Abstract
Groundwater contamination by fluoride is a typical problem associated with most of the regions in India. Mining of minerals can accelerate the dissolution of fluoride resulting in the further contamination of groundwater resources. The present study was undertaken to determine the concentration of fluoride in groundwater around the Bakhrija sandstone mine located in Haryana state, India. It was observed that the groundwater in immediate vicinity of the mine had relatively higher level of dissolved fluoride. The risk associated with consumption of fluoride contaminated groundwater was also observed to be higher in villages adjacent to the mines. The geochemical investigation suggested that dissolution of carbonate minerals may have resulted in solubilisation of fluoride in groundwater through the process of ion-exchange. The study concluded that fluoride level may rise in the other nearby regions if the intensity of mining increases. It may result in further spread of fluoride to other aquifers located around Bakhrija mine, if suitable environmental management plan is not developed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Fluoride in groundwater is one among the major pollutants that can affect human health adversely. Since fluorine is an abundantly present element in earth’s crust, it is prevalent as dissolved fluoride in groundwater around the globe. Whereas 0.7–1.0 mg/l of fluoride in drinking water is essential to prevent dental cavities and tooth decay, excess of fluoride (≥ 1.5 mg/l) may result in dental and skeletal fluorosis. Although the target organ of fluoride is bones, it is also known to interfere with brain development in children, reduced IQ (Xu et al. 2020), hypothyroidism, hyperglycaemia, infertility (Dey and Giri 2016), and osteosarcoma (Cohn 1992). The accumulation of fluoride in human body may result due to exposure through drinking water, fluoride-rich milk (Ullah et al. 2017), meat, tobacco (Yadav et al. 2007), dentifrice (Kanduti et al. 2016), and other food materials (Fein and Cerklewski 2001). There are a number of reports on fluoride exposure and risk analysis through food or drinking water, but most of the reports investigate the adverse effects related to fluoride-rich drinking water. Fluorite (CaF2) and/or fluorapatite (Ca5(PO4)3F) present as natural minerals in soil react with ground water, thereby resulting in contamination of drinking water, particularly in rural and remote areas (Haritash et al. 2018).
Most of the sources of groundwater in India have relatively higher concentration of fluoride since geographical distribution of fluoride-rich mineral is higher in Indian soil. The states like Andhra Pradesh (Adimalla et al. 2019), Rajasthan (Arif et al. 2013), Gujarat (Gupta et al. 2005), and Madhya Pradesh (Avtar et al. 2013) represent exceedance of fluoride level (> 1.0 mg/l), but the other states like Jharkhand (Pandey et al. 2012), Bihar (Kumar et al. 2018), Uttar Pradesh (Ali et al. 2017), and Haryana (Haritash et. al 2008) also represent dispersed pockets of fluoride-rich groundwater. Studies have revealed that almost 80 percent of total fluoride accumulated in human body (mg/kg/day) is through drinking water. Therefore, assessment of health risk associated with fluoride-rich ground water is pertinent. Out of the total fluoride ingested, about 60% is absorbed; while the absorption on empty stomach is about 100% (WHO 2004). The rate of dissolution of fluoride from soil increases if the conditions are acidic or time of soil–water interaction is more. Such conditions are found due to the release of acid-mine drainage and are seldom observed in mining areas. Sometimes, the collection of surface runoff in mine pits results in enhanced soil water interaction and higher percolation as well. Different mining operations (drilling and blasting) may result in formation of vertical cracks in subsurface impervious rock stratum resulting in an easy introduction of contaminants into the ground water (Armiento et al. 2016). Sandstone mining involves the use of heavy mechanical drills and explosives to fracture/fragment the rocks. Since fluoride is associated with such geological material, its interaction with outside environment increases upon excavation. It has been reported that the groundwater around sand stone mining remains contaminated by nitrate (NO3−), fluoride (F−), or pathogens (Kumar et al. 2017). Therefore, the present study was undertaken to determine fluoride level in groundwater around sandstone quarries located in Mahendragarh district of Haryana state, India. Further, the exposure assessment and risk were calculated as per the methodology suggested by USEPA (1993).
