays the p values of each parameter at the selected titer (0.5, 2.0, 4.0, and 7.0 g/L). At 0.5 g/L, all parameters have been shown to be statistically significant, as they all have p values of less than the alpha cutoff (0.05). Compared to the IEX I loading, all other parameters have more significant effect as they have much lower p values. At higher titers of 2, 4, and 7 g/L, the IEX I loading is no longer statistically significant while the other parameters continue to have p values of <0.0001 and thus statistically significant. IEX I loading has the least to none statistical significance because ion exchange resin is relatively inexpensive compared to protein A resin. In addition, in this case study, since the step is operated in flowthrough mode, the resin already has a high resin utilization rate of ∼200 g/L loading, any further increase in IEX I loading would only result in a marginal impact on downstream COGs. Although the DoE analysis in this study was able to screen statistically significant parameters, it did not give insight into the relative importance of these parameters as they all have the same p values of <0.0001. Additional analysis, such as PCA, would be helpful to elucidate the relative importance of each parameter on reducing downstream cost of goods. Since PCA is commonly used to reduce dimensionality of dataset, the results from the PCA analysis would enable us to rank the statistically significant parameters from the DoE study and thus to identify the top parameters for further optimization.
Principal Component Analysis to Confirm or Refine DoE Results
In order to verify and further refine the conclusions derived from the DoE analysis, the DoE data were processed through PCA. The first principal component captures ∼30% of the total variance in the downstream cost of goods. The loading plot (Fig. 5) from the first principal component confirmed that at all titers analyzed, the IEX loadings have lower contributions to the downstream cost of goods compared to chromatographic yields or protein A loading. As discussed in previously, IEX resin in general is less costly; therefore, increasing the resin utilization is unlikely to have a significant impact on the downstream COGs.
The loading plot shows that at 0.5 g/L, IEX II yield has the biggest contribution to the total variance. Furthermore, the chromatographic yields (ProA yield, IEX I yield, and IEX II yield) have higher loading scores, or bigger contribution, compared to chromatographic loading (ProA loading, IEX I loading and IEX II loading). This trend indeed aligns with what have been shown in previous literature research that at a lower titer, especially at <1.0 g/L, the purification cost is relatively lower [4]; thus, increasing the overall throughput is a more competitive strategy to reduce cost.
At higher titers of >2 g/L, protein A loading has the most influence on the downstream cost of goods; more importantly, its contribution to the total variance increases as the titer increases. The cost of protein A resin becomes more significant as titer increases, because more resin would be needed to purify the incoming stream, given the number of chromatography cycle is fixed in this study. The cost of this highly expensive resin is indeed ∼85% of the total consumable cost at 7 g/L titer. Hence, an increase in protein A loading would increase the resin utilization and therefore decrease the total amount of protein A resin consumption.
In addition, the loading plot shows the intra-relationship of parameters. For instance, as titer increases, the contribution of chromatographic yields (ProA yields, IEX I and II yield) decreases, while the contribution of chromatographic loading (ProA loading and IEX II loading) increases. This implies that the purification cost becomes more dominant at higher titer. Reduction in resin consumption, especially the protein A resin, is likely to decrease the downstream COGs significantly. This case study suggests that both protein A loading and IEX II yield have the most influence on the downstream cost of goods. Monte Carlo simulation was utilized to generate the frequency distribution of downstream COGs by optimizing either the protein A loading or the IEX II yield, and the results were compared with the average downstream COGs generated by deterministic approach.
Monte Carlo Simulation Results at Optimized Process Conditions
Similar to the Monte Carlo simulation setup in the previous section, representative triangular distributions indicated in Table 3 were assigned to the six key downstream parameters and titer. The fluctuations in these parameters are typically observed in large-scale manufacturing. Three hundred simulations were performed, and Fig. 6 shows the downstream COGs frequency distributions for [1] base case, in which all parameters were set at current process conditions, optimized protein A loading [2], in which the Protein A loading was operated at target condition, while the other parameters remained constant, and Optimized IEX II Yield [3], in which the IEX II yield was operated at target condition, while the other parameters remained constant. Protein A loading and IEX II yield were chosen for further study because of their strong contribution to the variance in downstream COGs through the PCA analysis.
At a lower titer of 0.5 g/L, the frequency distribution of the downstream COGs is positively skewed at both optimized conditions, although the mean is slightly lower when the IEX II yield is optimized. At 2 g/L, in the base case, the downstream COGs span from $47.5 to $70/g, whereas the cost distribution is much narrower in the optimized cases: with a range of $40–$57.5/g when the Protein A loading is optimized, and $42.5–$60/g when the optimized IEX II yield is optimized. In addition, compared to the base case, increase in IEX II yield reduces the mean downstream COGs by 11%, while a 14% reduction is achieved by increasing the protein A loading. This observation confirms our previous conclusion that protein A loading is more significant than IEX II yield in terms of their impact on downstream COGs. As the titer increases to 7 g/L, the mean downstream COGs is reduced by almost 20% at the optimized protein A loading condition, while 15% reduction is achieved at optimized IEX II yield. Moreover, the “risk,” which is measured by the standard deviation, is lower at the optimized protein A condition as compared to the base case or to the optimized IEX II yield condition.
