Abstract
Green synthesis of selenium nanoparticles (SeNPs) was achieved by a simple biological procedure using the reducing power of fenugreek seed extract. This method is capable of producing SeNPs in a size range of about 50–150 nm, under ambient conditions. The synthesized nanoparticles can be separated easily from the aqueous sols by a high-speed centrifuge. These selenium nanoparticles were characterized by UV–Vis spectroscopy, scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and elemental analysis by X-ray fluorescence spectrometer (XRF). Nanocrystalline SeNPs were obtained without post-annealing treatment. FTIR spectrum confirms the presence of various functional groups in the plant extract, which may possibly influence the reduction process and stabilization of nanoparticles. The cytotoxicity of SeNPs was assayed against human breast-cancer cells (MCF-7). It was found that SeNPs are able to inhibit the cell growth by dose-dependent manner. In addition, combination of SeNPs and doxorubicin shows better anticancer effect than individual treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the twenty-first centenary nanotechnology has become one of the promising approaches for innovations that lead to fulfill the human needs. Nanoparticles have generated intense interest because of their unique electronic, optical, photoresponsive, catalytic properties [1–4] and biomedical application [5, 6]. Rapid synthesis of nano-scale material using techniques like laser ablation, pyrolysis, lithography, chemical vapour deposition, sol–gel and electro deposition is very expensive and hazardous. Eco-friendly synthesis of nanoparticles using microbes, algae and plant materials are of great interest in the current research. Rapid biosynthesis of nanomaterials using plant materials has more advantages over other biological methods, because it is inexpensive and does not need any special conditions.
Fenugreek (Trigonella foenum-graecum L), a member of Fabaceae family, is used both as herb and spice. The plant is cultivated worldwide as a semi-arid crop. Fenugreek seeds are rich source of polysaccharide galactomannan. They are also a source of saponins such as diosgenin, yamogenin, gitogenin, tigogenin, and neotigogens [7]. Other bioactive constituents of fenugreek include mucilage, volatile oils, and alkaloids such as choline and trigonelline.
Selenium (Se) has unique properties and great potential in the field of physics, chemistry and biology [8–10]. Se has been successfully used in the production of photovoltaic cells, photographic exposure meters and xerography [11, 12]. In the past decades, many stable organic selenium compounds have been synthesized which are used as antioxidants, enzyme inhibitors, anti-tumor and anti-infective agents, cytokine inducers and immuno-modulators [13, 14]. The selenium at nano size acts as a potential chemo-preventive agent with reduced toxicity [15, 16].
A reproducible but simple method of preparation of stable selenium nanoparticles with biomedical application is still a challenge. Both reduction and oxidation techniques can be employed to prepare selenium nanoparticles. The main synthetic approach for preparing selenium nanoparticles is by chemical reduction, employing reducing agent and stabilizer. However, the use of stabilizer may hinder the normal utilization of synthesized nanoparticles in biological applications and further stabilizer may have toxic potential due to its chemical nature. The present study reports a simple phytochemical mediated green synthesis of selenium nanoparticles and evaluates its potential biological application as anticancer agent.
Materials and methods
Chemicals
Selenious acid, sodium alginate, ascorbic acid and ethanol were purchased from SRL (India). Fenugreek seeds were purchased from Nilgris super market, Puducherry, India and all other chemicals were obtained from Himedia and used as received.
Preparation and synthesis of selenium nanoparticles (SeNPs)
Preparation of fenugreek extract
Fenugreek seed were, obtained from commercial source was confirmed by Prof. Dr. P. Jayaraman, Director, Plant Anatomy Research Centre, National Institute of Herbal Science, Chennai, South India (PARC/2009/994). 1 % fenugreek extract was prepared in double-distilled water. The contents were mixed by stirring for 15 min. It was filtered using Whatman no. 1 filter paper. The filtered extract was used for the synthesis of nanoparticles.
Synthesis of selenium nanoparticles
For the ecofriendly synthesis of SeNPs, 1 ml of plant extract was mixed with 10 ml of 30 mM selenious acid solution, along with 200 μl of 40 mM ascorbic acid which was used as an initiator of reduction reaction. Standard positive control was maintained using 0.2 % sodium alginate + selenious acid and 200 μl of 40 mM ascorbic acid for the synthesis of selenium nanoparticles [17] while 1 % fenugreek extract + 200 μl of 40 mM ascorbic acid was used as negative control. The preparations were incubated at room temperature. At various time intervals, small aliquot (1 ml) of solution was used for the UV–Vis spectroscopy analysis. After 24 h of incubation, the preparation was centrifuged at 10,000 rpm for 30 min. The pellet was washed with double-distilled water and then with absolute ethanol three times, this washed ethanol pellet was dried overnight. The red SeNPs were suspended in PBS (pH 7.4) by ultra sonication and then centrifuged. The powder form of the extract was used for further analysis.
