Abstract
Motion at the knee joint is a complex mechanical phenomenon. Stability is provided by a combination of static and dynamic structures that work in concert to prevent excessive movement or instability that is inherent in various knee injuries. The anterior cruciate ligament (ACL) is a main stabilizer of the knee, providing both translational and rotatory constraint. Despite the high volume of research directed at native ACL function, pathogenesis and surgical reconstruction of this structure, a gold standard for objective quantification of injury and subsequent repair, has not been demonstrated. Furthermore, recent studies have suggested that novel anatomic structures may play a significant role in knee stability. The use of biomechanical principles and testing techniques provides essential objective/quantitative information on the function of bone, ligaments, joint capsule, and other contributing soft tissues in response to various loading conditions. This review discusses the principles of biomechanics in relation to knee stability, with a focus on the objective quantification of knee stability, the individual contributions of specific knee structures to stability, and the most recent technological advances in the biomechanical evaluation of the knee joint.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Motion at the knee joint is a complex mechanical phenomenon with displacements occurring across multiple planes of motion [1]. The inherent stability of the knee is provided by a combination of primary and secondary stabilizers, each serving as a relative restraint to motion across a specific plane of motion [2]. The anterior cruciate ligament (ACL) is one of the most frequently injured [3] and, likewise, most highly studied structures in the knee joint. As a key component of anterior–posterior and rotational knee stability [4], surgical reconstruction of the ACL is a critical step in returning individuals to function after injury. However, despite the volume of research in the field of ACL and other ligamentous knee injuries, no “gold standard” treatment protocol has been identified for these patients [5, 6]. Recent advances in the field of biomechanics have enabled scientists to perform comprehensive analyses of knee function, yielding objective kinematic assessments of knee motion in the intact, injured, and reconstructed states.
Biomechanical analyses provide essential information about the complexities of how bone, cartilage, and other soft tissues behave. Through biomechanical analyses, information such as mechanical properties of tissues and structural properties of bone-ligament-bone complexes, forces experienced by certain structures, contact pressures for osteoarthritis, and joint kinematics can be evaluated. Additionally, biomechanical analyses can be used to quantitatively determine the effectiveness of certain treatments and surgical techniques by assessing changes in biomechanical parameters. As a result, the understanding of basic biomechanics helps surgeons optimize their surgical techniques to improve outcomes for their patients.
This has allowed physicians to better tailor treatment regimens and provide “individualized” knee reconstruction surgery. Most recently, a large amount of research has focused on the role of objective assessment of knee instability, operative strategies to restore native knee motion, and the contribution of specific anatomic structures to knee stability and patient outcome. In order to properly characterize the motion at the knee, an understanding of biomechanical principles is needed. This review/chapter will highlight the most recent biomechanical research surrounding the restoration of native knee motion, both in anterior–posterior (a–p) and rotational planes with focus on the objective assessment of the “pivot shift” phenomenon, while also examining the role of the anterolateral knee structures in both a–p and rotational knee stability.
Basic theory of clinical knee biomechanics and objective assessment
In the context of clinical knee measurement, often a simplified but subjective terminology is used to describe physical observations about the knee. For example, qualifiers such as instability and laxity are used interchangeably to describe a pathologic deviation from normal knee function in the literature. However, the definitions of these terms are ambiguous and do not provide objective information regarding the kinematics and function of the knee [7]. From a biomechanical standpoint, laxity can most likely be described as “the passive response of a joint to an externally applied force,” whereas instability constitutes a “functional measure,” expressed by the patient [8]. Laxity and instability can be better quantified by understanding the stiffness of a complex. Stiffness allows one to understand how a tissue resists deformation in response to forces. As a result, higher tissues with higher stiffness have the effect of limiting overall joint motion. However, abnormally high stiffness in tissues can over-constrain joint motion, as is possible in procedures such as extra-articular tenodesis. Thus, an increased laxity is not tantamount to knee instability nor an inferior outcome, as patients compensate differently and experience differing levels of disability [9]. In order to achieve standardized comparison of technique across patient populations, recent research has focused on the objective measurement of validated, subjective clinical exams such as the “pivot shift test.” This specific clinical exam has been demonstrated as the most specific test for ACL deficiency, while also best correlating patient-reported outcome and the development of osteoarthritis [10–12]. Therefore, the objective reporting of this exam has significant value for both clinical and research outcomes.
The objective quantification of knee motion begins with the establishment of a suitable frame of reference, ideally directly comparable to the normal functional movement. With regard to the description of knee kinematics, a total of six independent degrees of freedom with three translations and three rotations (knee flexion/extension, external/internal rotation, adduction/abduction, anterior/posterior translation, compression/distraction, and medial/lateral shift) must be considered and reported. A fundamental framework for the knee joint is well described within the joint motion description proposed by Grood and Suntay [13], which allows for the kinematic output of numerous objective assessment tools, including robotic testing systems, navigation systems, and electromagnetic tracking, commonly utilized during the physical examination of in vivo and in vitro knee joints.
