Abstract
The monthly variations of below- and aboveground biomass of Spartina alterniflora were documented for a south Louisiana salt marsh from March 2004 to March 2005, and in March 2006 and 2007. The annual production rate above- and belowground was 1821 and 11,676 g m−2, respectively (Smalley method), and the annual production rate per biomass belowground was 10.7 g dry weight−1, which are highs along the latitudinal distributions of the plant’s range. The average root + rhizome/shoot ratio (R&R/S) was 2.6:1, which is lower than the R&R/S ratios of 4 to 5.1 reported for Spartina sp. marshes in the northeastern US. The belowground biomass increased from July to September and fluctuated between October and November, after which it declined until February when the growing season began. The belowground biomass was dominated by rhizomes, which declined precipitously in spring and then rose to a seasonal high in the month before declining again as the late summer rise in inflorescence began. Over half of the root biomass in a 30-cm soil profile was in the upper 10 cm, and in the 10- to 20-cm profile for rhizomes. The maximum March biomass above- and belowground was four to five times that of the minimum biomass over the four sampling years. The net standing stock (NSS) of N and P in live biomass aboveground compared to that in the belowground biomass was about 1.7 times higher and equal, respectively, but the NSS of N and P for the live + dead biomass was about six times higher belowground. The average nitrogen/phosphorous molar ratios of 16:1 aboveground is in agreement with the often tested N limitation of biomass accumulation aboveground, whereas the 37:1 belowground ratio suggests that there is an influence of P on R&R foraging for P belowground. Some implications for management and restoration are, in part, that salt marshes should be evaluated and examined using information on the plant’s physiology and production both below- and aboveground.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetlands, and particularly tidal salt marshes, have long been recognized for their high rates of aboveground primary production (Whittaker 1975). The belowground biomass component, however, has received only limited attention, primarily because harvest techniques are labor-intensive and may be subjective because of the difficult process of visually separating live and dead roots and rhizomes (White and Howes 1994). It is important to improve our knowledge of root and rhizome (R&R) productivity for several reasons. Roots and rhizomes, for example, are a considerable reservoir in the energy and material cycles in estuarine wetland communities (Schubauer and Hopkinson 1984) and inhibit erosion. The belowground biomass contributes to the volume, hence elevation of the marsh, and appears to be much more important than inorganic matter for a salt marsh to maintain itself once established (Turner et al. 2001). If the accumulation of organics is not sufficient, then a salt marsh with abundant aboveground plant growth might quickly become open water if sulfides accumulate and cause the demise of plants (Mendelssohn et al. 1981; Turner et al. 2004).
This study is about the seasonal variations in the above- and belowground plant biomass of Spartina alterniflora, a dominant, herbaceous, native, and warm-season perennial grass that forms dense vegetative colonies along shorelines and intertidal flats from Canada to the Gulf of Mexico. It is a robust and rapidly spreading plant tolerant of fluctuating water levels and salinities ranging from 0 to 35 psu. It spreads primarily by vegetative propagation, producing new stems from an extensive system of underground rhizomes. The width and thickness of a colony are controlled by a number of site-specific conditions such as elevation, shoreline slope, the frequency, depth, and duration of flooding, as well as a wide range of other conditions, including temperature and length of the growing season. Compared to plants in the southeastern U.S., for example, plants in the northeast have a short growing period, the aboveground shoots developing during the summer are dead in the fall, and the aboveground production is lower than in the southern US (Turner 1976; Gallagher and Seliskar 1976; Gallagher 1983). Fifty to ninety percent of the annual production of S. alterniflora in eastern US salt marshes occurs belowground as root and rhizomes (Valiela et al. 1976; Smith et al. 1979), with root/shoot ratios generally greater than 1:1 (Good et al. 1982). The quantity and timing of the storage of the underground reserves and their translocation for aerial growth would be expected to change within its distribution range. There are no previous studies of the simultaneous determination of seasonal variations in the above- and belowground biomass of Spartina alterniflora at the southern limits of its U.S. range. We attempted to fill this data gap by studying the changes in macrophyte biomass in a Louisiana salt marsh, and included tissue analyses and other complementary measurements, and compared the results to those from other locations. Our expectations were that: (1) the belowground production and production per unit biomass would be higher than the aboveground production, but we did not know how much higher, (2) the translocation of reserves from below- to aboveground would be less important in these relatively warm marshes, hence the standing stock of rhizomes would be lower, and, 3) changes in the standing stocks of nitrogen and phosphorus would mimic the seasonal variations in organic matter.
