Abstract
In this study, solid-phase extraction combined with dispersive liquid–liquid microextraction is presented for the simultaneous determination of trace amounts of deltamethrin and permethrin in honey by gas chromatography–mass spectrometry. First, permethrin and deltamethrin were extracted from polluted honey samples by ultrasound-assisted liquid–liquid extraction followed by solid-phase extraction (SPE). Then, the analytes were eluted from the SPE cartridge. The elution solvent containing trace amounts of analytes was applied in dispersive liquid–liquid microextraction (DLLME) step as disperser solvent for further purification and preconcentration of deltamethrin and permethrin. The important parameters such as the type and volume of the solvent for initial extraction of pesticides from honey matrix, the amount of honey, and type and volume of extraction solvent and elution solvent (disperser solvent) were investigated through the process. Under the optimal conditions, good linearity in the range of 0.2–800 ng g−1 with the correlation coefficient (r 2) >0.9986 and 0.9990, low limits of detection (LODs) of 0.02 and 0.04 ng g−1, enrichment factors of 4955 and 4925, and relative standard deviations (RSD%, n = 7) of 1.8 and 2 % were obtained for permethrin and deltamethrin, respectively. The proposed procedure showed satisfactory results for simultaneous determination of deltamethrin and permethrin in honey. Relative recoveries of 94–99.2 % were obtained for real sample analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pyrethroids have been increasingly used to replace organophosphate pesticides which are widely banned due to their highly toxic and persistent nature (Narendra et al. 2008; Weston et al. 2013). Pyrethroid insecticides disrupt the sodium channel which leads to the death of a variety of insects (Thatheyus and Selvam 2013). Permethrin and deltamethrin are two potent and common pyrethroids which are extremely toxic to aquatic life, bees, and wildlife. Permethrin, like other pyrethroids, disrupt nervous function by modifying the normal biochemistry and physiology of sodium channels in nerve membrane. Human exposure to permethrin can result in nausea, headache, muscle weakness, excessive salivation, respiratory dysfunction, and seizures (Svobodova et al. 2003). Deltamethrin is considered as one of the most toxic pyrethroids. Deltamethrin is a neurotoxin which can rapidly attack the nervous system. Bioaccumulation of deltamethrin can lead to convulsions, ataxia, dermatitis, diarrhea, tremors, and vomiting (Bouwman et al. 2006; Doi et al. 2006). Application of permethrin and deltamethrin is restricted by the US Environmental Protection Agency (EPA) due to their toxicity (de la Cruz et al. 2014). Honeybees may collect these insecticides while foraging blooming plants, resulting in the presence of insecticide residues in their honey, which would subsequently be consumed by humans (Pirard et al. 2007). Therefore, determination of pyretriod residues in honey is necessary to avoid health effects on human. Unfortunately, there are not sufficient studies to determine trace/ultra-trace amounts of pyretriods in honey owing to the complex biological matrix of honey. Time-consuming cleanup process and large volume of extraction solvent are the main disadvantages of previous methods (Al-Rifai and Akeel 1997; Driss et al. 1994; Pang et al. 2006). Furthermore, the obtained detection limits in some of these techniques are not sufficiently satisfactory (Albero et al. 2004). Different pretreatment techniques such as liquid–solid extraction coupled with magnetic solid-phase extraction (Jiang et al. 2013), liquid–liquid extraction (LLE) (Shen et al. 2011), solid-phase extraction (SPE) (Boonchiangma et al. 2012; Chen et al. 2011), single-drop microextraction (SDME) (Pinheiro et al. 2011), liquid-phase microextraction (LPME) (Lin et al. 2011), and homogeneous liquid–liquid microextraction via flotation assistance (HLLME-FA) (Haddadi et al. 2014) are reported for determination of pyrethroids in various matrixes. According to the scientific and technical point of view, SPE is a universal pretreatment technique which is used for separation and enrichment of trace analytes (Hennion 1999). Low solvent volume consumption, amenable to automation, high recoveries and good reproducibility, and proper selectivity are the advantages of SPE (Ulrich 2000; Żwir-Ferenc and Biziuk 2006). Recently, SPE combined with gas chromatography coupled with an ion trap mass spectrometer detector (GC-IT/MS) has been applied for determination of acaricides in honey samples which showed proper analytical performance such as detection limit of 