Abstract
Temperatures are increasing globally and causing species-specific geographic range expansions. In the Gulf of Mexico, mangroves are encroaching regions historically dominated by temperate salt marshes, changing animal communities and nutrient cycling in the intertidal zone. Marine systems are highly connected; therefore, we expect that changes in the intertidal will alter functions of adjacent subtidal seagrass meadows. We surveyed seagrass meadows adjacent to mangroves, salt marshes, and a mixture of the two and asked, do changes in intertidal plant composition influence (1) environmental conditions (subtidal water and sediment characteristics); (2) biogeochemical cycling (net oxygen and nitrogen gas fluxes); (3) seagrass meadow cover, biomass, and productivity; and (4) invertebrate community assemblage? There are clear differences in sediment organic matter and net nitrogen gas (N2) fluxes between adjacent intertidal habitats, but the magnitude or direction of change differs seasonally. We hypothesize that this seasonal pattern is due to outwelling from the intertidal, as mangroves senesce in fall, and marshes senesce later in winter. Therefore, changes in adjacent intertidal habitat can impact the timing of organic matter delivery. This also has implications for seagrass biomass. Thalassia testudinum belowground biomass adjacent to mangroves substantially decreased over the winter, suggesting vulnerability to stressors as the intertidal plant community shifts from marsh to mangrove dominance. Epifauna density and diversity did not vary among seagrass meadows based on adjacent intertidal habitats, but subtle differences in community assemblages associated with shifts in intertidal plant community were detected. This work demonstrates that impacts of species range expansions are far-reaching due to connectivity in marine systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global temperature increases impact ecosystems through both small-scale shifts in reproductive biology and phenology, and larger scale changes in geographic ranges, community composition, and ecosystem functions (McCarty 2002). In marine systems, greater habitat connectivity and increased rates of temperature changes facilitate relatively rapid range shifts (Burrows et al. 2011). Based on the understanding that connectivity is greater in marine habitats relative to terrestrial habitats (Burrows et al. 2011), changes in species distribution in marine systems may result in impacts on a larger spatial scale. For example, tropical grazers moving into temperate seagrass meadows alter seagrass canopy structure (Vergés et al. 2014; Heck et al. 2015). Increased grazing can directly and indirectly cause alterations in highly valuable seagrass functions and services, such as carbon sequestration, nursery habitat, nutrient uptake, and sediment trapping, which likely impact nearby systems (Scott et al. 2018).
A common example of tropical species’ range expansion is the poleward migration of mangroves into salt marshes (Saintilan et al. 2014; Cavanaugh et al. 2019). Although mangroves and salt marshes provide similar functions (e.g., habitat provision, erosion control, and nutrient cycling), their notable phenological and structural differences result in shifts to these highly valuable processes (Kelleway et al. 2017). Salt marsh grasses are herbaceous and die back in the winter, while mangroves have a persistent woody structure. Mangroves tend to have larger aboveground carbon storage, and because their larger structure results in more sediment accretion, sediment carbon storage is elevated (Bianchi et al. 2013; Doughty et al. 2016; Simpson et al. 2017). The fine root structure of salt marsh grasses, compared to mangroves, increases soil water content and decreases soil redox potentials (Perry and Mendelssohn 2009; Comeaux et al. 2012), impacting microbial community structure and rates of nitrogen fixation and denitrification (Barreto et al. 2018; Gao et al. 2019). While these processes result in larger pools of nitrogen in salt marsh soils, mangrove litter tends to be more labile, higher in nitrogen, and decomposes faster, resulting in faster mineralization rates and higher rates of primary production (Lee 1995; Lewis et al. 2014; Simpson et al. 2020).
While both mangroves and marshes share common faunal species and nursery functions, animal communities may differ slightly (Bloomfield and Gillanders 2005). For example, grass shrimp and infauna, such as polychaetes and crustaceans, tend to dominate salt marsh faunal communities, while crabs and fish are more abundant in mangroves (Smee et al. 2017). These invertebrates are prey for commercially important fish species, and therefore could significantly affect higher trophic levels (Glazner et al. 2020). However, there is some redundancy, as recent studies have shown no effects on predator-prey interactions in recently established mangroves compared to salt marshes (Walker et al. 2019); but this may change as mangroves become more mature and established (Scheffel et al. 2018).
Coastal ecosystems are highly interconnected through the coastal ecosystem mosaic (Sheaves 2009). In this mosaic, the intertidal and adjacent subtidal can directly and indirectly influence each other (Huxham et al. 2018). Intertidal plants (e.g., mangroves or salt marshes) can alter water, energy, and nutrient flow into the subtidal by acting either as a source or sink for nutrients (Odum 1968; Valiela and Cole 2002; Taillardat et al. 2019). In India, seagrass meadows adjacent to mangroves had higher density, biomass, and productivity compared to meadows adjacent to shorelines without mangroves (Mishra and Apte 2020). Faunal communities also link the intertidal and subtidal. Salt marsh fish communities depend on adjacent subtidal habitat—the abundance of pinfish in marshes increased with presence of seagrass (Irlandi and Crawford 1997); and richness of nursery species (e.g., Ocyurus chrysurus and Scarus iserti) in seagrasses was greater in meadows adjacent to mangroves (Nagelkerken et al. 2001).
In the northern Gulf of Mexico, seagrasses, an important ecosystem engineer, are impacted directly by tropicalization (e.g., increased direct consumption by a tropical fish, Heck et al. 2015), and may also be indirectly impacted through intertidal plant species changes. Because seagrasses are highly connected to adjacent intertidal systems, alterations in habitat function and biogeochemical pools and cycles associated with tropical mangrove expansion into salt marshes will likely spill over into seagrass meadows. Changes in intertidal nutrient and organic matter processing associated with mangrove expansion may alter rates of mineralization and assimilation in adjacent seagrasses though intertidal sequestration or outwelling of terrestrial-derived material (Fulweiler et al. 2013), possibly resulting in changes to plant productivity, radial oxygen loss, and sediment nitrification rates (Eyre et al. 2013a, b). Substantial changes in nutrient loading may also indirectly impact faunal communities. Nutrient loads can impact seagrass productivity, density, and species assemblage, all of which can change the habitat function (Terrados et al. 1999; Pérez et al. 2007; Ruiz-Halpern et al. 2008; Han et al. 2017). Furthermore, high nutrient loads are associated with high epiphyte cover, which is an important food source for many small invertebrates (Gil et al. 2006). Additionally, while seagrasses support a higher density and diversity of fishes and invertebrates than mangroves and salt marshes (approximately 5 and 108× higher densities, respectively; Bloomfield and Gillanders 2005), many of those fish species use both intertidal and subtidal habitats depending on the tide. Thus, changes in intertidal fish communities could alter faunal density or community composition of seagrass meadows. Large faunal community differences could alter grazing rates, with the potential to affect seagrass species composition, as Syringodium filforme and Halodule wrightii are more palatable than T. testudinum (Armitage and Fourqurean 2006; Prado and Heck 2011).
In this study, we investigate differences in seagrass meadows adjacent to salt marshes, mangroves, and a mixture of the two. Previous studies exploring implications associated with the formation of the mangrove-salt marsh ecotone focus on the intertidal zone (Stevens et al. 2006; Perry and Mendelssohn 2009; Cavanaugh et al. 2013; Yando et al. 2016; Simpson et al. 2017). However, to our knowledge, the impact of these changes on adjacent subtidal seagrass meadows remains unclear. We use seasonal surveys and sediment flux incubations to document differences in seagrass meadow form and function as related to adjacent intertidal plant community and hypothesize that (1) environmental conditions; (2) biogeochemical cycling; (3) cover, biomass, and productivity; and (4) invertebrate community assemblage will vary in seagrass meadows adjacent to mangroves, salt marshes, and a mixture of the two.
Methods
Site Description
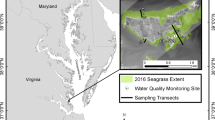
The study sites are located near Cedar Key, Florida, USA (25° 05′ 49″ N, 83° 03′ 58″ W); an estuarine system, in the northern Gulf of Mexico. The system is dominated by mixed siliciclastic-carbonate sediments (Wright et al. 2005) (Fig. 1), and experiences seasonal changes from warm summers (24 to 30 °C) to cooler winters (12 to 22 °C). Cedar Key is representative of the larger region, with high abundances of salt marsh and seagrass habitats, and expanding mangrove distributions. In the 1980s, freezes (i.e., air temperatures below − 6.3 and − 7.6 °C, Osland et al. 2017) caused mangroves to regularly die-off, allowing the historically dominant intertidal marsh species, Spartina alterniflora, to dominate the area. However, the last few decades of mild winters have allowed mangrove survival and expansion—predominantly Avicennia germinans, and Rhizophora mangle to a lesser extent (Stevens et al. 2006; Purtlebaugh et al. 2020). Seagrass meadows are common in the shallow subtidal. T. testudinum is the most common seagrass species; however, S. filiforme and H. wrightii are also present. The intertidal plant communities experience frequent flooding in this region (Sweet et al. 2018), as the tidal amplitude ranges about 0.86 m (NOAA 2019).