2 Materials and methods
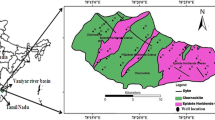
The present study was undertaken in Mahendragarh district, Haryana, India. The district has area of 1939 Km2 with total population of 922,088 (Census of India 2011). Climate of Mahendragarh district is hot in summers and cold in winters with unevenly distributed rainfall of 500 mm during monsoon. Mahendragarh district has nine major mining sites and Bakhrija stone mine is the largest with an area of about 66 km2. The location of Bakhrija mines is between 27°55′1″ and 27°54′6″ North latitude and 76°03′28.34″ to 76°03′27.56″ East longitude and it is famous for quarrying of calcite, limestone, and mica as chief minerals. Bakhrija stone mines are surrounded by Bakhrija, Dholera, Meghot Binja, Nujota, Meghot Halla and Khojpur Naglia villages (Fig. 1). In the present study, a total number of fifteen (15) groundwater samples were collected from bore wells located around the mining area. Samples were collected in pre-rinsed fresh Polypropylene bottles of 1.0 L capacity each. The samples were stored at low temperature in an ice box, and transported to the laboratory within 6 h for their chemical analysis. The samples were characterized for different parameters, in triplicates, following the standard methods as prescribed by APHA (2012). The fluoride concentration was determined using an ion-specific electrode (ISE—Orion Scientific, USA). Later, the exposure assessment and risk analysis were performed using the methodology as given by USEPA (Eq. 1). The suitability of sampled ground water sources was also evaluated comparing the observed values against standard prescribed values of Bureau of Indian Standards (BIS 2012) and WHO (2004).
2.1 Fluoride and human health
To estimate the risk and content of adverse effects of fluoride on human health, the procedure is divided into four phases (Selinus et al. 2016). These four phases are (i) Identification of hazard, (ii) Value selection on toxicity reference, (iii) Assessment of exposure and (iv) Characterisation of risk. Although there are alternate ways of exposure to fluoride i.e. drinking water, intake of other beverages and food, toothpaste, tea, pan masala and tobacco, etc. (Yadav et al. 2007), but drinking water is the prominent source of fluoride. For the study area, the health risk associated with the daily intake of fluoride-rich water was estimated using the following formula
where CDI is chronic daily intake of fluoride through drinking water (mg/Kg/day), Cw is the fluoride concentration in drinking water (mg/l), IR is ingestion rate of drinking water (3 L/day), EF is frequency exposure (365 day/year) and ED is exposure period (70 years), BW is average weight of the body (60 kg) and AT is average time of exposure (365 × 70 Days).
The non-carcinogenic risk to human health from fluoride toxicity is determined with the use of formula for hazard quotient (HQ).
RfD indicates the reference fluoride dosage in the formula above in mg/kg/day. RfD is used to determine fluoride’s risk to health during a defined pathway of exposure. According to USEPA’s Integrated Risk Information System (IRIS), RfD for drinking water is 0.05 mg/kg/day. The HQ value less than one is considered safe, while the HQ more than unitary value has potential possibility of non-carcinogenic health effects that can arise due to the consumption of water contaminated with fluoride.
3 Results and discussion
Based on the physico-chemical characterisation, it is noticed that the groundwater contains most of the cations and anions at or above measurable concentration (Table 1). All the samples of groundwater were observed to be slightly alkaline with respect to pH. Since natural minerals with basic nature (calcium, magnesium, carbonate and bicarbonate) dominantly get dissolved, the pH of ground water is generally alkaline. Based on TDS, most of the samples (93%) were found to be exceeding the prescribed limit of 500 mg/l; while based on total hardness, all the collected samples were classified as hard. Similar to this, all the collected samples exceeded the prescribed limit for calcium and magnesium, thus, confirming the hard nature of the groundwater. Unlike calcium and magnesium, chloride and sulphate exceeded the specified limit in 33% of samples collected, indicating that the hardness was dominantly contributed by carbonate and bicarbonate salts of the cations. Nitrate was within the permissible limit (< 45 mg/l) in all the ground water samples indicating that anthropogenic addition through fertilizer or waste water disposal is not resulting in ground water contamination in the study area. Fluoride is another important anion which may induce potential health effects over the exposed population. It was observed to be exceeding the level of 1.0 mg/l in 66% of the collected samples of ground water. Relatively higher values of fluoride at some locations are a cause of concern considering its toxicity and its health effect.