Physical examinations are a mainstay of the diagnosis and management of patient-reported injury and functional impairment. In the documentation of ACL injury, the identification of excessive rotational knee motion, i.e., the pivot shift test, is highly valued for its specificity and predictive value [14–16]. During the pivot shift maneuver, the anterior tibial translation of the lateral compartment exceeds the motion in the medial compartment. This translation has been shown to correlate with clinical grading of the pivot shift [17], making the objective quantification of this translation clinically significant. For the purpose of objective quantification of the pivot shift in both in vivo and in vitro settings, a variety of methods have been identified and described. These include electromagnetic tracking, surgical navigation, two-dimensional image analysis, and inertial sensors [18]. The techniques provide output that has historically been divided into four general categories, (1) translations, (2) rotations, (3) acceleration/velocity, and (4) calculated indexes, which aim to provide a standardized value for knee stability based on kinematic output. However, due to variability in performing physical examinations, attention must also be directed toward the use of standardized devices to simulate physical examinations and yield more consistent results [19]. From the in vitro setting, the use of custom-designed robotic mechanisms (Fig. 1) and industrial robotic manipulators has enabled researchers to standardize the magnitude and direction of forces and moments applied to the knee [19–23]. This has allowed the practice of repeatable forces applied to the joint under study. These devices have a wide range of capabilities such as applying complex loading conditions, measuring joint motion, and determining the force in tissues. To analyze knee biomechanics using these devices, either static or continuous measurements are made during testing. Static tests show how a tissue or joint is behaving at a specific load or joint position. While static tests have direct clinical applicability, the quantity of data obtained from these tests is limited. Conversely, continuous tests show how a tissue or joint behaves throughout a range of loading levels or joint positions. In other words, while a static test may show the function of the ACL at 30° and 60° of knee flexion, a continuous test will show the function of the ACL at 30° through 60° of knee flexion. As a result, continuous tests could be utilized to simulate clinical exams such as the pivot shift test in order to provide clinicians additional information about how the knee responds throughout the entire exam. The existence of this technology allows for the objective reporting of physical examination components and helps to shape clinical practice and operative strategies toward individualized patient outcomes. Specifically, the in vitro simulation of the pivot shift test typically includes application of valgus and internal rotation torques to the knee, and the resulting anterior tibial translation is reported as the primary outcome [19, 24, 25].
Several different clinical tools have been utilized to objectively report real-time subject kinematics in the clinical and operative settings. However, cost and ease of use have limited the widespread clinical applicability of these assessment devices. This has established the need for continued development and validation of new technologies to detect and quantify the pivot shift with ease of use for patients and clinicians alike. New technologies utilizing simple image analysis of anterior tibial translation and the measurement of lateral compartment acceleration during the tibial reduction have been utilized, and their output was shown to correlate with the clinical grade of pivot shift [26–29]. The most recent studies concerning the emerging techniques for objective quantification of the pivot shift and their clinical significance will be described within this chapter. Likewise, a description of the most recent biomechanical studies for “hot-topic” orthopedic issues, including the anterolateral structures and individualized ACL surgery paradigms, will be described.
Objective quantification of pivot shift phenomena
The pivot shift test determines dynamic rotatory knee laxity and has significant clinical implications. After the first historical description of the test by Galway and MacIntosh [30], many other variations have been described by physicians and researchers [31–33]. However, research has documented that the interpretation of the pivot shift test remains subjective and examiner dependent [17, 34–37]. The attempt of quantifying the pivot shift test has proven difficult given the multiple techniques for the exam and the associated large variation in kinematics [37]. In addition, a primary reason for the difficulty to establish an objective measurement system is the complexity of the pivot shift movement. The pivot shift test and resultant translation of the lateral compartment are movements composed of tibial internal–external (i–e) rotation, varus–valgus (v–v) rotation, and anterior–posterior (a–p) translation [38]. The development of a standardized technique by Hoshino et al. has increased the reproducibility of the exam and likewise increased the consistency of objective quantification [16]. The most recent research in the field of objective quantification of the pivot shift has focused on the use of a novel two-dimensional image analysis tablet software, inertial sensors (including accelerometers and gyroscopes), and novel knee imaging devices [29, 39].