Characterization of the synthesized SeNPs
UV–Vis absorbance spectroscopy analysis
The bioreduction of the selenious acid in solution was monitored by periodic sampling of aliquots (2 ml) and measuring the UV–Vis spectra of the solution with a Shimadzu 1,700 UV–Vis spectrophotometer at wavelength ranging between 200 and 1,000 nm with a scanning speed of 1,856 nm/min. The readings were recorded at 5, 30, 60, 180, 720 and 1,440 min.
Scanning electron microscope analysis
Scanning electron microscope (SEM) images were taken for the analysis of size and shape of SeNPs (Hitachi s-3400N) with resolution of 500 nm operated at 10 kV HV mode and detectors contain secondary electron; semiconductor BSE (Quad type).
Size-distribution analysis
Size distribution of SeNPs was analyzed by dynamic light scattering (DLS) method using Malvern Particle Size Analyzer MS2000.
Fourier transform infrared spectroscopy (FTIR) analysis
For FTIR measurements, the air-dried powder form of the samples was grinded with KBr pellets and analyzed on a Thermo Nicolet model 6700 spectrum instrument in the diffuse reflectance mode operating at a resolution of 4 cm−1. To obtain good signal/noise ratio, 512 scans were recorded. The peaks obtained were plotted as % transmittance in X axis and wave number (cm−1) in Y axis.
X-ray diffraction analysis
X-ray diffraction (XRD) patterns of SeNPs were obtained using Philips Panlytical X’pert Pro X-ray diffractometer using Cu Kα (1.54059 Å) radiation with the X-ray generator operating at 45 kV and 40 mA.
Wave length dispersive X-ray fluorescence (XRF) spectrometer analysis
For XRF measurements, the air-dried powder form of the samples was grinded with boric acid and analyzed on a Bruker (Model: S4 PIONEER) WD-XRF spectrometer. Rhodium was used as the standard anode material. The tube and generator are designed for a permanent output of 4 kW. The detector is scintillation counter and proportional counter. Collimators with a low resolution (1.5–2.0°) and high resolution (0.077°) were used for the detection of light and heavy elements, respectively.
Anticancer effect of SeNPs
Morphological study
MCF-7 breast-cancer cell lines were plated in cell culture plate with 1 × 104 cells/well (400 μl of culture medium). These cells were treated with synthesized selenium nanoparticles (green synthesis and stand methods) at the concentration of 25, 50, 75, and 100 μg/ml. After 24-h treatment at 37 °C and 5 % CO2, the cells were observed under the phase contrast microscope.
Lactate dehydrogenase assay
Lactate dehydrogenase (LDH) was estimated using a kit from Stanbio Laboratory (Boerne, Texas, USA).
MTT assay
Cell proliferation was measured by MTT assay [3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide, Himedia, India]. MTT was dissolved in sterile PBS at 5 mg/ml, sterilized by passing through a 0.22-μm filter and stored in the dark at 4 °C. MCF-7 cells (5 × 103 cells/well) were placed in 200 μl of culture medium and incubated overnight. After 24 h, cultures were treated with 0, 25, 50, 75 and 100 μg/ml of SeNPs in triplicates. MTT reagent (20 μl) was added at different time points and then incubated at 37 °C for 4 h. 100 μl of acid isopropanol (0.04 N HCl in isopropanol) was then added to the wells, and the dark crystals were dissolved by mixing thoroughly. The absorbance was measured at 570 and 630 nm.
Determination of apoptosis
Apoptotic cells were detected by staining with the DNA binding fluorescent dye, Hoechst 33342. Cells grown on glass coverslips (1 × 106 cm−2) were treated with SeNPs for indicated time points, washed with ice-cold PBS, and fixed in 4 % paraformaldehyde for 30 min at room temperature. After washing with cold PBS, the cells were stained with Hoechst 33342 in PBS (5 μg/ml) for 1 min and washed in deionized water. The cells were mounted in 50 % glycerol containing 20 mM citric acid and 50 mM disodium orthophosphate, and the nuclear staining was evaluated by fluorescent microscopy (Eclipse E600, Nikon). Those cells with brightly stained condensed chromatin or nuclei were identified as apoptotic cells. Ten fields per slide (each in triplicate) were assessed for determining the number of apoptotic cells.