Method and Materials
The study was conducted in Spartina alterniflora dominated salt marsh located about 0.5 km west of the Louisiana Universities Marine Consortium (LUMCON) laboratory, in Cocodrie, LA (29°15′N, 91°21′W). The tidal range during sampling period was −0.23 to 0.59 m with a mean of 0.18 m. The marsh floods at 0.35 m on the tide gage whose records are archived at http://www.lumcon.edu. Tides there occur once or twice daily, and the mean tidal range changes seasonally in response to cold front passages in the winter and storm events in summer. The salinity during the study ranged from 7 to 20 psu. The sampled marsh plots were 20 m from the edge of a 1.5-m deep tidal channel. Salt marshes in these microtidal environmental are typically described as being either a streamside marsh or inland marsh, rather than the high, middle, or low marshes of the east coast, U.S. Inland marshes have a lower canopy height and represent 80–90% of the entire salt marsh landscape (Turner and Gosselink 1975). Streamside marshes at this location are within 2 m of the water’s edge, and the mean live standing biomass in the inland marshes are about 59% of that in the streamside marshes (Kirby and Gosselink 1976).
The monthly variation of above- and belowground biomass of S. alterniflora was documented from March 2004 to March 2005, and again in March 2006 and March 2007. The monthly harvests were from different plots of a monospecific stand of S. alterniflora. We used a series of boardwalks to facilitate sampling and to minimize damage to the marsh, which was located inland of the 1- to 3-m wide streamside marsh. The aboveground S. alterniflora was harvested by clipping vegetation at the sediment surface in three adjacent replicate 0.25 m2 plots designated left, center, and right. All standing live and dead culms and litter were removed and placed into prelabeled plastic bags and transported to the Louisiana State University (LSU) processing laboratory. Dead shoots and leaves were identified by their yellowish or brownish coloration and separated from living material. The live or dead plant material was put into prelabeled paper bags, dried at 60°C for approximately 72 h, and weighed to the nearest 0.01 g. The annual net primary production was estimated using two harvest methods: the Smalley method corrects for mortality between samples by summing monthly changes in live biomass and in dead biomass (Smalley 1959), and the Max–Min method is based on the maximum difference in live biomass over the growing seasoning. Both methods underestimate the total production because plant biomass turnover between sampling is incompletely accounted for (Kaswadji et al. 1990).
The belowground biomass was collected using a 40-cm long stainless steel tube with sharpened edges. The 11-cm diameter × 30-cm long sediment cores were taken in the middle of each of three plots after the aboveground biomass sample was collected. The cores were extruded in the field and sliced into 0- to 10-cm, 10- to 20-cm, and 20- to 30-cm segments. Each segment was put in labeled plastic bags and placed in a cooler for transport to the LSU laboratory where they were refrigerated until processed. Each segment was washed in a 2-mm sieve over a 0.5-mm sieve to prevent the loss of dead and fine root material. Live roots and rhizomes were separated from dead material with a suture set under running water for better separation (live roots and rhizomes are white and turgid; dead materials are dark and flaccid). Root color can be variously tinged pink or orange, possibly because pigmentation zones contain feeding deterrents, i.e., roots colonized by arbuscular mycorrhizal fungi are often yellow. The physiological significance of these pigments is unclear (Fitter 2002). Discolored turgid roots were defined as live roots. Dead material included partially decayed root material. Live roots and dead belowground material were placed in paper bags, labeled, and dried to a constant weight at 60°C, and weighed to the nearest 0.01 g. All belowground materials (roots and rhizomes) were sorted by a single individual (F.A.D.) to assure continuity of sample processing, and to reduce potential sources of error. Monthly root + rhizome/shoot ratios (R&R/S) were determined by averaging the dry weight of the live above- and belowground biomass from the three plots. Dried plant and root material were ground in a grinder and sent to the LSU Soil Testing and Plant Analysis Lab where they were analyzed for their nitrogen and phosphorus content.