A good correlation between a–p translation of the lateral compartment and the results of the clinical pivot shift test grade as shown are the basis for image analysis software [17] (Fig. 2). This allowed for clinical marking of associated lateral compartment landmarks which could be captured with a high-resolution camera and processed accordingly. The bony landmarks of interest include the lateral epicondyle, Gerdy’s tubercle, and the fibular head. With a digital camera, the intersection between the connecting line of Gerdy’s tubercle and fibular head, and the perpendicular running connection to the lateral epicondyle is captured and deemed the “pivot point.” The ratio of the distances between the pivot point and Gerdy’s tubercle, and between Gerdy’s tubercle and fibular head is then used to calculate the femoral AP position from the Gerdy’s point. The anterior–posterior translation of the femoral AP position from the Gerdy’s tubercle dependent during the pivot shift over time illustrates a sudden decrease (Fig. 3), occurring simultaneously to the reduction of the tibia, in ACL-deficient knees. This sudden decrease is used for the quantification of the pivot shift. However, ACL-intact knees do not show such a decline of the femoral AP position. This highlights the biomechanical role of the native ACL in rotatory stability. Using electromagnetic tracking to digitize the actual bony movement, a correlation with the motion of the skin markers was confirmed, so that the principle of image analysis was shown to be valid. Using a novel tablet-based software, the analyzing time is approximately to 10–15 s, with an average error of less than 6 % in distances less than or equal to 175 cm between tablet computer and marker position. A user-friendly interface is implemented to simplify the clinical application. All alterations considered these adjustments make simple image analysis to an easily applicable tool for the daily clinical work [40, 41•].
Anatomic landmarks: a lateral femoral condyle, b fibular head, and c Gerdy’s tubercle. The intersection of the line between fibular head and Gerdy’s tubercle and the perpendicular connecting line to the femoral condyle is defined as the pivot point. By calculating the translation of the pivot point during examination, the lateral compartment translation can be quantified
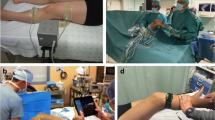
a The PIVOT software and KiRA accelerometer (red circle) in clinical use; b the anterior translation of the lateral knee compartment in the PIVOT software interface; c the measured acceleration curve per unit time is plotted by the KiRA software. The yellow labeling illustrates the range between maximum and minimum acceleration
In addition to image analysis made clinically affordable and easy to use with the development of tablet software, the use of inertial sensors has been found to provide real-time objective knee measurements [28]. With the use of a three-dimensional accelerometer and three perpendicularly arranged gyroscopes, acceleration about the lateral aspect of the proximal tibia, closed to Gerdy’s tubercle, is acquired with 110-Hz frequency and transmitted to a tablet computer device (Fig. 3). The developed KiRA software (Orthokey LLC, Lewes, DE, USA) analyzes the data, plots the acceleration in relation to the time, and saves the data to a secure patient-oriented server. Identifying the corresponding curve generated by the pivot shift, the software automatically calculates the maximum and minimum acceleration of the limb, the range between those values, and the curve’s slope as an indicator for smoothness [42•]. The reliability in using this device was evaluated [43], confirming the differences between ACL-intact and ACL-deficient knees, illustrating post-operative deceleration (relative to pre-operative state), and confirming a successful outcome. A recent study demonstrates a significant decrease in femoral acceleration at the point of tibio-femoral reduction, with strong correlation (r = 0.84, p < 0.0001) demonstrated between acceleration decrease and clinical grade [44]. Future study into the use of tibial and femoral acceleration values are needed to document ease of use, and focus on new technologies that decrease the cost of acceleration-based quantification systems is needed.
An additional device is in preliminary assessment for the documentation of ACL-deficient kinematics with open MRI technology and a specific positioning device. The KneeMRI (KneeM) device is a brace-like imaging setup which exerts an anterior drawer force of 100 N and an internal rotation force of 20 N throughout the range of flexion (0°, 20°, 40°, and 60°, respectively). This allows for characterization of tibio-femoral spatial relationships across the full range of flexion using high-sensitivity MRI imaging. Testing both ACL-deficient and healthy contralateral knees, a significant difference in the anterior translation of the lateral compartment at 0° and 20° of flexion (p = .005 and p = .002, respectively) was demonstrated [39]. In addition, the lateral plateau of ACL-deficient knees was found to translate 7.7 mm posteriorly between 20° and 40° of flexion, an important finding as this is where the pivot shift phenomenon has been documented to occur [45, 46]. This study suggests that measurement of tibiofemoral kinematic during flexion using the KneeM device was useful for quantifying rotatory laxity in ACL-deficient and may provide clinicians with enhanced understanding of knee stability on review of knee imaging. More studies are needed for continued validation and ease of use for this novel technology.