We chose 3 replicate sites in the subtidal T. testudinum dominated seagrass beds adjacent to mangroves, salt marshes, or an ecotone—a mixture of the two plant types (referred to as intertidal habitats). Sites were placed within continuous seagrass at least 5 m from the edge. At these sites, mudflat often occurs between the intertidal and the seagrass meadow, and sites had varying distances from the shoreline. To ensure that hydrology did not co-vary with intertidal habitat, we measured distance from shore, water depth, and exposure direction (N, S, E, W), and used an ANOVA to show that these metrics did not vary systematically. We also quantified plant species composition in each intertidal habitat by taking oblique photos at each site approximately 5 m away from the shoreline, and estimated percent cover (%) (n = 3) with quadrats randomly drawn on the image.
Environmental Conditions
From 2018 to 2019, we collected water chemistry and sediment samples in the adjacent subtidal seagrass meadow of each site a total of 5 times (once in each season, with summer sampled twice). For each sampling event, at each site, 3 replicate samples were collected at each site for a total of 27 samples. We measured water depth (m), dissolved oxygen (DO) (mg L−1 and % saturation), salinity (ppt), and temperature (°C) using a YSI Pro DSS data logger and attached probes. On each sampling date, we collected sediment cores—1 cm diameter by 5 cm depth (n = 3). Sediments were homogenized and analyzed for organic matter using the loss on ignition method (Heiri et al. 2001). Water and sediment nutrient content were measured 2 times over the sampling year in the summer and spring (to represent the end and beginning of the T. testudinum growing season). We collected 50-mL water samples (n = 3) to analyze NOx (nitrate + nitrite) using the automated cadmium reduction method via Bran + Luebbe Auto Analyzer 3 (MDL = 0.010 mg L−1). Sediment samples (bulk, not acidified) were analyzed for carbon (TC) and nitrogen (TN) content using a CE-Elantech Flash EA 1112 Nitrogen and Carbon Analyzer. Separate homogenized cores (1 cm diameter by 5 cm depth) were used to calculate bulk density.
Biogeochemical Cycling
On 2 October 2018 (summer, water temperature 29.6 °C (0.35 SE)) and 5 April 2019 (spring, water temperature 20.6 °C (0.09 SE)), we used sediment core incubations to quantify net oxygen (O2) and nitrogen (N2) fluxes across the sediment-water interface (Fig. 2). We extracted one core (9.5 cm diameter by 10 cm depth) from each site (n = 3 for each adjacent intertidal habitat). The sampling dates were chosen to represent the end and beginning of T. testudinum growing season. All cores contained intact T. testudinum above and belowground biomass.
We set up two water baths with five cores in each, containing 95 L of aerated water. Incubations were conducted indoors in dark conditions underneath fluorescent lights. Rotating magnets were placed inside each core, and cores were acclimated for 45 min before they were sealed with a gas tight lid fitted with two ports. One port acted as an outlet and one was connected to the replacement water line. Using a syringe, we removed 15 mL of overlying water from each core at 30-min intervals over 2 h in October, and 45-min intervals over 4.5 h in April. In October, while we measured linear change, the decrease in DO was less than the 2 mg L−1, so we increased the incubation time in April to ensure this recommendation was met (Fulweiler and Nixon 2012). Samples were preserved with 100 μL of saturated zinc chloride and stored in submerged exetainers below ambient temperature until N2, O2, and Argon (Ar) analysis.
We analyzed O2:Ar and N2:Ar ratios on water samples using a membrane inlet mass spectrometer (MIMS). Coefficients of variation for O2:Ar and N2:Ar were 0.10% and 0.06%, respectively. Fluxes were calculated as \( \frac{\Delta \mathrm{C}}{\Delta \mathrm{t}} \) * \( \frac{V}{A} \), where \( \frac{\Delta \mathrm{C}}{\Delta \mathrm{t}} \) is the slope of the concentration change in the overlying water over time, V is the volume of the overlying water, and A is the core surface area (flux units = μmol m−2 h−1). We only used data from samples where DO concentration was greater than 2 mg L−1 (above anoxic conditions). Fluxes were calculated only if the change in time occurs in a linear manner (R2 > 0.65) (Fulweiler et al. 2010).
After the incubation, live plant biomass, invertebrates, and sediment were removed and taken back to the laboratory for further analysis. Total seagrass biomass (g) was dried to a constant weight (60 °C for at least 48 h), and then weighed. We separated and counted all invertebrates in each core (in April only) as potential covariates. A subsample of sediment was also collected from each core and stored frozen at − 20 °C for organic matter content analysis as described above.
Seagrass Cover, Biomass, and Productivity
To observe potential intertidally driven changes in seagrass structure and function, we conducted seagrass surveys at each established sampling location seasonally, with August and September 2018 representing the summer, November 2018 for the fall, January 2019 for the winter, and April 2019 for the spring. The additional September summer survey was conducted to determine variability within a season. If September was not different from August, then data were considered one sampling event. Data were combined for all parameters except sediment organic matter and infauna. For our sampling events, we sampled on a rising tide and sites were sampled in an order that was not biased towards one adjacent intertidal habitat type.
At each site, we estimated percent cover and species composition in haphazardly placed 1-m2 quadrats (n = 3). We used 15 cm diameter by 15 cm depth cores (n = 3) to estimate plant biomass. In the laboratory, live above and belowground tissue for each species present was separated from each core. Tissue was dried to a constant weight (60 °C for at least 48 h), weighed, and ground to analyze TC and TN concentrations using a CE-Elantech Flash EA 1112 Nitrogen and Carbon Analyzer. Biomass per core parameters was converted in units per area (g m−2 using the core dimensions).
To quantify leaf growth, on 21 September 2018 and 17 April 2019 (representing the end and beginning of the growing season), every shoot present in a haphazardly selected 10 cm by 20 cm quadrat was marked by punching a hole 1 cm above the bundle sheath with a syringe needle, and collected approximately 1 week after punching to measure growth (Zieman and Wetzel 1980). For each leaf, of each shoot, new and old growth were separated, dried to constant weight (60 °C for at least 48 h), and weighed (g). Productivity was calculated as new growth (g m−2 d−1) and nitrogen assimilation (new growth * TN (%)) (gN m−2 d−1).
Invertebrate Community Assemblage
Seasonally, we quantified epifauna and infauna density, diversity, and community structure. For epifauna, we placed a 0.5-mm mesh bag over the seagrass and harvested all aboveground material into the bag (n = 3) (Reynolds et al. 2014). Infauna was separated from the biomass cores described above (n = 3). Organisms were separated from harvested material, preserved in scintillation vials with ethanol, and identified to species level. Total individual density (i.e., independent of species) and species richness were calculated in units per area (m−2). We also calculate species diversity as Shannon-Wiener’s Index (Shannon 1948)—H = −Σpilnpi, where pi is the proportion of ith species.
Statistical Analysis
Statistical analyses were performed using JMP Pro 14.1.0. To determine differences between seasons, a split plot repeated measures analysis was used (α = 0.05) for all response variables. Data were square root transformed when necessary to meet assumptions (split plot comparisons). Significant tests were followed with Tukey’s pairwise multiple comparison to further determine differences. For datasets with multiple zeros, the interaction between season and adjacent intertidal habitat was removed (α = 0.01), to better meet the assumptions of the model (only for S. filiforme biomass parameters). To determine differences within seasons between adjacent intertidal habitats, the non-parametric Wilcoxon test was used. Multiple comparisons were made using the non-parametric Steel-Dwass (α = 0.05) to determine differences between adjacent intertidal habitats and within seasons for each of the variables measured. We used non-parametric tests as the data was not normally distributed due to the small sample size in each period. In addition, Spearman rank correlations were used to look for correlations between measured parameters. To quantify community assemblages, compositions (Bray-Curtis similarity, square root transformed) were analyzed using a permutational multivariate analysis of variance (PERMANOVA) (α = 0.05). Furthermore, to determine contribution of species to Bray-Curtis index, in respect to adjacent intertidal habitat, a similarity percentage analysis (SIMPER) was performed. Community analyses were conducted in Primer-7 (6.1.5) software (Anderson and Walsh 2013; Clarke and Gorley 2015).
Results
Environmental Conditions
Hydrology is an important factor to consider in this region; however, in our study, water depth (F(2,7) = 0.088, P = 0.92) and distance to shoreline (X2 = 0.27, P = 0.88) did not differ between sites (Supplementary Table 1). Direction of exposure also did not vary by adjacent intertidal habitat (Fig. 1). Additionally, sites did not differ in environmental characteristics with adjacent intertidal habitat—temperature (F(2,33) = 0.52, P = 0.60), DO (mg L−1: F(2,32) = 0.0098, P = 0.99; %: F(2,33) = 0.040, P = 0.96), salinity (F(2,7) = 0.58, P = 0.58), and water column NOx (F(2,6) = 0.83, P = 0.48). As expected, temperature (F(3,33) = 327, P < 0.0001) and DO (F(3,32) = 11, P < 0.0001) varied with seasons (Table 1). The highest water temperature was in the summer (30.0 ± 0.33 °C), intermediate in the spring (22.9 ± 0.32 °C), and lowest in the fall (15.9 ± 0.48 °C) and the winter (15.3 ± 0.18 °C). DO was the highest in the fall (10.8 ± 0.59 mg L−1), intermediate in winter (8.04 ± 0.07 mg L−1) and spring (8.36 ± 0.45 mg L−1), and lowest in the summer (6.72 ± 0.46 mg L−1).