The N/P molar ratios in plant tissues were calculated to determine whether plant accumulation was likely to be N or P limited (Koerselman and Meuleman 1996) and to serve as a baseline measurement in a separate nutrient enrichment experiment (Darby and Turner 2008). A N/P molar ratio <33 indicates N limitation, whereas a N/P ratio >33 suggests, but does not prove, P limitation (Verhoeven 1996; USEPA 2002).
The results of a statistical analysis of monthly above- and of belowground biomass were compared to determine if the means were significantly different (significance level at p < 0.05 in all cases) based on Tukey’s adjustment. The analysis was carried out using the general linear model procedure (analysis of variance [ANOVA]; SAS 2002–2003). A logarithmic transformation was used on the data, which were then subject to a test for normality of distribution.
Results
Aboveground Biomass
The live aboveground biomass was lowest in March 2004 (114 g m−2) and increased steadily until the peak in September (877 g m−2; Fig. 1). The biomass in March 2005 (590 g m−2) was five times that of March 2004, and was statistically different. The live aboveground biomass was 276 and 303 g m−2 in March of 2006 and 2007, respectively (Fig. 2). The peak biomass of September (877 g m−2) was significantly different (p < 0.0001) from values for the summer and winter months.
The mean (± 1 SE) of three replicates of monthly aboveground live and dead biomass (upper panel) and the monthly belowground live and dead biomass of root and rhizomes (lower panel). Values represent the mean of three replicates (± 1 SE.). There were virtually no rhizomes in July. There were no statistically significant differences among the monthly dry weights of live R+R
A statistically significant difference in biomass (ANOVA; p < 0.0001) was measured from month to month. No discernable patterns were observed among months in the average number of stems or stem length (Fig. 3). Inflorescences appeared in October (58 per m2) and lasted until December (9 per m2; Fig. 4).
Monthly values of rhizomes biomass (g m−2; open circles) and number of stems with inflorescence (# m−2; closed circles). Values are the mean of three replicates ± 1 SD. The rhizome biomass increased after Julian day 190 (July) and peaked between 221 and 255 (August–September). A decline in rhizome biomass occurred as inflorescence production increased
The amount of dead aboveground biomass declined slightly from March (479 ± 100; μ ± 1 Std. error [SE]) to July (301 ± 9; μ ± 1 SE) as the aboveground live biomass increased (Fig. 1). The highest accumulation of aboveground dead biomass was in January (887 ± 3.8; μ ± 1 SE) and remained relatively high through March 2005. The estimates of annual aboveground production using Smalley and Max–min methods were 1281 and 762 g dry wt m−2 yr−1, respectively.
Belowground Biomass
A proliferation of live roots and rhizomes (R&R; 1110 and 550 g m−2, respectively) existed in March 2004 before the spring burst in aboveground vegetative growth, which was followed by a decrease in belowground biomass as the aboveground biomass increased (Fig. 1). The mean R&R/shoot (S) ratio was 2.6 ± 1, ranging from a minimum of 0.12 in midsummer (July) to a maximum of 14.5 in early spring (March 2004; Fig. 5). The belowground biomass in March 2004, 2005, 2006, and 2007 was 1660, 390, 317, and 1245 g m−2, respectively. The corresponding R&R/S ratios were 14.5, 0.7, 1.2, and 4.1 (Fig. 2).
The mean (± 1 SE) of three replicates of monthly root and rhizome biomass. Letters indicate the results of a Tukey’s Studentized Range Test for differences in rhizome biomass by month (g m−2). Means with the same letter are not significantly different from each other (significance level <0.05). No significant differences were seen in root biomass by month. The high error bar for day 225 is the result of one high value. The monthly root and rhizome/shoot ratios are indicated by the line. The root and rhizome/shoot ratio in March 2004 (day 78) equaled 14, then declined and remained stable for the remainder of the sampling period
There was a statistically significant difference between the mean of total live belowground biomass (P < 0.003; R&R) and the mean biomass of rhizomes (P < 0.001; log-transformation data). The majority of the live root biomass was in the upper 1–10 cm throughout the 1 year monthly sampling period (Table 1). In March, before the spring growth of aboveground biomass, the belowground biomass of roots was fairly uniform with depth, extending beyond 30 cm, and was sparse in the 20- to 30-cm layer. This result for the roots was in contrast to the seasonal patterns observed in the vertical profile of live rhizome biomass (Fig. 6). Rhizomes were noticeably absent from the 20- to 30-cm layer with the exception in February 2005 (398 g m−2). Rhizomes in the 10- to 20-cm layer increased from March to June from 136 to 431 g m−2. The rhizome biomass nearly disappeared from all three layers by July, but there was a second peak of belowground biomass in September (2337 g m−2) when rhizomes comprised 2185 g m−2 of the total belowground biomass (0–10 cm = 967 g m−2; 10–20 cm = 1218 g m−2). Rhizomes were present throughout the 0- to 30-cm profile from January through March 2005.
