Abstract
Mosquito-repellent textiles are in high demand as a disease-prevention solution for mosquito bites as mosquito-borne diseases proliferate over the world. In, this study selenium nanoparticles (SeNPs) conjugated by synthesized pyridotriazolprymidine derivative VI were utilized to treat 100% cotton, 65/35% cotton/PET, viscose and gauzy cotton fabrics, for mosquito repellency application. The synthesized triazole derivative and the nanoparticles were confirmed by different instrumental techniques. The presence of SeNPs@triazole derivative on the fabrics surface was examined using FT-IR, SEM and EDX. The modified fabrics were tested for repellency to Culex pipiens mosquitoes and the results indicated 100% repellency for 2 h and complete mortality after 1 day, the samples of fabrics are more durable by the ratio of 100% after the 9th washing but decreased for the 10th washing. The cytotoxicity of the treated fabrics showed enhanced compatibility with the human normal fibroblast cell line (BJ1) with no toxic effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Mosquito-borne illnesses are common in more than one hundred nations throughout the world, affecting more than seventy million people annually and resulting in health issues as they serve as vectors for harmful infections, causing millions of diseases and deaths in both humans and animals each year [1]. Culex has a fairly wide distribution in Egypt [2], and is regarded as the core vector of Rift Valley fever [3, 4], Wuchereria bancrofti and the Western Nile virus [2]. In addition, mosquitoes cause noising for human especially children. So, researchers search to solve mosquitoes problem in different ways such as pesticide [5, 6], and insect repellents by modification of fabrics [7,8,9]. Fabrics such as curtains, military uniforms, nets, and garments, can be coated or incorporated with insecticides or repellents materials and provide protection against mosquito [4]. However, these methods have low efficiency because the insecticide or repellents agent are detached upon washing resulting in low durability of the treated fabrics [10]. Modified fabric with good anti-mosquito substance has been prepared utilizing metal-organic framework [11]. Using of nanoparticles in the modification of fabrics was reported in several manuscripts [12,13,14]. Recently, nanoparticles were used in the modification of fabrics and it gives high and promising activity as antifungal, antibacterial, UV-stability, insect repellent, and anti-firing agent [7, 13, 15].
Selenium nanoparticle (SeNPs) is an important new material due to their low toxicity, bio-compatibility, its collaboration with proteins when compared to inorganic or organic selenium [16,17,18]. Selenium nanoparticle (SeNPs) are used for different applications such as photoelectric application, biology and medicine [19,20,21]. Synthesis of SeNPs can be performed using different techniques including chemical reduction, microwave-assisted technique, laser ablation, electro-deposition technique, solvo-thermal and green synthesis. [22,23,24]. Testing of inorganic nanoparticles such as SeNPs towards mosquito’s activity was studied. Green synthesized SeNPs using Clausena dentata plant leaf extract indicated high mortality using low concentrations of SeNP—extract; 240, 104 and 99 mg/L towards three mosquitos vector; A. stephensi, A. aegypti, and C. quinquefasciatus, respectively [25]. In another study SeNPs- conjuagated with—prickly pear peel waste extracts was synthesized and tested for the fecundity and hatchability of C. pipiens mosquito that showed decreased activity as applied concentrations increased [26].
Several fabrics were modified using organic compounds as antifungal and antibacterial heterocyclic compounds [27,28,29]. Triazole derivatives are a kind of heterocyclic organic compound that can be found in natural products or synthesized having diverse pharmacological characteristics such as antibacterial, anti-cancer, anti-tubercular and anti-malarial activities, and can be developed for new applications [30,31,32].
Many research papers tested heterocyclic compounds for their activity towards mosquito control application their results indicated high activity of these materials. A set of potentially effective “N-acylpiperidine derivatives” that work as mosquito repellent were prepared by the reaction of “acylbenzotriazoles” with “piperidines” followed by screening them as mosquito repellents by testing their repellency thru exposing them to cloth patches worn on the arms of human volunteers [33]. Several compounds had a repellent effect on mosquitoes that lasted up to three times longer than “N,N-diethyl-m-toluamide” (DEET), which is the most commonly used commercial repellent. A cationic dye with a heterocyclic structure was synthesized and utilized to treat the acrylic fabric to act as mosquito-repellent material, where 100% repellency against “Anopheles female mosquitoes” is obtained by this treated fabric [34].
Textile fabrics can be modified by nanoparticles using various finishing treatments including chemical and physical vapor deposition, dip coating, spraying, padding, printing, coating and plasma-induced grafting [35, 36]. Textile fabric can be modified for different applications such as water/oil repellence, fire resistant, UV protection, antimicrobial, and insect repellent [37,38,39]. The present study has planned to combine selenium nanoparticles conjugated with pyridotriazolprymidine derivative VI for modifying different types of fabrics 100% cotton, 65/35% cotton/PET, viscose and Gauzy cotton fabrics. The effect of fabric modification on repellency to Culex pipiens Mosquito, and adulticide activity in addition to fabric durability will be explored.