3–18 ng g−1 and the linear range of 0.030–10.0 μg g−1 (Notardonato et al. 2014). Dispersive liquid–liquid microextraction (DLLME) is one of the high-potential methods of liquid-phase microextraction techniques, which was first introduced by Rezaee in 2006 (Rezaee et al. 2006). DLLME has been widely used for extraction and determination of different pyrethroids (Boonchiangma et al. 2012; Wang et al. 2012). Simplicity, rapidity, microliter solvent extraction consumption, and significantly high enrichment factor are some of the advantages of DLLME which make this technique prominent for many analytical procedures (Zang et al. 2009). Combination of SPE and DLLME (SPE-DLLME) is one of the most effective extraction techniques which have the privileges of both SPE and DLLME. In recent years, this technique has been successfully carried out as an efficient pretreatment method for different analytes (Bai et al. 2013; Martinis et al. 2010; Samadi et al. 2012; Stanisz et al. 2014; Zgoła-Grześkowiak and Grześkowiak 2012). Since honey has a complex matrix with various kinds of chemical compounds, analysis of honey for determination of trace amounts of pyrethroids such as deltamethrin and permethrin becomes difficult. It seems that among all reported methods, SPE-DLLME can ideally be applied for determination of trace amount of deltamethrin and permethrin in complex matrix of honey. Hence, this paper explored the applicability and the potential of SPE-DLLME to separate/perconcentrate deltamethrin and permethrin in honey simultaneously. The effect of important variables, including the amount of honey, the type and volume of extraction and disperser solvents, ionic strength, and flow rate on the performance of the method, was studied.
Materials and Methods
Materials
Deltamethrin and permethrin with the highest purity were purchased from Merck (Darmstadt, Germany). A stock standard solution of deltamethrin and permethrin (1000 mg kg−1) was prepared in methanol. The interest working standard solutions were prepared by dilution of the standard solutions in deionized water and stored at 4 °C. Methanol, acetone, ethanol, chlorobenzene, dichloroethane, carbon tetrachloride, and acetonitrile were all in analytical grade and obtained from Merck. Honey samples were purchased from local markets. A stock solution of acetophenone (500 mg kg−1) was prepared as internal standard solution.
Instrumentation
A Centurion Scientific (Arundel, UK) model 1020D centrifuge was used. Shimadzu (GC-MS; model QP5050) equipped with a capillary column of CBP-5 (30-m length, 0.25-mm internal diameter, and 0.25 μm of film thickness) was applied for quantitative determination of permethrin and deltamethrin. Helium was used as carrier gas at flow rate of 2.2 ml min−1. The temperature program was used for the analysis. The GC injection port and the interface temperature were set at 2700 and 305 °C, respectively. The initial column temperature was set at 100 °C and held for 1 min. The temperature program followed by heating from 100 to 220 °C with the rate of 15 °C min−1 and holding for 2 min. Finally, the temperature increased to 300 °C with rate of 10 °C min−1 and held for 4 min.
Experimental
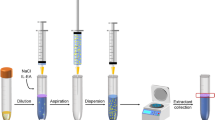
An amount of 1.5 g of weighted honey was spiked by 50 μL of 1000 ng g−1 solution of deltamethrin and permethrin. The mixture was stirred for 10 min, and it was left for 5 min to reach to the equilibrium state. Fifteen milliliters of acetonitrile/water at the volume ratio of 10:5 (v/v) was added to the spiked honey sample to extract the pesticides from honey matrix, and ultasonicated for 10 min. Then, the solution was filtrated and the lower phase was diluted by deionized water in a 50-mL volumetric flask. The solution was loaded on the C18 SPE cartridge at the flow rate of 10 mL min−1 by the vacuum pump. The C18 SPE cartridge was rinsed by deionized water to remove any residue contamination from the solution. Then, the cartridge was eluted by 1 mL of methanol as elution solvent. Methanol containing analytes was collected in a 2-mL sample vessel. Thirty microliters of carbon tetrachloride as extraction solvent was added to methanol. The mixture of methanol, carbon tetrachloride, and analytes was rapidly injected into a conical test tube containing 5 mL deionized water. A cloudy solution formed in the solution due to the dispersion of carbon tetrachloride fine droplets. Then, the mixture was centrifuged for 3 min at 5000 rpm. One microliter of the sedimentary carbon tetrachloride containing analytes was injected into the GC-MS. The peak area in selected ion monitoring mode (SIM) was considered as the analytical signal for quantification.