Anatomical and biomechanical implications of the anterolateral knee structures
The restoration of rotational knee stability has emerged as a major focus in most recent literature due to its association with optimal patient outcome and prevention of osteoarthritis. The contribution of the individual anterolateral knee structures (Fig. 4) to static and dynamic stability of the knee joint has been well described in past literature but has recently become a primary focus of rotatory knee stability [47]. Historically, the anterolateral capsule has been described as a secondary stabilizer of anterior translation and rotation of the lateral compartment [14]. This has been well documented in instances of combined injury to the anterior cruciate ligament and the anterolateral structures, where increased anterior translation and increased internal rotation at 90° of flexion has been quantified compared to isolated ACL injuries [48]. Furthermore, the addition of lateral capsule or ALL ligament injury to an isolated ACL injury has increased the magnitude of the reduction during the pivot shift test in cadaveric navigation and robot studies, establishing its role in the control of dynamic rotational laxity [14, 49]. Given this historical literature that has outlined the role of the lateral structures in rotatory stability [50–53], this proposed “ligamentous” structure and the “capsulo-osseous layer of the iliotibial band” appear to have similar roles in providing knee stability. Furthermore, in order to determine the in situ forces in the anterolateral structures as well as their contributions to joint stability, the principle of superposition must be applied appropriately during the use of robotic testing systems. The principle of superposition requires three fundamental assumptions: (1) There is no interaction between the structures of interest, (2) the bony tissue is rigid relative to the ligaments, and (3) the position of each bone is accurately reproduced [54]. Dissection of the anterolateral capsule or iliotibial band (ITB) or both results in increased rotational movement in ACL-deficient knees, indicating a secondary role of these structures in rotational stability. These results were obtained in cadaveric studies, with the individual use of a navigation system, electrogoniometer and robotic testing system to document objective knee movement [14, 48, 55, 56]. Recently, the ITB has been demonstrated to have large contributions in the restraint of lateral tibial translation and tibial internal rotation [57•]. However, research has shown that resection of the ITB may diminish reduction of the tibia during the pivot shift test [58]. In addition, the ITB acts as an ACL agonist, suggesting that using an ITB graft for extra-articular reconstruction may hinder its function as a restraint to the knee [56].
Despite the understanding of the anterolateral structures as a restraint, recent publications of a newly discovered “anterior longitudinal ligament” has engaged the research community in a re-examination of the anatomical, histological, and biomechanical components of the anterolateral capsule [59, 60]. Recent anatomic study has reported the dissections of 24 different animal species in which a double lateral collateral ligament (LCL) was observed in three types of primates, but no discrete ALL was ever discovered. Further, the dissection of several human fetuses aged 18–22 weeks also demonstrated no distinguishable anterolateral ligament within the capsule [61]. These observations suggest a negative selection against this feature in human evolution. The macroscopic anatomy, histology, and radiology of the anterolateral capsule in mature human cadaver specimens were also recently examined and revealed that only 30 % of the specimens showed a discrete thickening of 2–4 mm on magnetic resonance imaging (MRI) [62•]. However, no discrete thickening was observed upon arthroscopic trans-illumination of these same specimens. Furthermore, a comparative histologic analysis of this “capsular thickening” and the LCL was performed and the LCL was found to be comprised of a clear, linear alignment of collagen fibers, while the anterolateral capsule was less organized and did not display the characteristics of a “true ligament” [62•].
The anterolateral structures contribute biomechanical restraint to knee motion [14, 48, 55, 56, 57•]. However, there has been an inability to observe and document specific damage patterns to this structure during ACL injury. In a recent arthroscopic evaluation of 150 patients with an ACL injury, no damage to the anterolateral capsule and no discrete thickening or ligamentous structure were found. Tensile testing of this region of the anterolateral capsule demonstrated that the capsule has four times less stiffness, five times less ultimate load, and two times the ultimate elongation compared to other knee ligaments [63]. During a simulated pivot shift test using a robotic testing system, the contribution of this region of the anterolateral capsule to knee stability was negligible. Interestingly, the use of dynamic stereo X-ray to evaluate knee kinematics after ACL reconstruction has demonstrated increased tibial external rotation in the operated knee. This would suggest that an additional anterolateral restraint might further worsen the abnormal kinematics by increasing the external rotation and placing a non-anatomic constraint upon the knee joint. Furthermore, reconstruction of the ALL has not been shown to restore knee kinematics in knees with high rotational motion [64]. In fact, over-constraint of the knee joint has been demonstrated as a result of extra-articular stabilization procedures, resulting in a decrease in functional internal rotation after a combined ACL reconstruction and extra-articular tenodesis [65, 66]. Although the structure and function of the anterolateral capsule have been well documented, there continues to be a lack of evidence for a discrete anatomical structure. Likewise, the supporting biomechanical evidence for an extra-articular repair strategy has not been convincing. Therefore, continued biomechanical study into the individual components of the anterolateral knee structures and their individual role in establishing translational and rotational constraints is needed.
The restoration of knee kinematics; individualized ACL surgery
The aim of ACL reconstruction focuses on restoring patient-reported activity and optimizing outcome on an individual basis. From an objective standpoint, this is often qualified as the ability to eliminate the pivot shift, which documents a return to native rotational stability. Many structures and anatomical characteristics can influence the grading of the pivot shift test and are involved in the genesis and magnitude of rotatory instability after an ACL injury and subsequent repair. These include tibial slope, tunnel placement, and ACL graft selection among others. The objective quantification of the pivot shift may be able to categorize knee laxity and provide adequate information on which structures are affected besides the ACL. A new algorithm for rotational instability treatment utilizing objective knee kinematics data and patients’ unique anatomical characteristics is discussed here for an individualized, patient-centered treatment protocol.