2 Experimental
2.1 Materials
3-glycidyloxypropyltrimethoxysilane (GPTMS, 99%) and 2-propanol (99%) were supplied from Aldrich. 1-Methlylimidazole was provided by BASF. Fixapret® ECO (low formaldehyde react resin based on dimethylolethylene urea (DMDHEU) (BASF, Germany), 100% cotton 120 g/m2, Plain weaves of 65/35% cotton/polyester (PET) 160 g/m2, viscose 110 g/m2 and gauzy cotton fabrics were provided from Misr Spinning and Weaving Co., Mehalla El Kobra, Egypt. Hydrochloric acid (HCl) and other fine chemicals are of analytical grade. Selenious acid (H2SeO3) and ascorbic acid were delivered from (Aldrich). All solvents were dried before being used.
2.2 Chemistry
Thin-layer chromatography (TLC) was used to monitor each reaction. UV fluorescent silica gel (Merck Kiesel gel 60 F245) was utilized with aluminum sheets. Iodine vapour and a UV lamp were used to see it. Melting points of the prepared triazole derivative compounds were explored using an electro-thermal IA 9100 apparatus from Shimadzu, Japan. The Microanalytical Centre (Faculty of Science, Cairo University, Egypt) performed microanalyses. The Microanalytical Centre (Faculty of Science, Cairo University, Egypt) performed microanalyses. On the JASCO FT/IR 6100 Japan spectrometer (National Research Centre, Egypt), IR spectra were recorded using KBr discs. Using JEOL EX-270 ran for 1H NMR at 270 MHz, and run for 13C NMR at 126 MHz spectrometers. Tetramethylsilane (TMS), the internal standard, was used to monitor chemical changes, which were then quantified in parts per million (ppm). In hertz (Hz), J, the coupling constant, is expressed. Mass spectra were measured using the SSQ 7000 spectrometer on the GCMS Finnigan mat. To capture UV–Vis, we employed a (Shimadzu spectrophotometer). High-Resolution Transmission Electron Microscopy (HR-TEM, JEOL, JEM-2100) was utilized to investigate the size and shape of nanoparticles. Dynamic light scattering (Zeta Sizer, Nano-ZS, Malvern Ltd., UK) was used to assess the size and zeta potential of the synthesized nanoparticles. Scanning electron microscopy (Quanta, FEG, 250, SEM) was used to explore the surface topography of the treated and untreated fabrics. Energy dispersive X-ray spectroscopy (EDAX -AMETEK Inc.; Mahwah, NJ, USA) was used to examine the type of elements and their rough content in the tested samples.
2.3 Synthesis of compound IV: Ethyl 7-amino-5-(5-methylfuran-2-yl)-[1,2,4]triazolo-[1,5-a]pyrimidine-6-carboxylate
In a dried round flask, 3-amino-1,2,4-triazole (I) (0.083 g, 0.001 mol), was added to 5-methyl furfuraldehyde (II) (0.011 g, 0.001 mmol) in ethanol (25 ml) stirred for 2 h. Ethyl cyanoacetate (III) (0.001 mol) and a few drops of trimethylamine were added to the reaction, the color changed to yellow, and TLC was used to observe the reaction. The reaction was refluxed over 24 h. The obtained precipitate was filtered off, washed and recrystallized using ethanol.
Yellow powder, product yield 82%; melting point 163–164 °C, IR (KBr, ν, cm-1); 3420 (NH2), 1714 (C = O), 1H NMR (DMSO-d6, δ ppm): 8.01 (singlet, 1H, -CH, triazole ring), 7.45–7.46 (doublet, 1H, J = 2.5 Hz, -CH, furan ring), 6.55–6.56 (doublet, 1H, J = 2.5 Hz, -CH, furan ring), 4.24–4.30 (quartet, 2H, J = 5.3 Hz, -CH2CH3), 3.35 (singlet, 2H, -NH2, D2O exchangeable), 2.51 (singlet, 3H, -CH3), 1.27–1.30 (triplet, 3H, J = 5.3 Hz, -CH2CH3); MS (m/z, %) (287.30, 31.23%); Anal; Calcd; for, C13H13N5O3 (287.28): C, 54.35; H, 4.56; N, 24.38; Found: C, 54.25; H, 4.40; N, 24.60%.
2.4 Preparation of derivative V: Ethyl7-acetamido-5-(5-methylfuran-2-yl)-[1,2,4]triazole [1,5-a]pyrimidine-6-carboxylate
In a flask with a circular bottom, derivative IV (2.87 g, 0.01 mol) was added to acetic anhydride (10 mL). After refluxing the reaction for 4 h, till the starting materials were finished. The reaction mixture was poured into (100 ml) water after cooling. Filtration was used to collect the solid, which was dried and recrystallized using ethanol.
The product was brown crystals with a yield about 60%; mp 125–127 °C, IR (KBr, ν, cm−1); 3436 cm−1 related to an amine group (NH), 1711 and 1659 cm−1 corresponding to carbonyl bond (C=O), and 1054 cm−1 related to ring double bond (C=C); 1HNMR (DMSO-d6, δ ppm) (Fig. 1a), 8.99 (singlet, 1H, NH, D2O exchangeable), 7.93 (singlet, 1H,CH), 7.34–7.33 (doublet, 1H, J = 2.5 Hz, -CH, furan ring), 6.48–6.47 (doublet, 1H, J = 2.5 Hz, -CH, furan ring), 4.23 (quartet, 2H, J = 5.4 Hz, -CH2), 2.36 (singlet, 3H, -OCH3), 1.88 (singlet, 3H, -CH3), 1.23 (triplet, 3H, J = 5.4 Hz, CH3). 13C NMR (DMSO-d6, δ ppm), 172.49, 168.01, 163.05, 161.21, 157.84, 157.84, 147.51, 144.79, 138.96, 116.05, 112.03, 95.04, 62.40, 23.88, 22.62, 21.46; MS (m/z, %) (329, 21%); Anal. Calcd. C15H15N5O4 (M. wt; 329.32), C, H, N, 54.71; 4.59; and 21.27, respectively; Found: C, H, N, 54.60; 4.66; and 21.19, respectively.