Results and Discussions
In order to achieve the optimal conditions for simultaneous extraction and determination of trace amounts of deltamethrin and permethrin in honey by the proposed method of SPE-DLLME, the main factors were optimized. Univariate method was applied for optimization of the process.
Extraction of the deltamethrin and permethrin from honey matrix
To figure out the potential of SPE-DLLME in separation and determination of deltamethrin and permethrin, 1.5 g of clean and pure honey sample was spiked with 50 μL of 1000 ng g−1 solution of permethrin and deltamethrin. An ultrasound-assisted liquid–liquid extraction is essential to extract and separate permethrin and deltamethrin from honey. Hence, four extraction solvents of methanol, ethanol, acetone, and acetonitrile were considered. The solubility of honey in these solvents is in the order of methanol > acetone > acetonitrile > ethanol. The solubility of honey in ethanol is the least, because ethanol cannot dissolve sugar compounds in honey matrix and honey matrix forms a bullet-shaped structure in ethanol which reduces the extraction of interest pesticides from the matrix. Thus, ethanol cannot be a proper solvent at this step. Methanol dissolves honey completely. Moreover, due to the strong hydrogen bonding of methanol with analytes, the retaining efficiencies of analytes on the SPE cartridge decrease and the analytes cannot be preconcentrated and retained on the cartridge completely. Consequently, according to the obtained results in Fig. 1a, acetonitrile was chosen as initial extraction solvent for permethrin and deltamethrin from honey matrix. The volume of acetonitrile was investigated in the range of 5–20 mL as it is shown in Fig. 1b; the volume of 15 mL was selected. In order to study the effect of the polarity of acetonitrile on extraction efficiencies of two analytes, the volume ratios of acetonitrile to water of 15:0, 10:5, 7.5:7.5, and 5:10 were studied. The volume ratio of 10:5 (acetonitrile to water) showed the highest extraction efficiencies as shown in Fig. 1c. As water was added to acetonitrile, the polarity of solution increased which led to more solubility of honey, particularly analytes, in acetonitrile. Generally, as the previous studies show, the extraction of pesticides from food samples was carried out by different mixtures of acetonitrile, methanol, acetone and water. Furthermore, addition of small amount of water to methanol, acetone, and acetonitrile as extraction solvents would clearly improve the recoveries as compared to the extraction carried out only by organic solvents (Hernandez‐Borges et al. 2004; Ravelo‐Pérez et al. 2006).
Amount of Honey
The initial amount of honey is an important parameter which implies the potential and ability of method. The effect of the amount of honey within the range of 0.5–3 g was investigated. As results show in Fig. 2, the extraction efficiencies are quantitative for both analytes until 1.5 g, and by increasing the amount of honey, the extraction efficiency decreases. Therefore, 1.5 g was selected as optimal amount of honey for subsequent work.
Effect of Type and Volume of Elution Solvent (Disperser Solvent)
The choice of elution solvent is greatly important in SPE-DLLME method, because elution solvent not only should elute the analytes from the SPE cartridge with the highest efficiency but also should have the miscibility in both organic extraction solvent and aqueous phase. Moreover, elution solvent has the role of disperser solvent in DLLME step. Therefore, the formation of cloudy solution and dispersion of extraction solvent depends on the nature of disperser solvent (Kocúrová et al. 2012; Zang et al. 2009). Three solvents of acetone, methanol, and acetonitrile were studied in the process. Methanol can make strong hydrogen bonding with analytes and then elute them from cartridge with the highest efficiencies. Furthermore, methanol reacts as a suitable disperser solvent. The results in Fig. 3a indicate that methanol is the proper elution/disperser solvent.