Sediment organic matter (SOM) varied seasonally (F(4,24) = 7.7, P = 0.0004) and by adjacent intertidal treatment (X2 = 7.2, P = 0.027), ranging from 2.67 to 5.48%. SOM was the lowest in August (3.11 ± 0.23%) and increased over the winter with the highest values in the spring (4.50 ± 0.29%) (Fig. 3). There was an interaction between sampling event and adjacent intertidal habitat (F(8,24) = 2.4, P = 0.048). In the spring, meadows adjacent to the ecotone had the highest SOM content (5.48 ± 0.50%) and meadows adjacent to mangroves had the lowest (3.38 ± 0.47%) (X2 = 7.2, P = 0.027); while in the winter, there were no differences (X2 = 0.60, P = 0.74). Sediment C:N did not differ seasonally (F(1,6) = 1.3, P = 0.30); however, C:N did vary with adjacent intertidal habitat. In the summer, seagrass sediment C:N adjacent to the ecotone (15.7 ± 0.38) was higher compared to adjacent to mangroves (14.0 ± 0.40) and salt marshes (14.1 ± 0.51) (X2 = 6.4, P = 0.042), while there were no differences between adjacent intertidal habitats in the spring (X2 = 3.3; P = 0.19) (Fig. 4a). Bulk sediment TC and TN were tightly, positively correlated with sediment organic matter (n = 54; ρ = 0.82; P < 0.0001 and n = 54; ρ = 0.78; P < 0.0001, respectively) (Supplementary Fig. 1).
Biogeochemical Cycling
As expected, temperature varied between summer and spring incubations. Summer had higher water temperatures (X2 = 14, P = 0.0002) (summer = 29.6 ± 0.35 and spring = 20.6 ± 0.09 °C) and higher DO (X2 = 3.9, P = 0.049) (summer = 131 ± 10.2 and spring = 98 ± 1.12%) (Supplementary Table 2).
Net O2 fluxes (μmol m−2 h−1) varied by season, but not by adjacent intertidal habitat (F(2,12) = 0.29, P = 0.76) (Table 2 and Supplementary Table 3). All cores yielded negative net fluxes, indicating an oxygen demand by the sediments (e.g., decomposition of organic matter); but demand was greater in the summer (− 5066 ± 313 μmol m−2 h−1) than the spring (− 2217 ± 244 μmol m−2 h−1) (F(1,12) = 23, P = 0.0004).
Net N2 fluxes (μmol m−2 h−1) varied by season (F(1,12) = 10, P = 0.0077) (Fig. 5), but not by adjacent intertidal habitat (F(2,12) = 2.1, P = 0.16) (Supplementary Table 3). In the summer, on average across all adjacent intertidal habitats, the net N2 flux was negative (indicating net fixation in excess of denitrification) (− 184 ± 101 μmol m−2 h−1), while in the spring, the mean net flux across all adjacent intertidal habitats was positive (indicating denitrification in excess of nitrogen fixation) (123 ± 28.8 μmol m−2 h−1) (Table 2); however, meadows adjacent to mangroves were always net denitrifying (summer = 170 ± 33.3 and spring = 57.8 ± 28.0 μmol m−2 h−1). Net N2 fluxes interactively varied as a function of both season and adjacent intertidal habitat (F(1,12) = 6.4, P = 0.013) (Fig. 5). Seagrasses adjacent to marshes and the ecotone changed from net fixing to net denitrifying, with a net difference of 857 (F(1,10) = 15, P = 0.003) and 177 μmol m−2 h−1 (F(1,10) = 17, P = 0.002) respectively, while seagrass sediments adjacent to mangroves were net denitrifying in both seasons and decreased by only 112 μmol m−2 h−1 (F(1,10) = 6.7, P = 0.027).
As expected, total biomass (g) in the cores was higher in the summer (3.04 ± 0.25 g) than the spring (2.01 ± 0.24 g) (F(1,6) = 9.5, P = 0.022) (Table 2), but biomass per core did not differ between adjacent intertidal habitats (F(2,6) = 0.59, P = 0.58). Over both seasons, there was a negative relationship between total biomass and net O2 flux (n = 18; ρ = − 0.61; P = 0.0077) (Supplementary Fig. 2); however, there was no relationship between total biomass and net N2 flux (n = 18; ρ = − 0.0093; P = 0.97). Invertebrate total abundance did not differ between adjacent intertidal habitats (X2 = 2.8, P = 0.25), and there was a negative correlation between individual abundance and net O2 flux (only in the spring) (n = 9; ρ = − 0.73; P = 0.025) (Supplementary Fig. 3). There was no relationship between invertebrate density and net N2 flux (n = 9; ρ = 0.45; P = 0.22).
Seagrass Cover, Biomass, and Productivity
Seagrass cover and biomass varied seasonally (Fig. 6). Percent cover (F(3,27) = 35, P < 0.0001) and aboveground biomass (F(3,27) = 52, P < 0.0001) were the highest in the summer (66.8 ± 3.24% and 15.5 ± 1.07 g m−2, respectively), intermediate in the fall (31.5 ± 2.61% and 6.56 ± 0.40 g m−2, respectively) and spring (28.9 ± 3.15% and 8.68 ± 0.60 g m−2, respectively), and lowest in the winter (16.3 ± 2.40% and 3.85 ± 0.36 g m−2, respectively). However, belowground biomass (g m−2) did not vary by season (F(3,27) = 0.59, P = 0.63).
Thalassia testudinum biomass parameters throughout the year. a Percent cover (%), b aboveground biomass (g m−2), and c belowground biomass (g m−2), for their respective adjacent intertidal habitat, with error bars representing standard error. Asterisk indicates differences between intertidal habitat type within seasons (fall and winter) (α < 0.05)
Seagrass cover and biomass also varied by adjacent intertidal habitat. T. testudinum percent cover was the highest adjacent to the ecotone (53 ± 3.85% averaged across seasons), intermediate adjacent to salt marshes (37.9 ± 3.47% averaged across seasons), and lowest adjacent to mangroves (35 ± 4.66% averaged across seasons) (F(2,7) = 7.0, P = 0.023) (Fig. 6a). Aboveground and belowground biomass varied with adjacent intertidal habitat, but only in some seasons. In the fall and winter, aboveground biomass was highest adjacent to salt marshes (7.80 ± 0.65 and 4.94 ± 0.65 g m−2) (X2 = 7.8, P = 0.021 and X2 = 6.7, P = 0.034, respectively), while belowground biomass was highest adjacent to the ecotone (41.7 ± 3.89 and 41.1 ± 4.86 g m−2) (X2 = 9.6, P = 0.0084 and X2 = 7.2, P = 0.027, respectively). Biomass was always the lowest adjacent to mangroves (aboveground = 4.90 and 2.59 g m−2 (Fig. 6b), belowground = 23.1 and 21.6 g m−2 (Fig. 6c)). Seagrass biomass also correlated with sediment characteristics, as belowground biomass was positively correlated with sediment C:N (n = 54; ρ = 0.39; P = 0.0033) (Supplementary Fig. 4); however, aboveground biomass was not (n = 54; ρ = 0.060; P = 0.67).
Thalassia testudinum primary productivity, estimated as new growth (g m−2 d−1) (F(1,6) = 13, P = 0.011) varied seasonally, and was higher in the summer (0.17 ± 0.02 g m−2 d−1) than in the spring (0.09 ± 0.01 g m−2 d−1) (Fig. 7). Nitrogen assimilation (gTN m−2 d−1) was marginally different (summer = 0.004 ± 0.0004 and spring = 0.003 ± 0.0002 gTN m−2 d−1) (F(1,6) = 5.4, P = 0.061). Both new growth (F(2,6) = 0.058, P = 0.94) and nitrogen assimilation (F(2,6) = 0.042, P = 0.96) did not differ by adjacent intertidal habitat.
Syringdoium filiforme, another common seagrass species found in the northern Gulf of Mexico, was also identified in biomass cores. Relative contribution was variable and always lower than T. testudinum. Mean contribution was 9.2%, and maximum contribution was 47.7% between sites. Results show no patterns with season, adjacent intertidal habitat, or interactions (Supplementary Table 4).
Thalassia testudinum aboveground C:N ratio was greater in the summer (16.7 ± 0.32 g m−2) than the spring (13.8 ± 0.15 g m−2) (F(1,6) = 39, P = 0.0008) (Fig. 4b), but did not differ between adjacent intertidal habitats (F(2,6) = 0.060, P = 0.94). Additionally, T. testudinum aboveground tissue C:N was negatively correlated with SOM (%) and with sediment C:N (n = 54; ρ = − 0.40; P = 0.0025 and n = 54; ρ = − 0.32; P = 0.021, respectively) (Supplementary Fig. 5 and 6).
Invertebrate Community Assemblage
Epifauna density, species richness, and species diversity varied seasonally. Total individual density (m−2) (F(3,27) = 13, P < 0.0001), richness (m−2) (F(3,27) = 25, P < 0.0001), and diversity (H′) (F(3,27) = 3.0, P = 0.049) were the greatest in the spring (493 ± 104 m−2, 126 ± 12.2 m−2, and 2.03 ± 0.07), intermediate in the winter (360 ± 57.5 m−2, 99.4 ± 12.1 m−2, and 1.68 ± 0.08) and fall (265 ± 61.2 m−2, 80.3 ± 11.1 m−2, and 1.70 ± 0.08), and lowest in the summer (85.2 ± 13.3 m−2, 30.1 ± 3.35 m−2, and 1.66 ± 0.07) (Table 3). PERMANOVA analysis indicated that epifauna community assemblage differed by season (F(3,123) = 10, P = 0.001). SIMPER analysis showed that Mitrella lunata, Ampithoe longimana, and Palaeomonetes pugio were the primary drivers in each sample, but the relative contribution changed with season (Supplementary Table 5). The largest average dissimilarity was between the summer and winter (76.6%) (P = 0.001), and the smallest between the winter and spring (63.7%) (P = 0.001).