2.5 Preparation of derivative VI: 7-(3-methoxyphenyl)-8-methyl-5-(5-methylfuran-2-yl)pyrimido[5,4-e][1,2,4]triazolo[1,5-a]pyrimidin-6(7H)-one
For 6 h the mixture of derivative V (3.29 g, 0.01 mol) and p-Anisidine (0.01 mmol) in ethanol (20 mL) was refluxed, and TLC was used for the monitoring of the reaction. To obtain the desired product VI, the obtained precipitate was filtered, washed and recrystallized using ethanol.
Brown crystals, product yield 68%; Novel Sono-synthesized point 206–208 °C; IR (KBr, ν, cm−1); 1709 (C = O), 1066 (C=C); 1H NMR (DMSO-d6, δ ppm) (Fig. 1b): 8.07 (singlet, 1H, -CH, triazole ring), 7.92–8.03 (multiplet, 4H, aromatic protons), 7.55–7.58 (doublet, 1H, J = 2.5 Hz, -CH, furan ring), 6.50–6.53 (doublet, 1H, J = 2.5 Hz, -CH, furan ring), 3.58 (singlet, 3H, -OCH3), 2.37 (singlet, 3H, -CH3), 2.34 (singlet, 3H, -CH3); MS (m/z, %) (388, 46%); Anal; Calcd; for, C20H16N6O3 (388.39): C, 61.85; H, 4.15; N, 21.64; Found: C, 61.70; H, 4.02; N, 21.88%.
2.6 Synthesis of SeNPs using triazole derivative (VI)
In distilled water (80 mL), selenious acid (H2SeO3, 0.013 gm., 0.01 mmol.) was solublized. In 10 mL dimethyl sulfoxid, derivative VI (0.01 g) was dissolved separately and added to the selenious acid solution. At 60 °C the mixture of derivative VI and selenious acid was fixed under stirring for 1 h. As a catalyst, 200 µL ascorbic acid (40 mM) was utilized. The ruby-red SeNPs were formed as suspension. UV–vis spectrophotometer, dynamic light scattering, and TEM were used to validate and characterize the production of SeNPs.
2.7 Hydrolysis of 3-Glycidyloxypropyltrimethoxysilane (GPTMS)
The silica sol form was produced by adding 3-Glycidyloxypropyltrimethoxysilane (GPTMS, 10 ml) to 180 ml propanol and 1.22 ml HCl (0.01 M) while stirring at room temperature for an hour. The hydrolyzed combination that resulted was used in finishing the tested fabrics.
2.8 Fabric Finishing
Samples of fabric (20 cm × 20 cm) under study; 100%, cotton 120 g/m2, 65/35%, cotton/PET 160 g/m2, viscose 110 g/m2 and gauzy cotton fabrics, were immersed in the synthesized finishing bath containing100 ml hydrolyzed GPTMS as a cross-linking agent to make frame network and 20 mg from VI conjugation with SeNPs under magnetic stirring for 10 min. to get good solubility, then few drops of 1-Methyl imidazole was added. Fabric samples were squeezed to 80% wet pick-up, then dried at 100 °C for 3 min. followed by curing at 140 °C for 90 s. All tested fabrics were washed using pure water to remove the unreacted constituents followed by drying at r.t for a day.
2.9 Repellent and Adulticidal Activity
2.9.1 Tested Mosquitoes
The Medical Entomology Institute provided the Culex pipiens mosquitoes, which were then transported to the Entomology laboratory, Faculty of Science, Ain Shams University. Self-replicating colonies were generated and maintained throughout the current study, in accordance with the procedure reported by [40].
2.9.1.1 Test for Repellency Action
The experiment was performed as previously designated in the literature with minor modifications [41]. In brief, one side of a cage (30 × 30 × 30 cm3) was attached with cloth and the cage was occupied with adult mosquitos (100 individuals, 3 days old). Cages were monitored to see how long the mosquitos stayed away from the tested fabrics. The unmodified fabric was used as a control test.
2.9.1.2 Test of the Adulticidal activity
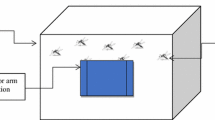
Adult Culex pipiens (3–4 days old) were exposed to various fabrics with synthetic material woven into them. Mortality counts were carried out at 24 and 48 h. For control adulticidal bioassays; insect tests were conducted in a WHO tube (Fig. 2) without the use of fabrics [7]. For adult feeding, a 10% glucose solution was supplied to each test replicate.