The volume of elution/disperser solvent should be sufficient for two main purposes: first, to completely elute the analytes from the SPE cartridge and, second, to increase the dispersion of extraction solvent in the aqueous phase (Herrera-Herrera et al. 2010; Zang et al. 2009). The volume of elution/disperser solvent was studied in the range of 0.5–2 mL. As the results indicate in Fig. 3b, the volume of 1 mL was chosen for the process.
Effect of Type and Volume of the Extraction Solvent
One of the most critical factors which directly affect the extraction efficiencies of the procedure is the type and volume of extraction solvent. High density for easy sedimentation after centrifugation and insolubility in water are the two considerable features of extraction solvent (Rezaee et al. 2010; Zang et al. 2009). Therefore, the effect of four solvents—carbontetrachloride, chlorobenzene, chloroform, 1,2-dichloroethane—were studied. As the results reveal in Fig. 3c, carbon tetrachloride had the most extraction efficiencies for both analytes and it was chosen as extraction solvent. The volume of extraction solvent has a determinative role in enrichment factor. The volume of extraction solvent was investigated in the range of 10–60 μL. According to the results in Fig. 3d, the optimal volume of 30 μL was obtained for further studies.
Effect of the Flow Rate of the Sample Solution
The flow rate of sample solution, loading on the SPE cartridge, is a leading factor which influences the time and extraction efficiencies of the process. The flow rate should be low enough for complete retention of analytes on the cartridge and also high enough to lower the extraction time (Liu et al. 2009). Hence, the flow rate was considered in the range of 5–25 mL min−1. The results in Fig. 4a indicate that from 5 to 15 mL min−1, the extraction efficiencies are nearly constant and, up to this range, the slight decreasing trend is observed. It seems that at high flow rates, the retention of analytes on the cartridge decreases. Therefore, the flow rate of 10 mL min−1 was selected as optimal flow rate.
Effect of Salt Addition
In order to consider the ability of the method to apply in saline matrixes, the effect of the ionic strength of the method was investigated by adding NaCl within the range of 0–8 % (w/v). The obtained results showed that by increasing NaCl concentration up to 4 %, the extraction efficiencies of the analytes would increase due to the salting-out effect. However, at salt concentrations higher than 4 %, the extraction efficiencies decrease due to the clogging of cartridge and also increasing the viscosity of the solution which made the dispersion difficult. Therefore, based on the results in Fig. 4b, the concentration of NaCl 4 % (w/v) was chosen as the optimal salt concentration for the further studies.
Analytical Performances
The analytical characteristics of the proposed method, including limits of detection (LODs) and quantification (LOQs), linear range, correlation coefficient, enrichment factor, and precision, were achieved by processing standard solution of permethrin and deltamethrin. The results are demonstrated in Table 1. The limits of detection and quantification were considered as 3S b/m and 10S b/m, respectively (where S b is the standard deviation of the blank signals and m is the slope of the calibration curve after extraction). The limits of detection of 0.02 and 0.04 ng g−1, and limits of quantification of 0.06 and 0.13 ng g−1 were obtained for permethrin and deltamethrin, respectively. The calibration curve linearity of 0.2–400 ng g−1 was obtained for both of analytes. The correlation coefficients (r 2) of 0.9986 and 0.9990, and the relative standard deviations for the solution of 2 ng g−1 of analytes were 1.8 and 2 % for permethrin and deltamethrin, respectively, which indicated good precision of the process. Typical GC-MS chromatogram in SIM mode is shown in Fig. 5. At the optimal conditions, the recovery of 98.9 and 99.4 % was achieved for the proposed method which shows the high potential and high recovery of SPE-DLLME for simultaneous preconcentration and separation of permethrin and deltamethrin.