3.1 Fluoride exposure and health implication
Health risk assessment is important for determining the extent and possibility of health consequences over the people living in the region since they are is vulnerable to life-threatening contaminants in drinking water. The oral ingestion of excess fluoride through drinking water plays a crucial role and can pose a non-carcinogenic health risk to the population. Accordingly, the non-carcinogenic risk of fluoride to human health is estimated in terms of daily intake or hazard quotient (Table 2). Hazard quotient of the fluoride for the collected samples lies between 0.4 and 8.8 and the mean F− concentration of the all collected samples is 2 mg/l which is higher than the permissible limit of BIS (2012).
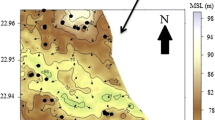
Fluoride concentration more than 1.5 mg/l has been found in groundwater of Bakhrija village (Fig. 2) with maximum concentration of 11 mg/l and significantly high value of HQ. Bakhrija is the nearest village from the mining area. Other villages viz. Dholera, Meghot Binja, and Meghot Halla represented groundwater fluoride concentration between 2.5 and 4.0 mg/l; and maximum concentration of 5.0 mg/l (Meghot Binja). Hazard quotient was in between 0.8 and 4.0 for these villages. Nujota and Khojpur Nagalia represented groundwater fluoride concentration within the permissible range. Human population living in the villages where the concentration of fluoride is recorded above the permissible limit is more likely to be exposed to potential health risks.
Fluorosis is a chronic disease involving the human population and is exacerbated through the intake of a higher concentration of fluoride through food and drink (Nuccio 2016). Children are more susceptible to higher fluoride as compared to adults. Lower body weight than adults could be the cause of higher risk from the exposure of fluoride (Kumar et al. 2016). Intake of fluoride (0.5–1.0 mg/l) is very important in early phase of life as it can stop dental caries, but higher level (> 1.5 mg/l) can lead to dental and skeletal fluorosis. Fluoride consumption for the first three years of life is the most important in fluorosis aetiology (Levy et al. 2002).
The groundwater of the study area was classified into three classes viz. 0–1 mg/l as safe; 1–4 mg/l causing dental fluorosis; and more than 5 mg/l causing skeletal fluorosis. About 33% samples fall in Class-I and can be considered safe for drinking; while 46% and 20% of groundwater samples come under Class II and Class III, respectively, and can cause dental and skeletal fluorosis (Fig. 3).
Chronic intake of excessive F− (i.e. 1.5–4.0 mg/l) can lead to fluorosis of the enamel and bone, and in severe cases (i.e. 4–10 mg/l), skeletal fluorosis associated with joint weakness, ligament calcification, and some osteosclerosis of the pelvis and vertebrae may be observed (Liang et al. 2017; Narsimha and Sudarshan 2016; Podgamy and McLaren 2015). This occurs mostly because F− is highly electronegative and has a comparable ionic radius (133 pm) to that of hydroxyl ion (140 pm), which contributes to hydrogen fluoride formation (Kumar et al. 2016). Fluoride in the human body, in fact, easily diffuses through the intestines, dissolves in the blood and accumulates in calcified tissues (Dey and Giri 2016). Health-related problems in and around the areas are primarily non-carcinogenic in nature, especially in the areas examined, where fluoride in drinking water does not reach 10 mg/l. However, higher doses (> 10 mg/l) can be correlated with debilitating fluorosis and carcinogenic risk (Ali et al. 2019). Confined studies on this aspect indicate that fluoride may allow cells to develop faster enough that will become cancerous over time, but it is controversial since there is no reliable correlation between fluoride and the influence of carcinogenicity (Bajpai 2013).
3.2 Genesis of groundwater and contamination
Based on the concentration (in meq/l) of selective dominant anions (Cl− and SO42−) and a cation (Na+), the prevailing dominant soil water interactions in the study area were identified based on the base-exchange indices. The classification of groundwater was done using the following equation (all units are in meq/l).