2.9.2 Durability of the Treated Fabrics Towards Longevity and Adulticidal Activity After Washing
The tested fabrics were washed with 2 g/l detergent and liquor ratio 1/20 from the weight of the fabric for 15 min at 40 °C with stirring then washed under tap water and let it dry. Evaluation of the adulticidal activity for the tested fabrics by the method mentioned before was performed.
2.9.3 Cytotoxic Effect of the Treated Fabrics
The modified textiles were tested for their cytotoxic effect on human normal fibroblast cel (BJ1) using an in vitro bioassay conducted at “the Bioassay-Cell Culture Laboratory, National Research Centre, Egypt”. The cell viability assessment was conducted by measuring the reduction of yellow “MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide)” to purple formazan, a process that is dependent on mitochondrial activity (Mosmann 1983). The aforementioned procedures were conducted in a sterile environment utilizing “a Laminar flow cabinet biosafety class II level (Baker, SG403INT, Sanford, ME, USA)”. The cells were placed in a solution containing “DMEM-F12” media, 1% antibiotic–antimycotic mixture (10,000U/ml “Potassium Penicillin”, 10,000 µg/ml “Streptomycin Sulfate” and 25 µg/ml “Amphotericin B”) and 1% “L-glutamine” at 37 ºC under 5% CO2. The cells were cultivated in batches for 10 days. After that, they were seeded at a concentration of 10 × 103 cells/well in a new complete growth medium. This was done in “96-well microtiter plastic plates”, which were placed in a water-jacketed carbon dioxide incubator: at a temperature 37 ºC for 24 h under 5% CO2 “Sheldon, TC2323, Cornelius, OR, USA”. The media was removed, fresh medium (without serum) was added. The cells were then incubated either by themselves (as a negative control) or with the sample being evaluated. After incubating for 48 h, the medium was removed and 40 microliters of “MTT” salt (2.5 μg/ml) were added to each well. The cells were then incubated for an additional 4 h at 37ºC under 5% CO2. To halt the reaction and dissolve the crystals that have formed, a solution of 200μL of 10% “Sodium dodecyl sulphate” (SDS) in deionized water was introduced to each well. The mixture was then incubated at 37 ºC for duration of one night. DOX were utilised as a positive control at a concentreation of 100 µg/ml, resulting in 100% lethality under identical circumstances [42]. The measurment of absorbance was conducted using a microplate multi-well reader “Bio-Rad Laboratories Inc., model 3350, Hercules, California, USA” at a wvelength of 595 nm, with a reference wavelength of 620 nm. The percentage of change in viability was determined using to the following formula:
A probit analysis was carried for IC50 and IC90 determination using SPSS 11 program.
3 Results & Discussion
3.1 Synthesis of Compound VI
As an extension of our previous work in fabric modification using nanoparticles, we start to use heterocyclic compound in the reduction of nanoselenium and modify the fabrics [7]. The synthesis of heterocyclic compounds such as triazolopyrimidine derivatives was reported [30], we synthesized compound VI starting from amino triazole derivatives as one pot reaction, using simple adding of an equal molar amount of amino triazole I, carbonyl compounds II and active methylene compounds III. All data was exact as reported before [43, 44] for the synthesis of triazolopyrimidines (Scheme 1).
Synthesis of selenium nanoparticles in the presence of triazolopyrimidine derivatives was performed to stabilize the nanoparticle and enhance the bioactivity performance of the nanoparticles. Indicating that SeNPs have been produced and distributed throughout the aqueous solution without forming any aggregates [45]. The formation of selenium nanoparticles was instantaneously confirmed by their red color formation during the preparation process (Fig. 3). Selenium colloidal nanoparticles either solely or loaded onto the synthesized heterocyclic compounds evaluation has also been confirmed by dynamic light scattering (DLS) and transmission electron microscopy (TEM) as clearly shown in Fig. (4). As displayed in the Fig. 4 a, b; the formed blank SeNPs showed small particles size around 8 nm with narrow distribution whereas SeNPs conjugated triazole derivative showed relative higher particle size around 40 nm. Figure 4c, d; the synthesized blank SeNPs showed a spherical shape within the size range of 10–20 nm, whereas the composite SeNPs triazole derivatives were observed in a spherical shape and exhibited some aggregates. These nanoparticles ranged in size from 20 to 90 nm.
3.2 Hydrolysis of GPTMS
In the following mechanism (Scheme 2 and 3), 3-glycidyloxypropyltrimethoxysilane (GPTMS), having methoxy groups undergo hydrolysis under acidic conditions. This process resulted in the groups of hydroxyl and a network of Si–O–Si having epoxy end groups formation. The epoxy groups will react and crosslink with the fabric's functional groups. Various fabrics were treated using sol–gel method in the presence of an anti-crease agent (DMDHEU) and GPTMS as co-crosslinking material. The hydrolyzed silane will react with hydroxyl groups within fabric specimen simultaneously with triazole derivative stabilized selenium nanoparticles. This technique was used to modify the Se nanoparticles with synthesized heterocyclic derivative on the fabrics surface to fix and enhance the durability of studied fabrics. The treated and untreated fabrics were evaluated using FTIR, SEM and EDX.