Comparison of Method with Other Techniques
The proposed method was compared to other techniques. The results are indicated in Table 2. The analytical performance of SPE-DLLME is obviously improved comparing to other studies owing to these reasons; combination of two extraction techniques helps to obtain low detection limits, acceptable linear range, and low RSD%. The proposed method showed high potential for determination of permethrin and deltamethrin in the complex matrix of honey with high recoveries.
Analysis of the Real Sample
In order to show the potential of the proposed method, SPE-DLLME-GC-MS was applied in Dena, Khomein honey, and a local honey sample to preconcentarte and separate trace amount of permethrin and deltamethrin. Reliability of the method was investigated either by spiking the sample. As the results show in Table 3, the obtained recovery is in the range 93–99 % for permethrin and 94–99 % for the deltamethrin. Moreover, no matrix effect was found in the analysis process. The obtained results confirm the potential of the method to be applied in complex matrixes like honey with high recoveries.
Conclusions
The present method was successfully and effectively applied for preconcentration and determination of permethrin and deltamethrin in honey with perfect accuracy and precision. The combination of SPE and DLLME led to higher sensitivity and extraction efficiency (%). Furthermore, the prominent advantages of the proposed method are very low use of organic solvent, high enrichment factor, low detection limits, easy, and rapid operation, and relatively short analysis time.
References
Albero B, Sánchez-Brunete C, Tadeo JL (2004) Analysis of pesticides in honey by solid-phase extraction and gas chromatography–mass spectrometry. J Agric Food Chem 52:5828–5835. doi:10.1021/jf049470t
Al-Rifai J, Akeel N (1997) Determination of pesticide residues in imported and locally produced honey in Jordan. J Apic Res 36:155–161. doi:10.1080/00218839.1997.11100943
Bai S-S, Li Z, Zang X-H, Wang C, Wang Z (2013) Graphene-based magnetic solid phase extraction dispersive liquid-liquid microextraction combined with gas chromatographic method for determination of five acetanilide herbicides in water and green tea samples. Chin J Anal Chem 41:1177–1182. doi:10.1016/S1872-2040(13)60672-6
Boonchiangma S, Ngeontae W, Srijaranai S (2012) Determination of six pyrethroid insecticides in fruit juice samples using dispersive liquid–liquid microextraction combined with high performance liquid chromatography. Talanta 88:209–215. doi:10.1016/j.talanta.2011.10.033
Bouwman H, Sereda B, Meinhardt HM (2006) Simultaneous presence of DDT and pyrethroid residues in human breast milk from a malaria endemic area in South Africa. Environ Pollut 144:902–917. doi:10.1016/j.envpol.2006.02.002
Chen L, Chen W, Ma C, Du D, Chen X (2011) Electropolymerized multiwalled carbon nanotubes/polypyrrole fiber for solid-phase microextraction and its applications in the determination of pyrethroids. Talanta 84:104–108. doi:10.1016/j.talanta.2010.12.027
de la Cruz E, Bravo-Durán V, Ramírez F, Castillo LE (2014) Environmental hazards associated with pesticide import into Costa Rica, 1977-2009 J Environ Biol 35
Doi H, Kikuchi H, Murai H, Kawano Y, Shigeto H, Ohyagi Y, Kira J (2006) Motor neuron disorder simulating ALS induced by chronic inhalation of pyrethroid insecticides. Neurology 67:1894–1895
Driss MR, Zafzouf M, Sabbah S, Bouguerra ML (1994) Simplified procedure for organochlorine pesticides residue analysis in honey. Int J Environ Anal Chem 57:63–71. doi:10.1080/03067319408033102
Haddadi H, Shirani M, Semnani A, Rezaee M, Mashayekhi HA, Hosseinian A (2014) Simultaneous determination of deltamethrin and permethrin in water samples using homogeneous liquid–liquid microextraction via flotation assistance and GC-FID. Chromatographia 77:715–721