Adjacent intertidal habitat only explained variation in epifauna density in the summer. Meadows adjacent to the ecotone had the greatest total individual density (117 ± 25.5 m−2), adjacent to mangroves were intermediate (97.8 ± 27.8 m−2), and adjacent to salt marshes were lowest (41.2 ± 6.68 m−2) (X2 = 7.5, P = 0.023) (Supplementary Table 6). PERMANOVA analysis indicated that epifauna community assemblage differed by adjacent intertidal habitat (F(3,123) = 2.4, P = 0.001), and there was no interaction between season and adjacent intertidal habitat (F(6,123) = 1.1, P = 0.20). SIMPER analysis also showed that epifauna community composition varied by adjacent intertidal habitat driven by A. longimana, M. lunata, Clibanarius vittatus, P. pugio, and Nassarius vibex, but relative contribution changed (Table 4). The smallest dissimilarity in epifauna assemblage was between meadows adjacent to salt marshes and mangroves (73.5%) (P = 0.07), while dissimilarities were greater between the ecotone with marshes (P = 0.001) and mangroves (P = 0.01).
Infauna total individual density (m−2) (F(4,24) = 51, P < 0.0001), species richness (m−2) (F(4,24) = 37, P < 0.0001), and species diversity (H′) (F(4,24) = 15, P < 0.0001) were the greatest in the spring (296 ± 23.6 m−2, 105 ± 5.49 m−2, and 1.94 ± 0.06), intermediate in the winter (133 ± 6.49 m−2, 80.0 ± 4.10 m−2, and 1.96 ± 0.06), and lowest in the fall (89.8 ± 8.49 m−2, 56.3 ± 4.11 m−2, and 1.61 ± 0.09) and summer months (August = 55.2 ± 6.31 m−2, 40.2 ± 3.58 m−2, and 1.25 ± 0.11, and September = 79.7 ± 5.64 m−2, 52.4 ± 4.01 m−2, and 1.54 ± 0.11) (Table 3). PERMANOVA analysis indicated that there were differences between sampling periods (F(4,120) = 8.4, P = 0.001). SIMPER analysis shows that infauna community composition varied between the months sampled, except for September and fall, in which differences were driven by the primary contributors N. vibex, Ampelisca abdita, and Microphiopholis atra, but relative contribution changed with season (Supplementary Table 7), with the largest dissimilarity being between August (summer) and the spring (87.9%) (P = 0.001), and the smallest being between September (summer) and the fall (67.8%) (P = 0.26).
Infauna differed by adjacent intertidal habitat only in the spring, where species diversity (H′) was the greatest in meadows adjacent to salt marshes (2.13 ± 0.08), intermediate to mangroves (1.94 ± 0.10), and lowest to the ecotone (1.76 ± 0.10) (X2 = 6.7, P = 0.034) (Supplementary Table 8). PERMANOVA and SIMPER analysis showed no differences in infauna community composition among meadows driven by adjacent intertidal habitats or an interaction (F(2,120) = 1.5, P = 0.06 and F(8,120) = 1.0, P = 0.37, respectively).
Discussion
Seagrass meadow environment (SOM); biogeochemical cycling (N2 and O2 fluxes); plant cover, standing biomass, and productivity; and invertebrate community (density, diversity, and assemblage) differed with adjacent intertidal habitat (mangroves, salt marshes, or ecotone). Importantly, the magnitude and direction of those differences varied seasonally. Other studies have linked seagrass form and function to impacts from adjacent intertidal habitat (Nagelkerken et al. 2001; Valiela and Cole 2002; Mishra and Apte 2020), and it is logical that a shift in intertidal function with mangrove encroachment could drive these changes. Although we did not directly measure it, the seasonality suggests that outwelling differences between mangroves and salt marshes may have driven the changes we observed in seagrass meadows. In the northern Gulf of Mexico, the timing of peak outwelling is expected to differ—mangroves senesce in September (Twilley et al. 1986; Dawes et al. 1999; Aké-Castillo et al. 2006), while salt marsh grass senesce in January (Kirby and Gosselink 1976; Lana et al. 1991; Pezeshki and DeLaune 1991; Darby and Turner 2008). That timing of organic matter delivery would explain our observed differences in SOM and gas fluxes. Meadows adjacent to mangroves and the ecotone had higher net denitrification (positive net N2 flux) rates, in the summer and spring, respectively, when organic matter delivery was the highest. Seagrass biomass also changed with intertidal plant community, as meadows adjacent to mangroves experienced a dramatic decrease in belowground biomass in the winter, while adjacent to marshes and the ecotone stayed consistent. Additionally, although epifauna density and diversity did not vary among adjacent intertidal habitats, meadows experienced subtle differences in community assemblage that may be correlated with adjacent intertidal habitat. Together these results suggest that shifts in intertidal species assemblage can scale-up and impact key ecosystem functions and services in seagrass meadows.
Environmental Conditions
Water chemistry within seagrass meadows did not vary by adjacent intertidal habitat; however, we observed a strong difference in SOM, which is a potential driver of our observed differences in net N2 and O2 fluxes. Mangroves senesce around September, and we observed an overall increase in SOM in seagrass meadows adjacent to mangroves from September to January, while meadows adjacent to the other two intertidal habitats showed a decrease in SOM (Fig. 3). Conversely, sediments in meadows adjacent to marshes and the ecotone increased in SOM from January to April, after salt marsh senesce. Concomitantly, we observed a decrease in meadows adjacent to mangroves. These results coincide with literature, from the Gulf of Mexico, documenting variability in porewater nutrient concentrations between marshes, mangroves, and the ecotone (Patterson and Mendelssohn 1991). The marshes and ecotone porewater nutrient concentrations peaked in early spring and declined late in the summer, while porewater nutrients in mangroves peaked in late summer and declined in early spring (Patterson and Mendelssohn 1991).
Other environmental factors, for example, hydrodynamic characteristics (e.g., tidal fluctuations and wave exposure), not measured as part of this study, may also explain our observed results. High wave action can impact organic matter delivery, nutrient concentrations and fluxes, the depth of the aerobic-anaerobic interface, and light availability (Koch and Gust 1999). More specifically, high energy can result in mixing, altering water column and sediment dissolved oxygen levels. These influences to organic matter and dissolved oxygen levels can scale-up to affect denitrification rates, biomass, plant growth, and faunal community assemblages, which result in consequences for altering key ecosystem functions observed in this study. However, our sites did not systematically vary in direction of exposure (a proxy for wave exposure) or water depth.
Nitrogen Cycling
In estuarine systems, habitat connectivity has been shown to be an influential driver in denitrification rates through organic matter subsidies (Smyth et al. 2015). Subsidies, in the form of senesced biomass, can stimulate denitrification by providing a source of nitrogen and labile carbon, in the form of organic matter, to denitrifying microbes in sediment (McMillan et al. 2010; Smyth et al. 2015). Likewise, the timing of senescence and subsequent outwelling of nutrients and organic matter can be a controlling factor in net N2 fluxes (Fulweiler et al. 2013).
Net N2 fluxes in the seagrass meadows studied here varied between summer and spring, and outwelling from the adjacent intertidal habitat may explain these seasonal differences. In October, just a month after expected mangrove senescence (Twilley et al. 1986; Dawes et al. 1999; Aké-Castillo et al. 2006), positive net N2 flux and increases in oxygen demand were observed adjacent to mangroves (Supplementary Table 3). In April, a few months after Spartina senescence (Kirby and Gosselink 1976; Lana et al. 1991; Pezeshki and DeLaune 1991; Darby and Turner 2008), positive net N2 flux and oxygen demands were greatest in meadows adjacent to the ecotone (Supplementary Table 3). The increase in oxygen demand suggests active organic matter mineralization and increased organic quality (Piehler and Smyth 2011; Eyre et al. 2013a, likely supported by senesced biomass from the adjacent intertidal habitat.
Our seagrass sediment data show differences in organic matter quantity and quality between adjacent intertidal habitats. Meadows adjacent to marshes and ecotones had higher SOM quantity, overall, but had lower quality (evidenced by higher sediment C:N), while meadows adjacent to mangroves had lower SOM quantity, but higher quality (evidenced by lower sediment C:N) (Fig. 8). The quality (C:N ratio, mixture of C:N ratios, and structure of the organic matter) can be more important than quantity in driving denitrification rates, and higher organic matter quality is typically observed in mangroves compared to marshes (Lee 1995; Eyre et al. 2013a; Lewis et al. 2014; Simpson et al. 2020).