3.3 FTIR Analysis
Characterization of the reactive functional groups of untreated and treated 100% cotton, 65/35% cotton/PET, viscose and cotton gauzy fabrics samples was performed using FT-IR as shown in Fig. 5. FTIR spectrum of blank cotton fabric displays characteristics peaks related to the cellulose molecules at 3310 cm−1 related to –OH stretching, 2900 cm−1 attributed to the –CH2 stretching vibration and 1320 cm−1 related to H–O-C symmetric bending vibration. IR spectra for treated fabrics indicated characteristics peak at 1730 cm−1 corresponding to -C = O in the function group in the suggested mechanism. The peak around 1120 cm−1 attributed to the Si–O bonds of treating media. By comparison of untreated and treated fabrics they showed the same peaks with a slight shift in some peaks due to the low concentration of treating chemicals used for modifying the fabrics. The peak near 3450 cm−1 is the stretching vibration absorption peak of O–H, due to the opening of the epoxy group results in the formation of a hydroxyl group, and the absorption peak at 1100 cm−1 indicate the -C–O–C-, the FTIR spectrum analysis confirms the presence of various functional groups including -OH, -CH3, -C–O–C-, -Si–O-, OCH3 and -NH groups These functional groups contribute to the desired properties.
3.4 Scanning Electron Microscope
SEM images of untreated 100% cotton, 65/35% cotton/PET, viscose and gauzy cotton fabrics samples are shown in Fig. 6. The blank untreated fabrics morphologies (Fig. 6 left) showed a clean and smooth surface with straight fibers arranged in parallel directions. Where the treated samples Fig. 6 right displayed deposited thin layer coats on the fiber surfaces with few deposits of modifying components. The network of the coat consisted of GPTMS/DMDHEU/SeNPs with various amount according to the nature of the fabrics. The appearance of the treated fabric was noticeably altered from the unmodified one. The elemental analysis of the viscose fabric surface (as an example of tested fabrics) before and after treatment was assessed using EDX measurement as displayed in Fig. 7. The viscose fabrics showed carbon and oxygen elements indicating the chemical composition of this fabric. After treatment of viscose fabric well characteristics peaks for selenium and silicate ions obviously appeared with concentrations of 4.9 and 1.71 wt %, respectively. The presence of selenium and silicate ions in EDX measurement confirmed the successful modification process of the tested fabric.
3.5 Repellency Action
The repellency of the treated and treated fabrics was assessed using the Cage test and WHO tube. The adult mosquitoes standing away from the cadge edge fixed with the treated fabrics used for experiments. The results for the longevity of the tested material on different types of fabrics showed a short period of repellency effect not extended for more than 2 h to all tested fabrics [11, 38, 39]. These results may be due to the stability of the triazole derivative and SeNPs with no volatile or penetrating odor. These results indicated that the novel tested compound has a moderate effect as a repellent action against mosquitoes.
3.6 Adulticidal Bioassay
The tested heterocyclic compound conjugated with selenium nanoparticles uploaded on 100% Cotton, 65/35% Cotton/PET, Viscose, and 100% cotton gauzy fabric appears adulticidal effect 100% after 24 and 48 h according to the used concentration on tested fabrics (Table 1). The effect of adult mortality may be due to the effect of selenium nanoparticles which tested before against larval stages of different genus of mosquitoes and found high activity [46, 47].
3.7 Longevity and Stability of Adulticidal Activity
The results in Table 2 showed the mortality and durability of tested material on different kinds of fabrics. The high mortality of the samples with 100% cotton fabrics and the low mortality of the samples with cotton gauzy fabrics may be due to the filaments not complying with each other. So, it has slightly contained selenium nanoparticles. But, in the case of, the different types of fabrics under study such as 100% cotton, 65/35% blended cotton /PET fabric and viscose after treatment showed high mortality after the 10th washing and then decreased to reach nearly 50% mortality after the 13th washing. These results may be attributed to the stability of the tested compound on the different types of fabrics during washing. We notice that in Table 2, the durability with washing is stable in the case of 100% cotton, 65/35% blended cotton /PET fabric and viscose after about 13th washing. This proves the mortality to reach nearly 50%, where, the numbers of washing times based on application and the utilizing field of fabrics. It can be in a cover bed or outdoors and curtains.
The prepared treated fabrics were compared with other materials previously published in the literature as shown in Table 3 and our results were promising as it was stable and durable for more than 10 cycles of washing for several kinds of fabric. Also the synthesized SeNPs triazole derivative has dual function repellent and killing action.
3.8 Cytotoxicity of the Treated Fabrics
The cytotoxicity was performed for one represented fabrics loaded with the nano-selenium conjugated with triazole derivative against a human normal fibroblast cell line (BJ1). The results are indicated in Table 3. The loaded fabric has no toxicity with negative value towards the human normal fibroblast cell line which promotes these fabrics for use as wound healing candidate. This test was performed to ensure the safety of these fabrics when in contact with human skin, however, these fabrics are targeted for outdoor curtains in hospitals and in arid zone Table 4. Cytotoxic effect of the treated fabrics against human normal fibroblast cell line (BJ1).