While the more traditional approach to estimate the average downstream COGs at optimized conditions is often a deterministic estimation, a stochastic approach with Monte Carlo simulation can sometimes offer a more accurate estimation for processes that are inherently random [9]. Figure 7 shows the cumulative probability plots of the downstream COGs for different process conditions, and the black line indicates the average downstream COGs obtained by deterministic approach. At 0.5 g/L, the deterministic estimates are close to the expected mean from the Monte Carlo simulation in all three conditions. However, as titer increases to 2.0 g/L, about 70% of the population is below the deterministic estimate with optimized protein A loading, while 80% of the population is above the with optimized IEX II yield. Hence, predicting the downstream COGs using a deterministic approach can sometimes overestimate or underestimate the mean, consequently distorting the ranking of parameters. The comparison between the two approaches at 7 g/L titer further emphasizes the limitation of the deterministic approach. The deterministic analysis suggests that optimizing IEX II yield is more effective, as indicated by lower expected downstream COGs, contradicting the conclusion from the PCA analysis.
Monte Carlos simulation results suggest that the deterministic approach overestimates the expected downstream COGs with optimized protein A loading and significantly underestimating the expected cost at optimized IEX II yield. These results highlight the drawback of using a deterministic approach in decision making and not accounting for process uncertainties.
Conclusion
This paper provides a systematic framework to identify parameters that have the most impact on downstream costs of goods and ultimately facilitate strategic decision making during process optimization for monoclonal antibody production. A design of experiments approach enables a screening of statistically significant parameters, based on the p values. Further analyzing the DoE data through principal component analysis confirmed the conclusion from the DoE study and, more importantly, offered insights into the relative contribution of each parameter to downstream of goods. Finally, a stochastic simulation was utilized to generate the frequency distribution of the downstream cost of goods by incorporating the process uncertainty. Although there were only six parameters in our study, this was intended to serve as an example of how we can implement the DoE-PCA and Monte Carlo simulation framework to enable decision making during second-generation process development. In other cases, there might be more parameters, and this approach can systematically identify strategies to maximize cost reduction under a compressed process development timeline.
References
Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC. Monoclonal antibody success in the clinic. Nat Biotechnol. 2005;23:1073–8.
Zider A, Drakeman DL. The future of monoclonal antibody technology. MAbs. 2010;2(4):361–4.
Costioli MD, Guillemot-Potelle C, Mitchell-Logean C, Broly H. Cost of goods modeling and quality by design for developing cost-effective processes. BioPharm Int. 2010;23(6):26–35.
Kelley B. Industrialization of mAb production technology the bioprocessing industry at a crossroads. MAbs. 2009;1:443–52.
Sommerfeld S, Strube J. Challenges in biotechnology production—generic processes and process optimization for monoclonal antibodies. Chem Eng Proc. 2005;44:1123–37.
Farid SS. Economic drivers and trade-offs in antibody purification processes. BioPharm Int Suppl. 2009;22:38–42.
Mitchell P. Next-generation monoclonals less profitable than trailblazers? Nature Biotechnol. 2005;23:906.
Suresh P, Hsu S–H, Akkisetty P, Reklaitis GV, Venkatasubramanian V. Onto MODEL: ontological mathematical modeling knowledge Management in Pharmaceutical Product Development. 1: conceptual framework. Ind Eng Chem Res. 2010;49(17):7758–67.
Blau G, Lasinski M, Orcun S, Hsu S-H, Caruthers J, Delgass N, Venkatasubramanian V. High Fidelity mathematical model building with experimental data: a Bayesian approach. Comp Chem Eng. 2008;32:971–89.
Zhao C, Jain A, Hailemariam L, Suresh P, Akkisetti P, Joglekar G, Venkatasubramanian V, Reklaitis GV, Morris K, Basu P. Towards intelligent decision support for pharmaceutical product development. J Pharm Innov. 2006;1(1):25–35.
Hailemariam L, Venkatasubramanian V. Purdue ontology for pharmaceutical engineering: part I. Conceptual framework. J Pharm Innov. 2010;5(3):88–99.
Babanova S, Artyushkova K, Ulyanova Y, Singhal S, Atanassov P. Design of experiments and principal component analysis as approaches for enhancing performance of gas-diffusional air-breathing bilirubin oxidase cathode. J Power Sources. 2013;245:389–97.
Farid SS, Washbrook J, Titchener-Hooker NJ. Decision-support tool for assessing biomanufacturing strategies under uncertainty: stainless steel versus disposable equipment for clinical trial material preparation. Biotechnol Prog. 2005;21:486–97.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Appendix 2
Rights and permissions
About this article
Cite this article
Li, Y.F., Venkatasubramanian, V. Integrating Design of Experiments and Principal Component Analysis to Reduce Downstream Cost of Goods in Monoclonal Antibody Production. J Pharm Innov 11, 352–361 (2016). https://doi.org/10.1007/s12247-016-9263-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-016-9263-8