A study recently reported upon the use of gait kinematics data from healthy individuals in a finite element (FE) model for prediction of ACL force across varying posterior slopes. Historically, a decreased tibial slope was found to be protective against anterior tibial translation in ACL-deficient knees [67, 68]. Likewise, another study [69] compared an ACL-deficient group (n = 100) with a control group (n = 100) and found that an increased tibial slope is associated with ACL rupture and higher grades in the pivot shift test. The findings of this most recent model state that ACL biomechanical forces increase with an increasing posterior tibial slope and are caused primarily by change in posterior tibial slope at the tibial plateau that carries a larger portion of joint contact force [70]. This report parallels recent studies that document a higher risk of ACL tear with increasing posterior tibial slope [71], as well as an increase risk factor for a high-grade pivot shift [72]. However, an additional recent biomechanical study demonstrated no increase in ACL strain with increasing posterior tibial slope (p < 0.05) and decreased internal rotation under combine flexion and internal rotation load with increasing posterior tibial slope [73]. These studies highlight the need for further in vivo and in vitro analysis of posterior slope in ACL-intact and ACL-deficient knees to understand the individual contribution to rotational instability.
In restoring native kinematics, the fixation of proper soft-tissue grafts is critical in providing individualized reconstruction. Recent study has focused on the use of quadriceps tendon to achieve restoration of anatomic knee kinematics. From a biomechanical standpoint, tensile tests demonstrated that cross-sectional area of the quadriceps tendon graft was nearly twice that of the bone-patella-bone graft with ultimate load to failure, and stiffness was also significantly higher for the QT graft. Recent in vitro study demonstrated quadriceps tendon to restore native kinematics to an ACL-deficient knee, with double-bundle quadriceps tendon restoring in situ ACL graft forces to more similar than that of the intact ACL [74].
Concluding remarks
In order to properly restore knee kinematics with surgical intervention, paradigms for the assessment of both in vitro and in vivo knee motion are needed. Despite the advances in assessment techniques, fundamental questions regarding static or continuous measurement have remained unclear. Recent work has highlighted the importance of continuous assessment of knee motion in robotic testing [75•]. Continuous monitoring allows for more data collection across the range of flexion, potentially elucidating trends across small subdivisions of the range of flexion that engage different constraints and anatomical points.
The focus for ACL-deficient knees should remain on optimizing patient outcome, with the goal of restoring the native anatomy. This native, uninjured state needs proper biomechanical characterization about the multiple degrees of freedom that comprise motion at the knee joint. Biomechanical principles, including the principle of superposition, need to be utilized properly and incorporated into research study design to determine the structure and function of knee ligaments and capsular structures. Research should continue to be directed to individual patient factors, including the presence of pivot shift and injury to anterolateral capsule, with patient-specific factors used for graft choice, adjustment of bony morphology, reconstruction method, and tunnel placement.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Grood ES, Noyes FR: Diagnosis of knee ligament injuries: Biomechanical precepts. In: Feagin JA, editor. The Crucial Ligaments. New York, Churchill Livingston 1994:245–60.
Kakarlapudi TK, Bickerstaff DR. Knee instability—isolated and complex. West J Med. 2001;174(4):266–72.
Gianotti SM et al. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12:622–7.
Ellison A, Berg E. Embryology, anatomy, and function of the anterior cruciate ligament. Orthop Clin North Am. 1985;16:3–14.
Hospodar SJ, Miller MD. Controversies in ACL reconstruction: bone-patellar tendon-bone anterior cruciate ligament reconstruction remains the gold standard. Sports Med Arthrosc. 2009;17(4):242–6.
Bonin M, Amendola A, Bellemans J, Mc Donald S, Ménétrey J (eds), The Knee Joint: Surgical Techniques and Strategies, Springer-Verlag 2012 Print.
Alter MJ: Science of flexibility. 3rd ed. Champaign, IL: Human Kinetics Publishers; 2004: 3–4.
Musahl V et al. Rotatory knee laxity and the pivot shift. Knee Surg Sports Traumatol Arthrosc. 2012;20:601–2.
Eastlack ME, Axe MJ, Snyder-Mackler L. Laxity, instability, and functional outcome after ACL injury: copers versus noncopers. Med Sci Sports Exerc. 1999;31(2):210–5.
Ayeni OR et al. Pivot shift as an outcome measure for ACL reconstruction: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):767–77.
Jonsson H, Riklund-Ahlström K, Lind J. Positive pivot shift after ACL reconstruction predicts later osteoarthrosis: 63 patients followed 5–9 years after surgery. Acta Orthop Scand. 2004;75(5):594–9.
Kocher MS et al. Relationships between objective assessment of ligament stability and subjective assessment of symptoms and function after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(3):629–34.
Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105(2):136–44.
Monaco E, Ferretti A, Labianca L, Maestri B, Speranza A, Kelly MJ, et al. Navigated knee kinematics after cutting of the ACL and its secondary restraint. Knee Surg, Sports Traumatol, Arthrosc: Off J ESSKA. 2012;20(5):870–7.