4 Conclusion
Our work is focused on the modification of cellulosic fabrics using selenium nanoparticle conjugated with heterocyclic triazoles (SeNPs@triazoles) for mosquito-repellent application. Treatment of four substrates of cellulosic fabrics (100% cotton, 65/35% cotton/polyester (PET), viscose and gauzy cotton fabrics) with SeNPs@triazoles has been performed using padding technique. Herein, SeNPs@triazoles were loaded into the hydrolyzed silane in the presence of anti-crease agent for coating the fabrics. Different characterization tools confirmed the successful synthesis of triazoles derivative, formation of nanoparticles and loading of this two conjugation into fabrics surface. The potential adulticidal activity of the modified fabric was in the following sequence; 100% Cotton > 65/35%Cotton/PET > Viscose > Cotton Gauzy. The mosquito mortality affected by the type of modified fabric and time of exposure. Briefly, the in-situ modification of fabric using SeNPs@triazoles suspension offers the development of a composite substance having promising anti-mosquito performance that does not involve the utilization of any harmful insecticide component.
Data Availability
Upon request, all of the data utilized and analyzed in the current study will be made available.
References
J.S. Lord, J.W. Hargrove, S.J. Torr, G.A. Vale, Climate change and African trypanosomiasis vector populations in Zimbabwe’s Zambezi Valley: a mathematical modelling study. PLoS Med. (2018). https://doi.org/10.1371/JOURNAL.PMED.1002675
M.M. El-Bahnasawy, E. Ebrahim, A. Fadil, T.A. Morsy, Mosquito vectors of infectious diseases: are they neglected health disaster in Egypt? J. Egypt. Soc. Parasitol. 43, 373–386 (2013). https://doi.org/10.21608/JESP.2013.94814
A.I. Youssef, S. Uga, Review of Parasitic Zoonoses in Egypt. Trop. Med. Health. 42, 3 (2014). https://doi.org/10.2149/TMH.2013-23
D.A. Medhat, H. Harry, Arboviruses infecting humans and lower animals in Egypt: a review of thirty years of research. J. Egypt. Public Heal. Assoc. [The] 56, 1–112 (1981)
R. Mulé, G. Sabella, L. Robba, B. Manachini, Systematic review of the effects of chemical insecticides on four common butterfly families. Front. Environ. Sci. 5, 32 (2017). https://doi.org/10.3389/FENVS.2017.00032/BIBTEX
E.H. Chio, Q.X. Li, Pesticide research and development: general discussion and spinosad case. J. Agric. Food Chem. 70, 8913–8919 (2022). https://doi.org/10.1021/ACS.JAFC.2C03821/ASSET/IMAGES/MEDIUM/JF2C03821_0005.GIF
A.A. El-Sayed, A. Amr, O.M.H.M. Kamel, M.M.T. El-Saidi, A.E. Abdelhamid, Eco-friendly fabric modification based on AgNPs@Moringa for mosquito repellent applications. Cellulose 27, 8429–8442 (2020). https://doi.org/10.1007/s10570-020-03355-8
A. Al Parvez, M.J. Hossain, M.Z. Hossain, M.S.H. Sohan, F. Hoque, M.H. Ahsan, M.S. Hoque, Mosquito repellent fabric: development and characterization of peppermint and garlic mixture finish on knitted fabric to examine mosquito repellency. Heliyon. 9, e15944 (2023). https://doi.org/10.1016/J.HELIYON.2023.E15944
M.D. Teli, P.P. Chavan, Modified application process on cotton fabric for improved mosquito repellency, 108, 915–921 (2016). https://doi.org/10.1080/00405000.2016.1204897.
V. Mb, T. Vg, P.S. Joshi, Study on larvicidal efficacy of Moringa Oleifera against the vector Anopheles Mosquitoes, Biosci. Discov. 9, 365–370 (2018). http://biosciencediscovery.com.
R.M. Abdelhameed, O.M.H.M. Kamel, A. Amr, J. Rocha, A.M.S. Silva, Antimosquito activity of a titanium-organic framework supported on fabrics. ACS Appl. Mater. Interfaces 9, 22112–22120 (2017). https://doi.org/10.1021/acsami.7b03164
S. Jadoun, A. Verma, R. Arif, Modification of textiles via nanomaterials and their applications. Front. Text. Mater. Polym. Nanomater. Enzym. Adv. Modif. Tech. (2020). https://doi.org/10.1002/9781119620396.CH6
N. Vigneshwaran, Modification of textile surfaces using nanoparticles. Surf. Modif. Text. (2009). https://doi.org/10.1533/9781845696689.164
D.K. Subbiah, S. Balasubramanian, A.J. Kulandaisamy, K. Jayanth Babu, A. Das, J.B.B. Rayappan, Surface modification of textiles with nanomaterials for flexible electronics applications. Adv. Funct. Finish. Text. (2020). https://doi.org/10.1007/978-981-15-3669-4_1
D.A. Marrez, A.E. Abdelhamid, O.M. Darwesh, Eco-friendly cellulose acetate green synthesized silver nano-composite as antibacterial packaging system for food safety. Food Packag. Shelf Life 20, 100302 (2019). https://doi.org/10.1016/j.fpsl.2019.100302
M.F. Mansour, A.H. Arisha, M. Al-Gamal, R. Yahia, A.A. Elsayed, S. Saad, K.M. El-Bohi, Moringa oleifera leaves extract ameliorates melamine-induced testicular toxicity in rats. Adv. Anim. Vet. Sci. 8, 47–54 (2020). https://doi.org/10.17582/journal.aavs/2020/8.s1.47.54
C.H. Ramamurthy, K.S. Sampath, P. Arunkumar, M.S. Kumar, V. Sujatha, K. Premkumar, C. Thirunavukkarasu, Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst. Eng. 36, 1131–1139 (2013). https://doi.org/10.1007/s00449-012-0867-1