The rhizome biomass declined by more than 50% from September to October when there was an increase in the production of inflorescences (58 ± 21 m−2; μ ± 1 SE; Fig. 4) indicating that there was some translocation from below- to aboveground. The changes in rhizome biomass from September to October represented the majority of the total annual belowground production and exceeded the maximum standing stock of aboveground production by five times, or more. The total belowground production (11,676 g m−2 y−1), obtained using the Smalley method, was five times the aboveground production.
The amount of dead belowground biomass was consistently larger than the pool of live biomass (Fig. 1). The dead biomass, for example, was 90% of the total biomass in May and 98% in July. The depth profile of the dead belowground biomass exhibited a different seasonal pattern than the live fraction. The increases in the percent biomass in the dead fraction coincided with decreases in the live belowground biomass. Unlike the live R&R fraction, the dead fraction of the belowground biomass was greatest in the 20- to 30-cm section.
Tissue Nutrient Content
The monthly standing stock of N (NSS; g m−2) was documented from March 2004 to February 2005. The NSS in the aboveground live biomass in March 2004 was 1.5 g N m−2, and ranged between 1.5 to 8.8 g N m−2 for the 12 months (Table 2). The NSS increased from 2.1 to 8.8 g N m−2 from April to February. The NSS in senescing leaves and litterfall was lower than in the live biomass and ranged from 4.0 g N m−2 in March 2004 to 6.7 g N m−2 in February 2005. The levels of NSS in the belowground live biomass were, in general, comparable to that of the aboveground live biomass, except for when the R&R biomass peaked in March and September. In March 2004, the NSS belowground was 21.4 g N m−2, and 1.5 g N m−2 aboveground, and in September the NSS was 21.4 g N m−2 in the belowground tissues and 6.4 g N m−2 in the aboveground tissues. However, the NSS in the belowground dead biomass was lowest in January 2005 (19.0 g N m−2) and highest in May 2004 (66.7 g N m−2). The standing stock of phosphorous (PSS) in the live aboveground biomass ranged from 0.23 to 1.7 g P m−2 for the entire sampling period (Table 2). The PSS was higher in the aboveground live biomass than in the aboveground dead biomass (maximum = 0.65 g P m−2 in January 2005; minimum = 0.08 g P m−2 in May). No apparent differences were seen in the PSS in the belowground live and dead biomass (range 0.07–1.4 g P m−2 in the belowground live biomass and 0.6–1.4 g P m−2 in the belowground dead biomass; Table 2). The nitrogen/phosphorus molar ratios ranged from 14.2 to 20.2 in the aboveground live biomass and was 26.7 to 45 in the belowground live biomass (Table 2). The average N/P molar ratios were <33 in the aboveground biomass and >33 in belowground biomass (Table 2).
Comparisons Among Marshes
The annual belowground productivity in this Louisiana salt marsh (11,767 g m−2 yr−1; Smalley method) is, by comparison, high relative to marshes from Nova Scotia to Georgia (Table 3). The measured R&R/S ratio in Louisiana (2.6) is within the range of values reported for all other locations, but lower than salt marshes in the northeastern US (Table 4).
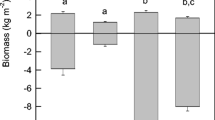
There are three environments where the belowground production per standing stock (end of season; P/SS) could be calculated in successive years using literature values, but each of the three data sets used a different method and so site-to-site comparisons of the absolute P/SS values cannot be made. All three examples demonstrate variability in belowground production between years, however, and each demonstrates higher production with increasing belowground standing stock (Fig. 7, left panel).