Statistical analysis
Statistical significance between the groups was determined by one-way ANOVA followed by a test for linear trend. A p value of <0.05 was considered statistically significant.
Results and discussion
Phytochemical screening was performed in aqueous extract of fenugreek seed. The present study shows the presence of alkaloids, flavonoids, amino acids and protein, carbohydrate, cardiac glycosides and saponins in aqueous extract of fenugreek seed (Supplementary Table 1). It is rich in polysaccharide galactomannan, having reducing functional groups [18], which might have played a role in reducing selenious acids to SeNPs. Fenugreek is also known to contain flavonol and phenol compounds [19], which might have acted as a stabilizer, similar to that of sodium alginate, a commercially available chemical [17]. It has been shown that plant extract containing phenol and flavonol derivatives act as reducing agents and nanoparticle stabilizer [20]. FTIR spectrum of fenugreek seed extract shows the presence of various functional groups (Supplementary Fig. 1).
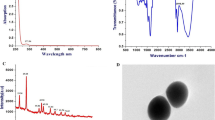
Biosynthesis of SeNPs from selenious acid was confirmed by UV–Vis spectra studies, due to color change from colorless selenious acid to ruby red color (SeNPs), having absorption maximum (λ max) at 200–400 nm. This color change may be due to the surface plasma resonance (SPR) with a broad peak, and this peak intensity of color change increased with the time from 5 to 1,440 min (Fig. 1a). The small peaks observed in the UV region (supplementary Fig. 2) may be due to the small organic molecules present in reaction mixture. As shown in Fig. 1b, the transparent and red mixed solution after the preparation could be observed. The red color of the Se colloids implied that selenium form was either amorphous or monodinic, since trigonal selenium is known to be black [18]. Comparing with the chemical method, fenugreek seed extract-mediated synthesis was similar.
a UV–Visible absorbance spectrum of SeNPs. b Photographs of different combinations of extract after 24 h. 1 30 mM selenious acid + 40 mM ascorbic acid; 2 1 % fenugreek extract + 40 mM ascorbic acid + 30 mM selenious acid; 3 1 % fenugreek extract + 30 mM selenious acid; 4 30 mM selenious acid; and 5 0.2 % sodium alginate + 40 mM ascorbic acid + 30 mM selenious acid
The size of the nanoparticles synthesized by fenugreek and ascorbic acid-mediated reduction of selenious acid were shown in Fig. 2. The SEM images of selenium nanoparticles synthesized by different combinations were oval in shape with smooth surface. The particle size was found to be around 50–150 nm. The average size of the green synthesis SeNPs was slightly larger than that of the chemical method (Fig. 2b). The size-distribution analysis is carried out by dynamic light scattering method, which correlates with SEM analysis (Supplementary Fig. 3). However, green synthesis of SeNPs was quite efficient compared to the chemical method, which may be due to the presence of various bioreducing functional groups in fenugreek [18, 19].
FTIR spectrum studies were carried out to investigate the possible bioreducing functional groups present in different combination of extracts. FTIR spectrum was recorded from 500- to 4,500-cm−1 wavelength region. FTIR spectrum of chemical (0.2 % sodium alginate + 40 mM ascorbic acid + 30 mM selenious acid) and biological (Fenugreek) reduction of selenious acid with ascorbic acid has vibrational and stretching of function at wave numbers 2,356.2, 1,618.7, 1,284.7, 2,360.1, 1,723.2 and 1,638 cm−1 were recorded. These peaks correspond to stretching and vibrational bending of C=C, NH2, COOH, CH2 and C=O, thus, indicating the presence of reducing groups responsible for the reduction of SeNps as shown in Fig. 3.
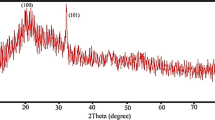
The crystal structure and the phase composition of selenium nanoparticles were determined, using XRD techniques shown in Fig. 4. The XRD pattern suggests that the sample is nanocrystalline in nature and matches very well with that of the standard selenium powder confirming the formation of selenium particles. The calculated lattice constants are a = 4.363 Å and c = 4.952 Å, which are in agreement with the literature value (JCPDS File No. 06-0362). We observed some noise background in biological method of SeNPs (Fig. 4a), which may be due to the presence of additional bioactive compound present in the fenugreek seed extract. The XRD pattern of powder shows that there is no requirement of post annealing to get desired crystalline phase.