Differences in organic matter delivery, both in terms of quantity (higher SOM) and quality (lower sediment C:N), may be greater than differences observed in this study and are likely to become more dramatic over time. The standing stock of SOM does not accurately represent the recent delivery, since organic matter in these systems can come from a variety of internal and external sources and may have long residence times. We integrated the top 5 cm in our samples, which may include contributions from several years ago, and mangrove encroachment into this region is relatively both recent and incomplete—mangroves have historically been small and patchy along the landscape. In future studies, measuring sediment properties in smaller vertical segments may increase understanding. Furthermore, as mangroves get larger and older, they become more different compared to salt marsh grasses, in terms of biomass and structure (C:N and % woody) (Osland et al. 2012; Kelleway et al. 2016), and therefore seasonal differences in organic matter delivery are likely to increase in magnitude. Applying a chronosequence approach would fill the knowledge gap of long-term impacts of mangrove encroachment on seagrass meadows. While we have anecdotal evidence that over the last few decades in Cedar Key mangroves have expanded and retracted multiple times, there are not enough frequent aerial images to efficiently study this in this region.
In the spring, net N2 fluxes adjacent to marshes were somewhat unexpected as they did not differ from zero, while seagrass sediments adjacent to ecotones had a positive net flux (Fig. 5). Our results show that meadows adjacent to marshes and the ecotone have the same seasonal fluctuations in SOM; however, we did not observe these patterns in gas fluxes. Marsh grass species can make a difference in retention and nutrient quality as low marsh species tend to decompose faster and are of higher organic matter quality than high marsh species, resulting in differences in leaching of soluble materials related to tidal elevation (Valiela et al. 1985). In the ecotone, mangrove species are more likely to colonize in the upper intertidal zone and outcompete high marsh species for space and nutrients (Alleman and Hester 2011; Peterson and Bell 2018). We measured fringing marsh species composition and biomass but not upper marsh species. Variation in high marsh species may also influence the quality and quantity of outwelled organic matter.
Although net N2 fluxes differed between summer and spring (Table 2), we observed the largest variation in meadows adjacent to salt marshes and the ecotone. Meadows adjacent to marshes and the ecotone shifted from net fixing to net denitrifying, from summer to spring, while meadows adjacent to mangroves were always net denitrifying (Fig. 5). Because these systems tend to be connected, a shift in meadows adjacent to marshes and the ecotone may be explained by intertidal outwelling, where marsh biomass senescence in the winter provided labile carbon to meadows, and increased denitrification rates. On the other hand, mangroves senesce in September, which explains net denitrification in the summer, but not in the spring. In the fall and winter, belowground biomass adjacent to mangroves experienced a dramatic decrease (Fig. 6c), while adjacent to marshes and the ecotone stayed consistent, which could explain why net fluxes adjacent to mangroves did not change. There was another nutrient source—internal (decomposing seagrass belowground biomass) rather than external. It is also possible these increased denitrification rates could have been driven by nitrate (Smyth et al. 2015). Although we did not measure it directly or extensively, organic matter availability is mineralized into ammonium, which will then undergo nitrification and increase nitrate concentrations. Through tight nitrification and denitrification coupling, N2 removal may be maximized (Piehler and Smyth 2011). Furthermore, this study only focused on the beginning and end of the growing season, and therefore it is possible that mangroves are net N2 fixing in another season not sampled here.
Seagrass Cover, Biomass, and Productivity
Seagrass cover and biomass varied by adjacent intertidal habitat and, like SOM and gas fluxes, the magnitude of the difference varied with season. Seagrass belowground biomass in meadows adjacent to mangroves was reduced in colder months, while roots and rhizome biomass was consistent over seasons adjacent to marshes and the ecotone. In these colder months, T. testudinum nitrogen uptake is dominated by the roots and rhizomes, while in the summer and fall, uptake is greater in the leaves (Lee and Dunton 1999). Furthermore, in the early growing season (when salt marsh grasses senesce, and mangroves do not), plants require high nutrient levels and use their belowground storage, which has been depleted over the winter (Lee and Dunton 1999). This may explain the decline in belowground biomass adjacent to mangroves in the winter since there is a reduction in outwelling of material to the seagrass meadow, while in the late summer and early fall, plants are under less nutrient stress as growth rates are declining; therefore, organic matter delivery from mangrove senescence has less impact on plant growth.
The influence of adjacent intertidal habitat on seagrass biomass was driven by differential shifts in belowground biomass. As expected, aboveground biomass was generally more responsive to seasonal change, while belowground biomass was generally more stable (Kaldy and Dunton 2000), except when seagrass meadows were adjacent to mangroves. The belowground biomass of seagrass meadows adjacent to mangroves decreased by almost half (42.9%) from summer to winter, while an order of magnitude less adjacent to the ecotone (1.4%), and marshes increased by less than half (20.2%) (Fig. 6c). Belowground material contains carbohydrate reserves and loss of these reserves may make seagrasses more vulnerable to stressors in general. Tropical systems with large, mature mangroves have a positive influence on seagrass density, biomass, and productivity through connectivity (Mishra and Apte 2020); however, our system is historically more temperate and mangroves here are patchy both spatially and temporally. The variability caused by colder temperatures, and possible winter freezes, hinders mangrove maturity, and therefore may drive instability in the system.
In this oligotrophic environment, organic matter and nutrient delivery of adjacent intertidal habitats to seagrasses potentially drives seagrass nutrient assimilation and primary productivity. Aboveground biomass C:N and production rates were greater in the summer than the spring, however did not vary among adjacent intertidal habitats (Fig. 4b and 7). Temperatures were much greater in the summer, compared to the spring (Table 1), and therefore seagrasses are assimilating nitrogen at greater rates to sustain higher rates of productivity (Fig. 7). Correlations between SOM, nutrients, and biomass indicate that, with increased organic matter, there will be higher mineralization and nitrification rates, which results in higher assimilation rates to sustain high growth rates (Supplementary Fig. 4–6). In this system, we expect seagrass meadows to respond to nutrient shifts that are potentially influenced by mangrove encroachment.
Invertebrate Community Assemblage
Our results suggest that, although seagrass epifauna community assemblage responded to adjacent intertidal habitat type, seasonality may be a more influential driver of faunal density and diversity. Both epifaunal and infaunal densities, diversities, and assemblage responded to seasonal changes, with the highest densities and diversities observed in the spring (Table 3). These findings are consistent with previous work in Gulf of Mexico H. wrightii beds, with invertebrate densities increased from the fall to the spring, peaking in April, and decreased gradually throughout the summer (Sheridan and Livingston 1983). Here, only epifaunal community assemblage differed by adjacent intertidal habitat.
Our results do show small but distinct differences in epifaunal species assemblage potentially related to indirect effects of adjacent intertidal habitat (Table 4). Previous work in intertidal communities show similar patterns, in which epifaunal community assemblages were driven by intertidal habitat (i.e., mangroves vs. marshes) (Smee et al. 2017; Scheffel et al. 2018). Although literature indicates that intertidal infauna vary between salt marshes and mangroves, those differences may not directly affect subtidal seagrass, because infauna tend to be less mobile than epifauna. Habitat connectivity can drive differences in species composition through migration between habitats (Sheaves 2009). We did not measure larger, more mobile organisms (e.g., fishes), but we expect changes in these organisms may regulate the invertebrate organisms we did measure through predation, as the presence of mangroves in marsh systems alter associated fish biomass and community structure (Caudill 2005; Smee et al. 2017; Scheffel et al. 2018).
Epifauna is more responsive to changes in standing seagrass biomass, while infauna are more dependent on the roots and rhizomes which are not as subjected to change with seasonal and environmental shifts (Tanner 2005). In our study, T. testudinum aboveground biomass differed by both adjacent intertidal habitat and seasons, while overall belowground biomass was typically more stable (Fig. 6b, c), as from summer to winter, aboveground biomass (g m−2) experienced an overall decrease by 75.2%, while belowground biomass (g m−2) only decreased by 9.14%. The water column is more dynamic than the sediment as it experiences greater seasonal variability, and therefore has a greater impact on their associated fauna. The variability of the aboveground biomass and water column, possibly influenced by adjacent intertidal habitat, indirectly influenced epifaunal community assemblage. Importantly, adjacent to mangroves, belowground biomass did significantly decline in winter but we did not see equal reductions in infauna density or biomass, maybe because that reduction was short in duration and during a time when predation was low.
Conclusion
Species range expansions, induced by warming temperatures, have a wide range of ecological implications, and those changes may spill over into adjacent, connected habitats. Our results show that seagrass meadows differed with adjacent intertidal plant communities (mangroves, salt marshes, and the ecotone—a mixture of the two habitats), suggesting that mangrove encroachment may drive changes to seagrass meadows. Mangroves are becoming more dominant along northern Gulf of Mexico shorelines (Osland et al. 2012; Saintilan et al. 2014; Cavanaugh et al. 2019) and are not only changing intertidal characteristics, but, as demonstrated here, also impacting subtidal habitats. Connectivity, specifically through the outwelling influence from the intertidal zone, is a critical component that is often overlooked. We observed changes in subtidal environmental conditions, gas fluxes, seagrass standing biomass, and epifaunal community assemblage that correlate with delivery of organic matter possibly though the outwelling of senesced biomass. Differences in SOM within the seagrass meadows suggest that timing of outwelling, from intertidal senesced biomass, influenced functions provided by meadows. Our results demonstrate that net N2 fluxes differed between adjacent intertidal habitats, but the magnitude and direction of these fluxes depended on seasonality. Peak denitrification rates (net positive N2 flux) within meadows coincided with when their respective adjacent intertidal habitat’s biomass senesced. Additionally, shifts from net fixing to denitrifying induces changes in nutrient regimes, which influenced seagrass biomass and their associated epifaunal communities.