As stated above (Eq. 3), the base-exchange values more than unitary (> 1) suggest that groundwater is NaHCO3− type; on the opposite, base exchange less than unitary (< 1), the groundwater represents Na+-SO42− type. The base exchange index plot shows that most of the samples (87%) belong to the Na+-SO42− type, while only 13% belongs to the Na+-HCO3− type in the study area. It is well known that the Na+-SO42− form of water accelerates the carbonate mineral deposition of calcite, and is linked with the release of fluoride from minerals in gneissic basement rocks and granite accumulation due to the dissolution of silicates and, therefore, a rise in the concentration of fluoride in groundwater. NaF(s) dissolves to release Na+ (aq), a conjugate base of strong acid fluoride that does not react with water. When the salt, NaF, is dissolved in water, the F-ion is formed (Chitrakshi and Haritash 2018; Mamatha and Rao 2010). The ion-exchange reactions followed by the mineral reaction are key factors in the study area that are responsible for a high level of fluoride.
Carbonate-rich rocks, such as limestone and dolomite, are major starting materials for carbonate weathering. The carbonates present in these rocks are dissolved in groundwater during water infiltration. Calcium (Ca2 +), magnesium (Mg2+), their molar ratio (Ca/Mg), and hydrogen carbonate (HCO3 −) are the major chemical parameters describing the groundwater carbonate equilibrium. Generally, the molar ratio in groundwater between calcium and magnesium depends on the lithological composition of groundwater recharge areas, i.e., if Ca/Mg molar ratio is equal to 1, it poses presence of dolomite while the higher molar ratio suggests dissolution of calcite minerals. The bivariant plot (Fig. 4) of Mg2+/Na+ verses Ca2+/Na+ clearly shows that carbonate dissolution is the dominant process which contributes to the chemical quality of the groundwater in the study area. Once again, the cross plot of verses (Ca2+ + Mg2 +) (HCO3 − + SO4 2−) (Fig. 4b) is observed and implies that most of the samples with more than one fluoride concentration are found above equiline, which further suggests the carbonate dissolution, weathering and the movement of ions in the region are responsible for higher F− and HCO3− concentrations in the groundwater. It is well known that an increase in pH (i.e., alkaline condition), sodium, and bicarbonate ion concentrations eventually raises the concentration of fluoride in groundwater as a result of the above reactions and mechanisms. In general, the longer interaction of rock water implies the weathering of fluoride-bearing minerals under alkaline conditions resulting in higher concentrations of fluoride in groundwater (Raj and Shaji 2017; Adimalla and Venkatayogi 2017; Cremisini and Armiento 2016).
4 Conclusion
The study indicates that groundwater adjacent to the mining area in Mahendragarh is rich in fluoride especially in the villages located in southeast direction. The exposure assessment study reveals high exposure in these regions resulting in the high risk of non-carcinogenic effects due to fluoride. The geochemical analysis reveals presence of silicate weathering in areas with higher fluoride level indicating that natural processes are resulting in dissolution of fluoride in groundwater and ion exchange is dominantly responsible. Further, it is important to mention that contamination of groundwater by fluoride may increase with time and with more intense rate of quarrying. It is recommended for regular monitoring of groundwater in the villages around mining area so that any possibility of fluoride contamination is timely noticed and checked.