Table 1 shows the different elements present along with selenium by XRF analysis; since XRF analysis can measure only element form and does not estimate salt or complex form. The data obtained clearly show the increased level of selenium element in fenugreek extract + 40 mM ascorbic acid + 30 mM selenious acid solution when compared to other combination of extracts. These results clearly indicate the reduction of selenium from selenious acid.
MCF7 cell lines were treated with different concentrations (25–100 μg/ml) of SeNPs synthesized by biological method, chemical method and selenious acid alone. As anticipated, SeNPs (chemical and biological method) increased (P < 0.01; P < 0.05) LDH release and decreased cell viability (P < 0.01; P < 0.05) in concentration dependent manner (Fig. 5a, b). Lowest (25 μg/ml) and highest (100 μg/ml), SeNPs concentrations used in this study exhibits statistically significant increased (P < 0.05) release of LDH and decreased (P < 0.05) cell viability at 36 and 6 h, respectively. We could not observe any different effect of SeNPs based on the synthetic process, i.e., chemical or biological method, they show similar biological effect. High concentration and longer duration treatment of selenious acid alone caused some cytotoxic effect as observed by LDH release and decrease (P < 0.05) in cell viability. As seen in Fig. 6 and Supplementary Fig. 4, the mode of cell death might be due to apoptosis [21, 22]. Even though we have well-characterized SeNPs, to rule out the possibility that phytochemical compounds present in fenugreek may attach to SeNPs, which may cause some effect in MCF 7 cells, we processed fenugreek extract alone in the same way as harvesting SeNPs (details are given in material and method section). The residue was exposed to MCF 7 cells. We did not observe any change in LDH and cell viability. Throughout the experiment we used doxorubicin as (5 μg/ml) a positive control. As seen in Fig. 7, 5 μg/ml of doxorubicin caused more than 50 % cell death at 24 h, whereas 2.5 μg/ml of doxorubicin caused only less than 20 % cell death at 24 h. Combination of SeNPs (25 μg/ml) + doxorubicin (2.5 μg/ml) showed more than 50 % cell death at 24 h. Furthermore, we tested whether selenious acid also had the same effect as SeNPs. Combination of selenious acid + doxorubicin did not have significant effect as SeNPs. Multidrug resistance is a common problem in cancer chemotherapy. Our observations indicate that individual drug either SeNPs or doxorubicin can initiate less extent of cell death. In the present study, we found that SeNPs can augment the cytotoxicity of doxorubicin. These results may be attributed to the fact that these SeNPs could readily interact with doxorubicin and form so-called conjugated nanocomposites, which could facilitate the cellular uptake of doxorubicin and, thus, enhance the cytotoxic effect on MCF 7 cells.
a Lactate dehydrogenase release in control and experimental MCF7 cells. Cells were treated with SeNPs [prepared by chemical (Ex. 1), and biological method (Ex. 2)], and selenious acid (Ex. 3) alone for various periods of time, after the experimental period LDH activity was measured. 1 P < 0.01; 2 P < 0.05, compared with respective control. b Cell death by MTT assay in control and experimental MCF7 cells. Cells were treated with SeNPs [prepared by chemical (Ex. 1), and biological method (Ex. 2)], and selenious acid (Ex. 3) alone for various periods of time, after the experimental period, MTT assay was performed. 1 p < 0.01; 2 p < 0.05, compared with respective control
The present study shows that the synthesis of SeNps is an eco-friendly approach using fenugreek seed extract compared to the other synthesis method. SeNps were characterized by UV–Vis spectrophotometer, SEM and FTIR analysis. To our knowledge, this study is the first to report that phytochemical present in the fenugreek seed extract can reduce metal complexes into metal nanoparticles. FTIR spectra showed that the vibrational, bond bending and stretching of different functional groups were responsible for selenious acid reduction. The significant MCF-7 cancer cell death was observed at 6 and 36 h in 100 μg/ml and 25 μg/ml, respectively. Combination of SeNPs with doxorubicin showed better anti-cancer effect when compared to individual ones. It has been observed that SeNps induces MCF 7 cell death through apoptosis. However, further studies are needed to elucidate the mechanisms involved.