Our study focused on seagrass meadows adjacent to different intertidal habitats, which meant that our spatial scale was relatively small. Despite this, our results showed differences in seagrasses form and function correlating with adjacent habitat, suggesting the importance of connectivity between the intertidal and subtidal. It is important to note that these differences may amplify on a larger spatial and temporal scale (Valiela and Cole 2002). Conversely, these impacts may be smaller in systems with a lower tidal amplitude, which occur in the northern Gulf of Mexico, where connectivity may be reduced. Likewise, small changes in nutrient availability result in observable changes in this oligotrophic environment. Impacts to system with a different nutrient history may differ in magnitude and direction. Importantly, the shifts we observed impact key ecosystem services—nitrogen cycling (Costanza et al. 1997; Waycott et al. 2009), blue carbon (Pendleton et al. 2012; Lavery et al. 2013), and food and recreation (McArthur and Boland 2006), that have further implications to other nearby habitats and even to coastal residents who rely on these services. Tropicalization, urbanization, and restoration are all changing intertidal habitats (Kennish 2001; Romañach et al. 2018; Kelleway et al. 2017; Scheffel et al. 2018), and connectivity can facilitate broad impacts on adjacent systems in these cases, as well.
References
Aké-Castillo, J.A., G. Vázquez, and J. López-Portillo. 2006. Litterfall and decomposition of Rhizophora mangle L. in a coastal lagoon in the southern Gulf of Mexico. Hydrobiologia 559 (1): 101–111. https://doi.org/10.1007/s10750-005-0959-x.
Alleman, L.K., and M.W. Hester. 2011. Refinement of the fundamental niche of black mangrove (Avicennia germinans) seedlings in Louisiana: Applications for restoration. Wetlands Ecology and Management, 19(1), 47–60. https://doi.org/10.1007/s11273-010-9199-6.
Anderson, M.J., and D.C.I. Walsh. 2013. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecological Monographs 83 (4): 557–574. https://doi.org/10.1890/12-2010.1.
Armitage, A.R., and J.W. Fourqurean. 2006. The short-term influence of herbivory near patch reefs varies between seagrass species. Journal of Experimental Marine Biology and Ecology 339 (1): 65–74. https://doi.org/10.1016/j.jembe.2006.07.013.
Barreto, C.R., E.M. Morrissey, D.D. Wykoff, and S.K. Chapman. 2018. Co-occurring mangroves and salt marshes differ in microbial community composition. Wetlands 38 (3): 497–508. https://doi.org/10.1007/s13157-018-0994-9.
Bianchi, T.S., M.A. Allison, J. Zhao, X. Li, R.S. Comeaux, R.A. Feagin, and R.W. Kulawardhana. 2013. Historical reconstruction of mangrove expansion in the Gulf of Mexico: Linking climate change with carbon sequestration in coastal wetlands. Estuarine, Coastal and Shelf Science 119: 7–16. https://doi.org/10.1016/j.ecss.2012.12.007.
Bloomfield, A.L., and B.M. Gillanders. 2005. Fish and invertebrate assemblages in seagrass, mangrove, saltmarsh, and nonvegetated habitats. Estuaries 28 (1): 63–77. https://doi.org/10.1007/BF02732754.
Burrows, M.T., D.S. Schoeman, L.B. Buckley, P. Moore, E.S. Poloczanska, K.M. Brander, C. Brown, J.F. Bruno, C.M. Durate, B.S. Halpern, J. Holding, C.V. Kappel, W. Kiessling, M.I. O’Connor, J.M. Pandolfi, S. Parmesan, F.B. Schwing, W.J. Sydeman, and A.J. Richardson. 2011. The pace of shifting climate in marine and terrestrial ecosystems. Science 334 (6056): 652–655. https://doi.org/10.1126/science.1210288.
Caudill, M. C. 2005. Nekton utilization of blank mangrove (Avicennia germinans) and smooth cordgrass (Spartina alterniflora) sites in southwestern Caminada Bay, Louisiana. MS Thesis. Louisiana State University.
Cavanaugh, K.C., J.R. Kellner, A.J. Forde, D.S. Gruner, J.D. Parker, W. Rodriguez, and I.C. Feller. 2013. Poleward expansion of mangroves is a threshold response to decreased frequency of extreme cold events. Proceedings of the National Academy of Sciences 111 (2): 723–727. https://doi.org/10.1073/pnas.1315800111.
Cavanaugh, K.C., E.M. Dangremond, C.L. Doughty, A.P. Williams, J.D. Parker, M.A. Hayes, W. Rodriguez, and I.C. Feller. 2019. Climate-driven regime shifts in a mangrove-salt marsh ecotone over the past 250 years. Proceedings of the National Academy of Sciences 116 (43): 21602–21608. https://doi.org/10.1073/pnas.1902181116.
Clarke, K.R., and R.N. Gorley. 2015. Getting started with PRIMER v7. PRIMER-E. Plymouth: Plymouth Marine Laboratory.
Comeaux, R.S., M.A. Allison, and T.S. Bianchi. 2012. Mangrove expansion in the Gulf of Mexico with climate change: Implications for wetland health and resistance to rising sea levels. Estuarine, Coastal and Shelf Science 96: 81–95. https://doi.org/10.1016/j.ecss.2011.10.003.
Costanza, R., R. d’Arge, R. de Groot, S. Farber, M. Grasso, B. Hannon, K. Limburg, S. Naeem, R.V. O’Neill, J. Paruelo, R.G. Raskin, R. Sutton, and M. van den Belt. 1997. The value of the world’s ecosystem services and natural capital. Nature 387 (6630): 253–260. https://doi.org/10.1038/387253a0.
Darby, F.A., and R.E. Turner. 2008. Below- and aboveground Spartina alterniflora production in a Louisiana salt marsh. Estuaries and Coasts 31 (1): 223–231. https://doi.org/10.1007/s12237-007-9014-7.
Dawes, C.J., K. Siar, and D. Marlett. 1999. Mangrove structure, litter and macroalgal productivity in a northern most forest of Florida. Mangroves and Salt Marshes 3 (4): 259–267. https://doi.org/10.1023/A:1009976025000.
Doughty, C.L., J.A. Langley, W.S. Walker, I.C. Feller, R. Schaub, and S.K. Chapman. 2016. Mangrove range expansion rapidly increases coastal wetland carbon storage. Estuaries and Coasts 39 (2): 385–396. https://doi.org/10.1007/s12237-015-9993-8.
Eyre, B.D., D.T. Maher, and P. Squire. 2013a. Quantity and quality of organic matter (detritus) drives N2 effluxes (net denitrification) across seasons, benthic habitats, and estuaries: Organic matter driven denitrification. Global Biogeochemical Cycles 27 (4): 1083–1095. https://doi.org/10.1002/2013GB004631.
Eyre, B.D., I.R. Santos, and D.T. Maher. 2013b. Seasonal, daily and diel N2 effluxes in permeable carbonate sediments. Biogeosciences 10: 1–15 http://www.biogeosciences.net/10/1/2013/.
Fulweiler, R.W., and S.W. Nixon. 2012. Net sediment N2 fluxes in a southern New England estuary: Variations in space and time. Biogeochemistry 111 (1-3): 111–124. https://doi.org/10.1007/s10533-011-9660-5.
Fulweiler, R.W., S.W. Nixon, and B.A. Buckley. 2010. Spatial and temporal variability of benthic oxygen demand and nutrient regeneration in an anthropogenically impacted New England estuary. Estuaries and Coasts 33 (6): 1377–1390. https://doi.org/10.1007/s12237-009-9260-y.
Fulweiler, R.W., S. Brown, S. Nixon, and B. Jenkins. 2013. Evidence and a conceptual model for the co-occurrence of nitrogen fixation and denitrification in heterotrophic marine sediments. Marine Ecology Progress Series 482: 57–68. https://doi.org/10.3354/meps10240.
Gao, G.F., P.F. Li, J.X. Zhong, Z.J. Shen, J. Chen, Y.T. Li, A. Isabwe, X.Y. Zhu, Q.S. Ding, S. Zhang, C.H. Gao, and H.L. Zheng. 2019. Spartina alterniflora invasion alters soil bacterial communities and enhances soil N2O emissions by stimulating soil denitrification in mangrove wetland. Science of the Total Environment 653: 231–240. https://doi.org/10.1016/j.scitotenv.2018.10.277.
Gil, M., A.R. Armitage, and J.W. Fourqurean. 2006. Nutrient impacts on epifaunal density and species composition in a subtropical seagrass bed. Hydrobiologia 569 (1): 437–447. https://doi.org/10.1007/s10750-006-0147-7.
Glazner, R., J. Blennau, and A.R. Armitage. 2020. Mangroves alter predator-prey interactions by enhancing prey refuge value in a mangrove-marsh ecotone. Journal of Experimental Marine Biology and Ecology 526: 151336. https://doi.org/10.1016/j.jembe.2020.151336.