The relationships between belowground production and belowground standing stock of biomass at the end of summer for consecutive years (left) and the belowground production per unit belowground biomass (right). Estimates are based on data in Conner and Chmura (2000; Nova Scotia, Canada), Blum and Christian (2005; VA, USA), Dame and Kenny (1986; SC, USA), Ornes and Kaplan (1989; SC, USA), Schubauer and Hopkinson (1984; GA, USA), this analysis (LA, USA), and Lana et al. (1991; Brazil)
There are five examples where the results of the same method (both the Smalley and Max–min methods) can be compared along a latitudinal gradient (Fig. 7, right panel). The data from both methods shows that the P/SS ratio is lowest at the colder end of the latitudinal distribution of S. alterniflora, and highest in the Gulf of Mexico salt marshes (10.7, dry weight basis).
Discussion
The seasonal aboveground biomass at the study site was lowest in March 2004 (114 g m−2) and highest in September (877 g m−2) and the annual aboveground production using the Smalley method was 1281 g dry wt m−2 yr−1. These values are within the range of previous estimates. Kaswadji et al. (1990), for example, reported a similar estimate of the annual aboveground production for Airplane Lake, LA, using the same method (1113 g m−2 yr−1), and the peak standing crop in their study occurred in August (831 g m−2). They also reported a low aboveground standing biomass in March of about 100 g m−2. Visser and Sasser (2006) reported on a study conducted from 1978 to 1995 in Louisiana in which they measured an average peak biomass at two sites that ranged from a high of 1698 and 1261 g m−2 (1986) at Airplane Lake and Lake Jessie (LA), respectively, and a low of 818 g m−2 (1980) at Airplane Lake and 473 g m−2 (1993) at Lake Jessie.
The Louisiana site had a lower average salinity than the other sites in Table 3, and this relative difference may be related to the higher production rates we measured in this Louisiana salt marsh. The warmer climate, microtidal environment, and organic soils may also be contributing factors. The variability in belowground production at one location, measured in two different ways and at two different locations (Fig. 7, left panel), suggests that variations in salinity, water level, or climate are more important than soil fertility in regulating differences in belowground production among years. The data from Brazil is the only belowground production data there are on S. alterniflora closer to the equator than in Louisiana. One of us (R.E.T.) has looked for natural stands of this plant along the Gulf of Mexico coast, from Central America to Texas, and never seen standing stocks of this plant that were more than about 50% of what is observed in Louisiana. Turner and Gosselink (1975) sampled a few sites in Texas that support this conclusion, although the site choices were conducted to determine height-to-biomass relationships, and not to sample representative salt marsh biomass values. We think that the interpretation of a peak in the latitudinal gradient in the northern Gulf of Mexico is, therefore, a conclusion that is supportable in a general way, and could be usefully tested or refined with some additional quantitative sampling of the live above- and belowground biomass during the late summer.
The seasonal variations in belowground biomass were more pronounced than the changes in the live aboveground biomass (Figs. 1 and 7) and the total belowground production and production per unit biomass in this salt marsh is the highest reported value for any S. alterniflora marsh (Table 3; Fig. 7). Most of this production is in the form of rhizome production. The seasonal values for rhizome biomass usually exceeded root biomass, the exceptions being in March (roots 61% vs. rhizome 39%) and in July (100% roots). Schubauer and Hopkinson (1984) also found that rhizomes made up a greater portion of belowground biomass for a Georgia salt marsh. The greatest variation in rhizome biomass among the three replicates was in September, which is the period of the greatest rise and fall between sampling dates (Fig. 4). The shape of the rhizome at this time is often curved, and returns to the surface layer where it is becomes a shoot. Some additional sampling variability is introduced in September because the geometry of the rhizome sampled can be the longer vertically aligned tissue, or the horizontal “U-shaped” curve of the rhizome at the deepest layer it penetrates. We did not experiment with the effect of increasing sample size throughout the year, but this might be helpful in the design of sampling standards.