References
Adimalla N, Venkatayogi S (2017) Mechanism of fluoride enrichment in groundwater of hard rock aquifers in Medak, Telangana State South India. Environ Earth Sci 76:45. https://doi.org/10.1007/s12665-016-6362-2
Adimalla N, Venkatayogi S, Das S (2019) Assessment of fluoride contamination and distribution: a case study from the rural part of Andhra Pradesh India. Appl Water Sci 9:94. https://doi.org/10.1007/s13201-019-0968-y-y
Ali S, Kumari M, Gupta S, Sinha A, Mishra B (2017) Investigation and mapping of fluoride-endemic areas and associated health risk—A case study of Agra Uttar Pradesh, India. Hum Ecol Risk Assess Internat J 23(3):590–604. https://doi.org/10.1080/10807039.2016.1255139
Ali S, Fakhri Y, Golbini M, Thakur S, Alinejad A, Parseh I, Shekhar S, Bhattacharya P (2019) Concentration of fluoride in groundwater of India: a systematic review, meta-analysis and risk assessment, Groundwater for Sustainable Development, 9. ISSN 100224:2352–2801. https://doi.org/10.1016/j.gsd.2019.100224
APHA (2012) Standard methods for the examination of water and waste water, 22nd edn. American Public Health Association American Water Works Association, Water Environment Federation
Arif M, Husain I, Hussain J, Kumar S (2013) Assessment of fluoride level in groundwater and prevalence of dental fluorosis in Didwana block of Nagaur district, Central Rajasthan India. Int J Occup Environ Med 4(4):178–184 (PMID: 24141866)
Armiento G, Angelone M, De Cassan M, Nardi E, Proposito M, Cremisini C (2016) Uranium natural levels in water and soils: assessment of the Italian situation in relation to quality standards for drinking water. Rend Fis Acc Lincei 27:39–50. https://doi.org/10.1007/s12210-015-0462-x
Avtar R, Kumar P, Surjan A (2013) Geochemical processes regulating groundwater chemistry with special reference to nitrate and fluoride enrichment in Chhatarpur area, Madhya Pradesh, India. Environ Earth Sci 70:1699–1708. https://doi.org/10.1007/s12665-013-2257-7
Bajpai J (2013) Fluoride carcinogenesis: the jury is still out! South Asian. J Cancer 2(4):192. https://doi.org/10.4103/2278-330X.119881
BIS (2012) Bureau of Indian standards, IS: 10500. Indian standard for drinking water specification
Census of India (2011) Haryana (Series 07), Part XII-B, District Census Handbook-Mahendragarh. Directorate of Census operations, Haryana. https://censusindia.gov.in/2011census/dchb/0616_PART_B_DCHB_MAHENDRAGARH.pdf
Chitrakshi HA (2018) Hydrogeochemical characterization and suitability appraisal of groundwater around stone quarries in Mahendragarh India. Environ Earth Sci 77:252. https://doi.org/10.1007/s12665-018-7431-5
Cohn P (1992) A brief report on the association of drinking water fluoridation and the incidence of osteosarcoma among young males. Department of Health Environment
Cremisini C, Armiento G (2016) High geochemical background of potentially harmful elements. The “geochemical risk” and “natural contamination” of soils and water: awareness and policy approach in Europe with a focus on Italy. Rend Fis Acc Lincei 27:7–20. https://doi.org/10.1007/s12210-015-0457-7
Dey S, Giri B (2015) fluoride fact on human health and health problems: a review. Med Clin Rev 2:2. https://doi.org/10.21767/2471-299X.100011
Fein N (2001) Fluoride content of foods made with mechanically separated chicken. J Agric Food Chem 49(9):4284–4286. https://doi.org/10.1021/jf0106300
Gupta S, Deshpande R, Agarwal M, Raval B (2005) Origin of high fluoride in groundwater in the North Gujarat-Cambay region, India. Hydrogeol J 13:596–605. https://doi.org/10.1007/s10040-004-0389-2
Haritash A, Kaushik C, Kaushik A, Kansal A, Yadav A (2008) Suitability assessment of groundwater in some villages of Rewari district in Haryana. Environ Monit Assess 145(1–3):397–406. https://doi.org/10.1007/s10661-007-0048-x
Haritash A, Aggarwal A, Soni J, Sharma K, Sapra M, Singh B (2018) Assessment of fluoride in groundwater and urine, and prevalence of fluorosis among school children in Haryana India. Appl Water Sci 8:52. https://doi.org/10.1007/s13201-018-0691-0
Kanduti D, Sterbenk P, Artnik B (2016) Fluoride: a review of use and effects on health. Materia Socio-Med 28(2):133–137. https://doi.org/10.5455/msm.28.133-137
Kumar S, Lata S, Yadav J, Yadav JP (2016) Relationship between water, urine and serum fluoride and fluorosis in school children of Jhajjar District, Haryana, India. Appl Water Sci 7:3377–3384. https://doi.org/10.1007/s13201-016-0492-2