Han, Q., L.M. Soissons, D. Liu, M.M. van Katwijk, and T.J. Bouma. 2017. Individual and population indicators of Zostera japonica respond quickly to experimental addition of sediment-nutrient and organic matter. Marine Pollution Bulletin 114 (1): 201–209. https://doi.org/10.1016/j.marpolbul.2016.08.084.
Heck, K.L., F. Fodrie, S. Madsen, C. Baillie, and D. Byron. 2015. Seagrass consumption by native and a tropically associated fish species: Potential impacts of the tropicalization of the northern Gulf of Mexico. Marine Ecology Progress Series 520: 165–173. https://doi.org/10.3354/meps11104.
Heiri, O., A.F. Lotter, and G. Lemcke. 2001. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. Journal of Paleolimnology 25 (1): 101–110. https://doi.org/10.1023/A:1008119611481.
Huxham, M., D. Whitlock, M. Githaiga, and A. Dencer-Brown. 2018. Carbon in the coastal seascape: How interactions between mangrove forests, seagrass meadows and tidal marshes influence carbon storage. Current Forestry Reports 4 (2): 101–110. https://doi.org/10.1007/s40725-018-0077-4.
Irlandi, E.A., and M.K. Crawford. 1997. Habitat linkages: The effect of intertidal saltmarshes and adjacent subtidal habitats on abundance, movement, and growth of an estuarine fish. Oecologia 110 (2): 222–230. https://doi.org/10.1007/s004420050154.
Kaldy, J., and K. Dunton. 2000. Above- and below-ground production, biomass and reproductive ecology of Thalassia testudinum (turtle grass) in a subtropical coastal lagoon. Marine Ecology Progress Series 193: 271–283. https://doi.org/10.3354/meps193271.
Kelleway, J.J., N. Saintilan, P.I. Macreadie, C.G. Skilbeck, A. Zawadzki, and P.J. Ralph. 2016. Seventy years of continuous encroachment substantially increases ‘blue carbon’ capacity as mangroves replace intertidal salt marshes. Global Change Biology 22 (3): 1097–1109. https://doi.org/10.1111/gcb.13158.
Kelleway, J.J., K. Cavanaugh, K. Rogers, I.C. Feller, E. Ens, C. Doughty, and N. Saintilan. 2017. Review of the ecosystem service implications of mangrove encroachment into salt marshes. Global Change Biology 23 (10): 3967–3983. https://doi.org/10.1111/gcb.13727.
Kennish, M.J. 2001. Coastal salt marsh systems in the U.S.: A review of anthropogenic impacts. Journal of Coastal Research 17: 731–748 http://www.jstor.org/stable/4300224.
Kirby, C.J., and J.G. Gosselink. 1976. Primary production in a Louisiana gulf coast Spartina alterniflora marsh. Ecology 57 (5): 1052–1059. https://doi.org/10.2307/1941070.
Koch, E., and G. Gust. 1999. Water flow in tide- and wave-dominated beds of the seagrass Thalassia testudinum. Marine Ecology Progress Series 184: 63–72. https://doi.org/10.3354/meps184063.
Lana, P., C. Guiss, and S.T. Disaró. 1991. Seasonal variation of biomass and production dynamics for above- and belowground components of a Spartina alterniflora marsh in the euhaline sector of Paranaguá Bay (SE Brazil). Estuarine, Coastal and Shelf Science 32 (3): 231–241. https://doi.org/10.1016/0272-7714(91)90017-6.
Lavery, P.S., M. Mateo, O. Serrano, and M. Rozaimi. 2013. Variability in the carbon storage of seagrass habitats and its implications for global estimates of blue carbon ecosystem service. PLoS One 8 (9): e73748. https://doi.org/10.1371/journal.pone.0073748.
Lee, S.Y. 1995. Mangrove outwelling: A review. Hydrobiologia 295 (1-3): 203–212. https://doi.org/10.1007/BF00029127.
Lee, K.S., and K.H. Dunton. 1999. Inorganic nitrogen acquisition in the seagrass Thalassia testudinum: Development of a whole-plant nitrogen budget. Limnology and Oceanography 44 (5): 1204–1215. https://doi.org/10.4319/lo.1999.44.5.1204.
Lewis, D.B., J.A. Brown, and K.L. Jimenez. 2014. Effects of flooding and warming on soil organic matter mineralization in Avicennia germinans mangrove forests and Juncus roemerianus salt marshes. Estuarine, Coastal and Shelf Science 139: 11–19. https://doi.org/10.1016/j.ecss.2013.12.032.
McArthur, L.C., and J.W. Boland. 2006. The economic contribution of seagrass to secondary production in south Australia. Ecological Modelling 196 (1-2): 163–172. https://doi.org/10.1016/j.ecolmodel.2006.02.030.
McCarty, J.P. 2002. Ecological consequences of recent climate change. Conservation Biology 15 (2): 320–331. https://doi.org/10.1046/j.1523-1739.2001.015002320.x.
McMillan, S.K., M.F. Piehler, S.P. Thompson, and H.W. Paerl. 2010. Denitrification of nitrogen released from senescing algal biomass in coastal agricultural headwater streams. Journal of Environment Quality 39 (1): 274–281. https://doi.org/10.2134/jeq2008.0438.
Mishra, A.K., and D. Apte. 2020. Ecological connectivity with mangroves influences tropical seagrass population longevity and meadow traits within an island ecosystem. Marine Ecology Progress Series 644: 47–63. https://doi.org/10.3354/meps13349.
Nagelkerken, I., S. Kleijnen, T. Klop, R. Van Den Brand, E.C. de La Moriniere, and G. Van der Velde. 2001. Dependence of Caribbean reef fishes on mangroves and seagrass beds as nursery habitats: A comparison of fish faunas between bays with and without mangroves/seagrass beds. Marine Ecology Progress Series 214: 225–235 https://www.int-res.com/abstracts/meps/v214/p225-235/.
NOAA. 2019. Tides & Currents. Center for operational oceanographic products and services. Available online: https://tidesandcurrents.noaa.gov/stationhome.html?id=8727520#info. Accessed 5 Jun 2020.
Odum, E. P. 1968. A research challenge: Evaluating the productivity of coastal and estuarine water. In Proceedings of the 2nd Sea Grant Conference (pp. 63-64). Graduate School of Oceanography, University of Rhode Island.
Osland, M.J., A.C. Spivak, J.A. Nestlerode, J.M. Lessmann, A.E. Almario, P.T. Heitmuller, M.J. Russell, K.W. Krauss, F. Alvarez, D.D. Dantin, J.E. Harvey, A.S. From, N. Cormier, and C.L. Stagg. 2012. Ecosystem development after mangrove wetland creation: Plant–soil change across a 20-year chronosequence. Ecosystems 15 (5): 848–866. https://doi.org/10.1007/s10021-012-9551-1.
Osland, M.J., R.H. Day, C.T. Hall, M.D. Brumfield, J.L. Dugas, and W.R. Jones. 2017. Mangrove expansion and contraction at a poleward range limit: Climate extremes and land-ocean temperature gradients. Ecology 98 (1): 125–137. https://doi.org/10.1002/ecy.1625.
Patterson, C.S., and I.A. Mendelssohn. 1991. A comparison of physicochemical variables across plant zones in a mangal/salt marsh community in Louisiana. Wetlands 11 (1): 139–161. https://doi.org/10.1007/BF03160845.
Pendleton, L., D.C. Donato, B.C. Murray, S. Crooks, W.A. Jenkins, S. Sifleet, C. Craft, J.W. Fourqurean, J.B. Kauffman, N. Marbà, P. Megonigal, E. Pidgeon, D. Herr, D. Gordon, and A. Baldera. 2012. Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS One 7 (9): e43542. https://doi.org/10.1371/journal.pone.0043542.
Pérez, M., O. Invers, J.M. Ruiz, M.S. Frederiksen, and M. Holmer. 2007. Physiological responses of the seagrass Posidonia oceanica to elevated organic matter content in sediments: An experimental assessment. Journal of Experimental Marine Biology and Ecology 344 (2): 149–160. https://doi.org/10.1016/j.jembe.2006.12.020.
Perry, C.L., and I.A. Mendelssohn. 2009. Ecosystem effects of expanding populations of Avicennia germinans in a Louisiana salt marsh. Wetlands 29 (1): 396–406. https://doi.org/10.1672/08-100.1.
Peterson, J., and S. Bell. 2018. Species composition of patches influences mangrove recruitment in a saltmarsh mosaic. Marine Ecology Progress Series 602: 103–116. https://doi.org/10.3354/meps12707.
Pezeshki, S.R., and R.D. DeLaune. 1991. A comparative study of above-ground productivity of dominant U.S. Gulf Coast marsh species. Journal of Vegetation Science 2 (3): 331–338. https://doi.org/10.2307/3235924.
Piehler, M.F., and A.R. Smyth. 2011. Habitat-specific distinctions in estuarine denitrification affect both ecosystem function and services. Ecosphere 2 (1): 1–17. https://doi.org/10.1890/ES10-00082.1.
Prado, P., and K. Heck. 2011. Seagrass selection by omnivorous and herbivorous consumers: Determining factors. Marine Ecology Progress Series 429: 45–55. https://doi.org/10.3354/meps09076.
Purtlebaugh, C., C.W. Martin, and M.S. Allen. 2020. Poleward expansion of common snook Centropomus undecimalis in the northeastern Gulf of Mexico and future research needs. PLoS One 15 (6): e0234083. https://doi.org/10.1371/journal.pone.0234083.