Rhizomes store and release large amounts of materials throughout the year, and quickly, whereas the amount of the smaller root biomass is relatively stable. Some have speculated that the extended growing season in southern marshes and the mild winter conditions there may minimize the amount of translocation between above- and belowground biomass pools (Good et al. 1982; McIntire and Dunstan 1976). We found, however, that the amount of roots and rhizomes (R&R) declined with the onset of the spring growth of aboveground biomass, indicating a substantial translocation of resources from below- to aboveground plant organs. Further, there was a decline in rhizome biomass in September as the production of inflorescence increased, indicating that there was a second large season translocation toward the aboveground biomass. The rise and fall of rhizome biomass at this time represented the majority of the annual belowground production. The highest turnover of R&R, measured using two methods, was highest in this Louisiana marsh, which is expected given that root turnover for forest, grasslands, shrubs, and wetlands is directly related to the mean annual temperature (Gill and Jackson 2000).
Seasonal patterns were observed in the depth profile of the live belowground biomass. Roots were concentrated in the top 0–10 cm for most of the 13-month sampling period, which may be related to higher rates of nitrogen fixation (Valiela et al. 1976), that the main nutrient source comes from the surface waters, or above a particular soil oxidation zone. Gross et al. (1991) noticed the same pattern in the R&R distribution in the soil profile for salt marshes ranging from Georgia to Nova Scotia. We conclude that, while the length and timing of the growing season varies along a latitudinal gradient, similar seasonal changes in root and rhizome biomass accumulations occur in the Gulf of Mexico and in Atlantic coast salt marshes.
The seasonal variations in NSS and in PSS in the aboveground live biomass had similar timing. The NSS and PSS increased during the fall through winter and decreased during the active growing season. The dilution of tissue nitrogen as aerial biomass increases is one possible cause of the lower NSS levels as plant biomass increased (Ornes and Kaplan 1989). The average NSS in belowground live biomass was 174% of the aboveground NSS, but the PSS in the belowground biomass was about equal to that of the aboveground biomass. The belowground PSS remained low in the fall and winter, and increased slightly during the growing season. The slight increase in belowground PSS may have been caused by an increase in reducing conditions resulting from higher summer tides and increased duration of flooding, which would make P more available.
The average N/P molar ratio in the aboveground plant tissue was 16:1, indicating nitrogen limitation of growth belowground. The average N/P molar ratios in the belowground tissue was 37:1, which may indicate a growth limitation of P on R&R biomass, or the resource allocation for R&R nutrient foraging in terms of biomass or metabolic pathway. Consequently, the often-tested conclusion that aboveground biomass accumulation is limited by N (Morris 1991) is supported, but the belowground accumulation of biomass appears to be more likely influenced by P, not N. This different sensitivity to N and P by above- and belowground biomass is discussed in a companion paper in which various combinations of N, P, and Fe were applied to plots located nearby to this sampling area (Darby and Turner, in press).
The amount of organic accumulation in these salt marsh sediments averages about 0.06 g cm−2 y−1 (Turner et al. 2001), which is about 10% of our estimate of the total annual belowground production. There are many ways to have a significant effect on the rate of organic matter accumulation, because relatively large amounts of nutrients and carbohydrates are translocated between above- and belowground tissues throughout the year. A small change in the biomass production belowground, perhaps resulting from a change in nutrient loading or increased flooding, could result in less (or more) biomass available to the aboveground plant tissues, and have a unfavorable effect on the long-term sustainability of the salt marsh. The factors influencing the tenuous balance between organic accumulation and loss deserve further attention because of the consequences to marsh stability.
The process of biomass translocation of nutrients from senescing leaves and shoots to belowground roots and rhizomes can be inferred from seasonal changes in the live biomass, whereas the changes in the dead biomass pool provide some indication of the decomposition rates and long-term accumulation of biomass (Conner and Chmura 2000; Gallagher 1983). But the timing of the peak and minimum biomass must be well-defined to understand the translocation and/or biomass storage dynamics. The sampling frequency must be sufficient to precisely tell when the translocation of nutrients occurs (Gallagher 1983). We recommend that sampling occur at least monthly to obtain accurate information about the recoverable underground reserves.