Kumar B, Singh U, Mukherjee I (2017) Hydrogeological influence on the transport and fate of contaminants in the groundwater India. JSM Biol 2(1–11):1009
Kumar S, Venkatesh A, Singh R, Udayabhanu G, Saha D (2018) Geochemical Signatures and isotopic systematics constraining dynamics of fluoride in groundwater across Jamui district, Indo-Gangetic alluvial plains, India. Chemosphere 205:493–505
Levy S, Hillis S, Warren J, Broffitt B, Mahbubul Islam A, Wefel J, Kanellis M (2002) Primary tooth fluorosis and fluoride intake during the first year of life. Community Dent Oral Epidemiol 30(4):286–295. https://doi.org/10.1034/j.1600-0528.2002.00053.x
Liang S, Nie ZW, Zhao M, Niu YJ, Xin KT, Cui XS (2017) Sodium fluoride exposure exerts toxic effects on porcine oocyte maturation. Sci Rep 7:17082. https://doi.org/10.1038/s41598-017-17357-3
Mamatha P, Rao S (2010) Geochemistry of fluoride rich groundwater in Kolar and Tumkur districts of Karnataka. Environ Earth Sci 61:131–142. https://doi.org/10.1007/s12665-009-0331-y
Matthess G (1982) The properties of ground water. Wiley
Narsimha A, Sudarshan V (2016) Contamination of fluoride in groundwater and its effect on human health: a case study in hard rock aquifers of siddipet, Telangana state, India. Water Sci 7:2501–2512. https://doi.org/10.1007/s13201-016-0441-0
Nuccio M (2016) Pollution of waters and soils by contaminants of magmatic origin. Rend Fis Acc Lincei 27:21–28. https://doi.org/10.1007/s12210-015-0474-6
Pandey A, Shekhar S, Nathawat M (2012) Evaluation of fluoride contamination in groundwater sources in Palamu District, Jharkhand India. J Appl Sci 12:882–887. https://doi.org/10.3923/jas.2012.882.887
Podgomy P, Mclaren L (2015) Public perception and scientific evidence for perceived harms/risks of community water fluoridation: an examination of online comments pertaining to fluoridation cessation in Calgary. Can J Pub Health 106(6):413–425. https://doi.org/10.17269/cjph.106.5031
Raj D, Shaji E (2017) Fluoride contamination in groundwater resources of Alleppey Southern India. Geosci Front 8(1):117–124. https://doi.org/10.1016/j.gsf.2016.01.002
Selinus O, Centeno J, Finkelman R (2016) Medical geology: impacts of the natural environment on public health. Geosciences 6(1):8. https://doi.org/10.3390/books978-3-03842-198-6
Ullah R, Zafar S, Shahani N (2017) Potential fluoride toxicity from oral medicaments: a review. Iran J Basic Med Sci 20(8):841–848
USEPA (1993) US environmental protection agency. Reference dose (RfD): description and use in health risk assessments, Background Document 1A. https://www.epa.gov/iris/reference-dose-rfd-description-and-use-health-risk-assessments
WHO (2004) World health organization guidelines for drinking water quality. World Health Organization
Xu K, An N, Huang H (2020) Fluoride exposure and intelligence in school-age children: evidence from different windows of exposure susceptibility. BMC Public Health 20:1657. https://doi.org/10.1186/s12889-020-09765-4
Yadav A, Kaushik C, Haritash A, Singh B, Raghuvanshi S, Kansal A (2007) Determination of exposure and probable ingestion of fluoride through Tea, Toothpaste, Tobacco and Pan Masala. J Hazard Mater 142:77–80. https://doi.org/10.1016/j.jhazmat.2006.07.051
Acknowledgements
The authors acknowledge the help of Mr. Rajesh Sehrawat, Mine inspector, Govt. of Haryana in several ways during this study.
Funding
The authors have no relevant financial or non-financial interests to disclose. The authors have no financial or proprietary interests in any material discussed in this article.
Author information
Authors and Affiliations
Contributions
SKA: Conceptualization, Methodology, Software, SKA: Data curation, Writing- Original draft preparation. AKH: Visualization, Investigation. AKH: Supervision: SKA: Software, Validation: SKA/AKH: Writing-Reviewing and Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Human and animal rights
Authors declare that no involvement of Human and Animals in the research work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ambastha, S.K., Haritash, A.K. Prevalence and risk analysis of fluoride in groundwater around sandstone mine in Haryana, India. Rend. Fis. Acc. Lincei 32, 577–584 (2021). https://doi.org/10.1007/s12210-021-00997-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-021-00997-z