A.P. Fernandes, V. Gandin, Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta Gen. Subj. 2015, 1642–1660 (1850). https://doi.org/10.1016/j.bbagen.2014.10.008
S. Dhanjal, S.S. Cameotra, Aerobic biogenesis of selenium nanospheres by Bacillus cereus isolated from coalmine soil. Microb. Cell Fact. 9, 52 (2010). https://doi.org/10.1186/1475-2859-9-52
S. Chhabria, K. Desai, Selenium Nanoparticles and Their Applications. Encycl. Nanosci. Nanotechnol. 20, 1–32 (2016)
S. Chaudhary, A. Umar, S.K. Mehta, Surface functionalized selenium nanoparticles for biomedical applications. J. Biomed. Nanotechnol. 10, 3004–3042 (2014). https://doi.org/10.1166/jbn.2014.1985
S.A. Wadhwani, U.U. Shedbalkar, R. Singh, B.A. Chopade, Biogenic selenium nanoparticles: current status and future prospects. Appl. Microbiol. Biotechnol. 100, 2555–2566 (2016). https://doi.org/10.1007/s00253-016-7300-7
N. Srivastava, M. Mukhopadhyay, Biosynthesis and structural characterization of selenium nanoparticles mediated by Zooglea ramigera. Powder Technol. 244, 26–29 (2013). https://doi.org/10.1016/j.powtec.2013.03.050
S. Ramya, T. Shanmugasundaram, R. Balagurunathan, Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J. Trace Elem. Med. Biol. 32, 30–39 (2015). https://doi.org/10.1016/j.jtemb.2015.05.005
P. Sowndarya, G. Ramkumar, M.S. Shivakumar, Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif. Cells Nanomed. Biotechnol. 45, 1490–1495 (2017). https://doi.org/10.1080/21691401.2016.1252383
A.H. Hashem, T.A. Selim, M.H. Alruhaili, S. Selim, D.H.M. Alkhalifah, S.K. Al Jaouni, S.S. Salem, Unveiling antimicrobial and insecticidal activities of biosynthesized selenium nanoparticles using prickly pear peel waste. J. Funct. Biomater. (2022). https://doi.org/10.3390/jfb13030112
H.F. Qian, X.L. Zhao, Y. Dai, W. Huang, Visualized fabric discoloration of bi-heterocyclic hydrazone dyes. Dye. Pigment. 143, 223–231 (2017). https://doi.org/10.1016/J.DYEPIG.2017.04.046
X. Dong, T. Xing, G. Chen, Durable antipilling modification of cotton fabric with chloropyrimidine compounds. Polymer 11, 1697 (2019). https://doi.org/10.3390/POLYM11101697
N. Ocal, A. Aker, H.N. Ergindemir, A. Hamitbeyli, Synthesis of novel heterocyclic Imine type UV absorbers for application on cotton based textile materials. J. Chem. (2016). https://doi.org/10.1155/2016/6387305
E.A. Elhefny, S. Swelam, Soliman, F.A. Elhag, A.A. El Rashedy, A.E. Soliman, Synthesis, characterization, antitumor evaluation and molecular docking of some triazolotriazine Derivatives, J. Pharma Res. 79–87 (2014). www.jprinfo.com.
A.A. El-Sayed, E.B. Pedersen, N.Y. Khaireldin, Thermal stability of modified i-motif oligonucleotides with naphthalimide intercalating nucleic acids. Helv. Chim. Acta 99, 14–19 (2016). https://doi.org/10.1002/hlca.201500140
J. Světlík, V. Kettmann, The chameleon-like behaviour of 3-amino-1,2,4-triazole in the Biginelli reaction: unexpected formation of a novel spiroheterocyclic system. Tetrahedron Lett. 99, 14–19 (2016). https://doi.org/10.1002/hlca.201500140
A.R. Katritzky, Z. Wang, S. Slavov, M. Tsikolia, D. Dobchev, N.G. Akhmedov, C.D. Hall, U.R. Bernier, G.G. Clark, K.J. Linthicum, Synthesis and bioassay of improved mosquito repellents predicted from chemical structure. Proc. Natl. Acad. Sci. U. S. A. 105, 7359–7364 (2008). https://doi.org/10.1073/pnas.0800571105
A. Singh, J. Sheikh, Synthesis of a novel cationic dye to impart mosquito-repellent and UV protection to an acrylic fabric. ACS Omega 8, 10214–10224 (2023). https://doi.org/10.1021/acsomega.2c07693
Q. Wei, Emerging approaches to the surface modification of textiles, in Surf. Modif. Text., 1st edn., ed. by Q. Wei (Elsevier, 2009), pp.318–323. https://doi.org/10.1533/9781845696689.318
N. Pan, E.G. Sun, Textile Institute (Manchester, Functional Textiles for Improved Performance, Protection and Health, 1st edn. (Woodhead Publishing, 2011)
A.E. Abdelhamid, E.H. Ahmed, H.M. Awad, M.M.H. Ayoub, Synthesis and cytotoxic activities of selenium nanoparticles incorporated nano-chitosan. Polym. Bull. (2023). https://doi.org/10.1007/s00289-023-04768-8
N.A. Ibrahim, A. Amr, B.M. Eid, multipurpose treatment of cellulose-containing fabrics to impart durable antibacterial and repellent properties. Fibers Polym. 21, 513–521 (2020). https://doi.org/10.1007/s12221-020-9221-4
N. Elhalawany, M.E. El-Naggar, A. Elsayed, A.R. Wassel, A.T. El-Aref, M.A. Abd Elghaffar, Polyaniline/zinc/aluminum nanocomposites for multifunctional smart cotton fabrics. Mater. Chem. Phys. 249, 123210 (2020). https://doi.org/10.1016/j.matchemphys.2020.123210
H.M.S. Kamel, O.M. Hassan, M.M. Abd El-Baky, S.M.M. Hafez, J.A., Synergistic and joint actions of some plant extracts on their larvicidal activity against the mosquito Ochlerotatus caspius (Diptera: Culicidae), Egypt. J. Acad. Soc. Environ. Dev. (A. Entomol. 6, 307–321 (2005). https://scholar.google.com/scholar?cluster=7753334096926294879&hl=en&oi=scholarr.