Hennion M-C (1999) Solid-phase extraction: method development, sorbents, and coupling with liquid chromatography. J Chromatogr A 856:3–54. doi:10.1016/S0021-9673(99)00832-8
Hernandez‐Borges J, Frias‐Garcia S, Cifuentes A, Rodriguez‐Delgado M (2004) Pesticide analysis by capillary electrophoresis. J Sep Sci 27:947–963
Herrera-Herrera AV, Asensio-Ramos M, Hernández-Borges J, Rodríguez-Delgado MÁ (2010) Dispersive liquid-liquid microextraction for determination of organic analytes TrAC. Trends Anal Chem 29:728–751. doi:10.1016/j.trac.2010.03.016
Jiang C et al (2013) Liquid–solid extraction coupled with magnetic solid-phase extraction for determination of pyrethroid residues in vegetable samples by ultra fast liquid chromatography. Talanta 114:167–175. doi:10.1016/j.talanta.2013.04.004
Kocúrová L, Balogh IS, Šandrejová J, Andruch V (2012) Recent advances in dispersive liquid–liquid microextraction using organic solvents lighter than water. Rev Microchem J 102:11–17. doi:10.1016/j.microc.2011.12.002
Li M, Zhang J, Li Y, Peng B, Zhou W, Gao H (2013) Ionic liquid-linked dual magnetic microextraction: a novel and facile procedure for the determination of pyrethroids in honey samples. Talanta 107:81–87. doi:10.1016/j.talanta.2012.12.056
Lin C-H, Yan C-T, Kumar P, Li H-P, Jen J-F (2011) Determination of pyrethroid metabolites in human urine using liquid phase microextraction coupled in-syringe derivatization followed by gas chromatography/electron capture detection. Anal Bioanal Chem 401:927–937. doi:10.1007/s00216-011-5122-0
Liu X, Li J, Zhao Z, Zhang W, Lin K, Huang C, Wang X (2009) Solid-phase extraction combined with dispersive liquid–liquid microextraction for the determination for polybrominated diphenyl ethers in different environmental matrices. J Chromatogr A 1216:2220–2226. doi:10.1016/j.chroma.2008.12.092
Martinis EM, Berton P, Monasterio RP, Wuilloud RG, Martinis EM, Berton P, Monasterio RP, Wuilloud RG (2010) Emerging ionic liquid-based techniques for total-metal and metal-speciation analysis TrAC. Trends Anal Chem 29:1184–1201. doi:10.1016/j.trac.2010.07.013
Narendra M, Kavitha G, Helah Kiranmai A, Raghava Rao N, Varadacharyulu NC (2008) Chronic exposure to pyrethroid-based allethrin and prallethrin mosquito repellents alters plasma biochemical profile. Chemosphere 73:360–364. doi:10.1016/j.chemosphere.2008.05.070
Notardonato I, Avino P, Cinelli G, Russo MV (2014) Trace determination of acaricides in honey samples using XAD-2 adsorbent and gas chromatography coupled with an ion trap mass spectrometer detector. RSC Adv 4:42424–42431. doi:10.1039/C4RA06822J
Pang GF et al (2006) Multi-residue method for the determination of 450 pesticide residues in honey, fruit juice and wine by double-cartridge solid-phase extraction/gas chromatography-mass spectrometry and liquid chromatography-tandem mass spectrometry. Food Addit Contam 23:777–810. doi:10.1080/02652030600657997
Pinheiro AS, da Rocha GO, de Andrade JB (2011) A SDME/GC–MS methodology for determination of organophosphate and pyrethroid pesticides in water. Microchem J 99:303–308. doi:10.1016/j.microc.2011.05.019
Pirard C et al (2007) Development and validation of a multi-residue method for pesticide determination in honey using on-column liquid–liquid extraction and liquid chromatography–tandem mass spectrometry. J Chromatogr A 1152:116–123. doi:10.1016/j.chroma.2007.03.035
Ravelo‐Pérez LM, Hernández‐Borges J, Rodríguez‐Delgado MÁ (2006) Pesticides analysis by liquid chromatography and capillary electrophoresis. J Sep Sci 29:2557–2577
Rezaee M, Assadi Y, Milani Hosseini M-R, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116:1–9
Rezaee M, Yamini Y, Faraji M (2010) Evolution of dispersive liquid–liquid microextraction method. J Chromatogr A 1217:2342–2357. doi:10.1016/j.chroma.2009.11.088