References
El-Sayed MA (2001) Some interesting properties of metals confined in time and nanometer space of different shapes. Acc Chem Res 34:257–264
McConnell WP, Novak JP, Brousseau LC, Fuierer RR, Tenent RC, Feldheim DL (2000) Electronic and optical properties of chemically modified metal nanoparticles and molecularly bridged nanoparticle arrays. J Phys Chem B 104:8925–8930
Liu C, Zhang ZJ (2001) Size-dependent superparamagnetic properties of Mn spinel ferrite nanoparticles synthesized from reverse micelles. J Chem Mater 13:2092–2096
Moreno-Mañas M, Pleixats R (2003) Formation of carbon–carbon bonds under catalysis by transition-metal nanoparticles. Acc Chem Res 36:638–643
Lanone S, Boczkowski J (2006) Biomedical applications and potential health risks of nanomaterials: molecular mechanisms. Curr Mol Med 6:651–663
Garnett MC, Kallinteri P (2006) Nanomedicines and nanotoxicology: some physiological principles. Occup Med 56:307–311
Sauvaire Y, Baissac Y, Leconte O, Petit P, Ribes G (1996) Steroid saponins from fenugreek and some of their biological properties. Adv Exp Med Biol 405:37–46
Drake EN (2006) Cancer chemoprevention: selenium as a pro-oxidant not an antioxidant. Med Hypotheses 67:318–322
Manna L, Scher EC, Alivisatos AP (2000) Synthesis of soluble and processable rod-, arrow-, teardrop-, and tetrapod-shaped CdSe nanocrystals. J Am Chem Soc 122:12700–12706
Poborchii VV, Kolobov AV, Tanaka K (1999) Photomelting of selenium at low temperature. Appl Phys Lett 74:215–217
Wang G, Yang X, Qian F, Zhang JZ, Li Y (2010) Double-sided CdS and CdSe quantum dot co-sensitized ZnO nanowire arrays for photoelectrochemical hydrogen generation. Nano Lett 10:1088–1092
Leonard KA, Hall JP, Nelen MI, Davies SR, Gollnick SO, Camacho S, Oseroff AR, Gibson SL, Hilf R, Detty MR (2000) A selenopyrylium photosensitizer for photodynamic therapy related in structure to the antitumor agent AA1 with potent in vivo activity and no long-term skin photosensitization. J Med Chem 43:4488–4498
Parnham MJ, Graf E (1991) Pharmacology of synthetic organic selenium compounds. Prog Drug Res 36:4–9
Sies H, Masumoto H (1997) Ebselen as a glutathione peroxidase mimic and as a scavenger of peroxynitrite. Adv Pharmacol 38:229–246
Zhang JS, Gao XY, Zhang LD, Bao YP (2001) Biological effects of a nano red elemental selenium. BioFactors 15:27–38
Wang H, Zhang J, Yu H (2007) Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med 42:1524–1533
Tan L, Jia X, Jiang X, Zhang Y, Tang H, Yao S, Xie Q (2009) In vitro study on the individual and synergistic cytotoxicity of adriamycin and selenium nanoparticles against Bel7402 cells with a quartz crystal microbalance. Biosens Bioelectron 24:2268–2272
Chowdhury S, Ahmed H, Chatterjee BP (1987) Purification and characterization of an α-d-galactosyl-binding lectin from Artocarpus lakoocha seeds. Carbohydr Res 159:137–148
Singh J, Gupta K, Arora SK (2002) Changes in the anti-nutritional factors of developing seeds and pod walls of fenugreek (Trigonella foenum graecum L.). Plant Foods Hum Nutr 46:77–84
Huang H, Yuan Q, Yang X (2004) Preparation and characterization of metal-chitosan nanocomposites. Colloids Surf B 39:31–37
Christensen MJ, Nartey ET, Hada AL, Legg RL, Barzee BR (2007) High selenium reduces NF-κB-regulated gene expression in uninduced human prostate cancer cells. Nutr Cancer 58:197–204
Huang G, Zhang Y, Zhang Q, Zhang B, Wen L (2010) Vacuolization and apoptosis induced by nano-selenium in HeLa cell line. Sci China Chem 53:2272–2278
Acknowledgments
The authors acknowledge the Department of Science and Technology (DST), Government of India, New Delhi, India for the financial support in the form DST-FIST. The authors thank Central Instrumentation Facility, Pondicherry University. C. H. Ram expresses his special thanks to CSIR, India for the financial assistance in the form of CSIR, SRF, (Acknowledgement No:09/559/(0084)/2012 EMR-I).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramamurthy, C., Sampath, K.S., Arunkumar, P. et al. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst Eng 36, 1131–1139 (2013). https://doi.org/10.1007/s00449-012-0867-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-012-0867-1