All of these observations on the monthly, seasonal, and interannual changes in biomass above- and belowground demonstrate that the evaluation of the salt marsh ecosystem will be incomplete if based exclusively on what is happening aboveground, or as though what happens aboveground is a satisfactory indicator of what is driving changes belowground—and vice versa. Monitoring programs, for example, could be improved if belowground soil processes were included, rather than excluded. Furthermore, it may be that because of the dominance of the changes in biomass pools belowground compared to aboveground, what happens belowground may be more influential to the long-term maintenance of the salt marsh than are changes in the aboveground components.
References
Blum, L., and R.R. Christian. 2005. Belowground production and decomposition along a tidal gradient in a Virginia salt marsh. In The ecomorphology of tidal marshes, eds. S. Fagherazzi, M. Marani, and L. K. Blum, 47–74. Washington, D.C.: American Geophysical Union.
Conner, R., and G.L. Chmura. 2000. Dynamics of above- and belowground organic matter in high latitude macrotidal saltmarsh. Marine Ecology Progress Series 204:101–110.
Dame, R.F., and P.D. Kenny. 1986. Variability of Spartina alterniflora primary production in the euhaline North Inlet estuary. Marine Ecology Progress Series 32:71–80.
Darby, F.A., and R.E. Turner. 2008. Below- and aboveground biomass of Spartina alterniflora: Response to nutrient addition in a Louisiana salt marsh. Estuaries and Coasts (in press).
Fitter, A. 2002. Characteristics and function of root systems. In Plant roots: the hidden half, eds. Y. Waisel, A. Eshel, and U. Kafkafi, 15–32. New York, Basel: Marcel Dekker, Inc.
Gallagher, J.L. 1974. Sampling macro-organic matter profiles in salt marsh plant root zones. Soil Science Society of America Proceedings 38:154–155.
Gallagher, J.L. 1983. Seasonal patterns in recoverable underground reserves in Spartina alterniflora Loisel. American Journal of Botany 70:212–215.
Gallagher, J.L., and D.M. Seliskar. 1976. The metabolism of senescing Spartina alterniflora. Annual Meeting Botany Society of America, New Orleans Abstract. p. 34.
Gill, R.A., and R.B. Jackson. 2000. Global patterns in root turnover for terrestrial ecosystems. New Phytologist 147:13–31.
Good, R.E., and B.R. Frasco. 1979. Estuarine evaluation study; a four year report on production and decomposition dynamics of salt marsh communities: Manahawkin marshes, Ocean County, New Jersey. Report to New Jersey Department of Environmental Protection, Division of Fish, Game, and Shellfisheries, Trenton, New Jersey, 105 pp.
Good, R.E., N.F. Good, and B.F. Frasco. 1982. A review of primary production and decomposition dynamics of the belowground marsh component. In Estuarine comparisons, ed. V.S. Kennedy, 139–158. New York: Academic Press.
Gross, M.F., M.A. Hardisky, P.L. Wolf, and V. Klemas. 1991. Relationship between aboveground and belowground biomass of Spartina alterniflora (Smooth Cordgrass). Estuaries 14:180–191.
Kaswadji, R.F., J.G. Gossenlnk, and R.E. Turner. 1990. Estimation of primary production using five different methods in a Spartina alterniflora salt marsh. Wetlands Ecology and Management 1:57–64.
Kirby, C.J., and J.G. Gosselink. 1976. Primary production in a Louisiana gulf coast Spartina alterniflora marsh. Ecology 57:1052–1059.
Koerselman, W., and A.F.M. Meuleman. 1996. The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. Journal of Applied Ecology 33:1441–1450.
Lana, P. da Cunha, C. Guiss, and S.T. Disaro. 1991. Seasonal variation of biomass and production dynamics for above- and belowground components of a Spartina alterniflora marsh in the euhaline sector of Paranaguá (SE Brazil). Estuarine, Coastal and Shelf Science 32:231–241.
McIntire, G.L., and W.M. Dunstan. 1976. Nonstructural carbohydrates in Spartina alterniflora Loisel. Botanica marina 19:93–96.
Mendelssohn, I.A., K.L. Mckee, and W.H. Patrick Jr. 1981. Oxygen deficiency in Spartina alterniflora roots: Metabolic adaptation to anoxia. Science 214:439–441.