Anderson CJ, Westerhaus BD, Pietrini SD, Ziegler CG, Wijdicks CA, Johansen S, et al. Kinematic impact of anteromedial and posterolateral bundle graft fixation angles on double-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2010;38(8):1575–83.
Kocher MS, Steadman JR, Briggs KK, Sterett WI, Hawkins RJ. Relationships between objective assessment of ligament stability and subjective assessment of symptoms and function after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(3):629–34.
Bedi A et al. Lateral compartment translation predicts the grade of pivot shift: a cadaveric and clinical analysis. Knee Surg Sports Traumatol Arthrosc. 2010;18:1269–76.
Lopomo N, Zaffagnini S, Amis AA. Quantifying the pivot shift test: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2013;21(4):767–83.
Musahl V, Voos J, O’Loughlin PF, Stueber V, Kendoff D, Pearle AD. Mechanized pivot shift test achieves greater accuracy than manual pivot shift test. Knee Surg, Sports Traumatol, Arthrosc: Off J ESSKA. 2010;18(9):1208–13.
Musahl V, Citak M, O’Loughlin PF, Choi D, Bedi A, Pearle AD. The effect of medial versus lateral meniscectomy on the stability of the anterior cruciate ligament-deficient knee. Am J Sports Med. 2010;38(8):1591–7.
Markolf KL, Park S, Jackson SR, McAllister DR. Simulated pivot-shift testing with single and double-bundle anterior cruciate ligament reconstructions. J Bone Joint Surg Am Vol. 2008;90(8):1681–9.
Debandi A, Maeyama A, Lu S, Hume C, Asai S, Goto B, et al. Biomechanical comparison of three anatomic ACL reconstructions in a porcine model. Knee Surg, Sports Traumatol, Arthrosc: Off J ESSKA. 2011;19(5):728–35.
Musahl V, Burkart A, Debski RE, Van Scyoc A, Fu FH, Woo SL. Accuracy of anterior cruciate ligament tunnel placement with an active robotic system: a cadaveric study. Arthrosc: J Arthrosc Relat Surg: Off Publ Arthrosc Assoc North Am Int Arthrosc Assoc. 2002;18(9):968–73.
Kanamori A, Zeminski J, Rudy TW, Li G, Fu FH, Woo SL. The effect of axial tibial torque on the function of the anterior cruciate ligament: a biomechanical study of a simulated pivot shift test. Arthrosc: J Arthrosc Relat Surg: Off Publ Arthrosc Assoc North Am Int Arthrosc Assoc. 2002;18(4):394–8.
Voos JE, Musahl V, Maak TG, Wickiewicz TL, Pearle AD. Comparison of tunnel positions in single-bundle anterior cruciate ligament reconstructions using computer navigation. Knee Surg, Sports Traumatol, Arthrosc: Off J ESSKA. 2010;18(9):1282–9.
Hoshino Y et al. In Vivo Measurement of the Pivot-Shift Test in the Anterior Cruciate Ligament–Deficient Knee Using an Electromagnetic Device. Am J Sports Med. 2007;35(7):1098–104.
Kopf S et al. A new quantitative method for pivot shift grading. Knee Surg Sports Traumatol Arthrosc. 2012;20:718–23.
Lopomo N et al. Quantitative assessment of pivot-shift using inertial sensors. Knee Surg Sports Traumatol Arthrosc. 2012;20:713–7.
Araujo PH et al. Comparison of three non-invasive quantitative measurement systems for the pivot shift test. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):692–7.
Galway HR, MacIntosh DL. The lateral pivot shift: a symptom and sign of anterior cruciate ligament insufficiency. Clin Orthop Relat Res. 1980;147:45–50.
Fleming BC, Beynnon BD, Tohyama H, Johnson RJ, Nichols CE, Renstrom P, et al. Determination of a zero strain reference for the anteromedial band of the anterior cruciate ligament. J Orthop Res: Off Publ Orthop Res Soc. 1994;12(6):789–95.
Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior-posterior motion of the knee. J Bone Joint Surg Am Vol. 1982;64(6):883–8.
Nakamura S, Kobayashi M, Asano T, Arai R, Nakagawa Y, Nakamura T. Image-matching technique can detect rotational and AP instabilities in chronic ACL-deficient knees. Knee Surg, Sports Traumatol, Arthrosc: Off J ESSKA. 2011;19 Suppl 1:S69–76.
Lipke JM, Janecki CJ, Nelson CL, McLeod P, Thompson C, Thompson J, et al. The role of incompetence of the anterior cruciate and lateral ligaments in anterolateral and anteromedial instability. A biomechanical study of cadaver knees. J Bone Joint Surg Am Vol. 1981;63(6):954–60.