Samadi S, Sereshti H, Assadi Y (2012) Ultra-preconcentration and determination of thirteen organophosphorus pesticides in water samples using solid-phase extraction followed by dispersive liquid–liquid microextraction and gas chromatography with flame photometric detection. J Chromatogr A 1219:61–65. doi:10.1016/j.chroma.2011.11.019
Shen C-y et al (2011) Determination of 17 pyrethroid residues in troublesome matrices by gas chromatography/mass spectrometry with negative chemical ionization. Talanta 84:141–147. doi:10.1016/j.talanta.2010.12.041
Stanisz E, Werner J, Zgoła-Grześkowiak A (2014) Liquid-phase microextraction techniques based on ionic liquids for preconcentration and determination of metals TrAC. Trends Anal Chem 61:54–66. doi:10.1016/j.trac.2014.06.008
Svobodova Z, Luskova V, Drastichova J, Svoboda M, Žlábek V (2003) Effect of deltamethrin on haematological indices of common carp (Cyprinus carpio L.) Acta Vet Brno 72:79-None
Thatheyus AJ, Selvam ADG (2013) Synthetic pyrethroids: toxicity and biodegradation. App Ecol Environ Sci 1:33–36
Ulrich S (2000) Solid-phase microextraction in biomedical analysis. J Chromatogr A 902:167–194. doi:10.1016/S0021-9673(00)00934-1
Walorczyk S, Gnusowski B (2009) Development and validation of a multi-residue method for the determination of pesticides in honeybees using acetonitrile-based extraction and gas chromatography–tandem quadrupole mass spectrometry. J Chromatogr A 1216:6522–6531. doi:10.1016/j.chroma.2009.07.045
Wang H, Yan H, Qiao J (2012) Miniaturized matrix solid-phase dispersion combined with ultrasound-assisted dispersive liquid–liquid microextraction for the determination of three pyrethroids in soil. J Sep Sci 35:292–298. doi:10.1002/jssc.201100753
Weston DP, Ding Y, Zhang M, Lydy MJ (2013) Identifying the cause of sediment toxicity in agricultural sediments: the role of pyrethroids and nine seldom-measured hydrophobic pesticides. Chemosphere 90:958–964. doi:10.1016/j.chemosphere.2012.06.039
Zang X-H, Wu Q-H, Zhang M-Y, Xi G-H, Wang Z (2009) Developments of dispersive liquid-liquid microextraction technique. Chin J Anal Chem 37:161–168. doi:10.1016/S1872-2040(08)60082-1
Zgoła-Grześkowiak A, Grześkowiak T (2012) Solid-phase extraction combined with dispersive liquid–liquid microextraction, fast derivatisation and high performance liquid chromatography–tandem mass spectrometry analysis for trace determination of short-chained dodecyl alcohol ethoxylates and dodecyl alcohol in environmental water samples. J Chromatogr A 1251:40–47. doi:10.1016/j.chroma.2012.06.056
Żwir-Ferenc A, Biziuk M (2006) Solid phase extraction technique—trends, opportunities and applications. Pol J Environ Stud 15:677–690
Acknowledgments
The financial support of this project by Shahrekord University and Isfahan Payame Noor University is appreciated. The authors were also partially supported by the Center of Excellence for Mathematics, Shahrekord University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There is no funding for this study.
Conflict of Interest
No conflict exists; author Mahboube Shirani declares that she has no conflict of interest. Author Hedayat Haddadi declares that he has no conflict of interest. Author Mohammad Rezaee declares that he has no conflict of interest. Author Abolfazl Semnani declares that he has no conflict of interest. Author E Saeed Habibollahi declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No humans are involved in this study.
Rights and permissions
About this article
Cite this article
Shirani, M., Haddadi, H., Rezaee, M. et al. Solid-Phase Extraction Combined with Dispersive Liquid–Liquid Microextraction for the Simultaneous Determination of Deltamethrin and Permethrin in Honey by Gas Chromatography–Mass Spectrometry. Food Anal. Methods 9, 2613–2620 (2016). https://doi.org/10.1007/s12161-016-0455-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0455-0