Morris, J.T. 1991. Effects of nitrogen loading on wetland ecosystems with particular reference to atmospheric deposition. Annual Review of Ecology and Systematics 22:257–279.
Ornes, W.H., and D.I. Kaplan. 1989. Macronutrient status of tall and short forms of Spartina alterniflora in a South Carolina salt marsh. Marine Ecology Progress Series 55:63–72.
Schubauer, J.P., and C.S. Hopkinson. 1984. Above- and belowground emergent macrophyte production and turnover in a coastal marsh ecosystem, Georgia. Limnology and Oceanography 29:1052–1063.
Smalley, A.E. 1959. The role of two invertebrate populations, Littorina irrorate and Orchelimum fidicinium, in the energy flow of a salt marsh ecosystem. Ph.D. thesis, University of Georgia, Athens.
Smith, K., R.R. Good, and N.F. Good. 1979. Production dynamics for above-belowground components of a New Jersey Spartina alterniflora tidal marsh. Estuarine Coastal Shelf Science 9:189–201.
Stout, J.P. 1978. An analysis of annual growth and productivity of Juncus roemerianus Sheele and Spartina alterniflora Loisel in coastal Alabama. Ph.D. dissertation, North Carolina State University, Raleigh.
Stroud, L.M. 1976. Net primary production of belowground material and carbohydrate patterns of two height forms of Spartina alterniflora in two North Carolina marshes. Ph.D. Dissertation, North Carolina State University, Raleigh.
Turner, R.E. 1976. Geographic variations in salt marsh macrophyte production: a review. Contributions in Marine Science 20:47–68.
Turner, R.E., and J.G. Gosselink. 1975. A note on standing crops of Spartina alterniflora in Florida and Texas. Contributions in Marine Science 19:113–118.
Turner, R.E., E.M. Swenson, and C.S. Milan. 2001. Contrasting organic and inorganic content in recently accumulated salt marsh sediments. In Concepts and controversies in tidal marsh ecology, eds. M. Weinstein, and D.D. Kreeger, 583–595. Dordrecht, The Netherlands: Kluwer Academic Publishers.
Turner, R.E., E.M. Swenson, C.S. Milan, J.M. Lee, and T.A. Oswald. 2004. Below-ground biomass in healthy and impaired marshes. Ecological Research 19:29–35.
United States Environmental Protection Agency. 2002. Methods for evaluating wetland conditions: vegetation-based indicators of wetland nutrient enrichment, Office of Water, U. S. Environmental Protection Agency, Washington, DC. EPA-822-R-02-024.
Valiela, I., J.M. Teal, and N.Y. Persson. 1976. Production and dynamic experimentally enriched salt marsh vegetation: belowground biomass. Limnology and Oceanography 21:245–252.
Verhoeven, J.T.A. 1996. Nitrogen- or phosphorus-limited growth in herbaceous, wet mire vegetation: Relations with atmospheric inputs and management regimes. Trends in Ecology and Evolution 11:494–497.
Visser, Y.M., and C.A. Sasser. 2006. The effect of multiple stressors on salt marsh end-of-season biomass. Estuaries and Coasts 29:331–342.
White, D.S., and B.L. Howes. 1994. Translocation, remineralization, and turnover of nitrogen in the roots and rhizomes of Spartina alterniflora (Graminae). American Journal of Botany 81:1225–1234.
Whittaker, R.H. 1975. Communities and ecosystems, 2nd edition. New York: Macmillan.
Acknowledgments
We thank J. Baustian, A. Darby, J. M. Lee, C. Milan, T. A. Oswald, J. Spicer, and E. M. Swenson for their assistance in the field sampling and laboratory support and L. Blum for her inspired mentoring. Two anonymous reviewers made many helpful comments on a draft manuscript. Support was provided by the NOAA Coastal Ocean Program MULTISTRESS Award No. NA16OP2670 to Louisiana State University and a Louisiana Board of Regents Fellowship to F.A.D.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Darby, F.A., Turner, R.E. Below- and Aboveground Spartina alterniflora Production in a Louisiana Salt Marsh. Estuaries and Coasts: J CERF 31, 223–231 (2008). https://doi.org/10.1007/s12237-007-9014-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-007-9014-7