Markolf KL, Willems MJ, Jackson SR, Finerman GA. In situ calibration of miniature sensors implanted into the anterior cruciate ligament part II: force probe measurements. J Orthop Res: Off Publ Orthop Res Soc. 1998;16(4):464–71.
Scanlan SF, Chaudhari AM, Dyrby CO, Andriacchi TP. Differences in tibial rotation during walking in ACL reconstructed and healthy contralateral knees. J Biomech. 2010;43(9):1817–22.
Sudasna S, Harnsiriwattanagit K. The ligamentous structures of the posterolateral aspect of the knee. Bull Hosp Joint Dis Orthop Inst. 1990;50(1):35–40.
Ajuied A, Wong F, Smith C, Norris M, Earnshaw P, Back D, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014;42(9):2242–52.
Tardy N, Marchand P, Kouyoumdjian P, Blin D, Demattei C, Asencio G. A preliminary in vivo assessment of anterior cruciate ligament-deficient knee kinematics with the KneeM device: a new method to assess rotatory laxity using open MRI. Orthop J Sports Med. 2014;13:2(3).
Hoshino Y et al. Quantitative evaluation of the pivot shift by image analysis using the iPad. Knee Surg Sports Traumatol Arthrosc. 2013;21:975–80.
Muller B, Hofbauer M, Rahnemai-Azar AA, Wolf M, Araki D, Hoshino Y, et al. Development of computer tablet software for clinical quantification of lateral knee compartment translation during the pivot shift test. Comput Methods Biomech Biomed Eng. 2016;19(2):217–28. This recent work reports the development of a computer tablet software to quantify anterior translation of the lateral knee compartment during the pivot shift test. The software is shown to provide reliable, objective, and quantitative data on translation of the lateral knee compartment during the pivot shift test and can be used without significant time delay in the clinical setting.
Zaffagnini S et al. Inertial sensors to quantify the pivot shift test in the treatment of anterior cruciate ligament injury. Joints. 2014;2(3):124–9. This recent work highlights the development of inertial sensors in the objective measurement of knee instability. This research may allow for information regarding the clinical significance of an ACL-injury to be collected in real-time, as past studies have documented the correlation between high acceleration values and clinical grade of pivot shift exam.
Berruto M et al. Is triaxial accelerometer reliable in the evaluation and grading of knee pivot-shift phenomenon? Knee Surg Sports Traumatol Arthrosc. 2013;21:981–5.
Labbé DR, Li D, Grimard G, de Guise JA, Hagemeister N. Quantitative pivot shift assessment using combined inertial and magnetic sensing. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2330–8.
Matsumoto H. Mechanism of the pivot shift. J Bone Joint Surg (Br). 1990;72(5):816–21.
Lane CG et al. In vivo analysis of the pivot shift phenomenon during computer navigated ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):487–92.
Guenther D, Griffith C, Lesniak B, Lopomo N, Grassi A, Zaffagnini S, et al. Anterolateral rotatory instability of the knee. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2909–17.
Wroble RR, Grood ES, Cummings JS, Henderson JM, Noyes FR. The role of the lateral extraarticular restraints in the anterior cruciate ligament-deficient knee. Am J Sports Med. 1993;21(2):257–62. discussion 263.
Rasmussen MT, Nitri M, Williams BT, Moulton SG, Cruz RS, Dornan GJ, Goldsmith MT, LaPrade RF. An In Vitro Robotic Assessment of the Anterolateral Ligament, Part 1: Secondary Role of the Anterolateral Ligament in the Setting of an Anterior Cruciate Ligament Injury. Am J Sports Med. 2016 Mar; 44(3):585-92.
Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities. Part I. The medial compartment and cruciate ligaments. J Bone Joint Surg Am. 1976;58(2):159–72.
Johnson LL. Lateral capsular ligament complex: anatomical and surgical considerations. Am J Sports Med. 1979;7(3):156–60.
LaPrade RF, Terry GC. Injuries to the posterolateral aspect of the knee. Association of anatomic injury patterns with clinical instability. Am J Sports Med. 1997;25(4):433–8.
Woods GW, Stanley RF, Tullos HS. Lateral capsular sign: X-ray clue to a significant knee instability. Am J Sports Med. 1979;7(1):27–33.
Rudy TW, Livesay GA, Woo SL, Fu FH. A combined robotic/universal force sensor approach to determine in situ forces of knee ligaments. J Biomech. 1996;29(10):1357–60.
Suero EM, Njoku IU, Voigt MR, Lin J, Koenig D, Pearle AD. The role of the iliotibial band during the pivot shift test. Knee Surg, Sports Traumatol, Arthrosc: Off J ESSKA. 2013;21(9):2096–100.
Yamamoto Y, Hsu WH, Fisk JA, Van Scyoc AH, Miura K, Woo SL. Effect of the iliotibial band on knee biomechanics during a simulated pivot shift test. J Orthop Res: Off Publ Orthop Res Soc. 2006;24(5):967–73.