J.G. Logan, N.M. Stanczyk, A. Hassanali, J. Kemei, A.E.G. Santana, K.A.L. Ribeiro, J.A. Pickett, A.J. Mordue, Arm-in-cage testing of natural human-derived mosquito repellents. Malar. J. 9, 1–10 (2010). https://doi.org/10.1186/1475-2875-9-239
M.I. Thabrew, R.D. Hughes, I.G. Mcfarlane, Screening of hepatoprotective plant components using a HepG2 cell cytotoxicity assay. J. Pharm. Pharmacol. 49, 1132–1135 (1997). https://doi.org/10.1111/j.2042-7158.1997.tb06055.x
R. Khattab, S. Swelm, A. Khalil, A. Elsayed Abdelhamid, A. Soliman, A. El-Sayed, Novel sono-synthesized triazole derivatives conjugated with selenium nanoparticles for cancer treatment. Egypt. J. Chem. 64, 4675–4688 (2021). https://doi.org/10.21608/ejchem.2021.81154.4018
A.E. Abdelhamid, A.A. El-Sayed, S.A. Swelam, A.M. Soliman, A.M. Khalil, Encapsulation of triazole derivatives conjugated with selenium nanoparticles onto nano-chitosan for overcoming drug resistant cancer cells, Egypt. J. Chem. (2022). https://doi.org/10.21608/EJCHEM.2022.147872.6401
V.R. Ranjitha, V.R. Ravishankar, Extracellular synthesis of selenium nanoparticles from an actinomycetes Streptomyces griseoruber and evaluation of its cytotoxicity on HT-29 cell line. Pharm. Nanotechnol. 6, 61–68 (2018). https://doi.org/10.2174/2211738505666171113141010
P. Sowndarya, G. Ramkumar, M.S. Shivakumar, Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif. Cells Nanomed. Biotechnol. An Int. J. 45, 1490–1495 (2016). https://doi.org/10.1080/21691401.2016.1252383
K. Meenambigai, R. Kokila, K. Chandhirasekar, A. Thendralmanikandan, D. Kaliannan, K.S. Ibrahim, S. Kumar, W. Liu, B. Balasubramanian, A. Nareshkumar, Green synthesis of selenium nanoparticles mediated by Nilgirianthus ciliates leaf extracts for antimicrobial activity on foodborne pathogenic microbes and pesticidal activity against Aedes aegypti with molecular docking. Biol. Trace Elem. Res 2006 200(2021), 2948–2962 (2021). https://doi.org/10.1007/S12011-021-02868-Y
F. Anwar, M. Abbas, M.H. Malik, A.A. Cheema, S. Tariq, W. Afzal, A. Khan, Development of mosquito-repellent camouflage fabric using eucalyptus oil with Moringa oleifera gum. ChemEngineering. 7, 1–12 (2023). https://doi.org/10.3390/chemengineering7040064
J. Mokhtari, A. Shams-Nateri, P. Ferdosi, Synthesis and characterization of novel reactive dyes with simultaneous insect-repellent and anti-bacterial properties. Fibers Polym. 15, 1369–1374 (2014). https://doi.org/10.1007/s12221-014-1369-3
M.D. Teli, P.P. Chavan, Synthesis of reactive dye to impart mosquito repellency to nylon. J. Text. Inst. 108, 226–232 (2017). https://doi.org/10.1080/00405000.2016.1161695
A. Singh, J. Sheikh, Synthesis and application of a novel cold brand reactive dye to impart colouration, mosquito repellency, and UV protection to nylon. Arab. J. Chem. 15, 104339 (2022). https://doi.org/10.1016/j.arabjc.2022.104339
Acknowledgements
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research through the project number IFP-IMSIU-2023040. The authors also appreciate the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) for supporting and supervising this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdelhamid, A.E., Rafea, M.A., Zaki, M.E.A. et al. Textile Finishing Using Triazole Derivatives and Selenium Nanoparticles as an Anti-mosquito. Fibers Polym 25, 2065–2079 (2024). https://doi.org/10.1007/s12221-024-00551-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-024-00551-2