Reynolds, P.L., J.P. Richardson, and J.E. Duffy. 2014. Field experimental evidence that grazers mediate transition between microalgal and seagrass dominance. Limnology and Oceanography 59 (3): 1053–1064. https://doi.org/10.4319/lo.2014.59.3.1053.
Romañach, S.S., D.L. DeAngelis, H. Lye Koh, Y. Li, S. Yean Teh, R.S. Raja Barizan, and L. Zhai. 2018. Conservation and restoration of mangroves: Global status, perspectives, and prognosis. Ocean & Coastal Management 154: 72–82. https://doi.org/10.1016/j.ocecoaman.2018.01.009.
Ruiz-Halpern, S., S.A. Macko, and J.W. Fourqurean. 2008. The effects of manipulation of sedimentary iron and organic matter on sediment biogeochemistry and seagrasses in a subtropical carbonate environment. Biogeochemistry 87 (2): 113–126. https://doi.org/10.1007/s10533-007-9162-7.
Saintilan, N., N.C. Wilson, K. Rogers, A. Rajkaran, and K.W. Krauss. 2014. Mangrove expansion and salt marsh decline at mangrove poleward limits. Global Change Biology 20 (1): 147–157. https://doi.org/10.1111/gcb.12341.
Scheffel, W.A., K.L. Heck, and M.W. Johnson. 2018. Tropicalization of the northern Gulf of Mexico: Impacts of salt marsh transition to black mangrove dominance on faunal communities. Estuaries and Coasts 41 (4): 1193–1205. https://doi.org/10.1007/s12237-017-0334-y.
Scott, A.L., P.H. York, C. Duncan, P.I. Macreadie, R.M. Connolly, M.T. Ellis, J.C. Jarvis, K.I. Jinks, H. Marsh, and M.A. Rasheed. 2018. The role of herbivory in structuring tropical seagrass ecosystem service delivery. Frontiers in Plant Science 9: 127. https://doi.org/10.3389/fpls.2018.00127.
Shannon, C.E. 1948. A mathematical theory of communication. Bell System Technical Journal 27: 379–423.
Sheaves, M. 2009. Consequences of ecological connectivity: The coastal ecosystem mosaic. Marine Ecology Progress Series 391: 107–115. https://doi.org/10.3354/meps08121.
Sheridan, P.F., and R.J. Livingston. 1983. Abundance and seasonality of infauna and epifauna inhabiting a Halodule wrightii meadow in Apalachicola Bay, Florida. Estuaries 6 (4): 407. https://doi.org/10.2307/1351400.
Simpson, L.T., T.Z. Osborne, L.J. Duckett, and I.C. Feller. 2017. Carbon storages along a climate induced coastal wetland gradient. Wetlands 37 (6): 1023–1035. https://doi.org/10.1007/s13157-017-0937-x.
Simpson, L.J., A. Cherry, R.S. Smith, and I.C. Feller. 2020. Mangrove encroachment alters decomposition rate in saltmarsh through changes in litter quality. Ecosystems. https://doi.org/10.1007/s10021-020-00554-z.
Smee, D.L., J.A. Sanchez, M. Diskin, and C. Trettin. 2017. Mangrove expansion into salt marshes alters associated faunal communities. Estuarine, Coastal and Shelf Science 187: 306–313. https://doi.org/10.1016/j.ecss.2017.02.005.
Smyth, A.R., M.F. Piehler, and J.H. Grabowski. 2015. Habitat context influences nitrogen removal by restored oyster reefs. Journal of Applied Ecology 52 (3): 716–725. https://doi.org/10.1111/1365-2664.12435.
Stevens, P.W., S.L. Fox, and C.L. Montague. 2006. The interplay between mangroves and saltmarshes at the transition between temperate and subtropical climate in Florida. Wetlands Ecology and Management 14 (5): 435–444. https://doi.org/10.1007/s11273-006-0006-3.
Sweet, W.V., D. Marcy, G. Duse, J.J. Marra, and M. Pendleton. 2018. 2017 state of U.S. high tide flooding with a 2018 Outlook. NOAA: https://repository.library.noaa.gov/view/noaa/20691. Accessed 12 Jan 2020.
Taillardat, P., A.D. Ziegler, D.A. Friess, D. Widory, F. David, N. Ohte, T. Nakamura, J. Evaristo, N. Thanh-Nho, T. Van Vinh, and C. Marchand. 2019. Assessing nutrient dynamics in mangrove porewater and adjacent tidal creek using nitrate dual-stable isotopes: A new approach to challenge the outwelling hypothesis? Marine Chemistry 214: 103662. https://doi.org/10.1016/j.marchem.2019.103662.
Tanner, J.E. 2005. Edge effects on fauna in fragmented seagrass meadows. Austral Ecology 30 (2): 210–218. https://doi.org/10.1111/j.1442-9993.2005.01438.x.
Terrados, J., C.M. Duarte, L. Kamp-Nielsen, N.S.R. Agawin, E. Gacia, D. Lacap, M.D. Fortes, J. Borum, M. Lubanski, and T. Greve. 1999. Are seagrass growth and survival constrained by the reducing conditions of the sediment? Aquatic Botany 65 (1-4): 175–197. https://doi.org/10.1016/S0304-3770(99)00039-X.
Twilley, R.W., A.E. Lugo, and C. Patterson-Zucca. 1986. Litter production and turnover in basin mangrove forests in southwest Florida. Ecology 67 (3): 670–683. https://doi.org/10.2307/1937691.
Valiela, I., and M.L. Cole. 2002. Comparative evidence that salt marshes and mangroves may protect seagrass meadows from land-derived nitrogen loads. Ecosystems 5 (1): 92–102. https://doi.org/10.1007/s10021-001-0058-4.
Valiela, I., J.M. Teal, S.D. Allen, R. Van Etten, D. Goehringer, and S. Volkmann. 1985. Decomposition in salt marsh ecosystems: The phases and major factors affecting disappearance of above-ground organic matter. Journal of Experimental Marine Biology and Ecology 89 (1): 29–54. https://doi.org/10.1016/0022-0981(85)90080-2.
Vergés, A., P.D. Steinberg, M.E. Hay, A.G.B. Poore, A.H. Campbell, E. Ballesteros, K.L. Heck, D.J. Booth, M.A. Coleman, D.A. Feary, W. Figueira, T. Langlois, E.M. Marzinelli, T. Mizerek, P.J. Mumby, Y. Nakamura, M. Roughan, E. van Sebille, A. Sen Gupta, D.A. Smale, F. Tomas, T. Wernberg, and S.K. Wilson. 2014. The tropicalization of temperate marine ecosystems: Climate-mediated changes in herbivory and community phase shifts. Proceedings of the Royal Society B: Biological Sciences 281 (1789): 20140846. https://doi.org/10.1098/rspb.2014.0846.
Walker, J.E., C. Angelini, I. Safak, A.H. Altieri, and T.Z. Osborne. 2019. Effects of changing vegetation composition and community structure, ecosystem functioning, and predator-prey interactions at the saltmarsh-mangrove ecotone. Diversity 11 (11): 208. https://doi.org/10.3390/d11110208.
Waycott, M., C.M. Duarte, T.J.B. Carruthers, R.J. Orth, W.C. Dennison, S. Olyarnik, A. Calladine, J.W. Fourqurean, K.L. Heck, A.R. Hughes, G.A. Kendrick, W.J. Kenworthy, F.T. Short, and S.L. Williams. 2009. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences of the United States of America 106 (30): 12377–12381. https://doi.org/10.1073/pnas.0905620106.
Wright, E.E., A.C. Hine, S.L. Goodbred, and S.D. Locker. 2005. The effect of sea-level and climate change on the development of a mixed siliciclastic-carbonate, deltaic coastline: Suwannee River, Florida, U.S.A. Journal of Sedimentary Research 75 (4): 621–635. https://doi.org/10.2110/jsr.2005.051.
Yando, E.S., M.J. Osland, J.M. Willis, R.H. Day, K.W. Krauss, and M.W. Hester. 2016. Salt marsh-mangrove ecotones: Using structural gradients to investigate the effects of woody plant encroachment on plant-soil interactions and ecosystem carbon pools. Journal of Ecology 104 (4): 1020–1031. https://doi.org/10.1111/1365-2745.12571.
Zieman, J.C., and R.G. Wetzel. 1980. Methods and rates of productivity in seagrasses, 87–116. New York: Handbook of seagrass biology Garland.
Acknowledgments
This paper is dedicated to Dr. Susan Williams, who was a role model and mentor to all but especially to women in this field. We thank Whitney Scheffel, Theresa Gruninger, Jamila Roth, and Christina Moreau for help with data collection. Additionally, we thank James Colee for help with statistical analysis advice. We thank 3 anonymous reviewers for their comments.
Funding
This work was funded by University of Florida, Institute of Food and Agricultural Sciences (FLA-SWS-005656).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Dan Friess
Supplementary Information
ESM 1
(DOCX 205 kb)
Rights and permissions
About this article
Cite this article
Sullivan, C.R., Smyth, A.R., Martin, C.W. et al. How Does Mangrove Expansion Affect Structure and Function of Adjacent Seagrass Meadows?. Estuaries and Coasts 44, 453–467 (2021). https://doi.org/10.1007/s12237-020-00879-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-020-00879-x