Kittl C, et al.: The Role of the Anterolateral Structures and the ACL in Controlling Laxity of the Intact and ACL-Deficient Knee, Am J Sports Med. 2015. This publication recently showed that the ‘anterolateral ligament’ and anterolateral capsule had a minor role in restraining internal rotation of knee, and therefore have a minimal role in the control of rotatory stability. The iliotibial tract was the primary restraint at 30° to 90° of flexion, and therefore should be the anatomic structure injured if an extra-articular repair is to be performed
Matsumoto H. Mechanism of the pivot shift. J Bone Joint Surg Br Volume. 1990;72(5):816–21.
Stijak L, Bumbasirevic M, Radonjic V, Kadija M, Puskas L, Milovanovic D, Filipovic B (2014) Anatomic description of the anterolateral ligament of the knee. Knee Surg Sports Traumatol Arthrosc. 2014 Nov 8.
Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321–8.
Musahl V, Rahnemai-Azar AA, van Eck CF, Guenther D, Fu FH. Anterolateral ligament of the knee, fact or fiction? Knee Surg Sports Traumatol Arthrosc. 2016;24(1):2-3.
Dombrowski ME, Costello JM, Ohashi B, Murawski CD, Rothrauff BB, Arilla FV, Friel NA, Fu FH, Debski RE, Musahl V. Macroscopic anatomical, histological and magnetic resonance imaging correlation of the lateral capsule of the knee. Knee Surg Sports Traumatol Arthrosc. 2015 Feb 4. This publication highlights the anatomical, histological and imaging characterization of the anterolateral knee structures. This paper provides strong objective evidence to the contrary of an anterolateral ligament structure that controls rotational stability of the knee
Rahnemai-Azar AA, Miller RM, Guenther D, Fu FF, Lesniak B, Musahl V, Debski RE. Structural Properties of the Anterolateral Capsule and Iliotibial Band of the Knee. Podium Presented in Summer Biomechanics, Bioengineering, and Biotransport Conference in June 2015 Utah and in American Academy of Orthopaedic Surgeons meeting; Orlando, FL. 2016.
Araujo PH et al. Individualized ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):1966–75.
Ahlden M, Araujo P, Hoshino Y, Samuelsson K, Middleton KK, Nagamune K, et al. Clinical grading of the pivot shift test correlates best with tibial acceleration. Knee Surg, Sports Traumatol, Arthrosc: Off J ESSKA. 2012;20(4):708–12.
Ahmed AM, McLean C. In vitro measurement of the restraining role of the anterior cruciate ligament during walking and stair ascent. J Biomech Eng. 2002;124(6):768–79.
Giffin JR et al. Effects of increasing tibial slope on the biomechanics of the knee. Am J Sports Med. 2004;32(2):376–82.
Senişik S et al. Posterior tibial slope as a risk factor for anterior cruciate ligament rupture in soccer players. J Sports Sci Med. 2011;10(4):763–7.
Brandon ML et al. The association between posterior-inferior tibial slope and anterior cruciate ligament insufficiency. Arthroscopy. 2006;22(8):894–9.
Marouane H, Shirazi-Adl A, Hashemi J. Quantification of the role of tibial posterior slope in knee joint mechanics and ACL force in simulated gait. J Biomech. 2015;48(10):1899–905.
Alentorn-Geli E et al. Prevention of anterior cruciate ligament injuries in sports. Part I: systematic review of risk factors in male athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):3–15.
Song GY, et al.: Risk Factors Associated With Grade 3 Pivot Shift After Acute Anterior Cruciate Ligament Injuries. Am J Sports Med. 2015 Nov 30.
Nelitz M et al. Increasing posterior tibial slope does not raise anterior cruciate ligament strain but decreases tibial rotation ability. Clin Biomech (Bristol, Avon). 2013;28(3):285–90.
Kim D, Asai S, Moon CW, Hwang SC, Lee S, Keklikci K, et al. Biomechanical evaluation of anatomic single- and double-bundle anterior cruciate ligament reconstruction techniques using the quadriceps tendon. Knee Surg Sports Traumatol Arthrosc. 2015;23(3):687–95.
Bell KM et al. Novel technique for evaluation of knee function continuously through the range of flexion. J Biomech. 2015;48(13):3737–40. This recent work highlights the importance of continuous assessment of knee motion during in vitro robotic testing. This manuscript shows that continuous monitoring allows for more data collection across the range of motion, potentially elucidating trends across small subdivisions of total motion that will engage different constraints and anatomical structures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jason P. Zlotnicki, Jan-Hendrik Naendrup, Gerald A. Ferrer, and Richard E. Debski declare that they have no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on ACL Update: Objective Measures on Knee Instability
Rights and permissions
About this article
Cite this article
Zlotnicki, J.P., Naendrup, JH., Ferrer, G.A. et al. Basic biomechanic principles of knee instability. Curr Rev Musculoskelet Med 9, 114–122 (2016). https://doi.org/10.1007/s12178-016-9329-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12178-016-9329-8